Abstract
The specific function of human skin-resident dendritic cell (DC) subsets in the regulation of immunity or tolerance is still a matter of debate. Langerhans cells (LC) induce anti-viral immune responses but, conversely to dermal DC, maintain tolerance to bacteria. However, the definite function of epidermal LC and cutaneous DC appears even more complex under inflammatory conditions. Here we investigated the immune responses of human immature monocyte-derived DC (MoDC) and LC-like cells (MoLC) upon stimulation with different Toll-like receptor ligands in the presence or absence of pro-inflammatory cytokines tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β). In MoDC, bacterial antigens selectively up-regulated CD83 and CD86 expression and induced the release of T helper type 1 (Th1) and Th17 cytokines and led to a higher CCR7-dependent migratory capacity compared with a low responsiveness of MoLC. Importantly, MoLC activation with lipopolysaccharide under inflammatory conditions strongly enhanced a phenotypically mature state, increased IL-12p70, IL-23 and IL-6 production and Th1 cytokine secretion by CD4+ T cells. Treatment with poly(I:C) specifically up-regulated surface expression of co-stimulatory molecules and increased release of IL-12p70 in MoLC and co-stimulation with TNF-α and IL-1β further elevated Th1 and Th17 cytokine production. Poly(I:C)-induced up-regulation of type I interferon mRNA levels in MoLC and MoDC was Toll-like receptor 3-dependent but not, or only weakly, modulated by pro-inflammatory cytokines. Our results indicate that inflammatory conditions greatly facilitate recognition of bacteria by MoLC. Furthermore, we suggest a critical involvement of both subsets in innate defence against viruses, whereas inflammatory skin environments additionally favour MoLC as potent inducers of Th1 and Th17 cytokines.
Keywords: dendritic cells, interleukin-1β, Langerhans cells, Toll-like receptors, tumour necrosis factor-α
Introduction
Dendritic cells (DC) are professional antigen-presenting cells and provide a link between the innate and adaptive immune systems by the recognition of invading pathogens and subsequent presentation of the processed antigens to naive T cells.1,2 The distinct DC subtypes are characterized by their particular localization and critical induction of immunity or tolerance, depending on the predetermined antigen specificity. Previous studies identified several subsets within human skin, demonstrating the existence of a complex network of dermal DC (DDC) and Langerhans cells (LC), a highly specialized population that is localized in the epidermis and so extensively exposed to microbial antigens.3,4
The analysis of DC maturation processes substantially extended our knowledge of the notable regulation of skin-associated immunity and tolerance. The recognition of invading pathogens is mediated by distinctive pattern recognition receptors, including Toll-like receptors (TLRs), triggering the maturation of skin-resident DC. Recent studies revealed a striking disparity in the expression of TLRs in various human subsets.5 Langerhans cells display a unique profile of TLRs and, as a result, exhibit a strongly impaired reactivity to whole bacteria or specified bacterial molecules, and so conversely to DDC, possibly contributing to the induction of tolerance towards the non-pathogenic commensal skin flora.6–9 In contrast, LC treated with antigens associated with viral infections, strongly promoted adaptive immune responses by activated CD4+ and CD8+ T cells.10,11
However, the selective stimulation of human DC subsets, according to the specific repertoire and functionality of pattern recognition receptors, insufficiently clarified their detailed reactivity towards pathogens in acute or chronic inflammatory skin environments. Here, a disturbed or abnormal barrier function facilitates the invasion of antigens and induces the release of tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) by surrounding keratinocytes and dermal fibroblasts.12,13 Notably, these inflammatory cytokines critically mediate a persistent and complex interplay between resident LC and DDC, skin-homing T cells and microbial pathogens. For instance, previous studies identified IL-1β as an IL-12-inducing agent in DC,14 whereas TNF-α was critically required to mature DC efficiently during virus-mediated stimulation.15 Therefore, it has been considered, that inflammatory environments possibly modulate the reactivity of skin-resident DC populations towards microbial challenge.
Research on pathological or inflamed human skin is evidently hampered by ethical aspects and difficulties in isolating pure and immature DC populations from diseased explants. As a consequence, the generation of genetically modified mice, selectively depleted of langerin+ DC, recently provided new insights into the discrete regulation of skin immunity in vivo.16–18 However, despite the differentiated characterization of LC and DDC, the transferability of murine infection models to human skin is still questionable.19,20 At present, little is known about the distinctive functional specialization of human DC subsets and the associated cellular mechanisms resulting in immunity or tolerance under steady-state and inflammatory skin conditions.
In this study, we investigated the maturation profile of in vitro generated human immature monocyte-derived DC (MoDC) and LC-like cells (MoLC) upon stimulation with TLR ligands and characterized the modulations in subsequent immunogenic processes in the absence or presence of pro-inflammatory cytokines. Treatment with recombinant human (rh) TNF-α and rhIL-1β, mimicking an inflammatory environment, strikingly modulated the maturation status, assessed by surface expression of co-stimulatory molecules, CCR7-dependent migratory activity and secretion of pro-inflammatory cytokines. Moreover, besides the initiation of adaptive immunity, a TLR3-dependent activation by poly(I:C) induced the up-regulation of type 1 interferon (IFN) mRNA levels in both subsets, suggesting a critical involvement in innate activity against viral pathogens. The evident modulation of maturation-related processes, induced by rhTNF-α and rhIL-1β, clearly indicates a distinctive reactivity of skin-resident DC populations under steady state and inflammatory skin conditions.
Materials and methods
Generation of LC-like cells and dendritic cells from human monocytes (MoLC and MoDC)
Both MoLC and MoDC were generated from plastic-adherent human monocytes as previously described.21 Peripheral blood mononuclear cells (PBMC) were obtained from buffy-coat donations from anonymous healthy volunteers (DRK-Blutspendedienst Ost, Berlin, Germany) after informed consent. All studies have been approved by the ethics committee of the Charité – Universitätsmedizin Berlin, Germany. Within 24 hr, density gradient centrifugation was performed using NycoPrepTM 1.077 (Axis-Shield, Olso, Norway), resulting in 10 × 107 to 25 × 107 PBMC in the interphase. After multiple washes in PBS (PAA Laboratories GmbH, Linz, Austria) without Ca2+/Mg2+, supplemented with EDTA (20 mm; Sigma Aldrich, Taufkirchen, Germany), monocyte purification was performed by PBMC adherence in 75-cm2 cell-culture flasks (Techno Plastic Products AG, Trasadingen, Switzerland) for 35 min. Adherent monocytes were subsequently differentiated by a 6-day culture in complete medium, consisting of RPMI-1640 (Sigma Aldrich) with 2 mm l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin (all from PAA Laboratories GmbH) and 10% heat-inactivated fetal calf serum (Biochrom, Berlin, Germany), supplemented with recombinant human granulocyte–macrophage colony-stimulating factor (100 ng/ml) and rhIL-4 (20 ng/ml) in the presence or absence of recombinant human transforming growth factor-β1 (rhTGF-β1; 20 ng/ml; all from Miltenyi Biotec, Bergisch Gladbach, Germany) to generate MoLC and MoDC, respectively. Non-adherent cells were used for the isolation of naive CD4+ T cells. At days 2 and 4, 50% of the medium was replaced with an equal volume of complete medium containing recombinant human granulocyte–macrophage colony-stimulating factor and either rhTGF-β1 for MoLC or rhIL-4 for MoDC. At day 6, the non-adherent cells were collected and additionally sorted by CD1a MicroBeads (clone HI149; Miltenyi Biotech). MACS was performed by passing the cells over large cell-separation columns (Miltenyi Biotech) according to the manufacturer's instructions, leading to immature CD1a+ CD207+ CD324+ TROP-2+ Axl+ MoLC and CD1a+ CD209+ CD207− MoDC, respectively, examined by a FACSCalibur flow cytometer (BD Bioscience, Heidelberg, Germany).
