Abstract
Recent studies of healthy human airways have revealed colonization by a distinct commensal bacterial microbiota containing Gram-negative Prevotella spp. However, the immunological properties of these bacteria in the respiratory system remain unknown. Here we compare the innate respiratory immune response to three Gram-negative commensal Prevotella strains (Prevotella melaninogenica, Prevotella nanceiensis and Prevotella salivae) and three Gram-negative pathogenic Proteobacteria known to colonize lungs of patients with chronic obstructive pulmonary disease (COPD) and asthma (Haemophilus influenzae B, non-typeable Haemophilus influenzae and Moraxella catarrhalis). The commensal Prevotella spp. and pathogenic Proteobacteria were found to exhibit intrinsic differences in innate inflammatory capacities on murine lung cells in vitro. In vivo in mice, non-typeable H. influenzae induced severe Toll-like receptor 2 (TLR2)-independent COPD-like inflammation characterized by predominant airway neutrophilia, expression of a neutrophilic cytokine/chemokine profile in lung tissue, and lung immunopathology. In comparison, P. nanceiensis induced a diminished neutrophilic airway inflammation and no detectable lung pathology. Interestingly, the inflammatory airway response to the Gram-negative bacteria P. nanceiensis was completely TLR2-dependent. These findings demonstrate weak inflammatory properties of Gram-negative airway commensal Prevotella spp. that may make colonization by these bacteria tolerable by the respiratory immune system.
Keywords: asthma, microbiota, chronic obstructive pulmonary disease, lung immunopathology, respiratory inflammation
Introduction
The role of commensal bacteria in health and disease is receiving increasing interest with the recognition that the microbiota plays a central role in shaping immune function, metabolism and protection from pathogenic microorganisms.1–3 The human lung has historically been considered sterile because of the absence of cultivable bacteria in bronchial alveolar lavage fluids from healthy individuals.4–6 However, culture-independent molecular methods for bacterial identification have recently been applied to characterize the human airway microbiota.7–11 Gram-negative anaerobic Prevotella species were found to be a prevalent bacterial colonizer of healthy airways,7,11 and reduced frequencies were reported in chronic obstructive pulmonary disease (COPD) and asthma.7 Prevotella species are considered commensal bacteria that rarely cause respiratory infections, and only few strains have been reported to be opportunistic in chronic infections, abscesses and anaerobic pneumonia.12–14.
Dysbiosis, a change in microbiota composition detrimental for host health, is associated with altered function of the immune system and inflammatory pathologies.15 It is becoming increasingly appreciated that respiratory bacterial pathogens commonly colonize the lower airways of patients with COPD and asthma.7,16,17 Both stable disease and exacerbation episodes in COPD are predominantly associated with the presence of pathogenic Gram-negative Proteobacteria (Haemophilus influenzae and Moraxella catarrhalis).18–20 The pathogenic Proteobacteria are believed to take part in disease progression of COPD by enhancing inflammatory processes and tissue degradation in the lower airways.21 Indeed, higher bacterial loads in the airways are associated with increased disease severity, exacerbation frequency, decreased lung function and increased production of inflammatory mediators.22–24 The notion of disease-promoting properties of bacteria is further supported by a recent interventional study showing that prophylactic antibiotic treatment reduced the frequency of bacterial colonization and exacerbations in COPD.25
A role for pathogenic Proteobacteria in asthma pathology remains controversial and few studies are found in the literature. Increased airway colonization by pathogenic Proteobacteria is associated with stable asthma in children and adults,7 exacerbation episodes in childhood26 and increased risk of developing asthma.17 We recently reported abnormal bacterial immune responses in infants later developing asthma – proposing that asthmatics exhibit decreased bacterial control and that divergent bacterial immune responses within the lung may contribute to asthma pathology in some disease endotypes.27
Studies using murine models of COPD demonstrated that H. influenzae enhances airway inflammation induced by exposure to cigarette smoke.28,29 Furthermore, mice challenged in the airways with H. influenzae lysates develop inflammatory features of COPD, including production of pro-inflammatory mediators tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6) and IL-1β, airway neutrophilia and lung tissue pathology.30
Commensal Prevotella species are frequent colonizers of the lower and upper airways; however, little is known about the inflammatory properties of these bacterial species in the respiratory system. As pathogenic Proteobacteria are recognized co-drivers of chronic airway inflammation in COPD and have been studied in relevant murine models of the disease, we here compared these to the commensal Prevotella spp. We demonstrate weak innate stimulatory properties of Gram-negative commensal Prevotella spp. that may make colonization by these bacteria tolerable by the respiratory immune system. In contrast, the innate stimulatory capacity of pathogenic Proteobacteria probably allow these bacteria to be specific co-drivers of disease progression in COPD and asthma patients.
Materials and methods
Mice
Wild-type (WT) C57BL/6J mice were obtained from Taconic (Silkeborg, Denmark). Toll-like receptor 2 (TLR2)-deficient mice on the C57BL/6J background were obtained from Jackson Laboratories (Bar Harbor, ME) and bred in-house. Mice were kept under specific pathogen-free conditions, according to national guidelines for experimental animal housing and under the daily care of animal technicians. All experiments were carried out using sex- and age-matched mice. The experimental protocols were approved by the Danish Animal Ethics Committee (permission number: 2007/561-1266).
Bacteria growth and preparation
Haemophilus influenzae B (KAK510), Haemophilus influenzae NT (KAK509) and Moraxella catarrhalis F48 (KAK508) reference strains were kindly provided by Karen A. Krogfelt and Jørgen Skov Jensen (Statens Serum Institut, Copenhagen, Denmark). Prevotella melaninogenica (DSM7089), Prevotella nanceiensis (DSM19126) and Prevotella salivae (DSM15606) were obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ), Braunschweig, Germany. Haemophilus and Moraxella strains were grown on chocolate agar plates (Statens Serum Institut) under 37° microaerobic (5% CO2) conditions. Prevotella strains were grown on anaerobic agar plates (Statens Serum Institut) under 30° anaerobic conditions. All strains were resuspended from plates with uniform growth and washed once in PBS. Bacteria were resuspended in PBS to optical density 1 and UV-irradiated for 45 min. UV killing was confirmed by plating. Dry weight of bacterial suspensions in PBS was determined on 3 × 1 ml portions after freeze-drying (subtracted by weight of PBS). Bacterial suspensions were frozen and stored at −80°.
