Abstract
A number of molecules have been shown recently to be involved in the pathogenesis and progression of immunoglobulin (Ig)A nephropathy (IgAN). Among these, we have selected C4d (complement lectin pathway involvement), CD3 (T cell marker, traducing interstitial inflammation), transglutaminase 2 (TGase-2, involved in tissue fibrosis development) and p-extracelluar-regulated kinase (ERK)1/2 (protein kinase intracellular signaling molecule) to perform a panel of immunohistological biomarkers and assess its predictive value for disease progression. Immunohistochemical staining of these biomarkers was performed in paraffin sections from 74 renal biopsy cases with the clinical diagnosis of IgAN. Association between score analysis of these parameters and disease course was assessed through univariate and multivariate analysis, including baseline clinical and histological data. Univariate analysis showed that glomerular C4d, tubulointerstitial TGase2 and CD3 scores were associated with baseline proteinuria and disease progression. Multivariate analysis showed that only baseline estimated glomerular filtration rate (eGFR), C4d and CD3 were associated independently with progressive kidney disease (decline of at least 50% in the eGFR or progression to end-stage renal disease (ESRD) during the follow-up period). Establishing an accurate prediction model for IgAN progression is still a matter of research in clinical nephrology. The complement system, particularly lectin pathway activation, and T cell activation, have been shown previously to be potential modifiers of the disease course. Here we show that the combination of two histological biomarkers (C4d and CD3) can be a powerful predictor of IgAN progression and a potential useful tool for the clinical approach of this disease.
Keywords: autoimmunity, complement, T cells
Introduction
Immunoglobulin A nephropathy (IgAN) is the most common primary glomerulonephritis in the world and is defined by the predominant deposition of IgA in the glomerular mesangium 1. It is characterized by a highly variable presentation and outcome, ranging from a majority of patients with a benign or reversible condition to a lower number (approximately 30%) with progressive kidney disease, leading eventually to renal replacement therapy 2,3. Both the variability of the clinical course and the different therapeutic options available (including supportive and immunosuppressive) justify the continuous demand for optimal biomarkers of disease progression.
Besides established clinical and pathological predictors of IgAN course, including hypertension, proteinuria >1 g/day, reduced glomerular filtration rate (GFR) at presentation 2, mesangial hypercellularity, segmental sclerosis and tubular atrophy/interstitial fibrosis 2,4, a number of molecules have been implicated recently in the pathogenesis and progression of the disease, suggesting that a panel of biomarkers can be a useful tool to predict the course of the disease 5.
Recent reports 6 postulate a four-piece axis that leads to mesangial IgA deposition and pathological damage causing IgAN. IgA1 would interact with soluble CD89 released by mononuclear cells and form IgA–CD89 complexes that interact with the transferrin receptor (CD71/TfR1) and transglutaminase 2 (TGase2) on mesangial cells, leading to a self-amplification loop that causes damage and inflammation in this disease. TGase2, a calcium-dependent enzyme that is involved in cross-linking proteins through the formation of ε(γ-glutamyl)-lysine bonds, has been implicated in renal fibrosis processes, and could play a role at multiple levels in mesangial cell activation leading to inflammation and fibrosis in IgAN.
The mitogen-activated protein kinase/extracellular-regulated kinase (MAPK/ERK) signal transduction pathway is implicated in several cellular functions, such as cytokine production, cell survival and inflammation 7–9. The dual-phosphorylated form of ERK (p-ERK) is an active kinase and is able to phosphorylate a number of transcription factor targets and thus alter the pattern of gene transcription 10. Recently, p-ERK1/2 was shown to be involved in the secretion of inflammatory cytokines in IgAN and also in renin–angiotensin (RAS)-dependent mesangial cell activation, leading to a mesangial cell–podocyte cross-talk that causes proteinuria 11.
Both systemic 12,13 and particularly local complement activation have been shown to be important factors in the amplification of the proliferative and inflammatory stimuli that lead to renal injury 2. Besides the alternative pathway well-established role, demonstrated by the almost universal detection of C3 in IgAN biopsies 2, previous works 14–16 have demonstrated the activation of the lectin pathway of complement in this disease, showing that C4d can, in this case, be a reliable marker of this pathway, and that it relates to a more severe prognosis.
As chronic tubulointerstitial changes are one of the strongest histopathological risk factors for poor outcome in IgAN, tubulointerstitial injury markers have also been studied and related to disease 17,18. In a study focusing on markers of tubulointersititial leucocyte and cytokine infiltration, Myllymaki et al. 19 showed that CD3, a T cell marker, had the strongest correlation with the progression of kidney disease.
