Abstract
Background
Coenzyme Q10 (CoQ10) supplementation is the most popular therapy for statin myalgia among both physicians and patients despite limited and conflicting evidence of its efficacy.
Objective
This study examined the effect of coenzyme Q10 (CoQ10) supplementation on simvastatin-associated muscle pain, muscle strength and aerobic performance in patients with confirmed statin myalgia.
Methods
Statin myalgia was confirmed in 120 patients with prior symptoms of statin myalgia using an 8-week randomized, double-blind crossover trial of simvastatin 20 mg/d and placebo. Forty-one subjects developed muscle pain with simvastatin but not with placebo and were randomized to simvastatin 20 mg/d combined with CoQ10 (600 mg/d ubiquinol) or placebo for 8 weeks. Muscle pain (Brief Pain Inventory [BPI]), time to pain onset, arm and leg muscle strength, and maximal oxygen uptake (VO2max) were measured before and after each treatment.
Results
Serum CoQ10 increased from 1.3±0.4 to 5.2±2.3 mcg/mL with simvastatin and CoQ10, but did not increase with simvastatin and placebo (1.3±0.3 to 0.8±0.2) (p<0.05). BPI pain severity and interference scores increased with simvastatin therapy (both p<0.01), irrespective of CoQ10 assignment (p=0.53 and 0.56). There were no changes in muscle strength or VO2max with simvastatin with or without CoQ10 (all p>0.10). Marginally more subjects reported pain with CoQ10 (14 of 20 vs 7 of 18; p=0.05). There was no difference in time to pain onset in the CoQ10 (3.0±2.0 weeks) vs. placebo (2.4±2.1 wks) groups (p=0.55). A similar lack of CoQ10 effect was observed in 24 subjects who were then crossed over to the alternative treatment.
Conclusions
Only 36% of patients complaining of statin myalgia develop symptoms during a randomized, double-blind crossover of statin vs placebo. CoQ10 supplementation does not reduce muscle pain in patients with statin myalgia.
Trial Registration
Keywords: statin, simvastatin, myalgia, ubiquinol, CoQ10, muscle pain, exercise, physical performance, muscle strength, aerobic performance
INTRODUCTION
Hydroxy-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors or statins reduce low-density lipoprotein (LDL)-cholesterol concentrations and cardiac events (1). Statins are well-tolerated but can produce myalgia in 1 to 25% of patients (2-4). In the Effect of Statins on Skeletal Muscle Function and Performance (STOMP) study, we observed a doubling of muscle complaints from 4.6 to 9.4% of subjects among 420 subjects treated with high dose atorvastatin (80mg/d) or placebo for 6 mos (3).
How statins produce muscular side effects is not clear, but depletion of the mitochondrial transport element ubiquinone, or coenzyme Q10 (CoQ10), is a possible mechanism since CoQ10 is also produced by the cholesterol metabolic pathway (5). The effects of CoQ10 supplementation on statin myalgia have not been extensively studied, and available studies are small and have produced conflicting results (6,7). Nevertheless, CoQ10 supplementation is used by many patients and recommended by many clinicians despite the absence of definitive results. A major limitation of prior studies is that statin myalgia was based on self-report. We designed the Co-Enzyme Q10 in Statin Myopathy study to evaluate the effect of CoQ10 supplementation in subjects whose muscle complaints were confirmed using a lead-in, double-blind, placebo comparison of statin vs placebo.
METHODS
Confirmation of Statin-Associated Myalgia
Men and women ≥20 yrs of age with a history of muscle complaints during statin treatment were recruited from the Cholesterol Management Center at Hartford Hospital, newspaper and radio advertisements, and contact with physicians’ offices. Subjects were excluded if they had had cancer within 5 years of entry, hypo- or hyperthyroidism (TSH > 5 or <0.01 IU/L), hepatic disease (ALT > 2 UNL), renal disease (creatinine> 2 mg/dL) or were using medications (such as corticosteroids) known to affect skeletal muscle metabolism. Subjects using supplemental CoQ10 discontinued supplementation for 2 months before the study. As previously detailed (8) (Figure 1), subjects’ cholesterol medications were discontinued for 4 weeks. Subjects then entered a randomized, double-blind, crossover, lead-in trial of simvastatin (20 mg/d) or placebo to confirm statin myalgia. Subjects were treated for 8 weeks or until muscle symptoms persisted for 1 week or were intolerable. Subjects then underwent a 4-week wash-out period, and were crossed over from simvastatin to placebo or vice versa. Only subjects who experienced muscle pain on simvastatin but not on placebo and whose pain resolved within 4 weeks off treatment were entered into the CoQ10 trial.