Colony forming units (CFU) were determined in bacterial suspensions before UV-irradiation using serial dilution. CFUs per bacterial dry weight were as follows: 7·81 × 105 CFU/μg (H. inf. B), 4·91 × 105 CFU/μg (H. inf. NT), 2·45 × 105 CFU/μg (M. cat.), 0·26 × 105 CFU/μg (P. mel.), 0·13 × 105 CFU/μg (P. nan.) and 0·15 × 105 CFU/μg (P. sal.).
Primary lung cell preparation and stimulation
Mice were anaesthetized using ketamine/xylazine (10/0·5 mg per 50 g body weight; Intervet, Boxmeer, Holland) and killed by heart puncture blood removal. PBS was perfused through the heart to remove systemic blood. Lungs were excised, cut into 1- to 2-mm pieces and incubated for 1 hr at 37° in PBS (Lonza, Basel, Switzerland) supplemented with 30 U/ml Clostridium histolyticum collagenase type II and 150 μg/ml DNAase (Sigma-Aldrich, Copenhagen, Denmark). The tissues were passed through a 70-μm cell strainer (BD Bioscience, San Jose, CA) to obtain a single-cell suspension of lung cells. Cells from lungs of three to five mice were pooled and separated into lung constituent cells (CD45−) and lung leucocytes (CD45+) using a CD45+ magnetic cell sorting kit (MACS; MiltenyiBiotec, Bergisch Gladbach, Germany) according to the manufacturer's recommendations. Purity was confirmed by flow cytometry. Cells were resuspended in complete RPMI-1640 medium [Lonza, Basel, Switzerland; supplemented with 2 mm l-glutamine (Cambrex, East Rutherford, NJ), 100 U/ml penicillin/streptomycin (Lonza) and 10% FCS (Lonza)] and plated in 96-well plates (2 × 105 cells/well). In experiments with mixed CD45− and CD45+ lung cells a ratio of 1 : 1 was used.
Primary lung cells were stimulated with 50 μg/ml of bacterial preparations supplemented with 50 μg/ml gentamycin (Sigma-Aldrich) to ensure no bacterial outgrowth. LPS (100 ng/ml) and medium alone were included to serve as a positive and negative control, respectively. All stimulations were performed in triplicates. Supernatants were harvested after 24 hr and stored at −80° until cytokine analyses.
Acute airway inflammation model
Mice were lightly sedated using ketamine/xylazine (5 mg/0·25 mg per 50 g body weight). While kept in a vertical position, 25 μl bacterial suspension or vehicle (PBS) alone was inhaled through each nostril giving a final dose of 22·5 μg bacteria in 50 μl (450 μg/ml). Mice were kept vertical for 1 min following inhalation.
Twenty-four hours after bacterial inhalation, the mice were anaesthetized using ketamine/xylazine (10 mg/0·5 mg per 50 g body weight) and killed by heart puncture. Bronchoalveolar lavage (BAL) cells were collected by perfusing lungs with 800 μl PBS five times. The right lung was snap frozen in liquid nitrogen and stored at −80° until measurement of tissue cytokines. The left lung was embedded in Tissue-tek OCT Compound (Sakura, Tokyo, Japan) and stored at −80° until histochemical analysis.
Tissue and supernatant cytokine measurements
Measurement of cytokine production in tissue was performed as previously described.31 Frozen lung tissues were homogenized in a mortar with 500 μl/mg tissue PBS contain 0·1% Tween-20 (volume/volume) and protease inhibitor cocktail (Complete, Roche; 1 tablet/50 ml PBS). Tissue homogenates were frozen in liquid nitrogen, thawed, sonicated for 30 seconds and centrifuged to remove debris. Lysates were stored at −80° until cytokine analysis by ELISA.
Cytokines were measured in lung tissue homogenates and supernatants from cell cultures using commercial ELISA kits [IL-5, IL-4 and IL-13 from eBioscience, San Diego, CA; macrophage inflammatory protein-2α (MIP-2α; IL-8), thymic stromal lymphopoietin (TSLP), TNF-α, IL-10, interferon-γ (IFN-γ), IL-17, CCL20, CXCL16, IL-1β, IL-6 and IL-10 from RnD Systems, Minneapolis, MN] according to the manufacturer's recommendations.
Flow cytometry
The composition of BAL cells was analysed by flow cytometry as previously described32 using the following anti-mouse fluorochrome-conjugated antibodies: anti-CD45/eFlour450, anti-CD11b/FITC, anti-CD11c/allophycocyanin (eBioscience), anti-Siglec-F/phycoerythrin (BD Bioscience), anti-Gr-1/allophycocyanin-Cy7 and anti-mF4/80/phycoerythrin-Cy7 (BioLegend, San Diego, CA). Staining was performed in PBS containing 1% fetal calf serum, 0·1% sodium azide and Fc-block (BD Bioscience) for 30 min at 4°. Cells were analysed on a BD FACSCanto™ II system running FACSdiva 6.0 software (BD Biosciences). Immune cells were identified among CD45+ leucocytes as follows: Eosinophils (CD11c−, Siglec-F+), lymphocytes (CD11b− CD11c−), macrophages (F4/80+ Gr-1−), neutrophils (F4/80− Gr-1+), monocytes (F4/80− Gr-1− CD11bint CD11c−), CD11b+ dendritic cells (CD11b+ DC; F4/80− Gr-1− CD11b+ CD11c+) and CD11b−/low dendritic cells (CD11b−/low DC; F4/80− Gr-1− CD11bint CD11c+). Flow cytometry data were analysed using FlowJo 7.6.5 (Tree Star, Ashland, OR).
Histochemical staining
Tissue-tek OCT Compound-embedded lung tissues were thawed overnight in 4% (volume/volume) paraformaldehyde/PBS at 4°. Tissues were embedded in paraffin and cut into 5-μm sections. Sections were stained by Mayer's haematoxylin & eosin (Pioneer Research Chemicals, Colchester, UK) and evaluated by microscopy. Two pictures were taken representing areas with most and least inflammation in each lung section. Both pictures were used to grade lung pathology giving an average score. Pathology scores were given on a relative scale 1–6 judging integrity of the bronchial epithelium, peribronchial/arterial inflammation, as well as alveolar integrity and inflammation. Pathology scoring was performed in a blinded manner.