Stimulation of MoLC and MoDC
At day 7, generated MoLC and MoDC were harvested, washed three times in PBS without Ca2+/Mg2+ (PAA Laboratories), supplemented with EDTA (20 mm; Sigma Aldrich) and seeded in a 24-well cell-culture plate (BD Bioscience) in complete medium without supplemented cytokines at a density of 106 cells/ml. Subsequently, the cells were treated with Pam3CSK4 (1 μg/ml), ultrapure lipopolysaccharide (LPS) from Escherichia coli serotype 0111:B4 (1 μg/ml), poly(I:C) (1 μg/ml, all from InvivoGen, San Diego, CA), rhTNF-α (20 ng/ml), rhIL-1β (30 ng/ml) or soluble rhCD40 ligand (CD40L; 500 ng/ml; all from eBioscience, Frankfurt, Germany) for 24 or 48 hr.
Blocking experiments
At day 7, generated MoLC and MoDC were harvested, washed three times in PBS and seeded in a 24 well cell-culture plate (BD Bioscience), pre-incubated with complete medium supplemented with chloroquine (20 μm; Sigma Aldrich) for 1 hr and subsequently stimulated with different agonists as described above for additional 24 hr in the presence of chloroquine (20 μm).
Isolation and culture of naive CD4+ T cells
To obtain naive human CD4+ T cells, negative isolation from non-adherent human PBMCs was performed using a Naïve CD4+ T Cell Isolation Kit II (Miltenyi Biotec), according to the manufacturer's instructions. Briefly, 10 × 107 cells were indirectly labelled with a cocktail of biotin-conjugated mouse anti human anti-CD8, -CD14,-CD15, -CD16, -CD19, -CD25, -CD34, -CD36, -CD45RO+, -CD56, -CD123, -TCR-γ/δ, -HLA-DR and -CD235a antibodies and subsequently anti-Biotin MicroBeads were added. Negative isolation was performed by passing the cells over large cell separation columns (Miltenyi Biotech). 5 × 106 to 10 × 106 cells were obtained and directly characterized by surface expression of CD4 (clone VIT4; Miltenyi Biotec) and CD45RA (clone T6D11; Miltenyi Biotec), in two-colour flow cytometry (BD Bioscience) leading to a > 90% CD4+, CD45RA+ double-positive fraction. A viability of > 95% was determined by Trypan blue exclusion assay. Isolated T cells were subsequently cultured in a 96-well cell culture plate with round bottom (Corning, Amsterdam, the Netherlands) at a density of 105 cells per 100 μl in RPMI-1640 containing 10% heat inactivated fetal calf serum (Biochrom) and 2 mm l-glutamine (PAA Laboratories). 104 immature or mature MoLC, stimulated with LPS (1 μg/ml) or rhTNF-α (20 ng/ml) and rhIL-1β (30 ng/ml) alone or in combination for 24 hr, were added and co-cultured with naive human CD4+ T cells. Experiments were performed in duplicate. At day 5, cell culture supernatant was collected and cytokine levels were measured by ELISA.
Flow cytometry
The cell surface expression of various proteins was analysed by two-colour flow cytometry. Cells were labelled with the following fluorophore-conjugated monoclonal antibodies: FITC-conjugated mouse anti-CD1a (clone HI149), anti-CD80 (clone 2D10.4; all from eBioscience), anti-CD86 (clone FM95; Miltenyi Biotec), anti-CD324 (clone 67A4; Biolegend, London, UK) and corresponding isotype control (eBioscience), phycoerythrin-conjugated mouse anti-CD207 (clone 10E2), anti-CD83 (clone HB15e; all from Biolegend), anti-CD209 (clone DCN47.5; Miltenyi Biotec) anti-CD197 (clone 150503; BD Bioscience), anti-CD184 (clone 12G5) and corresponding isotype control (all from eBioscience), Peridinin chlorophyll protein-conjugated mouse anti-CD14 (clone TÜK4) and corresponding isotype control (all from Miltenyi Biotec), Alexa Fluor-conjugated mouse anti-Axl (clone 108724) and corresponding isotype control (all from R&D Systems, Wiesbaden, Germany) and unconjugated mouse anti-TROP-2 (clone 162-46; BD Bioscience) and corresponding isotype control (eBioscience), followed by polyclonal phycoerythrin-conjugated goat F(ab’)2 anti-mouse IgG1 (R&D Systems) for secondary staining. Cells were examined using a FACSCalibur flow cytometer collecting a total of 10 × 103 to 20 × 103 events. Dead cells and debris were excluded by scatter gates and propidium iodide staining (1 μg/ml; Sigma Aldrich).
Intracellular cytokine staining
At day 7, generated MoLC and MoDC were harvested, washed three times in PBS and stimulated with different agonists, as described above. After 6 hr of incubation, brefeldin A or monensin solution (1 ×; all from Biolegend) was added to the medium to stop the vesicular transport. After an additional 18 hr, the production of various cytokines was analysed by flow cytometry. Briefly, the cells were washed and fixed with 2% paraformaldehyde in PBS at room temperature for 20 min and further stained with FITC-conjugated rat anti-IL-6 (clone MQ2-13A5; Biolegend), phycoerythrin-conjugated mouse anti-IL-12p40 (clone HP40; eBioscience) or phycoerythrin-conjugated rat anti-IL-10 (clone JES3-9D7; BD Bioscience) in the presence of BD Perm/Wash (1 ×; BD Bioscience). 10 × 103 to 20 × 103 cells were counted on a FACSCalibur flow cytometer. Cell debris was excluded by scatter gates.
Migration assay
At day 7, generated MoLC and MoDC were harvested, washed three times in PBS and stimulated with different agonists for 48 hr, as described above. After washing, 5 × 105 cells in complete medium were added to the upper well of a 24-well transwell plate with 8-μm pore size (BD Bioscience). Complete medium supplemented with rhCCL21 (100 ng/ml; Miltenyi Biotec) was added to the lower well. Cells were allowed to migrate for 3·5 hr. The migrated cells were harvested and counted in a FACSCalibur flow cytometer for 100 s. Cell debris was excluded by scatter gates.
ELISA
The cell culture supernatant was collected at the conclusion of the experiments and assayed for IL-6, IL-23, IL-12p70 and IFN-γ by using commercially available ELISA kits (DuoSet; R&D Systems and ELISA-Ready Set Go; eBioscience).
RNA isolation and quantitative RT-PCR
Total RNA isolation, cDNA synthesis and quantitative RT-PCR were performed as described previously.22 Primers (synthesized by TIB Molbiol, Berlin, Germany) with the following sequences were used: YWHAZ, IFN-α, IFN-β and TLR1–10 as published previously,23,24 and SDHA, 5′-TGGGAACAAGAGGGCATCTG-3′ and 5′-CCACCACTGCATCAAATTCATG-3′. Fold difference in gene expression was normalized to the housekeeping gene SDHA or YWHAZ, which showed the most constant level of expression.
