Abstract
MicroRNA (miRNA) are small, non-coding RNA molecules that have been linked with immunity through regulating/modulating gene expression. A role for these molecules in T-cell and B-cell development and function has been well established. An increasing body of literature now highlights the importance of specific miRNA in dendritic cell (DC) development as well as their maturation process, antigen presentation capacity and cytokine release. Given the unique role of DC within the immune system, linking the innate and adaptive immune responses, understanding how specific miRNA affect DC function is of importance for understanding disease. In this review we summarize recent developments in miRNA and DC research, highlighting the requirement of miRNA in DC lineage commitment from bone marrow progenitors and for the development of subsets such as plasmacytoid DC and conventional DC. In addition, we discuss how infections and tumours modulate miRNA expression and consequently DC function.
Keywords: dendritic cells, immune modulation, microRNAs
Dendritic cell subsets (human versus mouse)
Dendritic cells (DC) are important antigen-presenting cells for promoting immune responses to pathogens such as bacteria and viruses, as well as for maintaining self-tolerance.1 These cells link the innate and adaptive immune systems by presenting antigen to T cells, providing co-stimulation and cytokines required for antigen-specific T-cell activation. Many varieties of DC have been described in both human and mouse, each with a particular location, phenotypic morphologies and function.1–3 To summarize, the major DC categories in the mouse include, conventional/classic DC (cDC), Langerhans DC (LC), plasmacytoid DC (pDC) and the monocyte-derived DC (moDC). The cDC can be subdivided into migratory DC, found in the skin and lymph nodes or tissue-resident DC, found in spleen and lymph nodes. Tissue-resident DC consist of several prominent subsets including (i) the CD8α+ DC, which are important for cross-presenting antigen to CD8+ T cells as well as being the major interleukin-12 p70 (IL-12p70) producer, (ii) the CD11b+ cDC, a heterogeneous population of DC including; CD4+ DC, which are capable of presenting Class II restricted antigens to CD4+ T cells and (iii) pDC, which are the major producer of type 1 interferons during viral infections.1–3 In man, the equivalent of the mouse CD8α+ DC has been defined, as CD141+ (BDCA3+) DC,4 as has the pDC subset.5
Monocyte-derived DC are derived from monocytes under inflammatory conditions and are closely related to the CD11b+ DC. Murine bone marrow (BM) cells cultured in vitro in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4 develop into what many consider moDC, as do DC derived from human blood monocytes cultured in the presence of these cytokines.6 Their in vivo equivalent has yet to be identified; however, several murine moDC candidates have been described including DC-specific intercellular adhesion molecules-3 grabbing non-integrin (DC-SIGN/CD209a) -positive DC that appear in lymph nodes after Toll-like receptor (TLR) ligand challenge7 and tumour necrosis factor-α (TNF-α) and inducible nitric oxide synthase-producing DC that appear during pathogen-associated inflammation.8
Given the heterogeneity of DC subsets as well as the wide-ranging function of DC, the following question arises, how is DC differentiation and function regulated? Different growth factors, such as cytokines (FLT3 and GM-CSF) and transcription factors (Irf8, E2-2, Id2, E4Bp4, Batf3, Irf4 and Notch-2) control cDC development.9–11 There is now increasing evidence that microRNA (miRNA) play an important part in ‘fine tuning’ the development and function of all DC subsets and this review will focus on recent findings in this exciting field of research.
MicroRNA
MicroRNA are vital for controlling many processes within the immune system including cell differentiation and homeostasis, cytokine responses, interactions with pathogens and tolerance induction.12 Defects in the action of miRNA are associated with oncogenesis and other diseases.13–15
MicroRNA are small, non-coding RNA of around 19–24 nucleotides in length,16 which function to suppress protein synthesis by binding to complementary 3′-untranslated regions of mRNA17–19 and either inhibit translation or accelerate mRNA degradation.19 The process of miRNA synthesis is shown in Fig.1. Briefly, miRNA are first transcribed into a primary transcript (pri-miRNA) by RNA polymerase II. Pri-miRNA then bind DiGeorge syndrome critical region gene 8 (DGCR8) and undergo processing by the RNAase III enzyme activity of Drosha resulting in hairpin pre-miRNA transcripts.20–22 The RNase III enzyme, Dicer, further processes these structures in the cytoplasm, following export from the nucleus via Exportin-5. This results in mature, 19- to 24-bp miRNA species, which are incorporated into the RNA-induced silencing complex (RISC) containing the Argonaute (Ago) protein.23–25 Each miRNA has the ability to inhibit many mRNA, in fact a single miRNA has the ability to affect over 100 genes and one mRNA can be targeted by more than one miRNA.20,26 MicroRNA can also regulate the expression of other miRNA.27 For many miRNA the target 3′ untranslated region of specific mRNA have been identified. A summary of some key miRNA involved in DC development and function and their targets are highlighted in Table1.
Figure 1.