Data analysis and statistics
Statistical analysis was performed using GraphPad PRISM 5.01 (GraphPad Software, La Jolla, CA). Differences in BAL cell composition, cytokine levels in lung tissue and pathology scores were analysed by two-way analysis of variance and post-hoc by Bonferroni's multiple comparison test. Cytokine production in lung tissue was reported after subtracting mean levels measured in tissue from PBS-treated mice (background). Student's one-sample t-test was applied to test if a cytokine was induced in the lung tissue after bacterial challenge (testing if mean level different from 0 ng/g lung tissue). Differences in the capacity of pathogenic Proteobacteria and Prevotella spp. to induce cytokine production in primary lung cells in vitro was analysed in a compartmentalized manner using Student's t-test comparing data from the three bacteria in each phylum. Bar charts show mean and standard error of the mean (SEM). P-values < 0·05 were considered statistically significant. P-values are indicated as follows: *P < 0·05; **P < 0·01; ***P < 0·001.
Results
Lung commensal Prevotella spp. exhibit distinct inflammatory properties compared with COPD and asthma-associated Proteobacteria
Three bacterial strains representing the COPD and asthma-associated Proteobacteria, as well as three Prevotella spp. associated with healthy lungs, were chosen for an initial analysis of innate inflammatory properties on primary murine lung cells. Haemophilus influenzae and Moraxella catarrhalis (M. cat.) were selected as the key members of pathogenic Proteobacteria bacteria associated with COPD and asthma. It was decided to include the Haemophilus influenzae B (H. inf. B) and non-typeable Haemophilus influenzae (H. inf. NT) strains, as these common strains are structurally different because of the presence or absence of bacterial capsule, respectively.33,34 Prevotella melaninogenica (P. mel.), Prevotella nanceiensis (P. nan.) and Prevotella salivae (P. sal.) were included as representative commensal Prevotella spp. predominantly associated with healthy airways.7,11 The innate inflammatory capacities of the Proteobacteria and Prevotella spp. were addressed by stimulating primary CD45+ and CD45− cells isolated from murine lungs representing lung leucocytes and non-immune cells, respectively. The production of key acute-phase cytokines MIP-2α (IL-8) and TNF-α known to be involved in COPD and asthma pathology were analysed in supernatants.35 Interleukin-10 was analysed to address potential anti-inflammatory aspects of the bacterial response. TSLP production was addressed as a cytokine produced exclusively by epithelial cells, and involved in priming type 2 immune responses.36 In addition, cytokines IL-5, IL-4 and IL-13 were analysed to assess potential type 2 immune responses induced by the bacteria. Co-cultures of CD45+ and CD45− cells (1 : 1) were performed to examine possible cytokine-enhancing cross-talk between these cellular compartments.
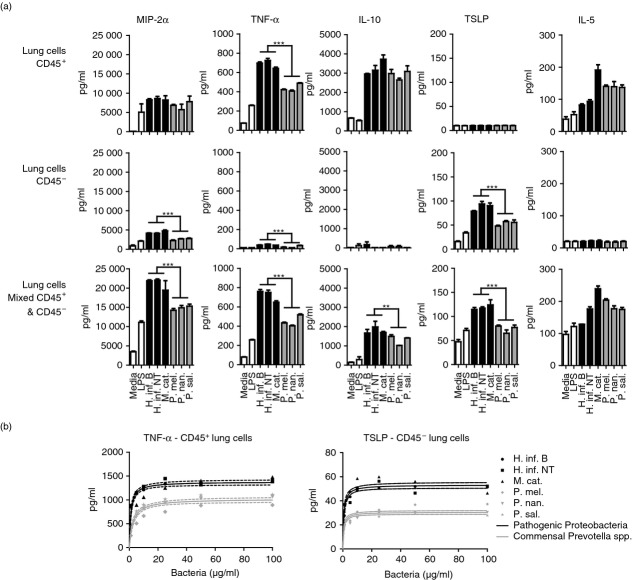
We observed significant differences between the cytokine profiles induced in primary murine lung cells by the COPD and asthma-associated Proteobacteria and the commensal Prevotella spp. (Fig.1a). Prevotella spp. generally induced lower cytokine production compared with Proteobacteria, but the observation was dependent on the cytokine investigated and the cellular compartment. In CD45+ lung cells, Prevotella spp. induced lower TNF-α production compared with the three Proteobacteria. Additionally, TNF-α, MIP-2α (IL-8) and TSLP were lower for Prevotella spp. in the CD45− compartment. These cytokines and IL-10 were also produced less in response to Pretovella spp. in 1 : 1 co-cultures of CD45+ and CD45− cells isolated from murine lungs (Fig.1a). Interestingly, measurements of MIP-2α (IL-8) production indicated a synergistic effect between CD45+ and CD45− cells, giving rise to generally higher MIP-2α (IL-8) levels in co-cultures. It was observed that production of some cytokines is restricted to a particular compartment. As expected, TSLP was only produced in CD45− lung cells, as this is an epithelially derived cytokine.36 Interleukin-5 production was absent in the CD45− compartment, indicating that this cytokine is only produced by lung immune cells. Furthermore, MIP-2α (IL-8), TNF-α and IL-10 were generally lower in the non-immune CD45− lung cell compartment. Production of IL-4 and IL-13 in response to the bacteria could not be detected in bacteria-exposed CD45+ or CD45− lung cells (data not shown).
Figure 1.
Inflammatory response of murine lung cells to three airway-related Proteobacteria and Prevotella spp. Macrophage inflammatory protein 2α [MIP-2α (IL-8)], tumour necrosis factor α (TNF-α), interleukin-10 (IL-10), thymic stromal lymphopoietin (TSLP) and IL-5 production by primary CD45+, CD45− or mixed CD45+/− (1 : 1) cells isolated from murine lungs in response to 24-hr stimulation with medium, lipopolysaccharide (LPS), three Proteobacteria or Prevotella spp. (a). Dose–response titration of Proteobacteria and Prevotella spp. on primary murine lung cells (b). Data represent one of four (a) and two (b) independent experiments (n = 3, mean + SEM). **P < 0·01; ***P < 0·001.