Immunofluorescence
MoLC and MoDC were harvested at day 7, washed in PBS and applied on a poly-lysine-coated slide (Thermo Fisher Scientific Bioscience, St Leon-Rot, Germany) using a cytospin procedure.25 Afterwards, the adherent monolayer was fixed with 4% paraformaldehyde (Carl Roth, Karlsruhe, Germany) for 10 min, washed with PBS and permeabilized with 0·5% Triton X-100 (Carl Roth) for a further 10 min, followed by washing with 0·0025% BSA (Aurion, Wageningen, the Netherlands) and 0·025% Tween (Carl Roth) in PBS three times for 5 min. After washing, slides were blocked with goat serum (1 : 20; Dianova, Hamburg, Germany) for 30 min and incubated with the following primary antibodies at 4° overnight: rabbit anti-E-cadherin (1 : 500), rabbit anti-CD1a (1 : 1000; all from Abcam, Boston, MA) and mouse-anti-Langerin (1 : 250; Dendritics, Dardilly, France). Secondary DyLight488-conjugated and DyLight594-conjugated anti-rabbit or anti-mouse antibodies (1 : 400; Dianova) were applied after washing for 1 hr at room temperature. All washing and antibody addition steps were performed with a combination of PBS, BSA and Tween. Cells were mounted in ImmunoSelect Antifading Mounting Medium with DAPI (Dianova). Images were obtained using a BZ-8000 fluorescence microscope (Keyence Deutschland GmbH, Neu-Isenburg, Germany).
Statistical analysis
Data are expressed as means + SEM or as box-and-whisker plots. Statistical significance of differences was determined by Kruskal–Wallis test followed by Dunn's multiple comparison test and considered significant at P < 0·05. Statistical analysis was conducted using GraphPad Prism software (San Diego, CA).
Results
MoLC highly up-regulate maturation markers in response to bacterial TLR ligands under inflammatory conditions
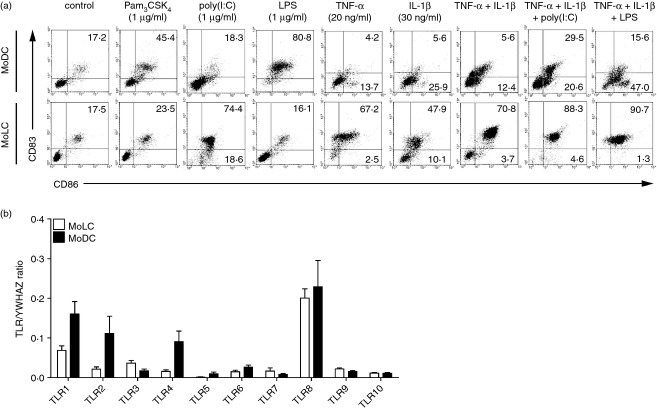
Freshly isolated human monocytes differentiate toward either MoLC or MoDC, depending on the presence or absence of TGF-β1.21 Phenotypic analysis revealed a distinct profile of surface proteins, used for the evaluation of differentiation and maturity (see Supporting information, Fig. S1a,b). The immature status of CD1a+ CD207+ CD324+ TROP-2+ Axl+ MoLC and CD1a+ CD209+ CD207− MoDC, was confirmed by the assessment of surface levels of HLA-DR, maturation marker CD83 and co-stimulatory proteins CD80 and CD86 (Fig. S1b,c). To address the reactivity of both populations towards bacterial antigens, we compared their ability to up-regulate CD83 and CD86 upon activation with ligands for TLR2/1 (Pam3CSK4) and TLR4 (LPS). Clearly, MoDC revealed a phenotypically mature state after 24 hr of stimulation (Fig.1a), whereas MoLC were less responsive and only marginally up-regulated CD83 and CD86 surface expression. This was in line with lower mRNA levels of TLR1, -2 and -4 of immature MoLC, compared with MoDC (Fig.1b) and confirmed previous findings of an attenuated activity of Langerhans cells to bacterial commensals.6–9 Conversely, stimulation with pro-inflammatory cytokines TNF-α and IL-1β alone, or in combination, highly induced the expression of CD83 and CD86 in MoLC. Accordingly, the addition of TNF-α and IL-1β to LPS mimicked a bacterial infection under inflammatory conditions and strongly enhanced the phenotypically mature state of stimulated MoLC, compared with TLR4 ligation alone. Considering the distinct maturation profile of both subsets, our results reveal a highly specialized tolerogenic function of MoLC towards bacterial commensals, which possibly converts into immunogenic activity in inflammatory skin environments.
Figure 1.
Monocyte-derived Langerhans cell-like cells (MoLC) and dendritic cells (MoDC) differentially up-regulate maturation marker CD83 and co-stimulatory molecule CD86 after stimulation with various agonists. (a) Immature cells were incubated with defined activation mixtures, containing Toll-like receptor (TLR) ligands, tumour necrosis factor-α (TNF-α) or interleukin-1β (IL-1β) for 24 hr. The specific concentrations and combinations of the distinct stimuli and percentage of stained cells are indicated. Surface expression of CD83 and CD86 was analysed by two-colour flow cytometry. Analysis of 10 000 cells of gated scatter plot from the same donor is representative of four to six independent experiments. (b) MoLC and MoDC differed quantitatively in the gene profile of TLR. Gene expression values of TLR1–10 are normalized to YWHAZ. Data represent mean values + SEM (n = 6).
Poly(I:C) specifically increases CD83 and CD86 expression in MoLC
Langerhans cells are ideally positioned in the epidermis to capture invading viruses, probably exerting effective anti-viral immunity.26 Hence, we determined the recognition of TLR3 ligand poly(I:C) alone, or in combination with TNF-α and IL-1β. Indeed, after 24 hr of stimulation, MoLC strongly up-regulated the expression of CD83 and CD86 (Fig.1a), indicating a high susceptibility against viral antigens. Although unexpected, but in line with lower TLR3 mRNA expression in MoDC compared with MoLC (Fig.1b), stimulated MoDC revealed a less mature state in response to poly(I:C) alone. However, MoDC up-regulated the surface levels of CD83 and CD86 in the presence of TNF-α and IL-1β and CD86 further increased when poly(I:C) was added. In summary, our results underline the possible involvement of MoLC in the induction of anti-viral immune responses and further support unique functional specializations of various DC subsets in human skin.27
TLR ligands induce production and release of Th1 and Th17 cytokines in MoLC in the presence of pro-inflammatory cytokines and enhance Th1 cytokine production by CD4+ T cells
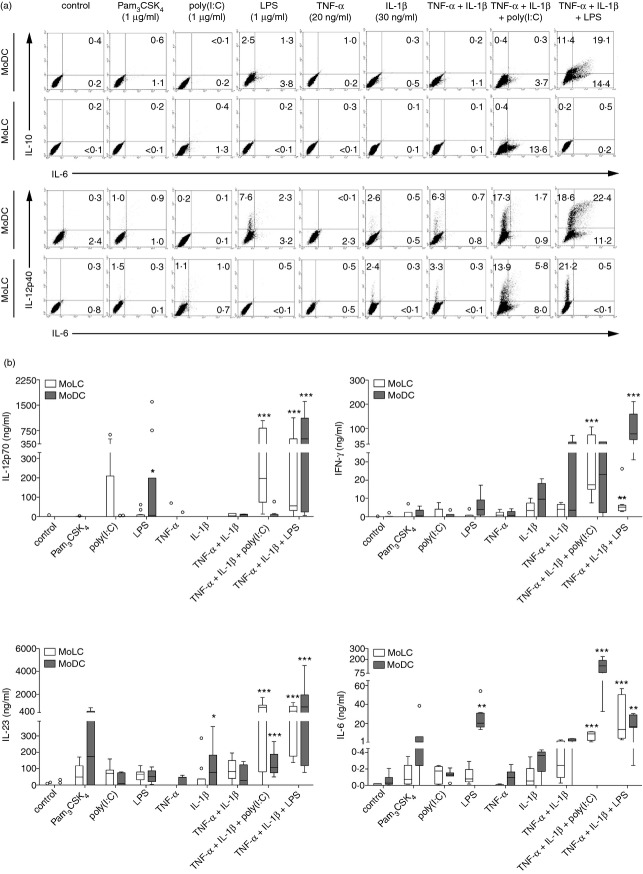
Activated DC subsets expressing distinct maturation markers are not necessarily functionally mature.28 Therefore, assessment of the surface levels of CD83 and CD86 on stimulated MoLC and MoDC is insufficient to completely characterize their functional properties. To evaluate the processes that are accompanied upon 24 hr of activation, we investigated the production of various immunoregulatory cytokines. Intracellular cytokine staining revealed a high increase of IL-12p40+ MoDC in the presence of LPS (Fig.2a), which was further amplified when stimulated in combination with pro-inflammatory cytokines. TNF-α and IL-1β enhanced the capacity to produce IL-6 but also remarkable amounts of IL-10. Therefore, besides the ability to initiate effective adaptive immunity against bacterial pathogens, DDC possibly permit the development of regulatory T cells under inflammatory skin conditions, hence negatively regulating the ongoing immune response and preventing an exaggeration and aggravation of inflammation.29 In contrast, MoLC failed to induce IL-10 production in response to TLR ligands alone or in combination with pro-inflammatory cytokines, but showed increased IL-12p40+ cells when activated with IL-1β alone and substantially when combined with TNF-α and LPS or poly(I:C), respectively.