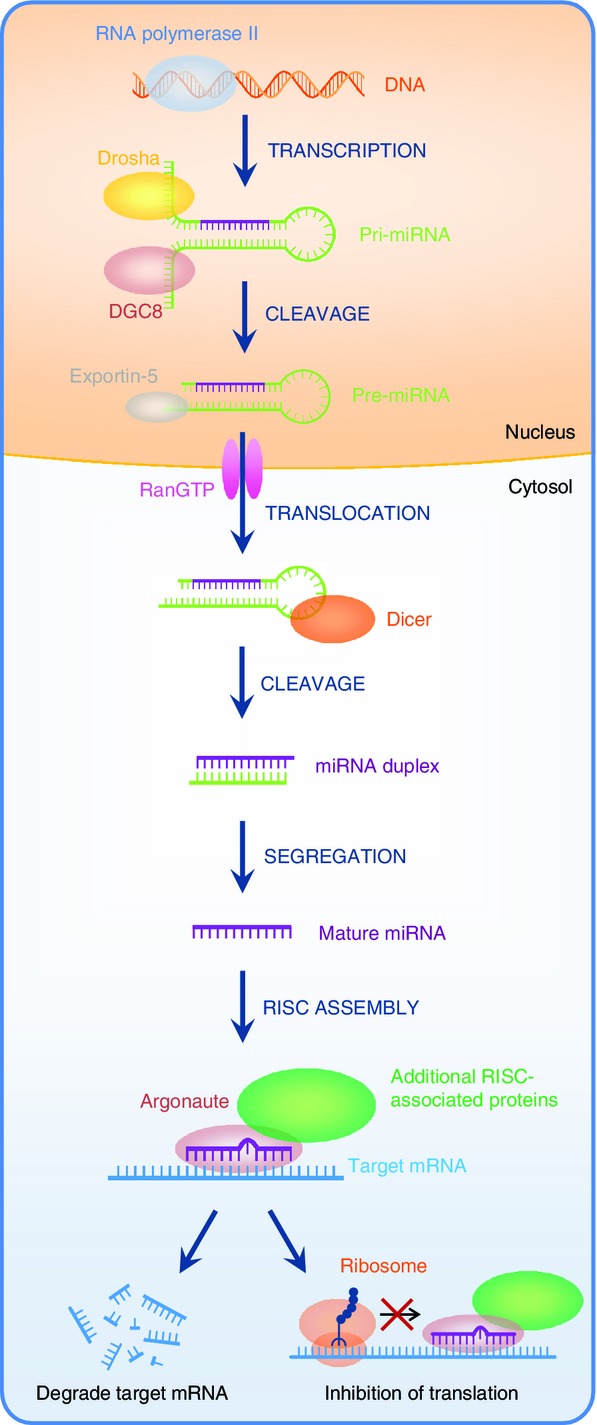
Biogenesis of microRNA (miRNA). In the nucleus, miRNAs are transcribed into primary transcript (pri-miRNAs) by RNA polymerase II. Pri-miRNAs then bind DiGeorge syndrome critical region gene 8 (DGCR8) and undergo processing by the RNAse III activity of Drosha resulting in hairpin pre-miRNA transcripts, which are transported to the cytoplasm via Exportin-5. The RNase III enzyme, Dicer, further processes these structures in the cytoplasm resulting in mature, 19- to 24-base-pair miRNA species, which are incorporated into the RNA-induced silencing complex (RISC) containing the Argonaute (Ago) protein. miRNA recognize the 3′ untranslated region of mRNA as part of the RNA-induced silencing complex (RISC).
Table 1.
MicroRNA (miRNA) regulate production of cytokines, differentiation and homeostasis of many dendritic cell subsets via affecting specific targets
| miRNA | Predicted/identified targets | References |
|---|---|---|
| miR-155 | TAB 2, PU.1, SOCS1, SHIP1, KPC1, csf1 gene, c-FOS, DC-SIGN, C/EBPβ | 33,37,44,45,47–49,71,82 |
| let-7 | BLIMP SOCS1 | 50,70 |
| miR-29a | P42.3 Lipoprotein lipase | 83,84 |
| miR-142-3p | IL-6 | 51 |
| miR-125a and miR- 99a | KLF13 | 36,85 |
| miR-148a and 148b miR152 | Calcium/calmodulin-dependent protein kinase IIa | 54 |
| miR-29b and miR-29c | Bcl-2 and Mcl-1 | 58 |
| miR-146a | IRAK1, IRAK2, TRAF6, TLR-4 | 41,86 |
| miR-126 | Tsc1 (which encodes a negative regulator of the kinase mTOR) | 52 |
| miR-34a | JAG1, WNT | 38 |
| miR-142 | 1,10 | |
| miR-21 | Jag1, PDCD4, IL-12p35 | 33 |
| miR-221 | P27kip1 | 33,39 |
| miR-22 | Irf8 | 40 |
| miR-23b | Notch 1 and NFKB | 62 |
| miR-107 | IL-23p19 | 66 |
| miR-301a | PTEN | 69,86 |
| miR-451 | YWHAZ/14-3-3z protein levels | 64 |
| miR-30b | Notch1 | 36 |
A key role for miRNA in regulating T- and B-cell immune cell development, immune homeostasis and controlling adaptive immune responses has been described.28–31 As for DC, Kuiper et al. observed that the conditional depletion of Dicer in mouse CD11c+ DC did not affect the short-lived resident DC present in lymph nodes or the spleen. However, they observed that both differentiation and function of LC was greatly affected, in as much as the lack of miRNA in these cells resulted in a selective loss of these cells in the epidermis and those that remained lacked the ability to mature and present antigen.32 Despite this finding, many publications now highlight the importance of miRNA in all murine DC subsets with a role for different miRNA in DC development and function now being well established. In fact, miRNA can ‘fine tune’ the immune response by inducing apoptosis, affecting homeostasis and changing cytokine profiles of DC.33,34
MicroRNA and DC differentiation
Dendritic cells are derived from haematopoietic stem cells (HSC) present in BM. HSC differentiate into myeloid progenitor cells and myeloid DC progenitor cells. The latter are the precursors of cDC progenitors, which upon leaving the BM seed peripheral secondary lymphoid tissues and non-haematopoietic tissues, where they give rise to the aforementioned immature DC subsets, which upon TLR stimulus, mature. Differential expression of miRNA has been observed throughout the developmental process of murine DC from HSC to immature and mature DC.35 Su et al.36 compared the miRNA profile of BM-derived DC (BMDC) HSC, immature and mature DC using next-generation deep sequencing. These authors demonstrated that 391 miRNA were differentially expressed during DC differentiation.36 However an overlap in miRNA expression between each developmental stage was also observed, for example miR-132 and miR-147 were highly expressed in immature and mature DC but not found in HSC.36 miRNA profiles changed significantly, between HSC and immature DC. GM-CSF expanded, immature and lipopolysaccharide-activated murine moDC also display distinct miRNA expression profiles.37 The requirement for miRNA for human moDC development from progenitors has also been shown with miR-21 and miR-34a both being implicated in this process.38
The observation that miRNA profiles vary greatly between progenitor and DC also extends to the different DC subsets.39 Mildner et al.10 observed that whereas some miRNA were highly expressed in all subsets of DC, for example miR-125, let-7 and miR-21, murine pDC and splenic cDC (both CD8α+ and CD4+) have defined clusters of miRNA signatures. Moreover, miR-22 was found to be highly expressed in cDC (CD4+, CD8α+ and CD4− CD8−) compared with pDC. Over-expression of this miRNA in DC progenitors both in vitro and in vivo preferentially expanded the CD11c+ CD11b+ B220− cDC subset.40
Development and maintenance of CD4+ DC has been linked to expression of miR-142.10 The miR-142 is highly expressed in FLT3-dependent CD4+ DC, but not CD8α+ or CD4− CD8α− DC. Mice deficient in miR-142 have a 60% reduction in Class II CD11chi DC owing to an increase in CD4+ DC apoptosis. In addition, miR-142-deficient BM cells failed to develop into CD4+ DC in vitro, in the presence of FLT3L; however, there was no inhibition of CD8α+ DC development. Interestingly, the loss of miR-142 in these mice only affected splenic CD4+ DC development, the gut CD4+ DC equivalents, the CD103+ CD11b+ DC, were found at normal numbers.10
