Abstract
CD38 is a 45 000 molecular weight transmembrane protein that is expressed in immature and mature lymphocytes. However, the expression and function of CD38 during B-cell differentiation in mice is poorly understood. Here, we report that CD38 is expressed from the earliest stages of B-cell development. Pre-pro-B, pro-B, pre-B and immature B cells from murine bone marrow all stained positive for CD38. Interestingly, CD38 expression increases with B-cell maturation. To assess the role of CD38 during B-cell maturation, CD38-deficient mice were analysed. CD38−/− mice showed a significant increase in both the frequency of B-lineage cells and the absolute numbers of pre-pro-B cells in bone marrow; however, no other differences were observed at later stages. CD38 cross-linking in Ba/F3 cells promoted apoptosis and marked extracellular signal-regulated kinase (ERK) phosphorylation, and these effects were reduced by treatment with the mitogen-activated protein kinase/ERK kinase inhibitor PD98059, and similar effects were observed in B-cell precursors from bone marrow. These data demonstrate that B-cell precursors in mouse bone marrow express functional CD38 and implicate the early ligation of CD38 in the ERK-associated regulation of the B-lineage differentiation pathway.
Keywords: bone marrow, CD38, extracellular signal-regulated kinase, mouse B-cell development
Introduction
CD38 is a type II transmembrane protein with both enzymatic and receptor functions. This protein is widely expressed at variable levels in various tissues and cells, particularly leucocytes. The extracellular domain of CD38 catalyses the conversion of nicotinamide adenine dinucleotide (NAD) to cyclic adenosine diphosphate ribose (cADPR), a potent metabolite that mobilizes calcium1 and efficiently hydrolyses cADPR to adenosine diphosphoribose (ADPR).2 Agonistic antibodies against CD38 induce a number of biological responses, such as cell proliferation, phosphorylation of signalling proteins, intracellular Ca2– mobilization, and induction and inhibition of apoptosis.3–5
CD38 expression fluctuates during haematopoietic differentiation. Although some studies have reported discrepancies in CD38 expression between mature mouse and human haematopoietic cells, the expression of CD38 in bone marrow (BM) is more stable.6–8 High levels of CD38 expression in early lymphoid cells suggest that it might play an important role in this developmental pathway.9
In humans, the expression of CD38 in B-cell and T-cell progenitors is similar to that in leukaemic cells from patients with acute lymphoblastic leukaemia.10 Anti-CD38 monoclonal antibodies have surprising effects on the viability, differentiation and proliferation of lymphoid progenitors. A marked reduction in the number of human B-lymphocyte progenitors was observed upon CD38 ligation in stromal cell co-cultures.11 This striking deleterious effect of anti-CD38 antibodies is also evident in cultured lymphoid and myeloid leukaemic cells.12 Although some of these effects have been attributed to Fc receptor cross-linking,13 there is increasing interest in using anti-CD38 monoclonal antibodies to treat leukaemia and other pathologies.14,15
In lymphocytes, several signalling events triggered by CD38 cross-linking have been characterized. In the Jurkat T-cell line, CD38 induces the phosphorylation and activation of CD3, Lck, Zap-70, AKT, phospholipase C-γ1 (PLC-γ1), extracellular signal-regulated kinase (ERK), LAT and c-Cbl.16,17 In these cells, CD38 is also detected and enriched in lipid rafts upon cell activation.18,19 In immature B lymphocytes, CD38 phosphorylates and activates CD19, Tec, Lyn, Syk, PLC-γ, c-Cbl and phosphoinositide 3-kinase;20–22 whereas in mature B lymphocytes, CD38 activates Btk, Lyn, Fyn, protein kinase C, phosphoinositide 3-kinase and PC-PLC/PLD and promotes IκB-α degradation and nuclear factor-κB nuclear trans-location.23–27 In addition, CD38 mutants lacking the cytoplasmic and transmembrane regions still induce signalling,28,29 suggesting that CD38-dependent signalling may depend on the physical/functional association of CD38 with other surface receptors.9
Accordingly, previous studies have shown that the surface expression of receptors, including the T-cell receptor, B-cell receptor and CD16, is required for the CD38-dependent activation of T cells, mature B lymphocytes and natural killer cells, respectively.16,30,31 Furthermore, in immature B-cell lines, CD38 phosphorylates and activates surface CD19 but not CD79a/b,20 suggesting that CD38 might bind to different receptors in specific cell subsets. This difference in receptor binding also suggests that CD38 could mediate differential signalling in various cell types or subsets, and although many CD38-dependent signalling events have been characterized, a comparative analysis of the specific signalling pathways in different cell types is lacking.
The mitogen-activated protein kinase (MAPK) cascade is one of the most ancient and evolutionarily conserved signalling pathways, and this pathway is important for many processes in the immune response.32 MAPK are part of a phospho-relay system. There are three major groups of MAPK in mammalian cells, p38 MAPK, c-Jun N-terminal kinase and ERK.32 The ERK cascade is activated by numerous stimuli and various internal processes such as proliferation, differentiation and development, and under certain conditions, in cell survival, migration, apoptosis, morphology determination and oncogenic transformation.33 Although the ERK signalling pathway is activated through CD38 in Jurkat cells, it is currently not known whether CD38 also activates this pathway in B lymphocytes.
The aim of this study was to analyse the role of CD38 in the BM of mice. First, by measuring the expression of CD38 in mouse BM, and second, by determining if its absence has an impact on B-cell development. Lastly, we used CD38 cross-linking to determine if CD38 has a receptor function in BM, as has been previously described. Here, we analysed the expression of CD38 in mouse BM throughout B-cell development. The functional evaluation of CD38 in B-cell precursors from BM and Ba/F3 cells suggested a signalling-associated role for this protein in early-stage B-cell development as a regulator of apoptosis.
Materials and methods
Mice
Eight- to twelve-week-old C57BL/6J and B6.129P2-Cd38tm1Lnd/J female mice were maintained at the animal facility of the Centre for Research and Advanced Studies (CINVESTAV). All experiments were approved by the Animal Care and Use Committee of CINVESTAV.
Isolation of BM cells
Bone marrow was isolated from the femurs of C57BL/6J mice using an 18-gauge needle. After passing the marrow through nylon mesh cell strainers to obtain a single-cell suspension in PBS containing 3% fetal calf serum (Invitrogen, Carlsbad, CA), the erythrocytes were depleted with ACK lysis buffer (Invitrogen). The BM cells were subsequently counted by trypan blue exclusion, and the total numbers of cells were calculated.