In the context of this possible multiple pathway pathogenesis of the disease, including proinflammatory and profibrotic stimuli, cell growth and proliferation, we aimed to establish a representative panel of immunohistological biomarkers that could assess the local status of this multi-event process, predict the outcome of the disease and help the clinician in choosing the therapeutic approach. For that we studied the immunostaining pattern of C4d, p-ERK1/2, TGase2 and CD3 in our cohort of IgAN biopsies.
Subjects and methods
Patients
We reviewed the renal pathology archives from January 2003 to December 2010 at Porto's Hospital São João in Portugal to retrieve those patients who were diagnosed as having IgAN by initial renal biopsy. IgAN was diagnosed when IgA was the sole or predominant glomerular immunofluorescence (IF) finding in the biopsy. The medical records were reviewed and the following information at the time of the renal biopsy was recorded: patient age, gender, presence or absence of hypertension, proteinuria, serum creatinine level, presence or absence of history of macroscopic haematuria, presence and type of comorbidities and number of glomeruli in the kidney biopsy. Progression of kidney disease and post-biopsy presence or absence of RAS blockade and/or immunosuppressive therapy record were evaluated through medical record review. Out of 115 patients, those aged <18 years (n = 2), having biopsies containing fewer than eight glomeruli (n = 13, including four cases excluded only after stainings were performed), missing clinical follow-up (n = 8) and having the diagnosis of Henoch–Schönlein purpura or with comorbid conditions such as diabetes mellitus, liver disease or systemic lupus erythematosus (n = 18) were excluded for analysis (total number of 41). Finally, 74 IgAN patients were enrolled into the study. The hospital's ethics committee approved the study protocol and the study was conducted in accordance with the principles that have their origin in the Declaration of Helsinki.
Clinical definitions
Using the above-mentioned data, the estimated glomerular filtration rate (eGFR) was determined by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Progressive kidney disease was defined as a decline of at least 50% in the eGFR or progression to end-stage renal disease (ESRD) during the follow-up period. Hypertension was defined as systolic blood pressure greater than 140 mmHg and/or diastolic blood pressure greater than 90 mmHg or usage of anti-hypertensive medication. Proteinuria was determined by measurements based on 24-h collection of urine. The follow-up ended at the time of a control visit performed during 2012–13, if the patient reached ESRD defined as chronic dialysis or renal transplantation or if the patient died. The median follow-up time after renal biopsy was 4 years (range 0·3–10).
Renal pathological evaluation
All renal biopsy specimens were processed routinely for light and electronic microscopy and IF. The paraffin-embedded tissue was serially sectioned at 3 μm in thickness and stained with haematoxylin and eosin, periodic acid-Schiff, silver methenamine and Masson trichrome. Direct immunofluorescence microscopy was used in all cases to detect deposition of IgG, IgA, IgM, C3, C1q and fibrinogen. Mesangial cellularity, glomerulosclerosis, tubular atrophy, interstitial fibrosis and inflammation, hyaline arteriolosclerosis and arterial intima fibrosis were evaluated and classified semiquantitatively as absent, mild, moderate and severe. All renal biopsies were reviewed and scored according to the Oxford classification. The four pathological variables of the Oxford classification were scored as follows: mesangial hypercellularity score <0·5 (M0) or >0·5(M1); segmental glomerulosclerosis absent (S0) or present (S1); endocapillary hypercellularity absent (E0) or present (E1); and tubular atrophy/interstitial fibrosis 0–25% (T0), 26–50% (T1) or >50% (T2). Concerning the latter, further simplification of the categorization to T0 versus T1–2 was performed to improve power in the statistical analysis, according to previous analysis 20.
Immunohistochemistry
Light microscopic immunoperoxidase staining was performed on 3 μm formaldehyde-fixed paraffin-embedded sections deparaffinized previously in xylene and rehydrated in graded ethanol. Sections were stained for polyclonal rabbit anti-human C4d (Biomedica, Vienna, Austria) (1:30), polyclonal rabbit anti-human CD3 (DakoCytomation, Glostrup, Denmark A/S) (1:50), polyclonal rabbit anti-human transglutaminase 2 antibody (Novus Biologicals, Inc., Littleton, CO, USA) (1:800) and monoclonal rabbit anti-human p-ERK1/2 (Thr202/Tyr204) (Cell Signaling Technology, Danvers, MA, USA) (1:200). Antigen retrieval was performed on rehydrated sections in a microwave oven for 10 min boiling, then 20 min cooling, using Tris ethylenediamine tetraacetic acid buffer as retrieval solution with CD3 and C4d antibodies (pH 9·0 and 8·5, respectively) and citrate and sodium citrate buffer (both pH 6·0) with TGase2 and p-ERK1/2, respectively (complemented in the latter with sodium acetate plus trypsin antigen retrieval at 37°C for 20 min).