The observed differences in innate immune stimulatory properties between the three COPD and asthma-associated Proteobacteria and the commensal Prevotella spp. could be due to differences in concentrations of specific innate ligands (microbe-associated molecular patterns; MAMPs) shared by the bacteria. To test this, we stimulated primary lung cells isolated from mice with increasing concentrations of the three Proteobacteria and Prevotella spp. Both TNF-α and TSLP were measured as representative cytokines with observed differences in production induced by the two groups of bacteria in the CD45+ and CD45− lung cell compartments, respectively. The cytokine response to increasing concentrations of bacteria from the Proteobacteria and Prevotella spp. fitted well to a one-site saturation binding equation (Fig.1b). There was a significant difference in the maximum capacity of the Proteobacteria and Prevotella spp. to induce innate stimulation in lung cells (TNF-α from CD45+ lung cells, Bmax 1378 pg/ml versus 1021 pg/ml, P < 0·0001; TLSP from CD45− lung cells, Bmax 53·2 pg/ml versus 30·5 pg/ml, P < 0·0001). These findings demonstrate intrinsic innate stimulatory divergence between the three COPD and asthma-associated Proteobacteria and commensal Prevotella spp., probably as the result of differences in composition of MAMPs with alternating capacity to activate innate immune receptors.
COPD and asthma-associated non-typeable H. influenzae, but not P. nanceiensis, potently induce airway inflammation and pathology in mice
Airway challenge with H. influenzae has been shown to induce inflammatory aspects of COPD in lungs of mice, including production of acute-phase cytokines TNF-α, IL-6 and IL-1β, airway neutrophilia and tissue pathology.30 We sought to investigate how the intrinsic differences in immune stimulatory capacity between the three Proteobacteria and Prevotella spp. may translate to the presentation of COPD- and/or asthma-like airway inflammation in vivo. We challenged mice with H. influenzae (H. inf. NT) and P. nanceiensis (P. nan.) as representative strains of the two groups of bacteria. Airways challenged with H. inf. NT induced predominant recruitment of neutrophils, as demonstrated by flow cytometry analysis of BAL cells (Fig.2a,b). No statistically significant recruitment of eosinophils, lymphocytes, macrophages, monocytes or dendritic cell subsets could be observed. We analysed a panel of acute-phase cytokines and chemokines [MIP-2α (IL-8), IL-6, IL-1β, TNF-α] known to be associated with airway inflammation.35,37 Furthermore, the analysis included cytokines and chemokines associated with type 17 (IL-17, CCL20) responses, type 1 (IFN-γ) responses, type 2 (IL-5, TSLP) responses, immune regulation (IL-10) and recruitment of T cells to the lung (CXCL16).38 The airway neutrophilia induced by H. inf. NT was accompanied by production of CCL20, CXCL16, IL-1β, IL-6 and MIP-2α (IL-8), and to lesser extent IFN-γ and TNF-α in lung tissue (significant induction by Student's one-sample t-test; Fig.2c). The induced cytokines and chemokines relate primarily to type 17 inflammation and neutrophil recruitment; however, induction of the prototype type 17 cytokine IL-17, as well as IL-10, TSLP and IL-5 were not detectable in the lung tissue. Immunohistochemical analysis revealed that the airway inflammation mediated by H. inf. NT was accompanied by lung pathology characterized by massive peri-bronchial inflammation, recruitment of immune cells and destruction of alveolar integrity (Fig.2d,e).
Figure 2.
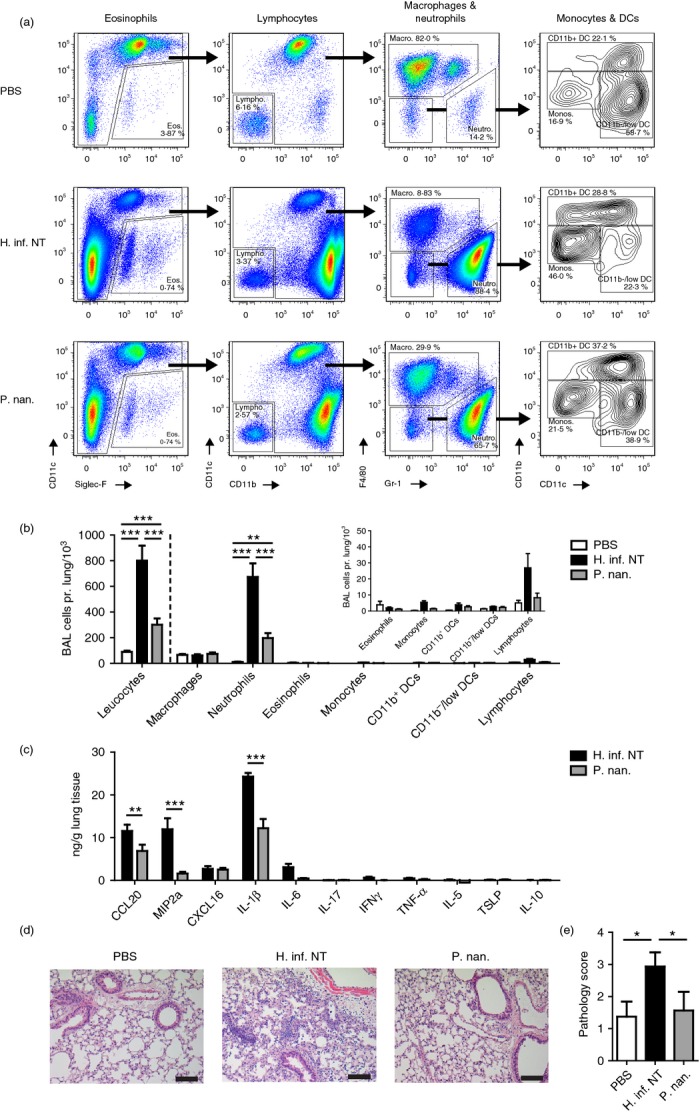
Airway inflammation following challenge with Haemophilus influenzae or Prevotella nanceiensis in mice. Flow cytometry of bronchoalveolar lavage (BAL) cells (a), absolute cell numbers in BAL (b), chemokine/cytokine levels in lung tissue homogenates (c), representative haematoxylin & eosin-stained lung tissue sections (d), and pathology scores (e) obtained from murine lungs 24 hr after airway challenge with vehicle (PBS), Haemophilus influenzae or Prevotella nanceiensis. Data represent two independent experiments (n = 8, mean + SEM). *P < 0·05; **P < 0·01; ***P < 0·001.