The development of pDC is also regulated by miRNA. Inhibiting miR-221 in BMDC progenitors led to the differentiation of pDC rather than cDC.33,39 Development of pDC has also been shown to require miR-126, which is highly expressed in both mouse and human pDC. Increased apoptosis of these cells was observed in the absence of this miRNA, suggesting that it is important for pDC survival.32,33 Another miRNA, miR-146a, has also been shown to affect pDC survival; over-expression of this miRNA in a pDC cell line induced apoptosis in these cells. This may reflect the fact that miR-146a blocks TLR-induced nuclear factor-κB (NFKB) activity by targeting IL-1 receptor-associated kinase 1 (IRAK1), leading to down-regulation of anti-apoptotic genes.41
MicroRNA and DC function
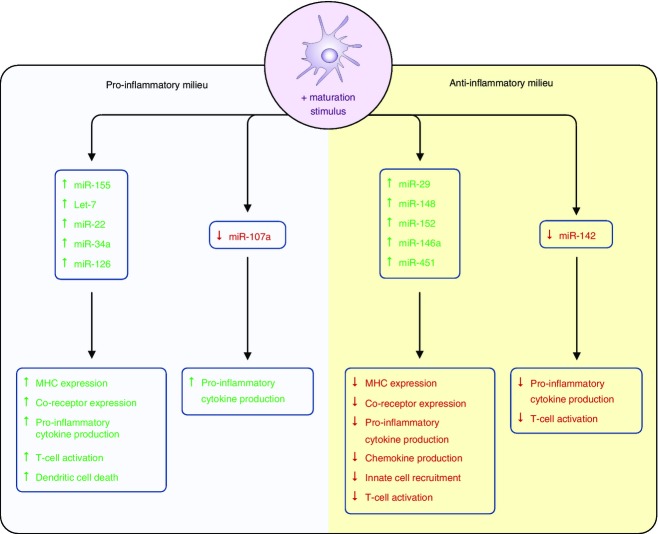
Cell intrinsic factors that form part of the anti-viral defence system, such as pattern recognition receptors (e.g. TLR, nucleotide-binding oligomerisation domain (NOD)-like receptor (NLR)), as well as cytokines, lipids, viruses, bacteria, parasites and tumours can modify the miRNA expression within DC.34,35,42 Each of these different stimuli was shown to induce or decrease expression of miRNA that influence, either positively or negatively, the ability of DC to process antigen, mature (expression of MHC, CD40, CD80, CD86 and DC-SIGN expression) and function (cytokine production and T-cell activation). Some of these miRNA are highlighted next and their effects on DC are summarized in Fig.2.
Figure 2.
MicroRNA (miRNA) expression during dendritic cell (DC) maturation. Activation of DC results in either an up-regulation or down-regulation of specific miRNAs that can modulate pro- or anti-inflammatory responses as well as T-cell activation and DC survival.
MicroRNA associated with DC maturation and function (miR-155, Let-7i and miR-126)
miR-155
Expression of miR-155 is induced rapidly in both human and mouse DC following maturation induced by either TLR activation (for example by dsRNA), cytokines [for example IL-1β, TNF-α and interferon-γ (IFN-γ)] as well as lipids (such as oxidized low-density lipoproteins and low-density lipoproteins).43 Expression of miR-155 by murine DC has been shown to be important for both their maturation and function, as highlighted by the observation that miR-155-deficient mice display impaired immune responses to pathogens.44,45 This observation being due in part to reduced expression of CD40 and CD86 following TLR activation.43 The DCs isolated from miR-155-deficient mice also have an impaired ability to activate antigen-specific T cells.46 Recently, the mechanism by which miR-155 modulates the ability of DC to activate T cells has been described.43 Dunand-Sauthier et al. found that the arginase, Arg-2, is a direct target of miR-155. Whereas miR-155-deficient BMDC have abnormally high levels of Arg-2 an increased expression of miR-155 in murine BMDC was associated with down-regulation of its expression. As arginine depletion by Arg-2 facilitates impaired T-cell proliferation, miR-155-induced repression of Arg2 expression appears critical for DC to activate T cells by controlling arginine availability in the extracellular environment.43
Increased levels of miR-155 in human moDC has been linked to DC co-receptor expression levels. The transcription factor PU.1, which regulates the expression of molecules such as DC-SIGN, is a direct target of miR-155.47 During DC maturation the increased expression of miR-155 results in decreased PU.1 levels and a subsequent reduction in DC-SIGN mRNA.47 The miR-155 has also been shown to modulate cytokine release.48,49 In human moDC, Ceppi et al.48 observed that inhibiting miR-155 expression in lipopolysaccharide-activated DC resulted in an increase in pro-inflammatory cytokine gene expression with IL-1α, IL-1β, IL-6, TNF-α and IL-23 being among the up-regulated genes found. These authors suggested that this miRNA could be an inhibitor of the inflammatory response given that down-regulation of these pro-inflammatory molecules occurred when miR-155 expression levels increased.48
Let-7i
miR-155 is not the only miRNA associated with DC maturation. For example, up-regulation of the miRNA let-7i in DC following TLR4 activation is important for normal DC maturation.50 When inhibited, expression of the co-stimulatory molecules CD80 and CD86, as well as pro-inflammatory cytokine production, were decreased. In addition, T-cell responses to antigen presented by DC were also reduced.50 The increased levels of miRNA let-7c and miR-155 in DC following maturation resulted in a pro-inflammatory phenotype, through the inhibition of the suppressor of cytokine signalling 1 (SOCS1), an inhibitor of Janus kinase/signal transducer and activator of transcription signalling after TLR activation.51
miR-126
Plasmocytoid DC maturation and function is also regulated by miRNA.52 Agudo et al.52 observed that miR-126 was highly expressed in both murine and human pDC compared with the cDC subsets. Mice lacking miR-126 were found to have reduced pDC-mediated activation, migration and IFN-γ release following TLR activation with CPG-A, suggesting that this miRNA was important for pDC function.52 These authors suggest that miR-126 regulated pDC function by directly targeting the mTOR pathway, which is required for TLR signalling by pDC,53 by controlling mRNA expression of tsc-1 a negative regulator of this pathway.52