The detection system included the following steps: endogenous peroxidases were blocked with 0·03% hydrogen peroxide for 30 min at room temperature, signal amplification was performed with goat anti-rabbit and rabbit anti-goat IgGs for TGase2 and CD3, biotinylated goat anti-rabbit IgG and the avidin–biotin complex (ABC) kit (Vector, Burlingame, CA, USA) for p-ERK1/2 (with previous avidin and biotin blocking steps) and the horseradish peroxidase (HRP) kit (Universal HRP Multimer kit; Ventana Medical Systems, Tucson, AZ, USA) for C4d. Diaminobenzidine was used as chromogen and haematoxylin as nuclear stain. The immunohistochemistry specificity was controlled by replacing the primary antibodies with irrelevant anti-sera.
Immunohistochemical score analysis was performed as follows: for C4d as negative (0) or positive (1). Patients were classified as ‘positive’ when diffuse (>50% of the glomeruli) staining for C4d was observed, as described in Maeng et al. 21. For glomerular and tubulointerstitial TGase2 (TGase2 G and TGase2 T, respectively) we evaluated the extent of the staining according to the aforementioned methods described in Ikee et al. and Kliem et al. 22,23. For glomerular p-ERK1/2, analysis was performed as described in Tamouza et al. 11. For tubulointerstitial CD3 score, pictures covering the whole slide length of cortical area were taken at ×200 magnification and analysed by two observers using Image J software version 1·47 (National Institutes of Health, Bethesda, MD, USA), and the mean score of all fields (except those presenting severe cortical scarring, not included) were considered as the patient's CD3 tubulointerstitial staining score. Due to the categorical nature of TGase2 T score (0 = absent staining, I = 1–5%; II = 5–25%; III = 25–50%; IV = 50–75%; V ≥ 75%) and the number of patients classified in each category, this variable was classified further as ‘low’ when the score for each slide was lower than 25% and ‘high’ when greater than 25%. This categorization was not performed for TGas2 G and p-ERK1/2, as their score was the mean value of each glomeruli score, or for CD3, as it was a continuous variable.
Statistical analysis
The software used for statistical analysis was spss version 21 (SPSS, Inc., Chicago, USA). The reported P-values are two-sided, but statistical significance was defined as one-sided P-values ≤ 0·05. Categorical variables are presented as percentages and numerical variables as mean ± standard deviation or as median (range). Univariate analysis comprised the Mann–Whitney U-test to compare groups for numerical variables and the χ2 test, or Fisher's exact test for categorical variables. A multivariate analysis was conducted to identify predictors of disease presentation and progression. Using logistic regression and Cox regression analysis we sought for covariates associated independently with progression. Multivariate models were constructed, including initially all correlated covariates identified in the univariate analysis. The non-significant variables were eliminated from the model one at a time. Survival to progressive state is also assessed through Kaplan–Meier survival analysis. Univariate survival comparisons were made using the log-rank test.
Results
Clinical data
Isolated urine abnormalities (haematuria and/or proteinuria) with normal renal function was the most common clinical presentation that led to renal biopsy (58% of the patients). In 42% of the patients, eGFR was lower than 60 ml/min. Average proteinuria was 2·4 g/day (75% had proteinuria >1 g) and 60·3% of patients had hypertension at the time of biopsy (Table 1). Progressive kidney disease was found in 20 patients (27%) during the follow-up period (mean 4·7 years). Clinical parameters at presentation that were associated with higher proteinuria were lower eGFR, older age and presence of hypertension. Lower eGFR at presentation was also associated with older age, hypertension and higher proteinuria. Clinical parameters that were associated with progression of kidney disease were baseline proteinuria, eGFR and hypertension (Table 2).
Table 1.
Clinical characteristics at the time of biopsy and follow-up in 74 patients with IgA nephropathy
| At the time of biopsy | Follow-up | ||
|---|---|---|---|
| Age (years) | 39 (18–78) | Duration of follow-up (months) | 48 (4–120) |
| Female | 24 (32·4) | ||
| Hypertension | 44 (60·3) | ||
| Previous macroscopic haematuria | 27 (38·6) | Treated with RAS blockade (ACEi and ARB) | 64 (95) |
| eGFR (ml/min per 1·73 m2) | 67·5 ± 36·5 | Immunosupression | 16 (24) |
| Stages 1, 2, 3, 4 CKD (KDIGO) | 32 (43), 15 (20), 18 (24) and 9 (12) | 50% decline in renal function | 20 (27) |
| Proteinuria (g/day) | 1·96 (0·17–9·8) | End-stage renal disease (dialysis or transplantation) | 16 (22) |
For quantitative variables, values are expressed as the mean ± standard deviation or the median (interquartile range) as appropriate. For qualitative variables, values are expressed as n (%). Immunosuppression includes prednisone and/or azathioprine and/or cyclophosphamide. RAS = renin-angiotensin system; ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; eGFR = estimated glomerular filtration rate.