Figure 2.
Monocyte-derived Langerhans cell-like cells (MoLC) and dendritic cells (MoDC) show distinct patterns of cytokine production and release in response to bacterial or viral Toll-like receptor (TLR) ligands in the presence or absence of tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β). (a) Immature cells were activated with the indicated TLR ligands, cytokines alone or in combination for 24 hr together with brefeldin A [interleukin-6 (IL-6) and IL-12p40] or monensin solution (IL-10), respectively. The specific concentrations and combinations of the tested compounds and percentage of stained cells are indicated. Detection of intracellular cytokines was examined by intracellular two-colour flow cytometry. Analysis of 20 000 counted cells of gated scatter plot from the same donor is representative of four to six independent experiments. (b) Immature MoLC and MoDC were stimulated with Pam3CSK4 (1 μg/ml), lipopolysaccharide (LPS; 1 μg/ml), poly(I:C) (1 μg/ml), recombinant human (rh) TNF-α (20 ng/ml) and rhIL-1β (30 ng/ml) alone or in combination for 24 hr. Levels of pro-inflammatory cytokines IL-12p70, interferon-γ, IL-23 and IL-6 in cell culture supernatants were measured by ELISA. Box plots represent the median (horizontal line) and interquartile range (box) and whiskers 1.5× the interquartile range. Outliers are indicated with (O). (n = 5–17). *P < 0·05, **P < 0·01, ***P < 0·001 vs. controls, Kruskal–Wallis test followed by Dunn's multiple comparison test.
We further determined the potential to induce distinct T-helper cell responses by analysing the release of Th1 (IL-12p70, IFN-γ) and Th17 restricted (IL-6, IL-23) cytokines in respective cell culture supernatants (Fig.2b). In response to TLR2/1 ligand Pam3CSK4, MoDC released high levels of IL-23 and IL-6. In addition, stimulation with LPS significantly increased IL-12p70 and IL-6 secretion. In accordance with the enhanced cytokine production in the presence of pro-inflammatory cytokines, the addition of TNF-α and IL-1β led to a significant increase of IFN-γ and IL-23 secretion and further enhanced IL-12p70 release by activated MoDC, hence indicating a potent Th1 and Th17 priming capacity. Furthermore, in line with the inability of MoLC to up-regulate CD83 and CD86 surface expression in response to bacterial TLR ligands alone, the addition of pro-inflammatory cytokines is crucial to significantly increase the release of IL-12p70, IFN-γ, IL-23 and IL-6. Regarding the specific profile of cytokine release induced by LPS together with TNF-α and IL-1β, our observations suggest a possible new role for MoLC in the recognition of bacteria under inflammatory skin conditions, in synergy with dermal DC.
To confirm this assumption, we subsequently analysed the capacity of MoLC to prime Th1 responses and co-cultured purified naive CD4+ T cells with activated MoLC, previously stimulated with the TLR4 ligand alone, rhTNF-α and rhIL-1β, as well as the combination of pro-inflammatory cytokines and LPS (Fig.3). In accordance with the low responsiveness of MoLC upon stimulation with LPS alone, the respective cell culture supernatants revealed low levels of IFN-γ, indicating no Th1 polarization. In contrast, the presence of pro-inflammatory cytokines greatly elevated IFN-γ production by activated T cells, which was even amplified when LPS was added. Although only one batch of generated MoLC showed reduced IFN-γ release upon LPS stimulation (data not shown), our results confirm the distinctive reactivity of MoLC towards bacterial antigens in the presence of TNF-α and IL-1β, possibly leading to an enhanced Th1 activity under inflammatory skin conditions.
Figure 3.
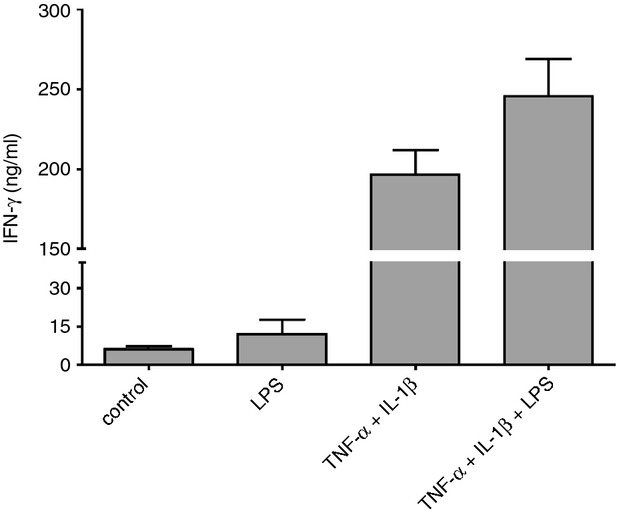
Monocyte-derived Langerhans cell-like cells (MoLC) activation with lipopolysaccharide (LPS) under inflammatory conditions enhances interferon-γ (IFN-γ) production by CD4+ T cells. MoLC were stimulated with LPS (1 μg/ml) or recombinant human tumour necrosis factor-α (rhTNF-α; 20 ng/ml) and recombinant human interleukin-1β (rhIL-1β; 30 ng/ml) alone or in combination for 24 hr and subsequently co-cultured for 5 days with naive CD4+ T cells, isolated from human peripheral blood mononuclear cells. Secretion of interferon-γ (IFN-γ) was measured by ELISA. Data represent mean values + SEM (n = 5).