Identification and purification of B-cell precursors by flow cytometry
Bone marrow cells (3 × 106) suspended in PBS containing 3% fetal calf serum were treated with a monoclonal antibody (clone 2.4G2) to block the Fc receptors, and then stained with the following antibodies: anti-CD19 allophycocyanin-Cy7 (clone ID3), anti-B220 Pacific Blue (clone RA3-6B2), anti-CD43 FITC (clone S7), anti-CD157 biotin (clone BP-3; Pharmingen, San Diego, CA), anti-IgM allophycocyanin (clone 1B4B1), anti-CD38 phyoerythrin (clone NIM-R5), and anti-mouse IgG2b FITC (Southern Biotechnology Associates, Birmingham, AL). Compensation was performed using single-stained cells for each of the fluorochromes used. Data were acquired using a Beckman Coulter CyAn flow cytometer (Brea, CA). Forward scatter-height versus forward scatter-area was used to gate single cells, and each subpopulation was analysed using FlowJo v.7.5 software (Tree Star, Inc., Ashland, OR). For functional assays, suspensions of 2 × 108 cells were stained with the above-mentioned antibodies and sorted using a MoFlo cell sorter (Beckman Coulter, Inc.). Each purified population was collected in 0·5 ml cold fetal calf serum. Cell viability was greater than 95%, and the cells were used immediately.
Ba/F3 cell culture
The IL-3-dependent murine pro-B cell line Ba/F3 was cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum and 10% (volume/volume) WEHI-3 supernatant (containing interleukin-3). CD38-transfected Ba/F3 cells were cultured under similar conditions.34
Annexin-V binding assay
CD38-transfected Ba/F3 cells (106) that were pre-incubated with 100 μm 8-bromo-cyclic adenosine diphosphate ribose (8-Br-cADPR) or left untreated for 4 hr at 37° were incubated with 50 µg/ml NIM-R5, 50 µg/ml NIM-R5 f(ab′)2 and 50 µg/ml anti-CD44 (NIM-R8).35 In the same way, FACS-sorted purified B-cell precursors (105) were incubated with the same concentration of NIM-R5 for 12 hr. After stimulation, the cells were transferred to FACS tubes for annexin-V/propidium staining. Briefly, Ba/F3 or BM cells were washed twice with cold PBS and resuspended in 100 µl Annexin-V binding buffer (0·1 mm HEPES, 137 mm NaCl, and 2·5 mm CaCl2, pH 7·2). The cells were incubated with 0·2 µg biotin-labelled human recombinant annexin-V36 for 10 min at room temperature, and then wash with 2 ml binding buffer and stained for 10 min with FITC-labelled streptavidin (Zymed, San Francisco, CA) and 2 µl of 50 µg/ml propidium iodide (PI; Sigma-Aldrich, St Louis, MO). After incubation, the cells were resuspended in 300 µl binding buffer and immediately captured in a FACSCalibur cytometer [Becton Dickinson (BD), Franklin Lakes, NJ]. Depending on the experiment, the cells were treated with the MAPK kinase inhibitor PD98059 (20 μm; Calbiochem, San Diego, CA) or vehicle (0·5% DMSO) for 30 min before cell activation. The data were analysed using FlowJo software.
Phosphorylation assay using flow cytometry
Total BM cells (2 × 106) or B-cell precursors were starved in serum-free AIMV medium (Life Technologies, Grand Island, NY) for 2 hr before incubation with several stimuli [PMA, 0·2 µg/ml; anti-CD38 antibody, 50 µg/ml (clone NIM-R5)] at 37°. After 5, 10, 15 or 20 min, the cells were fixed with 100 µl formaldehyde (1·5% final concentration) for 10 min. The cells were centrifuged, resuspended in cold methanol, and then incubated on ice for 20 min. The cells were washed in PBS-A (PBS plus 1% albumin and 0·1% sodium azide) and incubated with anti-CD45R Pacific Blue (clone RA3-6B2) and anti-ERK 1/2 pY202/pY204-phycoerythrin (clone 20A; BD Phosflow) antibodies for 30 min at room temperature. The cells were washed with PBS-A, and then analysed in a Beckman Coulter CyAn flow cytometer. The mean fluorescence intensity was obtained from three independent experiments, and a rat IgG-phycoerythrin antibody (Pharmingen) was used as a control. Each subpopulation was analysed using FlowJo v.7.5 software.
Western blot analysis
Total spleen, total BM or Ba/F3 cells (5 × 106) were stimulated for 5 or 10 min with the anti-CD38 antibody (50 µg/ml), PMA (0·2 µg/ml) or rat IgG (50 µg/ml). The cells were treated 30 min before cell activation, and subsequently treated throughout the experiment with the MAPK kinase inhibitor PD98056 (5 or 20 µm) or vehicle (0·5% DMSO). After stimulation, the cells were lysed in a buffer containing 10 mm Tris–HCl (pH 7·3; Merck, Darmstadt, Germany), 2 mm Na3VO4 (Sigma-Aldrich), 0·4 mm EDTA (Mallinckrodt Baker, Phillipsburg, NJ), 10 mm NaF (Sigma-Aldrich), 1 mm PMSF (Sigma-Aldrich), 2 µg/ml aprotinin (Sigma-Aldrich), 2 µg/ml leupeptin (Roche Diagnostics, Indianapolis, IN), and 1% Triton X-100 (volume/volume) (Sigma-Aldrich). Equal amounts of proteins were separated in a 12% SDS–polyacrylamide gel. After separation, the proteins were transferred onto nitrocellulose membranes, and incubated with an anti-phospho-p42/44 MAPK antibody (Thr202/Tyr204; Cell Signaling Technology, Danvers, MA), and then an anti-mouse horseradish peroxidase antibody (Zymed). As a loading control, the membrane was stripped, and total ERK-2 was detected with a polyclonal anti-ERK-2 antibody and anti-rabbit IgG-horseradish peroxidase (DakoCytomation, Dako North America, Carpinteria, CA). Protein signals were detected using the SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific, Waltham, MA).
Statistics
Intergroup comparisons were performed using the unpaired t-test. Two-tailed P-values < 0·05 were considered significant. To confirm statistical significance, one-way or two-way analysis of variance was used, depending on the experiment.