MicroRNA that prevent DC maturation (miR-148, miR-142, miR-146a and miR-29a)
Although some miRNA are increased in DC following activation, their expression has a negative effect on DC maturation. For example, miR-148 is up-regulated in murine BMDC following TLR4 activation. Increased levels of this miRNA led to reduced MHC Class II expression, inhibition of pro-inflammatory cytokine secretion and decreased DC-mediated CD4+ T-cell expansion.54 In addition, in human DC, miR-29 was found to be up-regulated in response to NOD2, a cytoplasmic pattern recognition receptor signal, which led to the down-regulation of the pro-inflammatory cytokine IL-23 by targeting IL-12p40 and IL-23p19.55
As mentioned earlier, pDC effector function (e.g. cytokine production) and the expression levels of co-stimulation molecules are also controlled by miRNA. Expression of miR-146a is induced by TLR ligation (TLR7/9) in human pDC.41 Increased expression of this miRNA (via lentiviral transduction) in a human pDC line impaired TLR-mediated maturation by inhibiting key components of the nuclear factor-κB pathway. In addition, increased miR-146a expression inhibited the up-regulation of CD40, CD80, CD86, HLA-DR and CCR7 molecules and the production of pro-inflammatory cytokines (IL-6 and IFN-γ) such that pDC-induced allogeneic T-cell responses were inhibited.41
miR-142
Maturation of DC also results in down-regulation of miRNA. miR-142 is constitutively expressed in immature BMDC and following lipopolysaccharide activation its expression is decreased.10,51 Down-regulation of miR-142 was found not to affect activation markers such as CD40, CD80 and CD86, all of which increase following TLR activation in its absence. However, despite these maturation changes, DC isolated from miR-142-deficient mice failed to induce a CD4+ T-cell response compared with normal DC. This observation was attributed to miR-142 directly controlling IL-6 mRNA and IL-6 production following lipopolysaccharide activation.51
MicroRNA that affect antigen presentation (miR-150 and miR-223)
In addition to regulating cytokine production and co-receptor levels, miRNA can affect the ability of DC to present antigen. Epidermal LC from miR-150-deficient mice have reduced soluble antigen cross-presentation abilities56 while deletion of miR-223 increases the capacity of LC to cross-present.57 How both of these miRNA affect the antigen presentation pathway has yet to be elucidated; however, miR-150-deficient LC do not have impaired phagocytic capacities, suggesting that this miRNA may affect antigen processing. Whether miRNA affect antigen presentation in different DC types has yet to be studied.
MicroRNA that affect DC survival (miR-155, miR-146, miR-126, miR-29b and miR-29c)
MicroRNA expression has also been linked with DC survival. BMDC derived from miR-155-deficient mice survived longer than DC isolated from normal BM after lipopolysaccharide activation, whereas over-expression of miR-155 in DC led to increased apoptosis of these cells.33 KPC1, which ubiquitinates p27kip1 for degradation, is a target of miR-155. The miR-155 inhibits KPC1 expression leading to enhanced p27kip1 levels and ultimately in DC death.33 Thereby expression of this miRNA following stimulation may help to resolve an immune response by inducing the death of antigen-presenting cells. Plasmacytoid DC apoptosis and cell survival is also regulated by miRNA, including miR-146,41 miR-29b and miR-29c,58 as well as miR-126.52
MicroRNA and DC in tolerance induction
Immature DC, cytokine (transforming growth factor-β; TGF-β) and/or drug-treated ‘tolerogenic’ DC can induce T-cell tolerance. ‘Tolerogenic’ DC produce less pro-inflammatory cytokines in favour of cytokines such as IL-10 and TGF-β.59 Several miRNA have been shown to inhibit pro-inflammatory cytokine production, including miR-21 (inhibits IL-12p35 production),60 miR-10 (inhibits IL-12/IL-23p40 chain)61 and miR-148a/152 which suppress both IL-6 and IL-12 production via calcium/calmodulin-dependent protein kinase IIα (CaMKIIα), an effector of calcium signalling pathways.54
Several miRNA have been linked with a ‘tolerogenic’ DC phenotype (Fig.3). Su et al.36 observed that miR-30b is significantly up-regulated in ‘tolerogenic’ DC (mature DC plus TGF-β) both in vitro and in vivo. Over-expressing miR-30b in DC led to increased IL-10 and NO production while inhibiting it reduced both. In addition, miR-125a and miR-99a expression increased in ‘tolerogenic’ DC in this study.36 miR-23b has also been associated with a ‘tolerogenic’ DC phenotype. Expressing miR-23b in mouse BMDC and human moDC, via transfection, resulted in DC with reduced IL-12 but increased IL-10 production capacity, as well as reduced Class II, CD80 and CD86 expression. Increased expression of Foxp3 was seen when CD4+ T cells were co-cultured in the presence of miR-23b expressing DC.62 Both Notch1 and nuclear factor-κB are inhibited by miR-23b.62
Figure 3.