Besides bacterial TLR ligands, stimulation with poly(I:C) greatly increased the number of IL-12p40+ and IL-6+ MoLC and MoDC, respectively, in the presence of TNF-α and IL-1β (Fig.2a). Analysing the observed cytokine production in more detail, poly(I:C) alone enhanced the secretion of IL-12p70 by activated MoLC (Fig.2b). Moreover, the combination with pro-inflammatory cytokines greatly induced the release of Th1- and Th17-restricted cytokines, which was in accordance with their phenotypically mature state (Fig.1) and further emphasized that MoLC critically exert skin-associated anti-viral immune responses. Compared with stimulation with TNF-α and IL-1β, we indeed confirmed a significant increase of IL-23 and IL-6 levels in cell culture supernatants of activated MoDC when poly(I:C) was added. Moreover, comparing the intense enhancement of the IL-12p40+ MoDC population with the secretion of respective cytokines simultaneously, we obtained no elevated levels of IL-12p70 and a slight, but significant increase of IL-23 in respective cell culture supernatants. Therefore, in comparison to the secreted cytokine pattern obtained by the activation mixture containing pro-inflammatory cytokines and LPS, MoDC revealed a reduced secretion of heterodimeric proteins in the presence of poly(I:C), indicating that dermal DC are possibly mainly responsible for the release of a monomeric p40 subunit in response to viral antigens, with critical chemoattractant properties and other, not yet fully identified, independent immunoregulatory functions.30
MoDC have a higher CCR7-dependent migratory capacity than MoLC
The chemokine receptor CCR7 has been identified as key regulator for DC migration from the periphery into skin-draining lymph nodes.31,32 Therefore, we investigated the migratory capacity of stimulated MoLC and MoDC towards the CCR7 ligand rhCCL21 in an in vitro transwell migration assay. After 48 hr of stimulation, enhanced migration of both subsets was observed (Fig.4a). Notably, activated MoDC obtained higher migratory potential than MoLC when stimulated with bacterial TLR ligands with or without pro-inflammatory cytokines, assuming a higher capacity to activate T cells in the lymphoid organs and favouring DDC as key regulators for the initiation of immune response towards bacterial pathogens. In accordance with the initially obtained less mature state of MoDC in response to poly(I:C) alone, we obtained a lower migratory capacity compared with co-stimulation with pro-inflammatory cytokines. Furthermore, the impaired migration of MoLC after 48 hr of activation corresponds to the two-step migration model of epidermal LC, which initially depends on the expression of chemokine receptor CXCR4 and the emigration from the epidermis into the dermis, whereas the up-regulation of CCR7 occurs at later stages of maturation.33 To confirm our hypothesis, we assessed the relative up-regulation of CXCR4 and CCR7 by activated MoLC after 24 and 48 hr, respectively (Fig.4b). Using soluble CD40L as potent inducer of DC migration and chemokine receptor expression,34,35 we found enhanced surface levels of CXCR4 in stimulated MoLC upon 24 hr of activation, compared with untreated cells, which was further increased after 48 hr and accompanied by the expression of CCR7. In contrast, activation with bacterial TLR ligands alone or in combination with pro-inflammatory cytokines only slightly increased the surface levels of both receptors, according to the low migratory potential towards rhCCL21. Therefore, our data confirm functional differences between MoLC and MoDC, including the distinctive potential of lymph node homing, hence leading to different contributions to adaptive immunity against microbial challenge.
Figure 4.
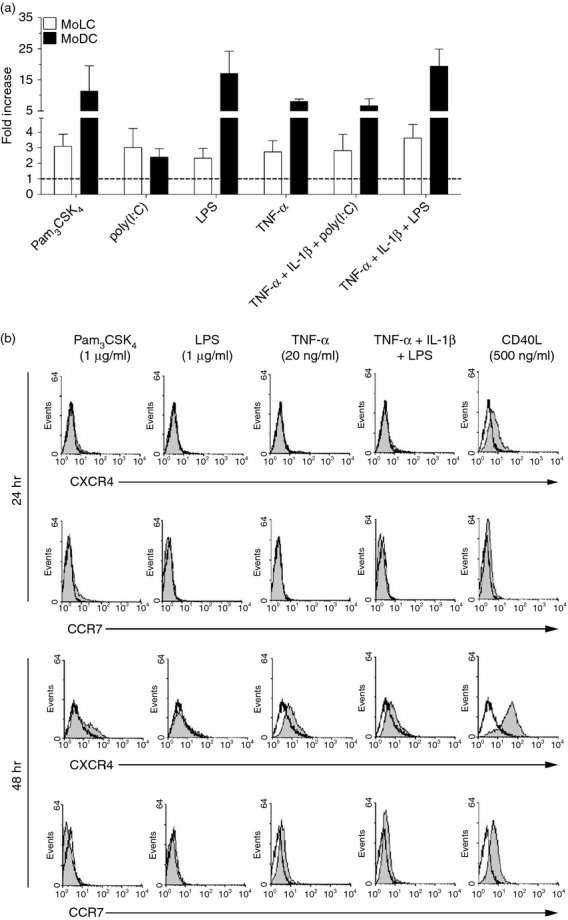
Stimulation of monocyte-derived Langerhans cell-like cells (MoLC) and monocyte-derived dendritic cells (MoDC) for 48 hr leads to altered migratory capacity towards CCR7 ligand CCL21. (a) MoLC and MoDC were stimulated with Pam3CSK4 (1 μg/ml), lipopolysaccharide (LPS; 1 μg/ml), poly(I:C) (1 μg/ml), recombinant human tumour necrosis factor-α (rhTNF-α; 20 ng/ml) and recombinant human interleukin-1β (rhIL-1β; 30 ng/ml) alone or in combination for 48 hr. After washing, cells were allowed to migrate for 3·5 hr in response to 100 ng/ml rhCCL21. Migrated cells in the lower chamber of the transwell plate were harvested and counted in a flow cytometer for 100 seconds. Migrated cells are relative to controls (control assigned as 1·0). Data represent mean values + SEM (n = 5 to 12). (b) Surface expression of chemokine receptors CXCR4 and CCR7 in MoLC, upon stimulation with Toll-like receptor ligands, pro-inflammatory cytokines or soluble CD40 ligand (CD40L) for 24 and 48 hr, was analysed by flow cytometry. The specific concentrations and combinations of the tested compounds are indicated. Histograms show surface expression of antigens (grey; control unfilled) of 10 000 counted cells of gated scatter plot. Analysis is representative of four independent experiments.
Poly(I:C) elicits TLR3-dependent up-regulation of type 1 IFN mRNA levels independent of pro-inflammatory cytokines
Our results suggest that inflammatory conditions influence the Th1/Th17 cytokine balance against viral pathogens by increasing Th1 and Th17 cytokine production by MoLC. In contrast, pro-inflammatory cytokines failed to increase poly(I:C)-induced CCR7-dependent migration in both subsets and poly(I:C) alone did not efficiently trigger critical processes of MoDC maturation. Therefore, we speculated whether both subsets possibly contribute to innate immune responses towards viral pathogens, rather than the induction of adaptive immunity.8 To confirm this assumption, we analysed the gene profile of potent anti-viral cytokines IFN-α and IFN-β in activated MoLC and MoDC, respectively. Indeed, both subsets significantly up-regulated the levels of IFN-β mRNA (Fig.5b) in response to poly(I:C), whereas MoDC additionally elevated IFN-α levels (Fig.5a). Therefore, we assume that the many immunological fates of skin-resident DC against viral pathogens include pivotal contributions to innate and adaptive immunity, presumably dependent on respective skin environments. To evaluate the induced modulation of gene levels in more detail, we activated both subsets in the presence of chloroquine to inhibit the endosomal acidification and activation of intracellular localized TLRs.36 Indeed, treatment with chloroquine markedly reduced the increase of type 1 IFN mRNA levels in both subsets and abrogated the poly(I:C)-dependent up-regulation of TLR3 mRNA in MoDC (Fig.5c), confirming that poly(I:C) is specifically recognized by endosomal TLRs.37 The addition of pro-inflammatory cytokines had no or little effect on the poly(I:C)-induced up-regulation of type I IFN expression.
Figure 5.