Table 2.
Clinical and pathological data according to the evolution of kidney disease
| Clinical and pathological data | Progressive kidney disease (n = 20) | Non-progressive kidney disease (n = 54) | P-value |
|---|---|---|---|
| Age | 45 ± 16 | 37 ± 13 | 0·053 |
| Female | 5 (25) | 19 (35·2) | 0·23 |
| Hypertensive | 18 (90) | 26 (49·1) | 0·001 |
| Previous macroscopic haematuria | 6 (33·3) | 21 (40·4) | 0·6 |
| eGFR (ml/min per 1·73 m2) | 31 ± 22 | 81 ± 31 | <0·005 |
| Proteinuria (g/day) | 3·907 ± 2·155 | 1·835 ± 1·372 | <0·005 |
| Immunosuppressive therapy | 5 (26·3) | 11 (23·4) | ≅1 |
| IgM positivity | 10 (50) | 18 (34) | 0·21 |
| IgG positivity | 5 (26·3) | 14 (26·9) | 0·96 |
| C1q positivity | 2 (10·5) | 8 (16) | 0·715 |
| S1 score | 8 (40) | 29 (53·7) | 0·295 |
| E1 score | 3 (15·0) | 5 (9·3) | 0·674 |
| T1–2 score | 17 (85) | 8 (15·4) | <0·005 |
| C4d positivity | 11 (55) | 14 (27·5) | 0·029 |
| TGase-2 G score | 1·35 ± 0·26 | 1·26 ± 0·29 | 0·19 |
| High TGase-2 T score | 16 (84·2) | 15 (33·3) | <0·005 |
| p-ERK1/2 score | 1·33 ± 0·68 | 1·32 ± 0·54 | 0·9 |
| CD3 score | 66 273 ± 39 311 | 19 322 ± 20 632 | <0·005 |
For quantitative variables, values are expressed as the mean ± standard deviation. For qualitative variables, values are expressed as n (%). Progressive kidney disease, defined as a decline of at least 50% in the glomerular filtration rate (GFR) or progression to end-stage renal disease (ESRD) during the follow-up period. Immunoglobulin (Ig)M, IgG and C1q refer to the results of routine immunofluorescence data. S1, E1 and T1–2 according to the Oxford classification reanalysis of the biopsies. High TGase-2 T score (>25% staining). eGFR = estimated glomerular filtration rate.
Pathology parameters
According to the Oxford classification, M1, S1 and E1 were present in 86, 50 and 11% of patients, respectively, and 35% of the biopsies were scored as having T1–2. Immunofluorescence data showed 38, 27, 80 and 11% positive biopsies for IgM, IgG, C3 and C1q, respectively. Among these data, only IgM, IgG, E1 and T1–2 were associated univariately with higher proteinuria, M1, E1 and T1–2 with lower eGFR and S1 with higher eGFR at presentation. Only T1–2 correlated with progressive kidney disease (Table 2).
Immunohistological panel
Glomerular C4d was positive in 25 patients (34%) (Fig. 1). C4d positivity was associated with higher proteinuria and progressive kidney disease (Fig. 2). It was also associated with the presence of T1–2 and IgG. TGase2 G and TGase2 T were both associated with lower eGFR, higher proteinuria and T1–2. TGase2 T correlated univariately with progression of kidney disease. Although there was a tendency to higher proteinuria and higher eGFR in patients with higher p-ERK1/2, these correlations were not statistically significant. Higher CD3 score was associated with T1–2, decreased eGFR and higher proteinuria at presentation and progressive kidney disease (Table 2, Fig. 3).
Figure 1.
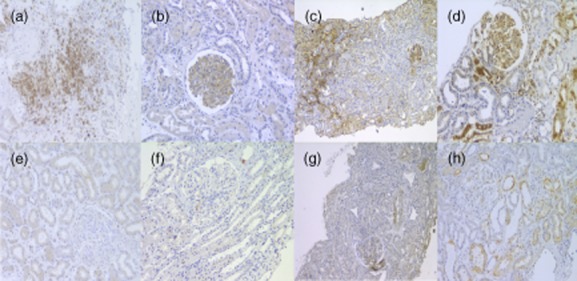
Renal tissue from patients with immunoglobulin A nephropathy (IgAN) was stained for the presence of CD3, C4d, transglutaminase2 (TGase 2) and protein kinase intracellular signalling molecule (p-ERK)1/2. Representative images are shown. (a,e) CD3 staining of infiltrating T cells in renal interstitium, severe and mild, respectively. (b,f) A representative glomerulus showing positive and negative staining for C4d, respectively. (c,g) Kidney tissue showing strong and mild glomerular and tubulointerstitial staining for TGase 2. (d,h) A glomerulus showing strong and mild staining for p-ERK1/2.