Results
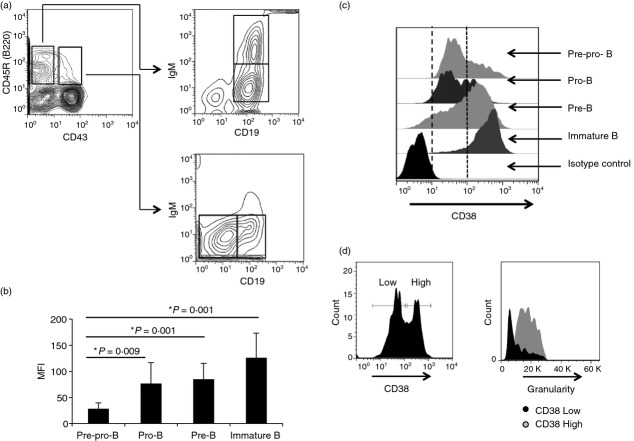
The distinct B-cell precursor populations in BM were identified according to Hardy's strategy.37 Pre-pro-B, pro-B, pre-B and immature B cells were distinguished as B220– CD43− CD19−, B220– CD43− CD19–, B220– CD43– CD19– IgM− and B220– CD43− CD19– IgM–, respectively (Fig.1a). CD38 expression was measured throughout B-lymphoid development. Although CD38 was displayed on cells at all developmental stages, its expression increased as differentiation progressed, and immature B cells had the highest CD38 expression (Fig.1b). Interestingly, CD38 expression was heterogeneous in both the pre-pro-B and pro-B cell populations, and these results were consistent across four different independent experiments (Fig.1c). Larger pro-B cells with high granularity expressed high levels of CD38, suggesting that active or proliferating precursor cells have increased CD38 expression (Fig.1d). Because CD38 has been previously implicated in the development of T1 and T2 B cells,38 and some differences in the frequencies and absolute numbers of T1 (increased) and T2 (decreased) cells in CD38−/− mice were observed, the abundance of sequential B-cell precursors from C57BL/6J and CD38−/− mice was analysed. No differences in absolute numbers or cell frequencies were observed, except in pre-pro-B cells (Fig.2a,b).
Figure 1.
Expression of CD38 on bone marrow (BM) B-cell precursors. (a) Bone marrow cells from C57BL/6 mice were stained with: anti-CD19, anti-CD45R, anti-IgM, anti-CD43, and anti-CD38 antibodies. Pre-pro-B (B220– CD43− CD19−), pro-B (B220– CD43− CD19–), pre-B (B220– CD43– CD19– IgM−), and immature B (B220– CD43− CD19– IgM–) cell fractions were analysed through flow cytometry. (b) CD38 expression within the pre-pro-B, pro-B, pre-B, and immature B-cell populations from murine BM. Values shown in mean and standard deviation (SD). (c) Median fluorescence intensity (MFI) of CD38 expression in different populations of BM B cells. (d) Analysis of granularity and CD38 expression of pro-B cells from murine BM, representative figure from four independent experiments.
Figure 2.
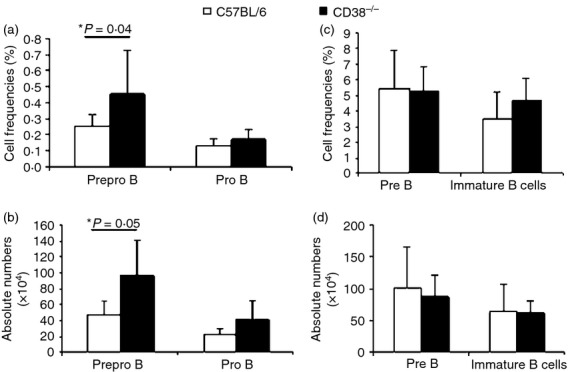
CD38 deficient mice show increased numbers of Pre-Pro-B cells. Cell frequencies (a, c) and absolute numbers (b, d) of pre-pro-B, pro-B, pre-B and immature B cells from the bone marrow (BM) of wild-type (WT) and CD38−/− mice. The average ± SD from 10 animals is shown.
CD157 is a member of the CD38 protein family;39 therefore, this protein might perform some CD38 functions. We analysed the expression of CD157 in different B-lymphocyte populations in BM from C57BL/6J and CD38−/− mice. As shown in Fig.3 and Supporting information (Fig. S4), CD157 was expressed on the membrane of B cells at all developmental stages; however, the expression of this protein decreased at the later stages of B-cell development. Notably, anti-CD157 antibody staining also exhibited the biphasic profile of a pro-B cell population, suggesting that this population may represent at least two intermediate stages of development (Fig.3c, left panel). Interestingly, similar to what was observed with CD38 expression, cells with increased CD157 expression also showed increased granularity (Fig.3c, right panel). Increased CD157 expression was not observed in CD38-deficient mice, suggesting that there is no compensatory effect of CD157 (see Supporting information, Fig. S4).
Figure 3.
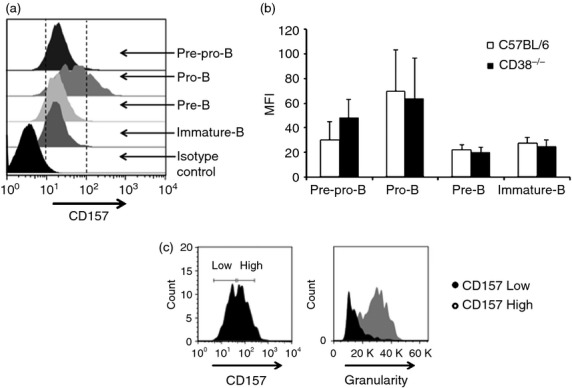
CD157 expression on pre-pro-B, pro-B, pre-B and immature B cells from the bone marrow (BM) of C57BL/6 and CD38−/− mice. (a) Expression of CD157 within the pre-pro-B, pro-B, pre-B and immature B cell populations from murine BM, [wild-type (WT) or CD38−/− mice]. (b) Mean fluorescence intensity (MFI) of CD38 expression in different bone marrow B-cell populations. The average ± SD from five independent experiments is shown. (c) Analysis of granularity and CD157 expression in pro-B cells from murine BM.
CD38 activates the ERK signalling pathway in Jurkat cells, a human T-cell line,17 and in transitional 1 (T1) B cells from the spleen.38 However, whether the activation of this pathway in B-cell lines or immature B cells in BM is CD38 dependent remains unknown. To determine whether ERK can be activated in B-cell lines, A Ba/F3 pro-B cell line that was stably transfected with full-length murine CD38 was stimulated with the NIM-R5 antibody. CD38 expression was detected on the surface of these cells following NIM-R5 antibody stimulation (Fig.4a), whereas no CD38 reactivity was observed in cells transfected with the empty vector (mock-transfected). Previously, we showed that stimulation with NIM-R5 increased protein tyrosine phosphorylation in CD38-expressing Ba/F3 cells, suggesting that CD38 induced signalling in these cells.31 Here, we show that CD38 cross-linking promoted strong ERK phosphorylation in CD38-transfected Ba/F3 cells, peaking 5–10 min after cross-linking (Fig.4b, upper panels). The mock-transfected cells showed low basal ERK phosphorylation, probably reflecting unspecific IgG-dependent activation, as was observed in cells treated with rat IgG only (Fig.4c). However, when the CD38-transfected cells were activated via CD38, PMA, or the isotype control rat IgG (Fig.4c), it was clear that CD38 induced strong ERK phosphorylation compared with that induced by PMA (positive control), suggesting that CD38 induced strong ERK activation in these cells (Fig.4c).
Figure 4.