The P. nan. was found to induce some airway neutrophil recruitment which was threefold to fourfold lower than the recruitment mediated by H. inf. NT (Fig.2b). The decreased airway inflammation by P. nan. was associated with significant decrease in the acute-phase cytokine IL-1β, and the neutrophilic chemokines CCL20 and MIP-2α (IL-8) compared with H. inf. NT (Fig.2c). No lung tissue pathology could be detected following airway challenge with P. nan. (Fig.2d,e). These findings suggest that Prevotella spp. associated with healthy lungs may be intrinsically tolerated by the respiratory immune system, whereas pathogenic Proteobacteria exhibit distinct properties that can mediate COPD-like inflammatory features.
A different role for TLR2 in airway inflammation mediated by non-typeable H. influenzae and P. nanceiensis
Toll-like receptors play an important role in mediating inflammation by recognizing conserved MAMPs. TLR4 is receptor for the molecule LPS that is classically viewed as the most potent MAMP found in Gram-negative bacteria. Activation of TLR4 and TLR2 has been reported to account for up to 90% of the inflammatory response to common pathogenic Gram-negative bacteria.39 Both the Proteobateria and Prevotella spp. analysed in this study are Gram-negative and contain LPS with the potential to activate TLR4. However, the two groups of bacteria may have different TLR4 activating properties. This is exemplified by the gut commensal Gram-negative Bacteroides fragilis of the same phylum as the Prevotella spp. (Bacteroidetes), which has been shown to activate TLR2, but not TLR4.40 It is possible to address the contribution of TLR2 using TLR2 knockout mice, and hence indirectly examine the contribution from other ligands, such as LPS (TLR4). The role of TLR2 in the airway inflammatory response to H. influenzae (H. inf. NT) and P. nanceiensis (P. nan.) was compared using WT and TLR2−/− mice. We found TLR2 to be dispensable for acute airway neutrophilia and lung tissue pathology mediated by H. inf. NT (Fig.3a,c). However, MIP-2α (IL-8) production in the lung in response to H. inf. NT was diminished in TLR2-deficient mice (Fig.3b), indicating that MIP-2α (IL-8) is not essential for full expression of airway neutrophilia.
Figure 3.
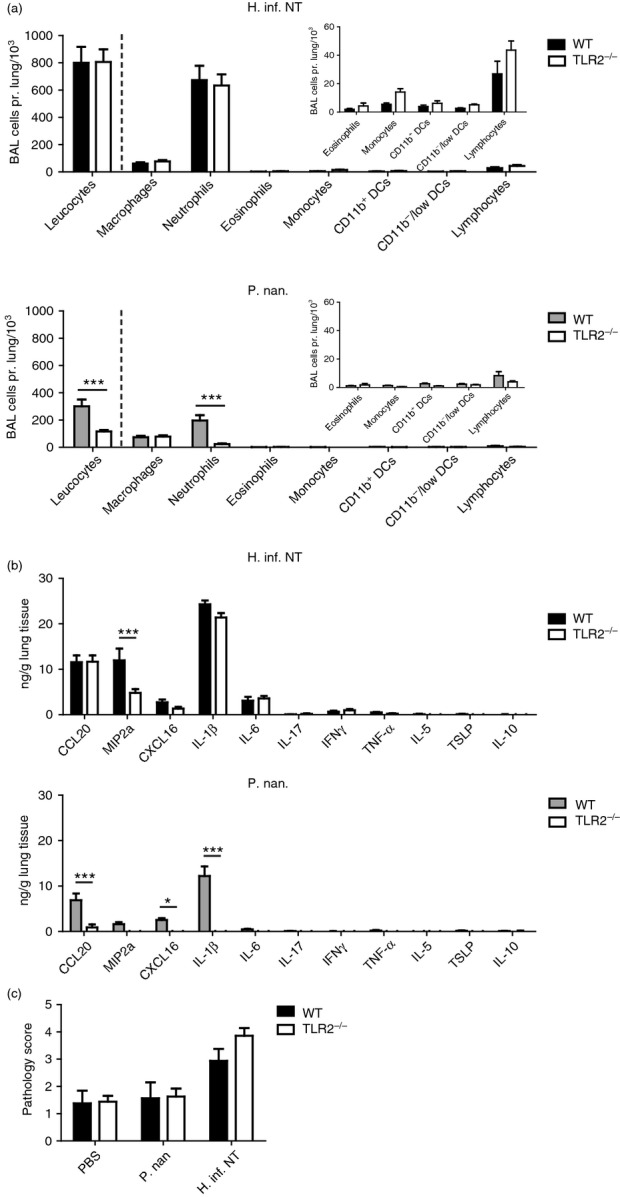
The role of Toll-like receptor 2 (TLR2) in the airway inflammatory response to Haemophilus influenzae or Prevotella nanceiensis in mice. Absolute cell numbers in bronchoalveolar lavage (BAL) (a), chemokine/cytokine levels in lung tissue homogenates (b), and pathology scores (c) obtained from wild-type (WT) or TLR2−/− murine lungs 24 hr after airway challenge with Haemophilus influenzae or Prevotella nanceiensis. Data represent two independent experiments (n = 8, mean + SEM). *P < 0·05; ***P < 0·001.
Airway inflammation mediated by P. nan. was found to be completely dependent on TLR2. Cellular recruitment in airways was absent (no difference when compared with PBS-treated group) and none of the analysed cytokines were found to be induced (tested by Student's one-sample t-test) in response to P. nan. in TLR2-deficient mice (Fig.3a,b). As expected, also TLR2-deficient mice showed no lung pathology in response to P. nan. (Fig.3c). These findings indicate that the innate stimulatory potential of Prevotella spp. is mainly related to TLR2 activation, whereas pathogenic Proteobacteria stimulate via other immune receptors that can fully express COPD-like inflammatory features.