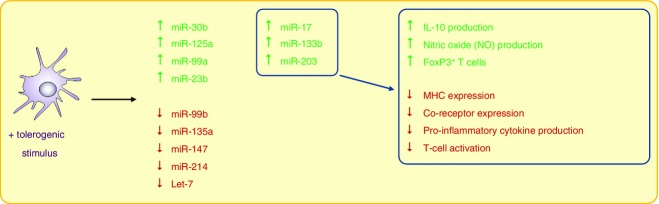
MicroRNA (miRNA) species linked with ‘tolerogenic’ dendritic cells (DC). Immature DC, cytokine (transforming growth factor-β) and/or drug-treated ‘tolerogenic’ DC can induce T-cell tolerance. ‘Tolerogenic’ DC produce less pro-inflammatory cytokines, in favour of cytokines such as interleukin-10, they also produce NO and can induce FoxP3+ T cells. Modification of specific miRNA has been linked to this ‘tolerogenic’ DC phenotype and function.
Stumpfova et al.63 analysed the expression of miRNA in ‘tolerogenic’ human moDC (IL-10- and TGF-β-treated) versus lipopolysaccharide-treated, IFN-γ-treated DC and immature DC. These authors showed that 27 miRNA, including miR-17, miR-133b and miR-203, were specifically increased in ‘tolerogenic’ DC when compared with mature DC. They also found four miRNA that were down-regulated in ‘tolerogenic’ DC, miR-99b, miR-135a, miR-147 and miR-214.63 Low levels of the miRNA let-7 in human moDC have been shown to favour the expansion of regulatory T cells, following interaction with these DC, again linking miRNA and tolerance induction.50
MicroRNA and DC during infections/tumours
Profiling murine splenic DC infected with influenza A virus in vitro revealed that miR-451 was induced by this ssRNA virus and not by dsRNA or lipopolysaccharide. Interestingly, following infection with this virus, myeloid cells present in the lung had increased expression of this miRNA. Expression of miR-451 in splenic DC reduced the production of a specific set of cytokines and chemokines including IL-6, TNF-α, IFN-γ, macrophage inflammatory protein-1α, CCL5 (involved in recruitment of T cells, eosinophils and basophils), CCL3 (involved in monocyte and neutrophil recruitment). This miRNA did not affect IL-10 or IL-1β production. Interestingly, inhibiting this miRNA had no effect on the expression levels of Class II and CD80 levels following activation. Taken together, it appears that viral infections may modulate miRNA expression to create an anti-inflammatory environment.64
Likewise, helminth worm antigens have also been shown to create an anti-inflammatory environment by modulating miRNA in human DC. Exposure of human moDC to secreted antigen from Taenia crassiceps reduced pro-inflammatory cytokine and chemokine production by inhibiting lipopolysaccharide-induced miRNA let-7 expression.65
Bacteria, including those of the intestinal microbiota, have been shown to have both a positive and a negative effect on host miRNA expression.61,66–68 Exposing germ-free mice to gut microbiota led to down-regulation of miR-10a in DC, and an increase in the pro-inflammatory IL-23p40 subunit.61 In addition, BMDC exposed to gut microbacteria such as Escherichia coli and flagellated A4 commensal bacteria have significantly lower miR-107a levels compared with untreated DC.66 Microbiota were also found to modulate miR-107a expression in gut CD11c+ myeloid cells leading to greater IL-23p19 production by these cells.66
Recently, it was reported that tumours modify miRNA expression in DC, creating an immune-suppressing and tumour-promoting environment.69 MicroRNA linked with tumour immune modulation such as miR-21, miR-222, miR-28 and miR-301a were up-regulated in DC exposed to tumour antigens. Over-expression of miR-301a in FLT3L-expanded BMDC did not affect Class II or CD80, CD86 or CD40 expression but inhibited IL-12, IL-6 and TNF-α production by DC and modulated T-cell responses. Although the interaction with miR-301a expressing DC did not affect antigen-specific CD4+ and CD8+ T-cell proliferation, cytokine release was modified; decreased IFN-γ from CD8+ CTL and CD4+ T cells while increased IL-3 and IL-17 were observed.69
What controls microRNA expression?
Several factors can regulate the expression of miRNA in DC, including transcription factors. For example, BLIMP affects the level of let-7c miRNA expression and miR-142, which has its own promoter, is targeted by the transcription factor PU.1.51,70
MicroRNA can also control the expression of other miRNA. For example, miR-155 has been shown to regulate the expression of miR-142. Increasing miR-155 expression following TLR ligation leds to reduced miR-142 expression, due to miR-155 targeting the PU.1 promotor. PU.1 is required for miR-142 expression.51 Conversely, knocking down miR-155 resulted in an increased miR-142 expression.51,62 Recently, it has been suggested that miR-155 is a ‘master’ miRNA regulator in DC.71 Comparing the miRNA profile in miR-155-deficient with miR-155 expressing DC, following a maturation stimulus, revealed miR155 ‘dependent’ and ‘independent’ miRNA. For example, miR-445-3p is induced following DC maturation only in the absence of miR-155 whereas miR-210-3p is not induced unless miR-155 is present. In addition, immature DC from miR-155-deficient mice lack miR-210-3p. It has been hypothesized that miR-155 regulates transcription factors, such as CCAAT/enhancer binding protein-β for example, that then bind to other miRNA promoters, such as miR-455, leading to up/down-regulation of these molecules.71
Intercellular transfer of microRNA
MicroRNA changes in DC may not always be due to intrinsic factors.72–75 In fact the intercellular transfer of miRNA between cells has been described through two pathways, gap junctions (GJ) and exosomes. At present the transfer of miRNA to DC via GJ has not been elucidated; however, this phenomenon has been described for several other cell types. Lim et al.73 demonstrated that miRNA could be transferred via GJ from BM-derived stromal cells into breast cancer cells where they caused a reduction in CXCL12 expression. In addition, Katakowski et al.72 elegantly showed miRNA transfer via GJ between miR-67-expressing gliosarcoma cells and target cells expressing a luciferase reporter containing an miR-67 binding site. They observed that following co-culture, luciferase expression was reduced, an effect that was reversed by the presence of carbenoxolone, a GJ uncoupler.72,76 Recently Aucher et al.77 found that miRNA were transferred from human macrophages to a hepatocarcinoma cell line. These studies suggest first that, miRNA transfer between cells can occur via GJ and second, that transferred miRNA are functional. Given that GJ formation occurs between T cells and DC during interaction at the immune synapse78 the possibility that the intercellular transfer of miRNA occurs between these cells warrants further research.