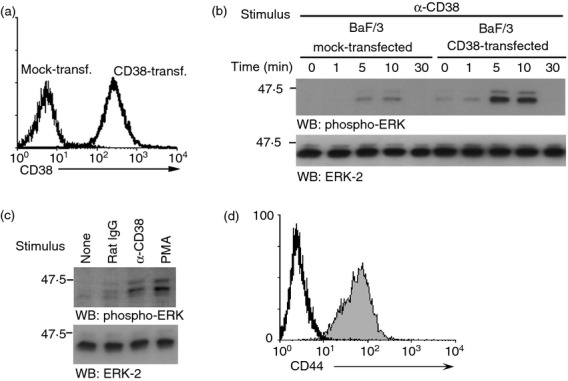
NIM-R5 induces extracellular signal-regulated kinase ( ERK) phosphorylation in the CD38-transfected pro-B-cell line Ba/F3. (a) CD38-transfected (right histogram) or mock-transfected (left histogram) Ba/F3 cells (106) were stained with the monoclonal antibody NIM-R5 followed by a goat anti-rat IgG2a-phycoerythrin-labelled antibody. The cell suspensions were analysed through FACS. (b) Ba/F3 cells stably transfected with murine CD38 or mock-transfected cells (107 cells/condition) were stimulated with anti-CD38 (NIM-R5) for 0, 1, 5 and 10 min at 37°. The cells were lysed for 30 min at 4° in a buffer containing 1% nonidet-40 and protease inhibitors, and 106 cell equivalents were resolved through SDS–PAGE and WB for phospho-ERK (upper), total ERK (lower). (c) CD38-transfected Ba/F3 cells were left untreated or were treated with rat IgG, anti-CD38 (NIM-R5) or PMA for 10 min at 37°. The cell lysates were resolved through SDS–PAGE and WB for phospho-ERK (upper) and total ERK (lower). (d) CD38-transfected Ba/F3 cells (106) were stained with the monoclonal antibody NIM-R8 (anti-CD44) followed by a goat anti-rat IgG2a-phycoerythrin-labelled antibody. The cell suspensions were analysed through FACS.
Stimulation of CD38 induced apoptosis in CD38-transfected Ba/F3 cells, and this phenomenon was dependent on tyrosine kinase activation.34 However, the signalling events involved in this process are poorly understood. To determine the participation of ERK in CD38-induced apoptosis, we stimulated Ba/F3 cells with the NIM-R5 antibody, and then we evaluated phosphatidyl serine translocation (based on annexin-V) and membrane permeability (PI incorporation) by FACS analysis in the presence or absence of the MAPK/ERK kinase inhibitor PD98059 (Fig.5). As shown in Fig.5(a), stimulation with the anti-CD38 antibody induced apoptosis (double Annexin-V/PI detection) in ∼ 70% of the CD38-transfected cells, whereas only a basal level of apoptosis (approximately 5%) was observed in un-activated cells or cells treated with NIM-R8,35 a rat IgG2a antibody similar to NIM-R5 that recognizes surface CD44, this antibody was used to rule out an unspecific effect mediated by an irrelevant molecule for this phenomenon (Fig.5a, upper panel). Pre-incubation of the cells with PD98059 significantly reduced the apoptotic response by approximately 40% in anti-CD38 treated cells (Fig.5a, lower panel and Fig.5b). These results indicated that CD38 activated the ERK signalling pathway, which partially contributed to the apoptosis induced through this receptor in Ba/F3 pro-B cells. The enzymatic activity of CD38 is involved in the chemotaxis of different leucocytes. Dendritic cells treated with 8-Br-cADPr mobilized significantly less Ca2– in response to CCR7 ligands, and treatment with an ADPr antagonist blocks Ca2– influx in leucocytes activated through chemokine receptors that also require CD38 and cADPr for activity.40,41 To determine whether the enzymatic activity of CD38 is involved in the apoptosis of BM B-lymphoid cells, we treated NIM-R5-stimulated Ba/F3 cells with the inhibitor 8-Br-cADPr. The results showed that 8-Br-ADPr treatment had no effect on NIMR5-induced apoptosis, suggesting that this phenomenon was independent of the enzymatic activity of CD38 (see Supporting information, Fig. S3). These results are similar to those of a previous report.34
Figure 5.
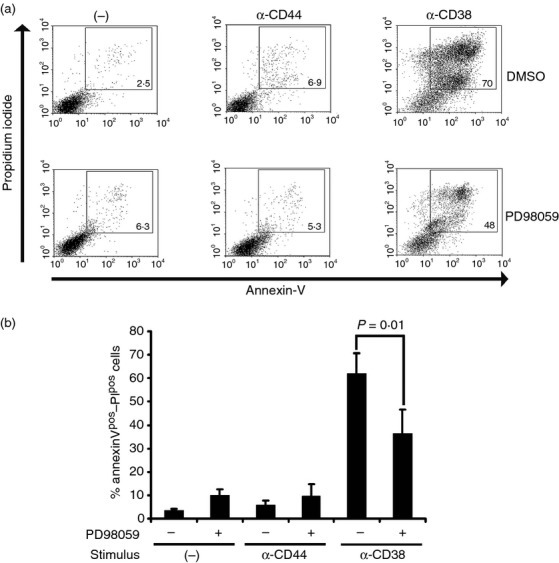
The extracellular signal-regulated kinase (ERK) pathway inhibitor PD98059 interferes with the CD38-induced apoptosis in CD38-transfected Ba/F3 cells. (a) CD38-transfected Ba/F3 cells (106) were pre-incubated for 30 min with either DMSO (upper panel) or the inhibitor PD98059 (20 µm, lower panel). The cells were stimulated with anti-CD38 (NIMR5), rat IgG or left untreated for 4 hr at 37° in the constant presence of vehicle or inhibitor. After stimulation, the cells were transferred to FACS tubes and Annexin-V/propidium iodide staining was performed as described in the Materials and methods. The cells were resuspended in 300 µl binding buffer and immediately captured using a FACSCalibur cytometer (Becton Dickinson). (b) Graph of percentages of Annexin-V/propidium iodide-positive cells. The averages ± SD from three independent experiments is shown. The groups were compared using unpaired Student's t-test and one-way analysis of variance, and P-values ≤ 0·05 were considered significant. PD98059 (−): absence of inhibitor; PD98059 (–): presence of inhibitor. Anti-CD44 was used as control (IgG2a).
It has been reported that activation of Fc receptor agonist antibodies induces apoptosis in immature B cells.13 It has also been observed that stimulation with anti-CD38 antibodies lacking the Fc portion does not induce apoptosis of immature leukaemic B cells;12 therefore, the NIM-R5 fraction F(ab′)2 was used to stimulate Ba/F3 cells. Figure S3 (see Supporting information) shows that the Fc portion of the NIM-R5 monoclonal antibody is not required for induction of apoptosis in this cell line.
To determine if CD38– primary B-cell precursors undergo apoptosis upon CD38 cross-linking, apoptosis was investigated in whole BM or highly purified immature and pre-B cells. Upon CD38 ligation with NIM-R5, apoptosis was observed both in pre-pro-B and immature B cells (Fig.6).
Figure 6.