Discussion
The present study demonstrates intrinsic differences in innate stimulatory and airway inflammatory properties between pathogenic Proteobacteria associated with chronic inflammatory airway disease (COPD and asthma) and commensal Prevotella spp. associated with healthy lungs.
Bacteria contain several conserved compounds including the MAMPs that cause immune activation via innate receptors. LPS is a well-known ubiquitous cell membrane constituent of all Gram-negative bacteria and is classically viewed as a potent MAMP for innate immune activation via TLR4. Studies have shown that TLR4 and TLR2 account for approximately 90% of the pro-inflammatory innate response of human leucocytes to common pathogenic Gram-negative bacteria.39 Here we found that airway inflammation driven by Prevotella spp. was completely dependent on TLR2. This finding suggests that Prevotella spp. do not contain immunostimulatory LPS that can mediate airway inflammation. Indeed, the difference in inflammatory potential between the Gram-negative COPD and asthma-associated Proteobacteria and commensal Prevotella spp. can perhaps be ascribed to differences in LPS structures.41 It is known that tetra- and penta-acylated LPS structures retain lower stimulatory activity on TLR4 than hepta- and hexa-acylated LPS.42,43 The prototypic hexa-acylated LPS of Escherichia coli seems to be the most biologically potent structure, whereas LPS with fewer and shorter acyl-chains have lower capacity to stimulate TLR4.44–46 Haemophilus influenzae and M. catarrhalis has been reported to contain hexa- and hepta-acylated LPS, respectively.47,48 In contrast, Prevotella spp. and other members of the Bacteroidetes phylum have been reported to contain penta-acylated LPS, which have implications for recognition of the bacteria by TLR4. LPS isolated from the oral commensal P. intermedia demonstrate approximately 10-fold reduced potency to induce IL-6 in murine macrophages compared with E. coli LPS.49 Furthermore, the gut commensal B. fragilis contain penta-acylated LPS,50 and this bacterium has been found to stimulate TLR2 but not TLR4.40 The presence of immune inactive LPS in all Prevotella spp. is supported by a recent bioinformatic-based analysis performed by our group. We analysed all sequenced bacterial genomes publicly available and studied the genes involved in LPS synthesis. We found that the LpxM gene encoding the enzyme (KDO)2-(lauroyl)-lipid IVA acyltransferase needed to add a sixth acyl chain to lipid A (important for TLR4 immune activity) is absent in all members of the Bacteroidetes phylum including Prevotella spp., but present in γ-Proteobacteria such as H. influenzae (Brix ., manuscript in revision).
In the present report, Prevotella spp. associated with healthy human lungs was well-tolerated in murine airways by inducing limited neutrophilia, chemokine and cytokine production, as well as provoking no detectable lung immunopathology compared with pathogenic Proteobacteria. Observational studies reporting lung dysbiosis with increased pathogenic proteobacteria and reduced Prevotella spp. in COPD and asthma7 suggest that the well-tolerated Prevotella spp. serves a disease-protective role. A protective role of Prevotella spp. could be ascribed to (i) a direct modulation of innate immune responses in the lung, (ii) induction of functional and developmental changes in the immune system, (iii) suppression of proteobacterial colonization or infection by taking up an ecological niche in the microbiota, and (iv) direct killing of Proteobacteria. Interestingly, a recent study in mice reported that the acquisition of a Bacteroidetes-rich lung microbiota during early life was associated with the establishment of tolerance in the lung and suppression of allergic inflammation, whereas Proteobacteria-rich lungs of neonatal mice showed exaggerated responses to allergen.51 Additionally, we have previously reported that Prevotella spp. modulates the in vitro inflammatory response of human dendritic cells to H. influenzae, suggesting that members of the commensal lung microbiota may directly limit the immune response to specific pathogens.41 We previously proposed41 that the penta-acylated LPS of Prevotella spp. might inhibit the activation of TLR4 in response to the immune active hexa-acylated LPS of pathogenic proteobacteria, since it has been demonstrated that penta-acylated LPS can block TLR4 signalling by sequestering MD-2.52,53 Additional studies are needed to establish if Prevotella spp. exhibit causal disease-protective effects.
We found that the three Proteobacteria associated with COPD and asthma potently mediated airway neutrophilia, inflammatory chemokine/cytokine production and tissue pathology in mice. This is in line with previous studies reporting that certain Proteobacteria can induce COPD-like inflammatory features and promote COPD disease in murine models.28–30 The results from murine studies support the notion of pathogenic Proteobacteria as potential co-drivers of COPD disease.21–24 We speculate that it is the specific immune active hexa-acylated structure of LPS in these bacterial strains that gives the bacteria their disease-promoting activity. Indeed, inhalation of LPS in humans mediates production of IL-1β, IL-6 and TNF-α, neutrophil recruitment and decreased lung function forced expiratory volume for 1 second (FEV1).54,55 Based on these findings it could be proposed to examine the applicability of inhaled TLR4 antagonists as an option for treatment of chronic inflammatory airway disease with involvement of pathogenic Proteobacteria.
Acknowledgments
The authors thank animal technician Pernille Wehler Güllich and laboratory technician Lisbeth Buus Rosholm for their excellent assistance and support during this study. Funding from the Lundbeck Foundation and the A. P. Møller Foundation for the Advancement of Medical Science is acknowledged. The funding bodies had no role in study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Glossary
- BAL
bronchoalveolar lavage
- COPD
chronic obstructive pulmonary disease
- H. inf. B
Haemophilus influenzae B
- H. inf. NT
non-typeable Haemophilus influenzae
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- M. cat.
Moraxella catarrhalis
- MAMP
microbial-associated molecular pattern
- MIP
macrophage inflammatory protein
- P. mel.
Prevotella melaninogenica
- P. nan.
Prevotella nanceiensis
- P. sal.