MicroRNA are also found in exosomes released by cells such as DC and T cells.74,79 The miRNA profiling of DC exosomes found that miRNA expression differs with the maturation status of the DC.75 Interestingly, miRNA present in DC-derived exosomes can be transferred to other DC in vitro and in vivo where they are functional.75 Recently, Mittelbrunn et al. observed that T-cell-derived exosomes also contain specific miRNA, which can be transferred to antigen-presenting cells, leading to modification of cell function.74,80,81 T-cell-derived exosomes are released during immune synapse formation, suggesting that during immune interactions directed release of miRNA-laden vesicles to DC during immune recognition may lead to immune modulation.80
Concluding remarks
It is clear that miRNA expression in BM progenitors drives DC differentiation, and in immature/mature DC miRNA expression helps to shape the adaptive immune response as well as resolve it through inducing death of the DC. Although miRNA plays an important role in DC function following interaction with infectious agents such as viruses, bacteria and parasites, little is known about whether cells of the immune system modulate DC miRNA during infection and tolerance induction either directly or via exosome release. Given the unique role of DC within the immune system, presenting antigen and shaping immune responses, understanding how cells of the adaptive immune system regulate DC miRNA is of vital importance.
The observation that both pathogens and tumours have evolved strategies that can modulate DC miRNA, creating either a non-inflammatory or inflammatory environment, is of clinical relevance and warrants further investigation in defined disease states.
Acknowledgments
This work was supported by a grant from the British Heart Foundation. This work was also supported by the Department of Health via the National Institute for Health Research Comprehensive Biomedical Research Center award to Guy's and St Thomas’ NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust.
Disclosures
The authors declare no financial or commercial conflict of interest.
References
- Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40:642–56. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol. 2014;14:417–28. doi: 10.1038/nri3683. [DOI] [PubMed] [Google Scholar]
- Dalod M, Chelbi R, Malissen B, Lawrence T. Dendritic cell maturation: functional specialization through signaling specificity and transcriptional programming. EMBO J. 2014;33:1104–16. doi: 10.1002/embj.201488027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CC, Ali N, Karagiannis P. Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. J Exp Med. 2012;209:935–45. doi: 10.1084/jem.20112583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong C, Matos I, Choi JH. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209+ dendritic cells for immune T cell areas. Cell. 2010;143:416–29. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Carbone FR, Heath WR. Cutting edge: conventional CD8α+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- Mildner A, Chapnik E, Manor O. Mononuclear phagocyte miRNome analysis identifies miR-142 as critical regulator of murine dendritic cell homeostasis. Blood. 2013;121:1016–27. doi: 10.1182/blood-2012-07-445999. [DOI] [PubMed] [Google Scholar]
- Murphy KM. Transcriptional control of dendritic cell development. Adv Immunol. 2013;120:239–67. doi: 10.1016/B978-0-12-417028-5.00009-0. [DOI] [PubMed] [Google Scholar]
- Chen CZ, Schaffert S, Fragoso R, Loh C. Regulation of immune responses and tolerance: the microRNA perspective. Immunol Rev. 2013;253:112–28. doi: 10.1111/imr.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro F, Lieberman J. Small RNAs guide hematopoietic cell differentiation and function. J Immunol. 2010;184:5939–47. doi: 10.4049/jimmunol.0902567. [DOI] [PubMed] [Google Scholar]
- Slezak-Prochazka I, Durmus S, Kroesen BJ, van den Berg A. MicroRNAs, macrocontrol: regulation of miRNA processing. RNA. 2010;16:1087–95. doi: 10.1261/rna.1804410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TY. The role of microRNAs in regulatory T cells and in the immune response. Immune Netw. 2011;11:11–41. doi: 10.4110/in.2011.11.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–98. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- Lai EC. Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet. 2002;30:363–4. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432:231–5. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–40. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293:1146–50. doi: 10.1126/science.1064023. [DOI] [PubMed] [Google Scholar]
- Dueck A, Ziegler C, Eichner A, Berezikov E, Meister G. microRNAs associated with the different human Argonaute proteins. Nucleic Acids Res. 2012;40:9850–62. doi: 10.1093/nar/gks705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–4. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek A, Grun D, Poy MN. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- Tang R, Li L, Zhu D. Mouse miRNA-709 directly regulates miRNA-15a/16-1 biogenesis at the posttranscriptional level in the nucleus: evidence for a microRNA hierarchy system. Cell Res. 2012;22:504–15. doi: 10.1038/cr.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Calado DP, Galler G. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131:146–59. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Thai TH, Calado DP, Casola S. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–8. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- Seddiki N, Brezar V, Ruffin N, Levy Y, Swaminathan S. Role of miR-155 in the regulation of lymphocyte immune function and disease. Immunology. 2014;142:32–8. doi: 10.1111/imm.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumjohann D, Ansel KM. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat Rev Immunol. 2013;13:666–78. doi: 10.1038/nri3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers H, Schnorfeil FM, Fehling HJ, Bartels H, Brocker T. Dicer-dependent microRNAs control maturation, function, and maintenance of Langerhans cells in vivo. J Immunol. 2010;185:400–9. doi: 10.4049/jimmunol.0903912. [DOI] [PubMed] [Google Scholar]