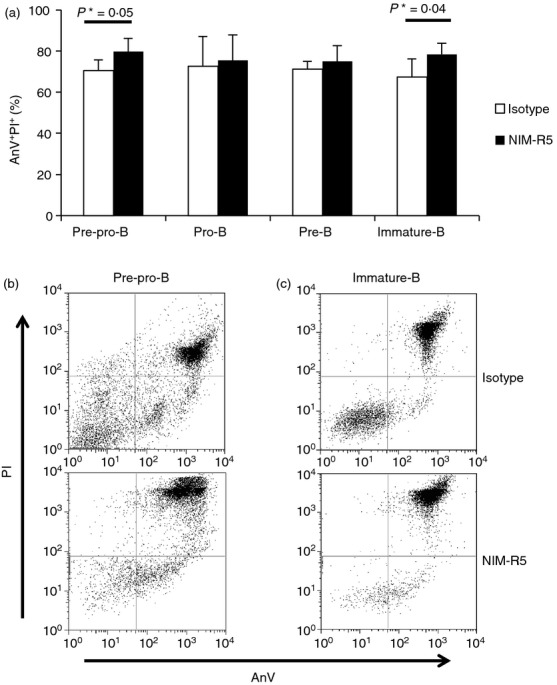
NIM-R5 induces apoptosis in B-cell precursors from bone marrow. (a) Purified B-cell precursors (Pre-pro-B, pro-B, pre-B and immature B) were stimulated for 12 hr in the presence 50 µg/ml anti-CD38 monoclonal antibody (NIM-R5) or an isotype control (rat IgG) antibody. Cells were then stained with annexin-V FITC and propidium iodide (AnV–PI) and analysed by FACS. AnV–IP– Percentage averages ± SD of three independent experiments are shown. (b) Representative dot plots of apoptosis of pre-pro-B cells and (c) immature B cells.
Moreover, to determine whether the ERK pathway is similarly functional and activated through CD38 cross-linking in primary immature B-cell precursors, total BM B cells were activated with the NIM-R5 monoclonal antibody, and PMA was used as a control (Fig.7). NIM-R5 induced modest but reproducible ERK phosphorylation (Fig.7b,d). The analysis of FACS-purified B-cell precursors from BM also showed ERK phosphorylation upon activation with the anti-CD38 mAb NIM-R5. These results indicate that CD38 cross-linking induces ERK-dependent cell activation in developing B-lineage cells (Fig.8). The specificity of this phenomenon is shown in Fig. S2 (see Supporting information) where NIM-R5 not induces phosphorylation of ERK in immature B cells from CD38-deficient mice.
Figure 7.
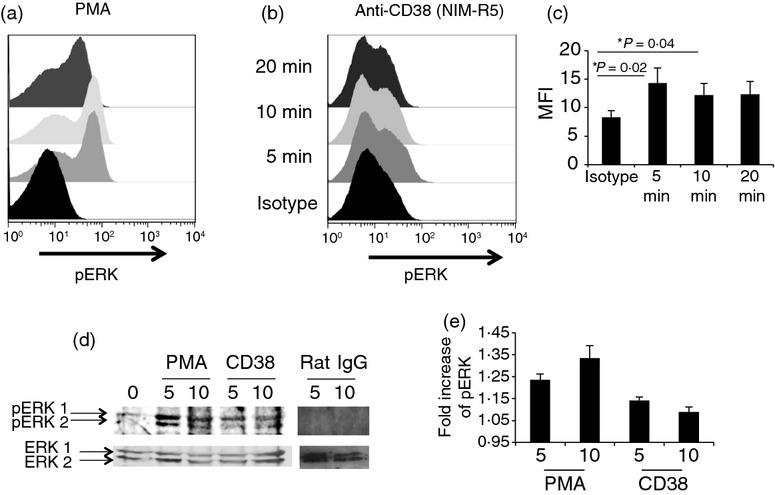
Extracellular signal-regulated kinase (ERK) is phosphorylated after CD38 ligation in total bone marrow B cells. Total bone marrow cells were stimulated with (a) PMA (0·2 µg/ml) as control for activation, (b) anti-CD38 (50 µg/ml) and an isotype control (rat IgG, 50 µg/ml) for 5, 10 or 20 min. After stimulation, pERK expression was measured in B cells using flow cytometry (c); mean fluorescence intensity (MFI) averages ± SD of three independent experiments are shown. (d) Western blot analysis and (e) fold increase of pERK.
Figure 8.
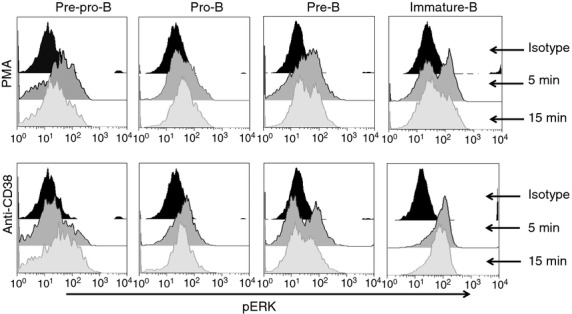
Extracellular signal-regulated kinase (ERK) is phosphorylated after CD38 ligation in B-cell precursors from bone marrow. Purified B-cell precursors (Pre-pro-B, pro-B, pre-B and immature B) were stimulated with PMA (0·2 µg/ml) as control for activation and anti-CD38 (50 µg/ml) or an isotype control (rat IgG) antibody for 5 and 15 min. After stimulation, pERK were measured using flow cytometry.
Discussion
CD38 is expressed on the membrane of B-lineage cells in BM from the earliest stages of differentiation, and distinct effects have been reported upon CD38 cross-linking, such as activation, proliferation, rescue from apoptosis, Ca2– influx and differentiation.5,25,38,42 However, none of these studies included a detailed analysis of the effects of CD38 deletion. In the present study, we observed that the expression of CD38 varies during the sequential stages of B-cell maturation; CD38 expression was detectable from the pre-pro-B stage, and its expression increased as differentiation progressed up to the immature B-cell stage, suggesting that CD38 might have an increasingly important role in maturation, particularly in the later stages of differentiation. Indeed, in a previous study, we showed increased numbers of splenic T1 B cells in CD38-deficient mice.38
The results obtained in the present study indicate that CD38 deletion leads to a defect at the pre-pro-B stage of differentiation, suggesting the importance of CD38 at this stage to continue the ontogeny of B cells in BM. However, no other apparent defect in B-cell differentiation was observed in the BM, probably reflecting the functional replacement of CD38 with the homologous molecule CD157, which is also expressed from the early stages of B-cell maturation (see Supporting information, Figs S1 and S4). Therefore, the increased number of T1 cells observed in the spleen is more likely the result of a delay in differentiation than of an increased supply of T1 cells from BM.38 In the thymus, CD157 is differentially expressed in T-cell precursors; CD157 is expressed on more immature precursor cells, whereas CD38 is expressed later in maturation.43–45 Most studies have described the function of CD157 as an enzyme, and only a few studies have provided evidence for its role as a receptor.