Figure 2.
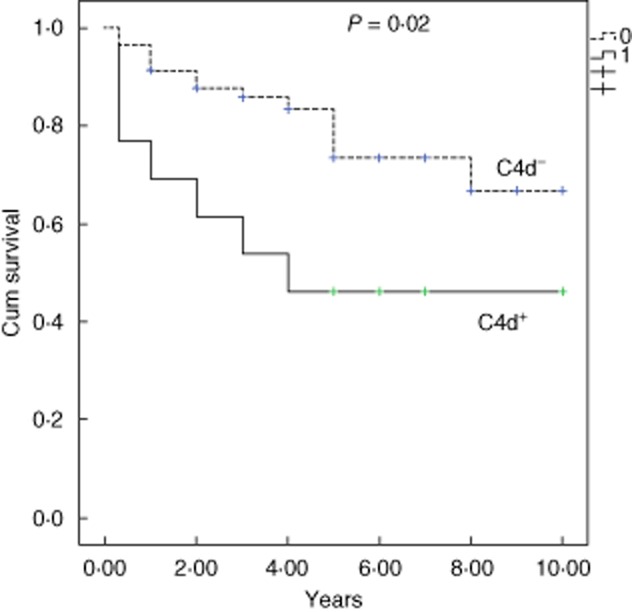
Kaplan–Meier renal survival [defined as decline of at least 50% in the glomerular filtration rate (GFR) or progression to end-stage renal disease (ESRD)] according to C4d-positive (+) and negative (−) staining.
Figure 3.
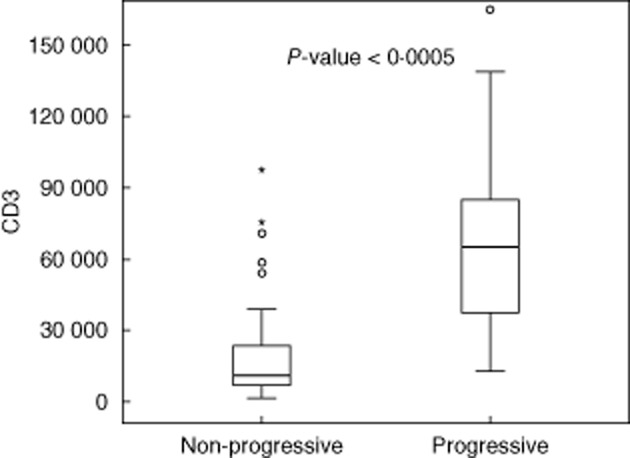
CD3 score according to progressive status of kidney disease.
Multivariate analysis including clinical, pathological and immunohistological parameters
Starting with a multi-step multivariate analysis to analyse the effect of both clinical and pathological parameters (not including the immunohistological biomarkers) on the progressive state of kidney disease, only eGFR and T1–2 were associated independently with the latter. Concerning the immunohistological biomarkers, a multivariate model with those (TGase2 T, CD3 and C4d) that showed a univariate correlation with progressive kidney disease revealed that they remained associated independently with progression. In a final multivariate analysis, including the previous three biomarkers with eGFR and T1–2, only eGFR, C4dG and CD3 were associated independently with progressive kidney disease (Table 3).
Table 3.
Multivariable logistic and Cox regression analysis – independent predictors of progressive state of kidney disease
| Variables |
Logistic regression |
Cox regression |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Clinical and pathological parameters | ||||
| eGFR | 0·96 (0·93–0·99) | 0·018 | 0·95 (0·93–0·98) | <0·0005 |
| Proteinuria (g/day) | 1·26 (0·75–2·13) | 0·379 | 1·19 (0·93–1·54) | 0·172 |
| T1–2 score | 8·72 (1·64–46·40) | 0·011 | 4·37 (1·19–16·1) | 0·027 |
| Immunohistological biomarkers | ||||
| High TGase-2 T score | 7·95 (0·97–64·84) | 0·053 | 3·88 (1·03–14·6) | 0·045 |
| C4d positivity | 7·68 (1·176–50·132) | 0·033 | 3·68 (1·3–10·43) | 0·014 |
| CD3 score* | 1067 (1027–1109) | 0·001 | 10017 (1007–1027) | 0·001 |
| Clinical, pathological and immunohistological biomarkers | ||||
| eGFR | 0·96 (0·925–0·991) | 0·012 | 0·95 (0·929–0·975) | <0·0005 |
| C4d positivity | 9·29 (1·18–73·25) | 0·034 | 3·35 (1·25–8·95) | 0·016 |
| CD3 score* | 1005 (1016–1096) | 0·006 | 1016 (1006–1026) | 0·003 |
*CD3 score – per 1000-units increment. T1–2 according to the Oxford classification reanalysis of the biopsies. High TGase-2 T score (>25% staining). CI = confidence interval; eGFR = estimated glomerular filtration rate.