- Lu C, Huang X, Zhang X. miR-221 and miR-155 regulate human dendritic cell development, apoptosis, and IL-12 production through targeting of p27kip1, KPC1, and SOCS-1. Blood. 2011;117:4293–303. doi: 10.1182/blood-2010-12-322503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Wu L. Functional regulation of monocyte-derived dendritic cells by microRNAs. Protein Cell. 2012;3:497–507. doi: 10.1007/s13238-012-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner ML, Schnorfeil FM, Brocker T. MicroRNAs regulate dendritic cell differentiation and function. J Immunol. 2011;187:3911–7. doi: 10.4049/jimmunol.1101137. [DOI] [PubMed] [Google Scholar]
- Su X, Qian C, Zhang Q. miRNomes of haematopoietic stem cells and dendritic cells identify miR-30b as a regulator of Notch1. Nat Commun. 2013;4:2903. doi: 10.1038/ncomms3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riepsaame J, van Oudenaren A, den Broeder BJ, van Ijcken WF, Pothof J, Leenen PJ. MicroRNA-mediated down-regulation of M-CSF receptor contributes to maturation of mouse monocyte-derived dendritic cells. Front Immunol. 2013;4:353. doi: 10.3389/fimmu.2013.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimi ST, Fulcher JA, Chang MH, Gov L, Wang S, Lee B. MicroRNA profiling identifies miR-34a and miR-21 and their target genes JAG1 and WNT1 in the coordinate regulation of dendritic cell differentiation. Blood. 2009;114:404–14. doi: 10.1182/blood-2008-09-179150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers H, Schnorfeil FM, Brocker T. Differentially expressed microRNAs regulate plasmacytoid vs. conventional dendritic cell development. Mol Immunol. 2010;48:333–40. doi: 10.1016/j.molimm.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Li HS, Greeley N, Sugimoto N, Liu YJ, Watowich SS. miR-22 controls Irf8 mRNA abundance and murine dendritic cell development. PLoS One. 2012;7:e52341. doi: 10.1371/journal.pone.0052341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrich JJ, Jachimowski LC, Libouban M. MicroRNA-146a regulates survival and maturation of human plasmacytoid dendritic cells. Blood. 2013;122:3001–9. doi: 10.1182/blood-2012-12-475087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch M, Zernecke A. microRNAs in the regulation of dendritic cell functions in inflammation and atherosclerosis. J Mol Med (Berl) 2012;90:877–85. doi: 10.1007/s00109-012-0864-5. [DOI] [PubMed] [Google Scholar]
- Dunand-Sauthier I, Irla M, Carnesecchi S, Seguin-Estevez Q, Vejnar CE, Zdobnov EM, Santiago-Raber ML, Reith W. Repression of arginase-2 expression in dendritic cells by microRNA-155 is critical for promoting T cell proliferation. J Immunol. 2014;193:1690–700. doi: 10.4049/jimmunol.1301913. [DOI] [PubMed] [Google Scholar]
- Dunand-Sauthier I, Santiago-Raber ML, Capponi L. Silencing of c-Fos expression by microRNA-155 is critical for dendritic cell maturation and function. Blood. 2011;117:4490–500. doi: 10.1182/blood-2010-09-308064. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Vigorito E, Clare S. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–11. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao CP, He L, Tsai YC. In vivo microRNA-155 expression influences antigen-specific T cell-mediated immune responses generated by DNA vaccination. Cell Biosci. 2011;1:3. doi: 10.1186/2045-3701-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Nunez RT, Louafi F, Friedmann PS, Sanchez-Elsner T. MicroRNA-155 modulates the pathogen binding ability of dendritic cells (DCs) by down-regulation of DC-specific intercellular adhesion molecule-3 grabbing non-integrin (DC-SIGN) J Biol Chem. 2009;284:16334–42. doi: 10.1074/jbc.M109.011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceppi M, Pereira PM, Dunand-Sauthier I, Barras E, Reith W, Santos MA, Pierre P. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci USA. 2009;106:2735–40. doi: 10.1073/pnas.0811073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Huang X, Cui H, Luo X, Tang Y, Chen S, Wu L, Shen N. miR-155 and its star-form partner miR-155* cooperatively regulate type I interferon production by human plasmacytoid dendritic cells. Blood. 2010;116:5885–94. doi: 10.1182/blood-2010-04-280156. [DOI] [PubMed] [Google Scholar]
- Zhang M, Liu F, Jia H. Inhibition of microRNA let-7i depresses maturation and functional state of dendritic cells in response to lipopolysaccharide stimulation via targeting suppressor of cytokine signaling 1. J Immunol. 2011;187:1674–83. doi: 10.4049/jimmunol.1001937. [DOI] [PubMed] [Google Scholar]
- Sun Y, Sun J, Tomomi T, Nieves E, Mathewson N, Tamaki H, Evers R, Reddy P. PU.1-dependent transcriptional regulation of miR-142 contributes to its hematopoietic cell-specific expression and modulation of IL-6. J Immunol. 2013;190:4005–13. doi: 10.4049/jimmunol.1202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agudo J, Ruzo A, Tung N. The miR-126-VEGFR2 axis controls the innate response to pathogen-associated nucleic acids. Nat Immunol. 2014;15:54–62. doi: 10.1038/ni.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, Pulendran B. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI3K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–64. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhan Z, Xu L, Ma F, Li D, Guo Z, Li N, Cao X. MicroRNA-148/152 impair innate response and antigen presentation of TLR-triggered dendritic cells by targeting CaMKIIα. J Immunol. 2010;185:7244–51. doi: 10.4049/jimmunol.1001573. [DOI] [PubMed] [Google Scholar]
- Brain O, Owens BM, Pichulik T. The intracellular sensor NOD2 induces microRNA-29 expression in human dendritic cells to limit IL-23 release. Immunity. 2013;39:521–36. doi: 10.1016/j.immuni.2013.08.035. [DOI] [PubMed] [Google Scholar]