CD38 and CD157 are also differentially expressed on B-cell precursors in BM. Although both are expressed by immature precursors (pre-pro-B and pro-B), upon maturation, CD38 expression increases, whereas CD157 expression decreases (see Supporting information, Figs S1 and S4). Hence, CD157 may compensate for the absence of CD38, and despite their differences in expression, in the later stages of development, the low level CD157 expression might be sufficient to facilitate B-cell differentiation. However, this idea needs to be further evaluated using a CD38, CD157 double knockout. We also observed that both molecules are expressed at similar levels in more granular pro-B stage cells (see Supporting information, Fig. S1) during initiation of DJ rearrangement of the immunoglobulin heavy chain genes. However, it remains unknown whether either or both of these molecules indirectly participate in this phenomenon. The natural, CD38-activating ligand present in BM has yet to be identified.
The MAPK pathway plays important regulatory roles in cell proliferation, cell cycle arrest, migration, differentiation, senescence and apoptosis.46 In the present study, we provide evidence that CD38 induces the activation of the MAPK p42/p44 (ERK) in murine immature B cells. ERK activation through CD38 was observed in both CD38-Ba/F3 cells and BM-derived immature B cells, suggesting that CD38 signalling occurs during B-cell maturation.
CD38 differentially affects the maturation of immature murine B-cell subpopulations in the spleen, and stimulation with an anti-CD38 antibody induces the proliferation and differentiation of transitional 2 (T2) B cells. In contrast, anti-CD38 antibody stimulation promotes apoptosis in immature transitional 1 (T1) B-cell populations. These effects were independent of the enzymatic activity of CD38.38
Previous studies have shown that CD38 cross-linking induces apoptosis of immature human and mouse B cells.11,12,33 The analysis of Ba/F3 cells was especially illuminating because it clearly demonstrated that blocking CD38 with an inhibitor of its enzymatic activity was able to deliver an apoptotic signal similar to that observed with the anti-CD38 monoclonal antibody alone. Therefore, the enzymatic function of CD38 can be separated from its receptor activity.
Despite not finding evidence of CD38 enzymatic participation in inducing apoptosis (see Supporting information, Fig. S3); there are several papers where the enzymatic activity of CD38 is important to regulate apoptosis of immune cells. For example, external NAD– can be converted to ADPR and cADPR by CD38, these metabolites are physiological co-activators of TRPM2 contributing to Ca2– influx signalling in immune cells. Influx of Ca2– through TRPM2 results in activation of extrinsic and intrinsic cell death pathways.47–49 Apoptotic cell death can be induced by several mechanisms, therefore our results point to a receptor-induced activation but the ADPR/cADPR-TRPM2 pathway may also participate in this phenomenon, an issue that was not analysed in this work.
CD38 promoted ERK activation in the pro-B cell line Ba/F3 and in B-cell precursors from BM. It has been previously demonstrated that CD38 induces apoptosis in pro-B-cell lines, including Ba/F3 cells and primary BM-derived B cells,11,28 and we observed a similar phenomenon with pre-pro-B and immature B cells. However, the signalling mechanisms that trigger apoptosis in these cells are largely unknown. Using the specific ERK inhibitor PD98059, we demonstrated that CD38-induced apoptosis requires ERK activation in Ba/F3 cells. Although apoptosis promotion is not a classical ERK function, previous studies have suggested that ERK participates in apoptosis in B lymphocytes.50,51 For example, the B-cell receptor-induced apoptosis of WEHI-231 cells has been associated with specific ERK activation, but not p38 or c-Jun N-terminal kinase activation.51 In addition, some studies have shown that B-cell lymphoma apoptosis induced through mitochondrial toxicants such as mC1CCP involves ERK and p38 activation,52 suggesting that ERK activation is promoted under mitochondrial damage. It would be interesting to determine the role of mitochondria in CD38-induced ERK activation and apoptosis in Ba/F3 cells and other pro-B-cell lines. Moreover, the analysis of CD38 signalling in immature B cells from BM revealed that CD38 reproducibly activated ERK.
CD38 is expressed abundantly in various leukaemias. For this reason, it has been considered as a candidate therapeutic target.14,15 Understanding its function and signalling mechanisms would provide more tools to look for additional therapeutic targets, in addition to the use of monoclonal antibodies.
In summary, the results of this study showed that CD38 is tightly regulated during B-cell maturation, suggesting that CD38 might function as a receptor during B-cell differentiation in the BM. Furthermore, these results suggest that CD38 is a versatile glycoprotein that has varied and complex receptor functions in B cells, depending on the cell type and/or maturation stage.
Acknowledgments
This work was supported by grants from Consejo Nacional de Ciencia y Tecnologia (CONACYT) (Nos. 153733 and 56836). The authors thank Victor Hugo Rosales-Garcia for his technical assistance, Dr Juan Carlos Rodriguez-Alba for his suggestions and MVZ Ricardo Gaxiola-Centeno for taking care of the mice. Héctor Romero Ramírez was a fellow from Consejo Mexiquense de Ciencia y Tecnología (COMECYT) (#12BCD0059-I).
Disclosure
The authors have no financial or commercial conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
CD38 and CD157 are also differentially expressed on B-cell precursors in the bone marrow.
Extracellular signal-regulated kinase (ERK) is not phosphorylated in immature B cells CD38.by NIM-R5.
Apoptosis induced by NIM-R5 in pro-B BAF/3-CD38 cell line is independent of activity enzymatic and fraction Fc.
Expression of CD38 and CD157 in B cells precursors of bone marrow from wild-type (WT) and CD38 mice.