Discussion
Although clinical and histopathological risk factors have classically been applied in clinics to establish a therapeutic plan for IgAN, biomarkers that can predict its prognosis most accurately are still a matter of research. As multiple pathophysiological pathways have been documented to play a role in the aetiology and different course of the disease 5,6,15,24, a demand for a more refined diagnostic approach that can detect their activation status seems justifiable. Immunohistochemistry is a widely available technique in clinical practice that can answer some of the questions demanding a pathophysiological approach to the diagnosis. Hereby we tried to establish a panel of biomarkers with some of the players that had been shown to explain the heterogeneity of the disease, including C4d, CD3, TGase2 and p-ERK1/2, and we found that the combination of C4d and Cd3 can powerfully predict its course.
This study could confirm the predictive role of some of the established risk factors for disease progression, including lower eGFR, higher proteinuria, hypertension and T1–2 score according to the Oxford classification 2,4. However, proteinuria and hypertension did not remain associated independently with disease progression when included on a multivariate analysis with clinical and pathological parameters, as observed in other studies similar to ours 16,19. We could not find an association between mesangial hypercellularity and segmental sclerosis with disease outcome as shown in the Valiga validation of the Oxford classification 20. The lower number of patients in our study, exclusion of the paediatric population and a slightly more aggressive clinical presentation found in our cohort (higher mean proteinuria and lower eGFR) could explain these differences.
IF data analysis showed no value in predicting the course of disease, but IgM and IgG detection were related to higher proteinuria at presentation. The latter was not found in the immunostaining study performed in the original Oxford classification cohort 25, which was a significantly different cohort in terms of ethnicity and age, and IgM was not related to the presence of segmental sclerosis, as reported previously 26. Adding this to the fact that both IgG and IgM positivity, but not C1q, were associated with the presence of glomerular C4d (P = 0·055 in the case of IgM) could reinforce the hypothesis of a previously described antibody-mediated role for lectin pathway activation 27, occurring particularly in IgAN, and adding new value for the IF interpretation in this disease.
This study confirms the previous reports showing glomerular C4d as a marker of lectin pathway activation in IgAN and its association with more severe presentation of disease and outcome 15,16,28. Unlike Espinosa et al.'s 16 findings, it showed no association with segmental sclerosis but remains associated with T1–2, supporting a potential causal role for disease progression rather than just a marker of glomerular damage. It also first validates its power as a biomarker when included in an analysis with clinical and histopathological parameters, described recently in the study by Espinosa et al. 28.
Previous studies have shown that mesangial and tubular epithelial cells are the main sources of TGase2 in glomerular and tubulointerstitial compartments, respectively 29. It has been shown to be an early marker of subsequential progressive renal scarring in conditions such as chronic allograft nephropathy 30. This makes TGase2 a potentially valuable marker for IgAN pathological and clinical evolution. A study by Ikee et al. 22, performing immunohistochemical analysis in a small number of IgAN biopsied patients, confirmed its association with more severe clinical and pathological presentation. In this study, we confirmed these previous results in a bigger cohort of patients, and first showed that TGase2 T correlates with a worse outcome of the disease. Due to the close association of both TGase2 G and T to T1–2, we could not confirm or exclude the hypothesis of Berthelot et al. 6 that establishes TGase-2 as a trigger for overt IgAN. A cohort including a higher number of early disease patients could probably elucidate this role further in human biopsies. For the same reason, the characteristics of our cohort could explain the absence of correlations between p-ERK1/2 mesangial expression and clinical presentation and outcome, as p-ERK1/2 activation has been described as an early process in the disease 11. The same authors describe a high inhibitory effect of renin–angiotensin blockade in this pathway, and the lack of complete data on the RAS blockade therapy status at the time of biopsy in our cohort precluded an adjusted analysis to its effect on mesangial p-ERK1/2 expression, introducing a significant bias to our results.
Tubulointerstitial injury 31, and particularly inflammation 19,32, have been implicated as very important factors in the prognosis of IgAN. Evaluation of CD3+ cell infiltration, an antigen expressed typically in T cells, has been used to quantify the intensity of inflammation in human tissue 17,19, and has proved to be the best among tubulointerstitial injury markers to predict progression of disease 19. In this study we confirm the same results, but we show that it is also superior to glomerular immune markers of the disease, reinforcing the role of tubulointerstitial injury in this so-called glomerular disease.