- Mi QS, Xu YP, Qi RQ, Shi YL, Zhou L. Lack of microRNA miR-150 reduces the capacity of epidermal Langerhans cell cross-presentation. Exp Dermatol. 2012;21:876–7. doi: 10.1111/exd.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi QS, Xu YP, Wang H, Qi RQ, Dong Z, Zhou L. Deletion of microRNA miR-223 increases Langerhans cell cross-presentation. Int J Biochem Cell Biol. 2013;45:395–400. doi: 10.1016/j.biocel.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Wu J, Zhao J. miR-29b and miR-29c are involved in Toll-like receptor control of glucocorticoid-induced apoptosis in human plasmacytoid dendritic cells. PLoS One. 2013;8:e69926. doi: 10.1371/journal.pone.0069926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JR, Ma Y, Churchman L, Gordon SA, Dawicki W. Regulatory dendritic cells for immunotherapy in immunologic diseases. Front Immunol. 2014;5:7. doi: 10.3389/fimmu.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TX, Hartner J, Lim EJ. MicroRNA-21 limits in vivo immune response-mediated activation of the IL-12/IFN-γ pathway, Th1 polarization, and the severity of delayed-type hypersensitivity. J Immunol. 2011;187:3362–73. doi: 10.4049/jimmunol.1101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, Feng T, Yao S, Wolf KJ, Liu CG, Liu X, Elson CO, Cong Y. Microbiota downregulates dendritic cell expression of miR-10a, which targets IL-12/IL-23p40. J Immunol. 2011;187:5879–86. doi: 10.4049/jimmunol.1100535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Jiang HY, Li J. MicroRNA-23b promotes tolerogenic properties of dendritic cells in vitro through inhibiting Notch1/NF-κB signalling pathways. Allergy. 2012;67:362–70. doi: 10.1111/j.1398-9995.2011.02776.x. [DOI] [PubMed] [Google Scholar]
- Stumpfova Z, Hezova R, Meli AC, Slaby O, Michalek J. MicroRNA profiling of activated and tolerogenic human dendritic cells. Mediators Inflamm. 2014;2014:259689. doi: 10.1155/2014/259689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger CM, Podyminogin RL, Navarro G, Zhao GW, Askovich PS, Weiss MJ, Aderem A. miR-451 regulates dendritic cell cytokine responses to influenza infection. J Immunol. 2012;189:5965–75. doi: 10.4049/jimmunol.1201437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrazas LI, Sanchez-Munoz F, Perez-Miranda M, Mejia-Dominguez AM, Ledesma-Soto Y, Bojalil R, Gomez-Garcia L. Helminth excreted/secreted antigens repress expression of LPS-induced Let-7i but not miR-146a and miR-155 in human dendritic cells. Biomed Res Int. 2013;2013:972506. doi: 10.1155/2013/972506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, Cao AT, Cao X. Downregulation of microRNA-107 in intestinal CD11c+ myeloid cells in response to microbiota and proinflammatory cytokines increases IL-23p19 expression. Eur J Immunol. 2014;44:673–82. doi: 10.1002/eji.201343717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmasso G, Nguyen HT, Yan Y, Laroui H, Charania MA, Ayyadurai S, Sitaraman SV, Merlin D. Microbiota modulate host gene expression via microRNAs. PLoS One. 2011;6:e19293. doi: 10.1371/journal.pone.0019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L, Jay F, Nomura K, He SY, Voinnet O. Suppression of the microRNA pathway by bacterial effector proteins. Science. 2008;321:964–7. doi: 10.1126/science.1159505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyfferoen L, Mestdagh P, Vergote K, De Cabooter N, Vandesompele J, Lambrecht BN, Vermaelen KY. Lung tumours reprogram pulmonary dendritic cell immunogenicity at the microRNA level. Int J Cancer. 2014;135:2868–77. doi: 10.1002/ijc.28945. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Gregersen PK, Diamond B. Regulation of dendritic cell activation by microRNA let-7c and BLIMP1. J Clin Invest. 2013;123:823–33. doi: 10.1172/JCI64712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueck A, Eichner A, Sixt M, Meister G. A miR-155-dependent microRNA hierarchy in dendritic cell maturation and macrophage activation. FEBS Lett. 2014;588:632–40. doi: 10.1016/j.febslet.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Katakowski M, Buller B, Wang X, Rogers T, Chopp M. Functional microRNA is transferred between glioma cells. Cancer Res. 2010;70:8259–63. doi: 10.1158/0008-5472.CAN-10-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PK, Bliss SA, Patel SA. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71:1550–60. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecalvo A, Larregina AT, Shufesky WJ. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–66. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink PR, Valiunas V, Gordon C, Rosen MR, Cohen IS. Can gap junctions deliver? Biochim Biophys Acta. 2012;1818:2076–81. doi: 10.1016/j.bbamem.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Aucher A, Rudnicka D, Davis DM. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J Immunol. 2013;191:6250–60. doi: 10.4049/jimmunol.1301728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Naranjo A, Bouma G, Pereda C. Functional gap junctions accumulate at the immunological synapse and contribute to T cell activation. J Immunol. 2011;187:3121–32. doi: 10.4049/jimmunol.1100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli AE, Larregina AT, Shufesky WJ. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–66. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- Choudhuri K, Llodra J, Roth EW. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature. 2014;507:118–23. doi: 10.1038/nature12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell RM, Kahn D, Gibson WS. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33:607–19. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Li Z, Tu J. MicroRNA-29a regulates pro-inflammatory cytokine secretion and scavenger receptor expression by targeting LPL in oxLDL-stimulated dendritic cells. FEBS Lett. 2011;585:657–63. doi: 10.1016/j.febslet.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Cui Y, Su WY, Xing J. MiR-29a inhibits cell proliferation and induces cell cycle arrest through the downregulation of p42.3 in human gastric cancer. PLoS One. 2011;6:e25872. doi: 10.1371/journal.pone.0025872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Cui H, Xie N. miR-125a-5p regulates differential activation of macrophages and inflammation. J Biol Chem. 2013;288:35428–36. doi: 10.1074/jbc.M112.426866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–6. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]