References
- Zocchi E, Franco L, Guida L, Benatti U, Bargellesi A, Malavasi F, Lee HC, De Flora A. A single protein immunologically identified as CD38 displays NAD– glycohydrolase, ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities at the outer surface of human erythrocytes. Biochem Biophys Res Commn. 1993;196:1459–65. doi: 10.1006/bbrc.1993.2416. [DOI] [PubMed] [Google Scholar]
- Lee HC. Structure and enzymatic functions of human CD38. Mol Med. 2006;12:317–23. doi: 10.2119/2006-00086.Lee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Argumedo L, Teixeira C, Perece G, Kirkham P, Parkhouse RME. A B lymphocyte surface molecule mediating activation and protection from apoptosis via calcium channels. J Immunol. 1993;151:3119–30. [PubMed] [Google Scholar]
- Lund FE, Solvanson N, Cooke MP, Health AW, Grimaldi JC, Parkhouse RM, Goodnow CC, Howard MC. Signaling through murine CD38 is impaired in antigen receptor-unresponsive B cells. Eur J Immunol. 1995;25:1338–45. doi: 10.1002/eji.1830250531. [DOI] [PubMed] [Google Scholar]
- Lund FE. Signaling properties of CD38 in the mouse immune system: enzyme-dependent and -independent roles in immunity. Mol Med. 2006;12:328–33. doi: 10.2119/2006-00099.Lund. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima F, Deguchi T, Laver JH, Zeng H, Ogawa M. Reciprocal expression of CD38 and CD34 by adult murine hematopoietic stem cells. Blood. 2001;97:2618–24. doi: 10.1182/blood.v97.9.2618. [DOI] [PubMed] [Google Scholar]
- Higuchi Y, Zeng H, Ogawa M. CD38 expression by hematopoietic stem cells of newborn and juvenile mice. Leukemia. 2003;17:171–4. doi: 10.1038/sj.leu.2402785. [DOI] [PubMed] [Google Scholar]
- Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–91. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- Vences-Catalán F, Santos-Argumedo L. CD38 through the life of a murine B lymphocyte. IUBMB Life. 2011;63:840–6. doi: 10.1002/iub.549. [DOI] [PubMed] [Google Scholar]
- Lamkin T, Brooks J, Annett G, Roberts W, Weinberg K. Immnuno-phenotypic differences between putative hematopoietic stem cells and childhood B cell precursor acute lymphoblastic leukemia cells. Leukemia. 1994;8:1871–8. [PubMed] [Google Scholar]
- Kumagai M, Coustain-Smith E, Murray DJ, Silvennoinen O, Murti KG, Evans WE, Malavasi F, Campana D. Ligation of CD38 suppresses human B lymphopoiesis. J Exp Med. 1995;181:1101–10. doi: 10.1084/jem.181.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todisco E, Susuki T, Srivannaboon K, Coustan-Smith E, Raimondi SC, Behm FG, Kinanaka A, Campana D. CD38 ligation inhibits normal and leukemic myelopoiesis. Blood. 2000;95:535–42. [PubMed] [Google Scholar]
- Suzuki T, Coustan-Smith E, Mihara K, Campana D. Signals mediated by FcγRIIA suppress the growth of B-lineage acute lymphoblastic leukemia cells. Leukemia. 2002;16:1276–84. doi: 10.1038/sj.leu.2402523. [DOI] [PubMed] [Google Scholar]
- de Weers M, Tai YT, van der Veer MS. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–8. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- Chillemi A, Zaccarello G, Quarona V, Ferracin M, Ghimenti C, Massaia M, Horenstein AL, Malavasi F. Anti-CD38 antibody therapy: windows of opportunity yielded by the functional characteristics of the target molecule. Mol Med. 2013;19:99–108. doi: 10.2119/molmed.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra M, Zubiaur M, Terhorst C, Sancho J, Malavasi F. CD38 is functionally dependent on the TCR/CD3 complex in human T cells. FASEB J. 1998;12:581–92. doi: 10.1096/fasebj.12.7.581. [DOI] [PubMed] [Google Scholar]
- Zubiaur M, Izquierdo M, Terhorst C, Malavasi F, Sancho J. CD38 ligation results in activation of the Raf-1/mitogen-activated protein kinase and the CD3-ζ/ζ-associated protein-70 signaling pathways in Jurkat T lymphocytes. J Immunol. 1997;159:193–205. [PubMed] [Google Scholar]
- Zubiaur M, Fernandez O, Ferrero E, Salmeron J, Malissen B, Malavasi F, Sancho J. CD38 is associated with lipid rafts and upon receptor stimulation leads to Akt/protein kinase B and Erk activation in the absence of the CD3-ζ immune receptor tyrosine-based activation motifs. J Biol Chem. 2002;277:13–22. doi: 10.1074/jbc.M107474200. [DOI] [PubMed] [Google Scholar]
- Munoz P, Navarro MD, Pavon EJ, Salmeron J, Malavasi F, Sancho J, Zubiaur M. CD38 signaling in T cells is initiated within a subset of membrane rafts containing Lck and the CD3-ζ subunit of the T cell antigen receptor. J Biol Chem. 2003;278:50791–802. doi: 10.1074/jbc.M308034200. [DOI] [PubMed] [Google Scholar]
- Kitanaka A, Ito C, Coustan-Smith E, Campana D. CD38 ligation in human B cell progenitors triggers tyrosine phosphorylation of CD19 and association of CD19 with lyn and phosphatidyl 3-kinase. J Immunol. 1997;159:184–92. [PubMed] [Google Scholar]
- Kitanaka A, Ito C, Nishigaki H, Campana D. CD38-mediated growth suppression of B-cell progenitors requires activation of phosphatidylinositol 3-kinase and involves its association with the protein product of the c-cbl proto-oncogene. Blood. 1996;88:590–8. [PubMed] [Google Scholar]
- Silvennoinen O, Nishigaki H, Kitanaka A. CD38 signal transduction in human B cell precursors. Rapid induction of tyrosine phosphorylation, activation of syk tyrosine kinase, and phosphorylation of phospholipase C-γ and phosphatidylinositol 3-kinase. J Immunol. 1996;156:100–7. [PubMed] [Google Scholar]
- Kaku H, Horikawa K, Obata Y, Kato I, Okamoto H, Sakaguchi N, Gerondakis S, Takatsu K. NF-κB is required for CD38-mediated induction of Cγ1 germline transcripts in murine B lymphocytes. Int Immunol. 2002;14:1055–64. doi: 10.1093/intimm/dxf072. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Yasue T, Miyake K, Kimoto M, Takatsu K. CD38 ligation induces tyrosine phosphorylation of Bruton tyrosine kinase and enhanced expression of interleukin 5-receptor α chain: synergistic effects with interleukin 5. Proc Natl Acad Sci USA. 1995;92:11814–8. doi: 10.1073/pnas.92.25.11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Garcia ME, Lopez-Bojorques LN, Zentella A, Humphries LA, Rawlings DJ, Santos-Argumedo L. CD38 signaling regulates B lymphocyte activation via a phospholipase C (PLC)- γ2-independent, protein kinase C, phosphatidylcholine-PLC, and phospholipase D-dependent signaling cascade. J Immunol. 2005;174:2687–95. doi: 10.4049/jimmunol.174.5.2687. [DOI] [PubMed] [Google Scholar]
- Santos-Argumedo L, Lund FE, Heath AW, Solvason N, Wu WW, Grimaldi JC, Parkhouse RM, Howard M. CD38 unresponsiveness of xid B cells implicates Bruton's tyrosine kinase (btk) as a regular of CD38 induced signal transduction. Int Immunol. 1995;7:163–70. doi: 10.1093/intimm/7.2.163. [DOI] [PubMed] [Google Scholar]