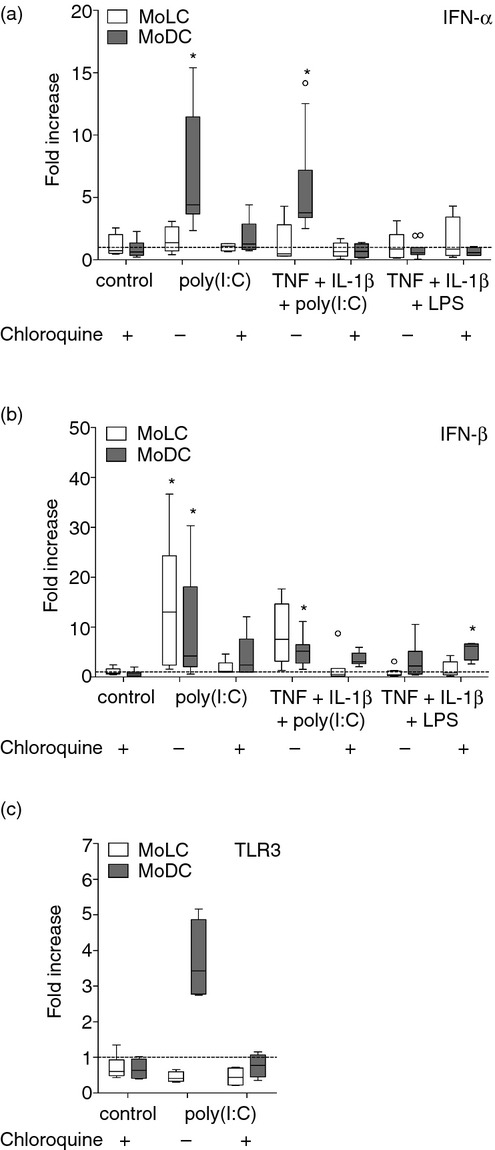
Monocyte-derived Langerhans cell-like cells (MoLC) and dendritic cells (MoDC) differently up-regulate mRNA levels of effective antiviral cytokines interferon-α (IFN-α) and IFN-β upon activation. MoLC and MoDC were stimulated with lipopolysaccharide (LPS; 1 μg/ml) in combination with pro-inflammatory cytokines or with poly(I:C) (1 μg/ml) alone or together with recombinant human tumour necrosis factor-α (rhTNF-α) (20 ng/ml) and recombinant human interleukin-1β (rhIL-1β; 30 ng/ml) for 24 hr with or without chloroquine (20 μm). Gene expression values of (a) IFN-α and (b) IFN-β are normalized to YWHAZ and SDHA and are relative to controls (control assigned as 1·0). Box plots represent the median (horizontal line) and interquartile range (box) and whiskers 1.5× the interquartile range. Outliers are indicated with (O). (n = 4 to 10). *P < 0·05 vs. controls without chloroquine, Kruskal–Wallis test followed by Dunn's multiple comparison test. (c) Chloroquine treatment abrogates poly(I:C)-induced up-regulation of Toll-like receptor 3 (TLR3) in activated MoDC. Gene expression values of TLR3 are normalized to YWHAZ and SDHA and are relative to controls (control assigned as 1·0). Box plots represent the median (horizontal line) and interquartile range (box) and whiskers 1.5× the interquartile range. (n = 4–6).
Discussion
Mouse infection models substantially extended our current knowledge on the distinctive functions of DC populations under pathological skin conditions. At present, the many functions of human subsets are mainly derived from genetically modified mice, despite various physiological differences, including origin and distribution of murine DC, compared with human counterparts.38,39 Hence, little is currently known about the distinctive functional contributions of human LC and DDC against pathogenic challenge within steady-state conditions compared with inflammatory skin environments.
In our study, we demonstrate substantial differences in the maturation process of human MoLC and MoDC generated in vitro, in response to TLR agonists in the presence or absence of rhTNF-α and rhIL-1β. Compared with MoDC, immature MoLC were mainly unresponsive towards bacterial antigens, namely Pam3CSK4 (TLR2/1) or LPS (TLR4), assessed by unaltered surface expression of CD83 and CD86 and a nominal cytokine release. These findings were in accordance with the obtained gene profile of TLRs and confirmed the previously established concept of an attenuated reactivity against bacterial molecules by human LC or in vitro generated counterparts.6–9 Conversely, co-stimulation with rhTNF-α and rhIL-1β, mimicking inflammatory skin conditions, strongly induced the expression of co-stimulatory molecules by MoLC and significantly elevated the production and release of Th1- and Th17-restricted cytokines in response to LPS. Consistent with this, we clearly demonstrated that activated MoLC showed an enhanced Th1 priming capacity in response to pro-inflammatory cytokines and additionally induced IFN-γ release by activated T cells, when LPS was present. The capacity to stimulate and prime naive CD4+ T cells under inflammatory conditions implies a critical immunoregulatory activity of MoLC towards bacterial antigens, in contrast to the recognized tolerogenic function of epidermal LC in healthy skin. Moreover, the elevated release of IL-23 and IL-6 by activated MoLC in response to rhTNF-α, rhIL-1β and LPS, indicates that MoLC might also regulate Th17 activity, thereby critically contributing to the pathogenesis of inflammatory disorders, such as psoriasis vulgaris.
Interestingly, compared with the freshly isolated monocytic progenitors, MoLC and MoDC strongly down-regulated the surface levels of TLR2 and TLR4 during the process of differentiation (data not shown). However, despite the low expression of important TLR proteins, MoDC remained clearly susceptible to bacterial antigens and evolved a highly mature state after stimulation with Pam3CSK4 or LPS, suggesting that specific TLR-dependent downstream signalling pathways, including MyD88- and TRIF-mediated activation of nuclear factor-κB, substantially multiply the different molecular mechanisms involved in maturation-associated cellular processes.40 Immature MoLC, usually unresponsive to bacterial antigens, dramatically induced a phenotypically and functionally mature state in the presence of LPS when pro-inflammatory cytokines were added. Therefore, our findings further indicate that the low immunogenic capacity of MoLC towards bacterial pathogens is not necessarily caused by a lack of specific pattern recognition receptors, but an insufficient activation of related signalling pathways. This effect is possibly mediated by TGF-β1, the key regulator of LC differentiation, which has previously been reported to dampen the susceptibility of DC to various stimuli.41 Receptor binding of rhTNF-α and rhIL-1β on MoLC might cross-regulate the TLR4-dependent activation by LPS, reducing the molecular mechanisms involved in the induction of functional tolerance. Indeed, the IL-1 receptor family shares common similarities and significant homologies with TLR2/1 and TLR4, leading to the activation of pivotal transcription factors mediating the inflammatory response.42 Hence, our data indicate that the induction of tolerance to microbial commensals is evidently important under steady-state conditions, whereas inflammatory skin environments critically drive MoLC to contribute to the maintenance and regulation of immunity, in synergy with DDC as considerable inducers of potent immune responses against bacterial pathogens.