Prevotella salivae
- TLR
Toll-like receptor
- TNF
tumour necrosis factor
- TSLP
thymic stromal lymphopoietin
- WT
wild-type
Author Contributions
JML conceived the study, designed experiments, analysed data and wrote the manuscript. HSM and TMB performed experiments and analysed data. CI performed histological staining and scoring. SB supervised the study. All authors provided important intellectual contributions to the study and writing of manuscript.
Disclosures
The authors have no financial or commercial conflicts of interest to disclose in relation to publication of the manuscript.
References
- Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–41. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N, Seo S-U, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–35. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–9. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- Kahn FW, Jones JM. Diagnosing bacterial respiratory infection by bronchoalveolar lavage. J Infect Dis. 1987;155:862–9. doi: 10.1093/infdis/155.5.862. [DOI] [PubMed] [Google Scholar]
- Baughman R, Thorpe J, Staneck J, Rashkin M, Frame P. Use of the protected specimen brush in patients with endotracheal or tracheostomy tubes. Chest. 1987;91:233–6. doi: 10.1378/chest.91.2.233. [DOI] [PubMed] [Google Scholar]
- Thorpe JE, Baughman RP, Frame PT, Wesseler TA, Staneck JL. Bronchoalveolar lavage for diagnosing acute bacterial pneumonia. J Infect Dis. 1987;155:855–61. doi: 10.1093/infdis/155.5.855. [DOI] [PubMed] [Google Scholar]
- Hilty M, Burke C, Pedro H. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ES, Chen J, Custers-Allen R. Disordered microbial communities in the upper respiratory tract of cigarette smokers. PLoS One. 2010;5:e15216. doi: 10.1371/journal.pone.0015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb-Downward JR, Thompson DL, Han MK. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One. 2011;6:e16384. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JK, De Groote MA, Sagel SD. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc Natl Acad Sci U S A. 2007;104:20529–33. doi: 10.1073/pnas.0709804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, Yadav A, Bushman FD, Collman RG. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med. 2011;184:957–63. doi: 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy E. Anaerobic infections: update on treatment considerations. Drugs. 2010;70:841–58. doi: 10.2165/11534490-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Brook I. Anaerobic pulmonary infections in children. Pediatr Emerg Care. 2004;20:636–40. doi: 10.1097/01.pec.0000139751.63624.0b. [DOI] [PubMed] [Google Scholar]
- Brook I. Microbiology of common infections in the upper respiratory tract. Prim Care. 1998;25:633–48. doi: 10.1016/s0095-4543(15)30006-3. [DOI] [PubMed] [Google Scholar]
- Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 2011;10:311–23. doi: 10.1016/j.chom.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goleva E, Jackson LP, Harris JK. The effects of airway microbiome on corticosteroid responsiveness in asthma. Am J Respir Crit Care Med. 2013;188:1193–201. doi: 10.1164/rccm.201304-0775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgaard H, Hermansen MN, Buchvald F. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–95. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- Murphy TF, Sethi S, Niederman MS. The role of bacteria in exacerbations of COPD. A constructive view. Chest. 2000;118:204–9. doi: 10.1378/chest.118.1.204. [DOI] [PubMed] [Google Scholar]
- Monsó E, Rosell A, Bonet G, Manterola J, Cardona PJ, Ruiz J, Morera J. Risk factors for lower airway bacterial colonization in chronic bronchitis. Eur Respir J. 1999;13:338–42. doi: 10.1034/j.1399-3003.1999.13b20.x. [DOI] [PubMed] [Google Scholar]
- Zalacain R, Sobradillo V, Amilibia J, Barrón J, Achótegui V, Pijoan JI, Llorente JL. Predisposing factors to bacterial colonization in chronic obstructive pulmonary disease. Eur Respir J. 1999;13:343–8. doi: 10.1034/j.1399-3003.1999.13b21.x. [DOI] [PubMed] [Google Scholar]
- Papi A, Luppi F, Franco F, Fabbri LM. Pathophysiology of exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:245–51. doi: 10.1513/pats.200512-125SF. [DOI] [PubMed] [Google Scholar]
- Hill AT, Campbell EJ, Hill SL, Bayley DL, Stockley RA. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am J Med. 2000;109:288–95. doi: 10.1016/s0002-9343(00)00507-6. [DOI] [PubMed] [Google Scholar]
- Wilkinson TMA, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1090–5. doi: 10.1164/rccm.200210-1179OC. [DOI] [PubMed] [Google Scholar]
- Sethi S, Maloney J, Grove L, Wrona C, Berenson CS. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:991–8. doi: 10.1164/rccm.200509-1525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert RK, Connett J, Bailey WC. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689–98. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgaard H, Hermansen MN, Bønnelykke K. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. doi: 10.1136/bmj.c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JM, Brix S, Thysen AH, Birch S, Rasmussen MA, Bisgaard H. Children with asthma by school age display aberrant immune responses to pathogenic airway bacteria as infants. J Allergy Clin Immunol. 2014;133:1008–1013. doi: 10.1016/j.jaci.2014.01.010. e4. [DOI] [PubMed] [Google Scholar]
- Gaschler GJ, Skrtic M, Zavitz CCJ, Lindahl M, Onnervik P-O, Murphy TF, Sethi S, Stämpfli MR. Bacteria challenge in smoke-exposed mice exacerbates inflammation and skews the inflammatory profile. Am J Respir Crit Care Med. 2009;179:666–75. doi: 10.1164/rccm.200808-1306OC. [DOI] [PubMed] [Google Scholar]
- Gaschler GJ, Zavitz CCJ, Bauer CMT, Stämpfli MR. Mechanisms of clearance of nontypeable Haemophilus influenzae from cigarette smoke-exposed mouse lungs. Eur Respir J. 2010;36:1131–42. doi: 10.1183/09031936.00113909. [DOI] [PubMed] [Google Scholar]