Figure 1.
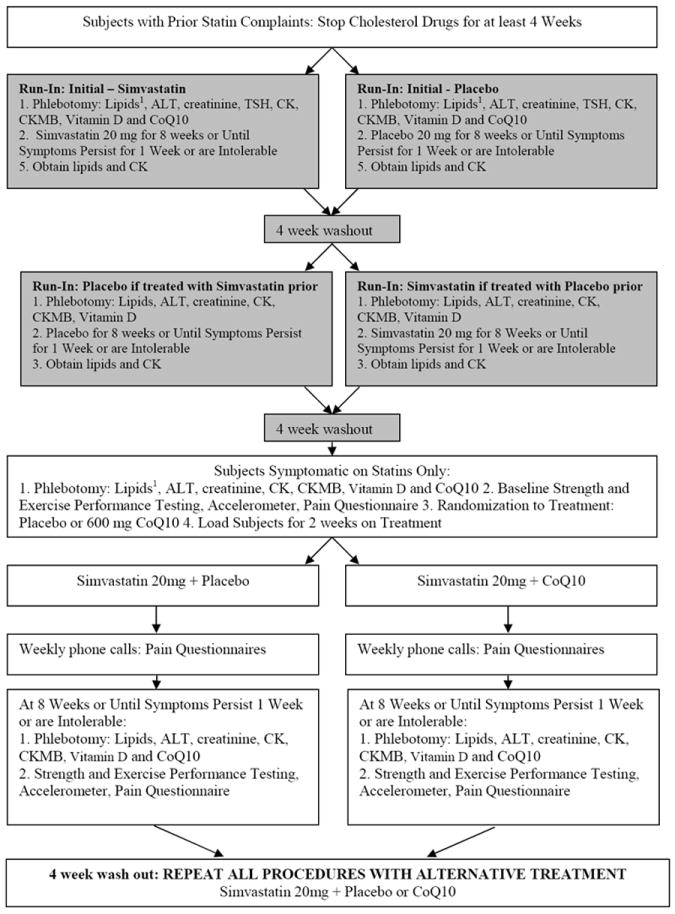
Protocol for Coenzyme Q10 (CoQ10) in Statin Myopathy study. For all study visits, procedures are performed in the order listed. 1Lipids: total cholesterol, low density lipoprotein (LDL)-cholesterol, high density lipoprotein (HDL)-cholesterol, triglycerides. ALT = alanine aminotransferase, CK = creatine kinase, CK-MB = creatine kinase heart-specific isoform, TSH = thyroid-stimulating hormone.
Study pharmacists compounded identical simvastatin and placebo capsules and subjects were randomized in a 1:1 fashion according to www.randomization.com. Simvastatin tablets were obtained from a single supplier, cut, covered with lactose secundum artem, and placed into opaque capsules. Placebo tablets were filled with lactose alone. Muscle symptoms were documented weekly by telephone.
CoQ10 Treatment Study
Forty one subjects with confirmed statin myalgia (Figure 2) entered another 4-week wash-out period and were then loaded for 2 weeks with either CoQ10 600 mg daily or placebo to ensure adequate CoQ10 levels before simvastatin treatment. After loading, subjects were randomized by study pharmacists again using www.randomization.com to simvastatin 20 mg/d and CoQ10 600 mg/d or simvastatin and placebo for 8 weeks or until muscle symptoms persisted for 1 week or were intolerable. One of the CoQ10 and 2 of the placebo group subjects failed to complete the study because of unrelated changes in medical or personal circumstances that made them discontinue study treatment. A subset of subjects (n=24) who completed the study then entered another 4 week washout and crossed over from statin/CoQ10 to statin/placebo or vice versa. The cross-over phase was added after some patients had completed the study because of the lower number of confirmed myalgics qualifying for the treatment phase; therefore only 24 of the 38 subjects who completed the first parallel treatment phase were crossed over to the alternative treatment. Pain intensity was recorded weekly, and subjects underwent phlebotomy as well as measurements of pain, muscle strength, VO2max, and physical activity level, at the beginning and end of each treatment phase. CoQ10 and placebo were obtained in identical matching 300 mg soft gelatin capsules from Tishcon Corporation (Waterbury, New York) according to standards for ubiquinol formation (CoQH2) delineated under the Investigational New Drug (IND) number assigned to the study (IND106208). All investigators and patients were blind to drug order and identity throughout the study. The study was approved by the Institutional Review Board at Hartford Hospital and a Data Safety and Monitoring Board (DSMB) composed of two physicians and a statistician oversaw the project with biannual meetings.