This is the first study to establish a reliable panel of biomarkers suggesting immunological activity both in glomerular and tubulointerstitial compartments of IgAN biopsies with a powerful predictive power, superior to previously used risk factors. Already applied to clinical practice in some situations, both C4d and CD3 immunohistochemical staining is an inexpensive and easy-to-perform method for the analysis of renal biopsies. Good sensitivity and specificity in paraffin sections 33,34 make the staining for these antibodies a reliable and useful tool, particularly when treatment discussion demands a more refined approach than the one provided by routine biopsy processing and analysis. C4d has the advantage of a simpler classification between positive and negative, but it is not totally clear what role chronic lesions can have in C4d results. For a rigorous CD3 evaluation, software analysis and eventually the establishment of a cut-off value for each laboratory makes this a slightly more complex approach, but also more accurate than C4d. We propose the combination of both to establish a more targeted medical treatment for IgAN patients.
Acknowledgments
The work that led to this paper was supported by a grant from the ERA–EDTA (European Renal Association–European Dialysis and Transplantation Association), on behalf of its Research Fellowship Program. The authors would also like to acknowledge Dr Ivan Moura and Dr Jonathan Chemouny for their valuable help in the p-ERK1/2 staining, Dr Anita Meter for general technical support and Professor Fátima Carneiro, Dr Susana Sampaio and Dr Pedro Rodrigues Pereira for facilitating the access to the Hospital São João's Kidney Biopsy Bank.
Disclosure
The authors have no conflicts of interest to declare.
References
- D'Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med. 1987;64:709–727. [PubMed] [Google Scholar]
- Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- Floege J, Eitner F. Current therapy for IgA nephropathy. J Am Soc Nephrol. 2011;22:1785–1794. doi: 10.1681/ASN.2011030221. [DOI] [PubMed] [Google Scholar]
- Cattran DC, Coppo R, Cook HT. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- Daha MR, van Kooten C. Deposition of IgA in primary IgA nephropathy: it takes at least four to tango. Nephrol Dial Transplant. 2013;28:794–797. doi: 10.1093/ndt/gfs445. [DOI] [PubMed] [Google Scholar]
- Berthelot L, Papista C, Maciel TT. Transglutaminase is essential for IgA nephropathy development acting through IgA receptors. J Exp Med. 2012;209:793–806. doi: 10.1084/jem.20112005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara T, Moroi Y, Uchi H, Furue M. Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J Dermatol Sci. 2006;42:1–11. doi: 10.1016/j.jdermsci.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Koj A. Initiation of acute phase response and synthesis of cytokines. Biochim Biophys Acta. 1996;1317:84–94. doi: 10.1016/s0925-4439(96)00048-8. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Masaki T, Stambe C, Hill PA, Dowling J, Atkins RC, Nikolic-Paterson DJ. Activation of the extracellular-signal regulated protein kinase pathway in human glomerulopathies. J Am Soc Nephrol. 2004;15:1835–1843. doi: 10.1097/01.asn.0000130623.66271.67. [DOI] [PubMed] [Google Scholar]
- Tamouza H, Chemouny JM, Raskova Kafkova L. The IgA1 immune complex-mediated activation of the MAPK/ERK kinase pathway in mesangial cells is associated with glomerular damage in IgA nephropathy. Kidney Int. 2012;82:1284–1296. doi: 10.1038/ki.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda K, Ohi H, Tamano M. Hypercomplementemia in adult patients with IgA nephropathy. J Clin Lab Anal. 2007;21:77–84. doi: 10.1002/jcla.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwirner J, Burg M, Schulze M. Activated complement C3: a potentially novel predictor of progressive IgA nephropathy. Kidney Int. 1997;51:1257–1264. doi: 10.1038/ki.1997.171. [DOI] [PubMed] [Google Scholar]
- Hisano S, Matsushita M, Fujita T, Endo Y, Takebayashi S. Mesangial IgA2 deposits and lectin pathway-mediated complement activation in IgA glomerulonephritis. Am J Kidney Dis. 2001;38:1082–1088. doi: 10.1053/ajkd.2001.28611. [DOI] [PubMed] [Google Scholar]
- Roos A, Rastaldi MP, Calvaresi N. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724–1734. doi: 10.1681/ASN.2005090923. [DOI] [PubMed] [Google Scholar]