- Moghaddam SJ, Clement CG, De la Garza MM. Haemophilus influenzae lysate induces aspects of the chronic obstructive pulmonary disease phenotype. Am J Respir Cell Mol Biol. 2008;38:629–38. doi: 10.1165/rcmb.2007-0366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonefeld CM, Larsen JM, Dabelsteen S, Geisler C, White IR, Menné T, Johansen JD. Consumer available permanent hair dye products cause major allergic immune activation in an animal model. Br J Dermatol. 2010;162:102–7. doi: 10.1111/j.1365-2133.2009.09417.x. [DOI] [PubMed] [Google Scholar]
- Dyer KD, Garcia-Crespo KE, Killoran KE, Rosenberg HF. Antigen profiles for the quantitative assessment of eosinophils in mouse tissues by flow cytometry. J Immunol Methods. 2011;369:91–7. doi: 10.1016/j.jim.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman M. Variation and type specificity in the bacterial species Haemophilus influenzae. J Exp Med. 1931;53:471–92. doi: 10.1084/jem.53.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geme JWS. Molecular and cellular determinants of non-typeable Haemophilus influenzae adherence and invasion. Cell Microbiol. 2002;4:191–200. doi: 10.1046/j.1462-5822.2002.00180.x. [DOI] [PubMed] [Google Scholar]
- Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-α in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153:530–4. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- Soumelis V, Reche PA, Kanzler H. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2001;34:50s–9s. [PubMed] [Google Scholar]
- Freeman CM, Curtis JL, Chensue SW. CC chemokine receptor 5 and CXC chemokine receptor 6 expression by lung CD8+ cells correlates with chronic obstructive pulmonary disease severity. Am J Pathol. 2007;171:767–76. doi: 10.2353/ajpath.2007.061177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson G, Dunn-Siegrist I, Daubeuf B, Pugin J. Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood. 2007;109:1574–83. doi: 10.1182/blood-2006-06-032961. [DOI] [PubMed] [Google Scholar]
- Alhawi M, Stewart J, Erridge C, Patrick S, Poxton IR. Bacteroides fragilis signals through Toll-like receptor (TLR) 2 and not through TLR4. J Med Microbiol. 2009;58:1015–22. doi: 10.1099/jmm.0.009936-0. [DOI] [PubMed] [Google Scholar]
- Larsen JM, Steen-Jensen DB, Laursen JM, Søndergaard JN, Musavian HS, Butt TM, Brix S. Divergent pro-inflammatory profile of human dendritic cells in response to commensal and pathogenic bacteria associated with the airway microbiota. PLoS One. 2012;7:e31976. doi: 10.1371/journal.pone.0031976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F, Normark S, Schweda EKH, Oscarson S, Richter-Dahlfors A. Structural requirements for TLR4-mediated LPS signalling: a biological role for LPS modifications. Microbes Infect. 2003;5:1057–63. doi: 10.1016/s1286-4579(03)00207-7. [DOI] [PubMed] [Google Scholar]
- Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- Lebeer S, Vanderleyden J, De Keersmaecker SCJ. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 2010;8:171–84. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- Caroff M, Karibian D. Structure of bacterial lipopolysaccharides. Carbohydr Res. 2003;338:2431–47. doi: 10.1016/j.carres.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Caroff M, Karibian D, Cavaillon JM, Haeffner-Cavaillon N. Structural and functional analyses of bacterial lipopolysaccharides. Microbes Infect. 2002;4:915–26. doi: 10.1016/s1286-4579(02)01612-x. [DOI] [PubMed] [Google Scholar]
- Schweda EKH, Twelkmeyer B, Li J. Profiling structural elements of short-chain lipopolysaccharide of non-typeable Haemophilus influenzae. Innate Immun. 2008;14:199–211. doi: 10.1177/1753425908095958. [DOI] [PubMed] [Google Scholar]
- Masoud H, Perry MB, Richards JC. Characterization of the lipopolysaccharide of Moraxella catarrhalis. Structural analysis of the lipid A from M. catarrhalis serotype A lipopolysaccharide. Eur J Biochem. 1994;220:209–16. doi: 10.1111/j.1432-1033.1994.tb18616.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Asai Y, Tamai R, Jinno T, Umatani K, Ogawa T. Chemical structure and immunobiological activity of lipid A from Prevotella intermedia ATCC 25611 lipopolysaccharide. FEBS Lett. 2003;543:98–102. doi: 10.1016/s0014-5793(03)00414-9. [DOI] [PubMed] [Google Scholar]
- Erridge C, Pridmore A, Eley A, Stewart J, Poxton IR. Lipopolysaccharides of Bacteroides fragilis Chlamydia trachomatis and Pseudomonas aeruginosa signal via toll-like receptor 2. J Med Microbiol. 2004;53:735–40. doi: 10.1099/jmm.0.45598-0. [DOI] [PubMed] [Google Scholar]
- Gollwitzer ES, Saglani S, Trompette A. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat Med. 2014;20:642–7. doi: 10.1038/nm.3568. [DOI] [PubMed] [Google Scholar]
- Coats SR, Reife RA, Bainbridge BW, Pham TT-T, Darveau RP. Porphyromonas gingivalis lipopolysaccharide antagonizes Escherichia coli lipopolysaccharide at toll-like receptor 4 in human endothelial cells. Infect Immun. 2003;71:6799–807. doi: 10.1128/IAI.71.12.6799-6807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats SR, Pham T-TT, Bainbridge BW, Reife RA, Darveau RP. MD-2 mediates the ability of tetra-acylated and penta-acylated lipopolysaccharides to antagonize Escherichia coli lipopolysaccharide at the TLR4 signaling complex. J Immunol. 2005;175:4490–8. doi: 10.4049/jimmunol.175.7.4490. [DOI] [PubMed] [Google Scholar]
- Hernandez ML, Herbst M, Lay JC, Alexis NE, Brickey WJ, Ting JPY, Zhou H, Peden DB. Atopic asthmatic patients have reduced airway inflammatory cell recruitment after inhaled endotoxin challenge compared with healthy volunteers. J Allergy Clin Immunol. 2012;130:869–76. doi: 10.1016/j.jaci.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller W, Heimbeck I, Hofer TPJ, Khadem Saba G, Neiswirth M, Frankenberger M, Ziegler-Heitbrock L. Differential inflammatory response to inhaled lipopolysaccharide targeted either to the airways or the alveoli in man. PLoS One. 2012;7:e33505. doi: 10.1371/journal.pone.0033505. [DOI] [PMC free article] [PubMed] [Google Scholar]