Figure 2.
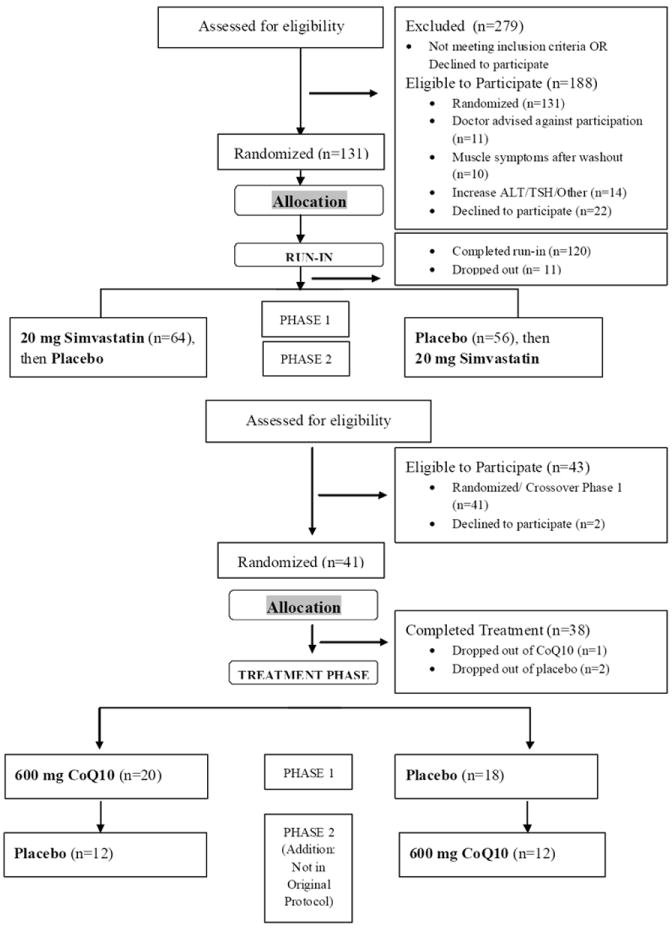
CoQ10 Participant Flow Diagram. Study flow diagram detailing numbers (n) of participants who were screened, randomized, completed and analyzed in the study.
Serologic Markers
Creatinine and thyroid stimulating hormone (TSH) levels were measured before the lead-in trial. Blood was drawn at each testing point during the lead-in and CoQ10 section of the study for measurement of serum lipids (total, HDL, and LDL-cholesterol and triglycerides), creatine kinase (CK), vitamin D, and alanine aminotransferase (ALT) levels. Blood CoQ10 levels were measured as described (9) at the Children’s Hospital Medical Center in Cincinnati, OH at each timepoint during both the myalgia confirmation phase and the CoQ10 trial.
Measurement of Muscle Pain
Subjects were contacted weekly during the confirmation and CoQ10 treatment portions of the study to inquire about muscle symptoms using the Brief Pain Inventory Short Form (BPI-SF) (10). A pain severity score (PSS) was calculated by averaging scores on 4 pain-intensity items, and a pain interference score (PIS) was calculated by averaging scores on 7 pain-interference items.
Muscle Strength and Endurance
Muscle strength and exercise performance were measured only during the treatment phase of the study. Subjects were tested at the start using one practice and one formal testing visit. Testing was repeated at the end of the statin/CoQ10 and statin/placebo portions of the study in one visit. Handgrip strength was measured using a handgrip dynamometer. Elbow flexor and knee extensor isometric and isokinetic force was measured using the Biodex System 3 isokinetic dynamometer (Biodex Medical, Shirley, New York). All measurements were taken on the dominant hand side of the body with measurements performed as described (11).
Measurement of Aerobic Exercise Performance
VO2max and the ventilatory threshold were measured at the beginning of the CoQ10 section and after each treatment phase using a breath-by-breath gas analysis and a Parvomedics system (Parvomedics, Sandy, UT) during a modified Astrand exercise protocol.
Medication Compliance Assessment
Subjects were instructed to take three study pills (1 simvastatin and either 2 CoQ10 or 2 placebo tablets) daily. Subjects were queried regarding missed pills and problems taking medications during weekly phone calls during both the confirmation trial and CoQ10 portion of the study. Subjects returned unused pills after each portion of the study.