- Espinosa M, Ortega R, Gómez-Carrasco JM. Mesangial C4d deposition: a new prognostic factor in IgA nephropathy. Nephrol Dial Transplant. 2009;24:886–891. doi: 10.1093/ndt/gfn563. [DOI] [PubMed] [Google Scholar]
- Falk MC, Ng G, Zhang GY. Infiltration of the kidney by alpha beta and gamma delta T cells: effect on progression in IgA nephropathy. Kidney Int. 1995;47:177–185. doi: 10.1038/ki.1995.21. [DOI] [PubMed] [Google Scholar]
- Silva GE, Costa RS, Ravinal RC. Renal macrophage infiltration is associated with a poor outcome in IgA nephropathy. Clinics (São Paulo) 2012;67:697–703. doi: 10.6061/clinics/2012(07)01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllymäki JM, Honkanen TT, Syrjänen JT. Severity of tubulointerstitial inflammation and prognosis in immunoglobulin A nephropathy. Kidney Int. 2007;71:343–348. doi: 10.1038/sj.ki.5002046. [DOI] [PubMed] [Google Scholar]
- Coppo R, Troyanov S, Bellur S. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 86:828–836. doi: 10.1038/ki.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng YI, Kim MK, Park JB. Glomerular and tubular C4d depositions in IgA nephropathy: relations with histopathology and with albuminuria. Int J Clin Exp Pathol. 2014;6:904–910. [PMC free article] [PubMed] [Google Scholar]
- Ikee R, Kobayashi S, Hemmi N. Involvement of transglutaminase-2 in pathological changes in renal disease. Nephron Clin Pract. 2007;105:139–146. doi: 10.1159/000098646. [DOI] [PubMed] [Google Scholar]
- Kliem V, Johnson RJ, Alpers CE. Mechanisms involved in the pathogenesis of interstitial fibrosis in 5/6-nephrectomized rats. Kidney Int. 1996;49:666–678. doi: 10.1038/ki.1996.95. [DOI] [PubMed] [Google Scholar]
- Kim MJ, McDaid JP, McAdoo SP. Spleen tyrosine kinase is important in the production of proinflammatory cytokines and cell proliferation in human mesangial cells following stimulation with IgA1 isolated from IgA nephropathy patients. J Immunol. 2012;189:3751–3758. doi: 10.4049/jimmunol.1102603. [DOI] [PubMed] [Google Scholar]
- Bellur SS, Troyanov S, Cook HT, Roberts IS. Immunostaining findings in IgA nephropathy: correlation with histology and clinical outcome in the Oxford classification patient cohort. Nephrol Dial Transplant. 2011;26:2533–2536. doi: 10.1093/ndt/gfq812. [DOI] [PubMed] [Google Scholar]
- Nasri H, Sajjadieh S, Mardani S. Correlation of immunostaining findings with demographic data and variables of Oxford classification in IgA nephropathy. J Nephropathol. 2013;2:190–195. doi: 10.12860/JNP.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daha NA, Banda NK, Roos A. Complement activation by (auto-)antibodies. Mol Immunol. 2011;48:1656–1665. doi: 10.1016/j.molimm.2011.04.024. [DOI] [PubMed] [Google Scholar]
- Espinosa M, Ortega R, Sánchez M. Association of C4d deposition with clinical outcomes in IgA nephropathy. Clin J Am Soc Nephrol. 2014;9:897–904. doi: 10.2215/CJN.09710913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TS, El-Koraie AF, Skill NJ. Tissue transglutaminase and the progression of human renal scarring. J Am Soc Nephrol. 2003;14:2052–2062. doi: 10.1097/01.asn.0000079614.63463.dd. [DOI] [PubMed] [Google Scholar]
- Johnson TS, Abo-Zenah H, Skill JN. Tissue transglutaminase: a mediator and predictor of chronic allograft nephropathy? Transplantation. 2004;77:1667–1675. doi: 10.1097/01.tp.0000131171.67671.3c. [DOI] [PubMed] [Google Scholar]
- D'Amico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis. 2000;36:227–237. doi: 10.1053/ajkd.2000.8966. [DOI] [PubMed] [Google Scholar]
- Freese P, Norden G, Nyberg G. Morphologic high-risk factors in IgA nephropathy. Nephron. 1998;79:420–425. doi: 10.1159/000045087. [DOI] [PubMed] [Google Scholar]
- Nadasdy GM, Bott C, Cowden D, Pelletier R, Ferguson R, Nadasdy T. Comparative study for the detection of peritubular capillary C4d deposition in human renal allografts using different methodologies. Hum Pathol. 2005;36:1178–1185. doi: 10.1016/j.humpath.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Anderson C, Rezuke WN, Kosciol CM, Pastuszak WT, Cartun RW. Methods in pathology. Identification of T-cell lymphomas in paraffin-embedded tissues using polyclonal anti-CD3 antibody: comparison with frozen section immunophenotyping and genotypic analysis. Mod Pathol. 1991;4:358–362. [PubMed] [Google Scholar]