- Yasue T, Nishizumi H, Aizawa S. A critical role of Lyn and Fyn for B cell responses to CD38 ligation and interleukin 5. Proc Natl Acad Sci USA. 1997;94:10307–12. doi: 10.1073/pnas.94.19.10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitanaka A, Suzuk T, Ito C, Nishigaki H, Coustan-Smith E, Tanaka T, Kubota Y, Campana D. CD38-mediated signaling events in murine pro-B cells expressing human CD38 with or without its cytoplasmic domain. J Immunol. 1999;162:1952–8. [PubMed] [Google Scholar]
- Lund FE, Muller-Steffner HM, Yu N, Stout CD, Schuber F, Howard MC. CD38 signaling in B lymphocytes is controlled by its ectodomain but occurs independently of enzymatically generated ADP-ribose or cyclic ADP-ribose. J Immunol. 1999;162:2693–702. [PubMed] [Google Scholar]
- Deaglio S, Zubiaur M, Gregorini A, Bottarel F, Ausiello CM, Dianzani U, Sancho J, Malavasi F. Human CD38 and CD16 are functionally dependent and physically associated in natural killer cells. Blood. 2002;99:2490–8. doi: 10.1182/blood.v99.7.2490. [DOI] [PubMed] [Google Scholar]
- Lund FE, Yu N, Kim KM, Reth M, Howard MC. Signaling through CD38 augments B cell antigen receptor (BCR) responses and is dependent on BCR expression. J Immunol. 1996;157:1455–67. [PubMed] [Google Scholar]
- Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- Shaul YD, Seger R. The MEK/ERK cascade:from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–26. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Lund FE, Muller-Steffner H, Romero-Ramirez H. CD38 induces apoptosis of a murine pro-B leukemic cell line by a tyrosine kinase-dependent but ADP-ribosyl cyclase-and NAD glycohydrolase-independent mechanism. Int Immunol. 2006;18:1029–42. doi: 10.1093/intimm/dxl037. [DOI] [PubMed] [Google Scholar]
- Santos-Argumedo L, Kincade PW, Partida-Sanchez S, Parkhouse RM. CD44-stimulated dendrite formation (‘spreading’) in activated B cells. Immunology. 1997;90:147–53. doi: 10.1046/j.1365-2567.1997.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SR, Mancini M, Rosen A, Schlissel MS. Annexin V binds to viable B cells and colocalizes with a marker of lipid rafts upon B cell receptor activation. J Immunol. 2000;164:1322–32. doi: 10.4049/jimmunol.164.3.1322. [DOI] [PubMed] [Google Scholar]
- Hardy R, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of Pro-B and Pre-Pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–25. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Alba JC, Moreno-Garcia ME, Sandoval-Montes C, Rosales-Garcia VH, Santos-Argumedo L. CD38 induces differentiation of immature transitional 2 B lymphocytes in the spleen. Blood. 2008;111:3644–52. doi: 10.1182/blood-2007-08-107714. [DOI] [PubMed] [Google Scholar]
- Czura AW, Czura CJ. CD38 and CD157: biological observations to clinical therapeutic targets. Mol Med. 2006;12:309–11. doi: 10.2119/2007-00006.Czura. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partida-Sanchez S, Goodrich S, Kusser K, Oppenheimer N, Randall TD, Lund FE. Regulation of dendritic cell trafficking by the ADP-ribosyl cyclase CD38: impact on the development of humoral immunity. Immunity. 2004;20:279–91. doi: 10.1016/s1074-7613(04)00048-2. [DOI] [PubMed] [Google Scholar]
- Partida-Sanchez S, Gasser A, Fliegert R. Chemotaxis of mouse bone marrow neutrophils and dendritic cells is controlled by ADP-ribose, the major product generated by the CD38 enzyme reaction. J Immunol. 2007;179:7827–39. doi: 10.4049/jimmunol.179.11.7827. [DOI] [PubMed] [Google Scholar]
- Manjarrez-Orduño N, Moreno-García ME, Fink K, Santos-Argumedo L. CD38 cross-linking enhances TLR-induced B cell proliferation but decreases IgM plasma cell differentiation. Eur J Immunol. 2007;37:358–67. doi: 10.1002/eji.200636453. [DOI] [PubMed] [Google Scholar]
- Ortolan E, Vacca P, Capobianco A, Armando E, Crivellin F, Horenstein A, Malavasi F. CD157, the Janus of CD38 but with a unique personality. Cell Biochem Funct. 2002;20:309–22. doi: 10.1002/cbf.978. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Kobune Y, Okuyama Y, Itoh M, Lee BO, Muraoka O, Hirano T. Stage-specific expression of mouse BST-1/BP3 on the early B and T cell progenitors prior to gene rearrangement of antigen receptor. Int Immunol. 1996;8:1395–404. doi: 10.1093/intimm/8.9.1395. [DOI] [PubMed] [Google Scholar]
- Vicari A, Bean A, Zlotnik A. A role for BP-3/BST-1 antigen in early T cell development. Int Immunol. 1996;8:183–91. doi: 10.1093/intimm/8.2.183. [DOI] [PubMed] [Google Scholar]
- Wada T, Peninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–49. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kozai D, Kobayashi R, Maximilian E, Mori Y. Roles of TRPM2 in oxidative stress. Cell Calcium. 2011;50:279–87. doi: 10.1016/j.ceca.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Massullo P, Sumoza-Toledo A, Bhagat H, Partida-Sanchez S. TRPM channels, calcium and redox sensors during innate immune responses. Semin Cell Dev Biol. 2006;17:654–66. doi: 10.1016/j.semcdb.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Kolisek M, Beck A, Fleig A, Penner R. Cyclic ADP-ribose and hydrogen peroxide synergized with ADP-ribose in the activation of TRPM channels. Mol Cell. 2005;18:61–9. doi: 10.1016/j.molcel.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Gauld SB, Blair D, Moss CA, Reid SD, Harnett MM. Differential roles for extracellularly regulated kinase-mitogen-activated protein kinase in B cell antigen receptor-induced apoptosis and CD40-mediated rescue of WEHI-231 immature B cells. J Immunol. 2002;168:3855–64. doi: 10.4049/jimmunol.168.8.3855. [DOI] [PubMed] [Google Scholar]
- Lee JR, Koretzky GA. Extracellular signal-regulated kinase-2, but not c-Jun NH2-terminal kinase, activation correlates with surface IgM-mediated apoptosis in the WEHI 231 B cell line. J Immunol. 1998;161:1637–44. [PubMed] [Google Scholar]
- O'Brien KA, Muscarella DE, Bloom SE. Differential induction of apoptosis and MAP kinase signaling by mitochondrial toxicants in drug-sensitive compared to drug-resistant B-lineage lymphoid cell lines. Toxicol Appl Pharmacol. 2001;174:245–56. doi: 10.1006/taap.2001.9215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CD38 and CD157 are also differentially expressed on B-cell precursors in the bone marrow.
Extracellular signal-regulated kinase (ERK) is not phosphorylated in immature B cells CD38.by NIM-R5.
Apoptosis induced by NIM-R5 in pro-B BAF/3-CD38 cell line is independent of activity enzymatic and fraction Fc.
Expression of CD38 and CD157 in B cells precursors of bone marrow from wild-type (WT) and CD38 mice.