Regarding the early onset of skin-associated immunity, mature MoDC revealed a strong CCR7-dependent migratory capacity when stimulated with Pam3CSK4 or LPS, independent of pro-inflammatory stimuli, so emphasizing the crucial contribution of DDC in the initiation of skin immunity against bacterial pathogens. Moreover, the low migratory capability of activated MoLC was in accordance with the recently established two-step model of LC migration in vivo, highlighting the initial dependence on CXCR4 to induce the migration from the epidermis into dermis. The transient period when activated LC are in the dermis possibly leads to critical interactions with DDC, indicating a potent mechanism to amplify the initiated immune response, until the subsequent up-regulation of CCR7 surface expression facilitates the migration to skin-draining lymph nodes.43 Indeed, the low expression of chemokine receptors confirmed the immature state of MoLC, whereas stimulation with soluble CD40L, a member of the TNF superfamily and T-cell-dependent activator of antigen-presenting cells, clearly enhanced the surface levels of CXCR4 and CCR7 on MoLC after 24 and 48 hr, respectively. The observation that bacterial TLR ligands, in the presence or absence of pro-inflammatory stimuli only slightly altered the expression of chemokine receptors, indicated that MoLC are not primarily contributing to the initiation of adaptive immune responses towards bacterial antigens, but possibly play an emerging immunoregulatory role at later onset of immunity. The CD40L-dependent induction of CXCR4 and CCR7 surface expression suggests that skin-homing T effector cells, as a consequence of previous DC interaction in lymphoid organs, activate a potent migratory capacity on MoLC. Indeed, besides the expression of chemokine receptors, soluble CD40L highly induces a phenotypically mature state, but fails to enhance the release of Th1- and Th17-restricted cytokines in activated MoLC44 (data not shown). The functional properties of DC in this so called ‘semi-mature’ state mainly differ from the immunogenic capacity obtained by stimulation with microbial antigens.45,46 Hence, MoLC might exert an important tolerogenic function in the presence of CD40L, characterized by functional advantages over general immature cells through enhanced potential of lymph node homing and antigen presentation, resulting in a higher capacity to activate and prime regulatory T-cell subsets, so possibly down-regulating the immune response against bacterial pathogens.
Interestingly, considering the reactivity of MoLC and MoDC against viral antigens, MoLC strongly induced Th1 cytokines in response to stimulation with the TLR3 ligand poly(I:C). Similarly to in vivo counterparts, which are ideally positioned to capture invading viruses and initiate a potent anti-viral immune response,26 MoLC strongly induced the surface expression of CD83 and CD86 after 24 hr of stimulation and elevated the release of IL-12p70. In contrast, MoDC indeed up-regulated mRNA levels of TLR3, confirming a specific activation by poly(I:C), but revealed a phenotypically and functionally less mature state, compared with MoLC and only exerted a low migratory capacity towards rhCCL21, after 48 hr of stimulation.47 These rather unexpected findings were supplemented by the notable up-regulation of mRNA levels of potent antiviral cytokines in both subsets. Regarding the low CCR7-dependent migration in response to poly(I:C), together with the modulation of IFN-β mRNA levels in MoLC and IFN-α and IFN-β in MoDC, our findings are in accordance with previous studies, suggesting that epidermal LC and dermal DC exhibit direct anti-viral activity,8 possibly in immunological synergy with pDC, which are known to effectively induce type 1 IFNs in response to viral pathogens.48 In contrast, the poly(I:C)-dependent activation in the presence of TNF-α and IL-1β significantly induced IL-6 secretion in MoDC, whereas MoLC elevated the release of Th1 and Th17 restricted cytokines. Again, the functional abilities of both subsets differed distinctly under the influence of inflammatory stimuli, where the initiation of a potent adaptive immunity was strongly driven due to the presence of pro-inflammatory cytokines, whereas steady-state conditions primarily favoured a type 1 IFN-dependent innate immune response.
In summary, our study revealed a substantial specialization of monocyte-derived human DC subsets in the regulation of skin-associated tolerance and immunity against microbial pathogens. Under steady-state conditions, epidermal LC and cutaneous DC act as sentinels, capturing invading pathogens depending on the expression and functionality of specific pattern recognition receptors, whereas our study reveals that inflammatory environments have a determining influence on the regulation of immunity in MoLC and MoDC. It seems, however, questionable whether the use of primary monocytes, obtained from human buffy coat samples to differentiate MoDC and MoLC ex vivo, adequately represents immunological functions of skin resident DC in vivo. Nevertheless, monocytes are mainly recruited to the skin under inflammatory conditions and act as progenitors for various DC populations, including Langerhans cells, to repopulate the dermis and epidermis.49,50 The distinctive reactivity of these ex vivo-derived DC subsets therefore might in part resemble the immunoregulatory properties of recently described inflammatory DC subsets, found in inflammatory skin diseases like atopic dermatitis and psoriasis vulgaris.51 Surrounding keratinocytes and dermal fibroblasts might act as pathophysiological sources of inflammatory cytokines, thereby possibly modulating the pivotal role of MoLC and MoDC in the skin-associated immune system. Further studies are required to understand the exact molecular mechanisms balancing the immunoregulatory role of DC subsets in human skin present under steady-state or inflammatory conditions.
New strategies for immunotherapies and vaccinations recently targeted skin-resident DC subsets as potent inducers of adaptive T-cell responses;52,53 our current study indicates that the efficiency of prophylaxis in microbial infections is strictly dependent on the microenvironment, determining the induction of cell-mediated immunity or tolerance.
Author contributions
AS, SB and GM performed the experiments and analysed data. GW supervised the work. AS and GW conceived and designed the experiments and wrote the manuscript.
Disclosures
The authors declare no conflicts of interest.
Glossary
- DC
dendritic cell
- DDC
dermal dendritic cell
- LC
Langerhans cell
- MoDC
monocyte-derived DC
- MoLC
monocyte-derived LC
- LPS
lipopolysaccharide
- Pam3CSK4
palmitoyl-3-cysteine-serine-lysine-4
- poly(I:C)
polyinosinic:polycytidylic acid
- PRR
pattern recognition receptor
- TLR
Toll-like receptor
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. In vitro generated monocyte-derived Langerhans cell-like cells (MoLC) and dendritic cells (MoDC) display typical features of immature human counterparts.
References
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Steinman RM. Dendritic cells: understanding immunogenicity. Eur J Immunol. 2007;37(Suppl. 1):S53–60. doi: 10.1002/eji.200737400. [DOI] [PubMed] [Google Scholar]
- Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17:273–83. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–47. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- Schreibelt G, Tel J, Sliepen KH, Benitez-Ribas D, Figdor CG, Adema GJ, de Vries IJ. Toll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1573–82. doi: 10.1007/s00262-010-0833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi J, Watari E, Shinya E. Down-regulation of Toll-like receptor expression in monocyte-derived Langerhans cell-like cells: implications of low-responsiveness to bacterial components in the epidermal Langerhans cells. Biochem Biophys Res Commun. 2003;306:674–9. doi: 10.1016/s0006-291x(03)01022-2. [DOI] [PubMed] [Google Scholar]
- Flacher V, Bouschbacher M, Verronese E. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J Immunol. 2006;177:7959–67. doi: 10.4049/jimmunol.177.11.7959. [DOI] [PubMed] [Google Scholar]
- Renn CN, Sanchez DJ, Ochoa MT. TLR activation of Langerhans cell-like dendritic cells triggers an antiviral immune response. J Immunol. 2006;177:298–305. doi: 10.4049/jimmunol.177.1.298. [DOI] [PubMed] [Google Scholar]
- van der Aar AM, Sylva-Steenland RM, Bos JD, Kapsenberg ML, de Jong EC, Teunissen MB. Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J Immunol. 2007;178:1986–90. doi: 10.4049/jimmunol.178.4.1986. [DOI] [PubMed] [Google Scholar]
- Furio L, Billard H, Valladeau J, Peguet-Navarro J, Berthier-Vergnes O. Poly(I:C)-treated human langerhans cells promote the differentiation of CD4+ T cells producing IFN-γ and IL-10. J Invest Dermatol. 2009;129:1963–71. doi: 10.1038/jid.2009.21. [DOI] [PubMed] [Google Scholar]
- van der Aar AM, de Groot R, Sanchez-Hernandez M, Taanman EW, van Lier RA, Teunissen MB, de Jong EC, Kapsenberg ML. Cutting edge: virus selectively primes human Langerhans cells for CD70 expression promoting CD8+ T cell responses. J Immunol. 2011;187:3488–92. doi: 10.4049/jimmunol.1101105. [DOI] [PubMed] [Google Scholar]
- Lisby S, Faurschou A, Gniadecki R. The autocrine TNFα signalling loop in keratinocytes requires atypical PKC species and NF-κB activation but is independent of cholesterol-enriched membrane microdomains. Biochem Pharmacol. 2007;73:526–33. doi: 10.1016/j.bcp.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Mauviel A, Temime N, Charron D, Loyau G, Pujol JP. Induction of interleukin-1β production in human dermal fibroblasts by interleukin-1α and tumor necrosis factor-α. Involvement of protein kinase-dependent and adenylate cyclase-dependent regulatory pathways. J Cell Biochem. 1991;47:174–83. doi: 10.1002/jcb.240470211. [DOI] [PubMed] [Google Scholar]
- Wesa AK, Galy A. IL-1β induces dendritic cells to produce IL-12. Int Immunol. 2001;13:1053–61. doi: 10.1093/intimm/13.8.1053. [DOI] [PubMed] [Google Scholar]
- Trevejo JM, Marino MW, Philpott N, Josien R, Richards EC, Elkon KB, Falck-Pedersen E. TNF-α-dependent maturation of local dendritic cells is critical for activating the adaptive immune response to virus infection. Proc Natl Acad Sci USA. 2001;98:12162–7. doi: 10.1073/pnas.211423598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley K, Igyarto BZ, Ortner D, Bobr A, Kashem S, Schenten D, Kaplan DH. Langerhans cells require MyD88-dependent signals for Candida albicans response but not for contact hypersensitivity or migration. J Immunol. 2012;188:4334–9. doi: 10.4049/jimmunol.1102759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elftman MD, Gonzalez-Hernandez MB, Kamada N, Perkins C, Henderson KS, Nunez G, Wobus CE. Multiple effects of dendritic cell depletion on murine norovirus infection. J Gen Virol. 2013;94:1761–8. doi: 10.1099/vir.0.052134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneschal J, Jiang X, Kupper TS. Langerin dermal DC, but not Langerhans cells, are required for effective CD8-mediated immune responses after skin scarification with vaccinia virus. J Invest Dermatol. 2014;134:686–94. doi: 10.1038/jid.2013.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–56. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp Med. 1998;187:961–6. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindl G, Castello F, Schäfer-Korting M. Evaluation of anti-inflammatory and atrophogenic effects of glucocorticoids on reconstructed human skin. Altern Lab Anim. 2011;39:173–87. doi: 10.1177/026119291103900212. [DOI] [PubMed] [Google Scholar]
- Weindl G, Naglik JR, Kaesler S, Biedermann T, Hube B, Korting HC, Schaller M. Human epithelial cells establish direct antifungal defense through TLR4-mediated signaling. J Clin Invest. 2007;117:3664–72. doi: 10.1172/JCI28115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi S, Schäfer-Korting M, Weindl G. TLR2/1 and sphingosine 1-phosphate modulate inflammation, myofibroblast differentiation and cell migration in fibroblasts. Biochim Biophys Acta. 2014;1841:484–94. doi: 10.1016/j.bbalip.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Sisino G, Bellavia D, Corallo M, Geraci F, Barbieri R. A homemade cytospin apparatus. Anal Biochem. 2006;359:283–4. doi: 10.1016/j.ab.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Cunningham AL, Carbone F, Geijtenbeek TB. Langerhans cells and viral immunity. Eur J Immunol. 2008;38:2377–85. doi: 10.1002/eji.200838521. [DOI] [PubMed] [Google Scholar]
- Klechevsky E, Morita R, Liu M. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–83. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–31. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28:33–8. doi: 10.1016/j.it.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Ohl L, Mohaupt M, Czeloth N. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–88. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Villablanca EJ, Mora JR. A two-step model for Langerhans cell migration to skin-draining LN. Eur J Immunol. 2008;38:2975–80. doi: 10.1002/eji.200838919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodycliffe AM, Shreedhar V, Ullrich SE, Walterscheid J, Bucana C, Kripke ML, Flores-Romo L. CD40–CD40 ligand interactions in vivo regulate migration of antigen-bearing dendritic cells from the skin to draining lymph nodes. J Exp Med. 2000;191:2011–20. doi: 10.1084/jem.191.11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandella E, Men Y, Gillessen S, Förster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–61. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol. 2011;186:4794–804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Guo M, Wang X. TLR3 activation efficiency by high or low molecular mass poly I:C. Innate Immun. 2013;19:184–92. doi: 10.1177/1753425912459975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. 2012;119:3383–93. doi: 10.1182/blood-2011-11-370130. [DOI] [PubMed] [Google Scholar]
- Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology. 2013;140:22–30. doi: 10.1111/imm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi H, Akira S. TLR signalling and the function of dendritic cells. Chem Immunol Allergy. 2005;86:120–35. doi: 10.1159/000086657. [DOI] [PubMed] [Google Scholar]
- Ohtani T, Mizuashi M, Nakagawa S, Sasaki Y, Fujimura T, Okuyama R, Aiba S. TGF-β1 dampens the susceptibility of dendritic cells to environmental stimulation, leading to the requirement for danger signals for activation. Immunology. 2009;126:485–99. doi: 10.1111/j.1365-2567.2008.02919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Ouwehand K, Santegoets SJ, Bruynzeel DP, Scheper RJ, de Gruijl TD, Gibbs S. CXCL12 is essential for migration of activated Langerhans cells from epidermis to dermis. Eur J Immunol. 2008;38:3050–9. doi: 10.1002/eji.200838384. [DOI] [PubMed] [Google Scholar]
- Peiser M, Wanner R, Kolde G. Human epidermal Langerhans cells differ from monocyte-derived Langerhans cells in CD80 expression and in secretion of IL-12 after CD40 cross-linking. J Leukoc Biol. 2004;76:616–22. doi: 10.1189/jlb.0703327. [DOI] [PubMed] [Google Scholar]
- Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- Lutz MB. Therapeutic potential of semi-mature dendritic cells for tolerance induction. Front Immunol. 2012;3:123. doi: 10.3389/fimmu.2012.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Nouen C, Hillyer P, Winter CC, McCarty T, Rabin RL, Collins PL, Buchholz UJ. Low CCR7-mediated migration of human monocyte derived dendritic cells in response to human respiratory syncytial virus and human metapneumovirus. PLoS Pathog. 2011;7:e1002105. doi: 10.1371/journal.ppat.1002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Caux C, Ait-Yahia S, Chemin K. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin Immunopathol. 2000;22:345–69. doi: 10.1007/s002810000053. [DOI] [PubMed] [Google Scholar]
- Seré K, Baek JH, Ober-Blöbaum J, Müller-Newen G, Tacke F, Yokota Y, Zenke M, Hieronymus T. Two distinct types of Langerhans cells populate the skin during steady state and inflammation. Immunity. 2012;37:905–16. doi: 10.1016/j.immuni.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Segura E, Amigorena S. Inflammatory dendritic cells in mice and humans. Trends Immunol. 2013;34:440–5. doi: 10.1016/j.it.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Bonifaz LC, Bonnyay DP, Charalambous A. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–24. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch L, Ronchese F, Romani N. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci USA. 2006;103:7783–8. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. In vitro generated monocyte-derived Langerhans cell-like cells (MoLC) and dendritic cells (MoDC) display typical features of immature human counterparts.