Statistical Analyses
Sample size was based on an expected 20% between-group difference in pain (6,7). This yielded a sample size of 40 subjects on CoQ10 and placebo to reject the null hypothesis and to detect possible interactions with gender, with power 0.9, and a type I error of 0.05. We aimed to identify 100 confirmed myalgics from the confirmation trial to assign 50 each to the CoQ10 and placebo arms of the trial. We estimated that 75% of recruited subjects would qualify as having confirmed statin myalgia. Consequently, we sought to recruit 135 myalgic subjects to enter the confirmation lead trial, which would yield approximately 100 subjects with confirmed myalgia to be randomly assigned to either the COQ10 or placebo groups. The confirmation trial yielded fewer confirmed myalgics than anticipated. Therefore, we added a cross-over phase to the CoQ10 treatment study, where confirmed myalgics were treated with both CoQ10 and placebo. This permitted within-subjects comparisons and reduced the sample size needed to detect a treatment effect.
Standard diagnostics were performed to ensure that basic statistical assumptions, including variance homogeneity, normality, and the absence of outliers, were met prior to data analyses. Resultant statistical analyses on the outcome were conducted with a linear mixed-effects model that accounts for the correlation among repeated measurements on an individual. In this repeated measures cross-over design there were two within subject factors (drug treatment and time). We examined the influence of other potential variables of interest (baseline serum CoQ10 levels, changes in serum CoQ10 levels, ethnicity, body mass index) as either between-subject factors with interaction terms in the Analysis of Variance (ANOVA) model or as continuous predictors in an Analysis of Covariance (ANCOVA) model. Comparisons of responses in the cross-over portion of the study were conducted with a paired-t test of individual change scores on CoQ10 vs. placebo.
RESULTS
Data were collected from October 2009-2012. Characteristics of subjects who completed both the placebo and simvastatin phase of the confirmation trial and either did (n=43) or did not (n=77) develop myalgia are presented in Table 1. Subjects with confirmed myalgia who subsequently entered the treatment phase of the study had a higher BMI and greater CK levels at study entry. Patients who received statin therapy first in the confirmation trial were also more likely to develop myalgia (28 of 64) than those who received it second (15 of 56) (χ2= 3.7; p = 0.05). In addition, while there were no differences in the characteristics of myalgia between groups (i.e., involved muscle groups, location), time to pain onset after starting simvastatin was significantly shorter in the confirmed myalgics relative to the subset of non-myalgic patients who experienced pain on simvastatin (1.7 ± 1.4 weeks vs. 3.0 ± 1.8 weeks; p < 0.01).
Table 1.
Baseline Characteristics of Subjects Who Were or Were Not Confirmed for Myalgia
| Confirmed (n=43) | Not Confirmed (n=77) | |
|---|---|---|
| Women (n) | 16 | 35 |
| Age (yr) | 58 ± 11 | 61 ± 9 |
| BMI (kg/m2) | 29.6 ± 5.3* | 27.8 ± 4.2 |
| SBP (mmHg) | 122 ± 14 | 124 ± 14 |
| DBP (mmHg) | 75 ± 7 | 75 ± 6 |
| CK (U/L) | 152 ± 89* | 117 ± 68 |
| Vitamin D (ng/mL) | 31 ± 12 | 31 ± 10 |
| Total C (mg/dL) | 255 ± 53 | 255 ± 49 |
| LDL-C (mg/dL) | 166 ± 49 | 165 ± 43 |
| HDL-C (mg/dL) | 52 ± 16 | 55 ± 15 |
| Triglycerides (mg/dL) | 186 ± 128 | 173 ± 111 |
BMI = body mass index; SBP = systolic blood pressure; DBP = diastolic blood pressure; CK = creatine kinase; C = colesterol; LDL = low density lipoprotein; HDL = high density lipoprotein.
indicates significant difference between groups at p < 0.05
Subjects receiving simvastatin with CoQ10 in the treatment phase increased total serum CoQ10 from 1.3 ± 0.4 to 5.2 ± 2.3 mcg/mL whereas serum CoQ10 decreased in the simvastatin and placebo group from 1.3 ± 0.3 to 0.8 ± 0.2; p < 0.05 for comparison. In addition, in the subjects who returned for the cross-over phase of the CoQ10 trial, serum CoQ10 also increased with CoQ10 treatment (Δ 4.3 ± 2.0) and decreased with placebo treatment (Δ -0.5 ± 0.3; p < 0.01). LDL-C decreased similarly in both the CoQ10 or placebo plus statin groups (Tables 2 and 3).
Table 2.
Treatment Responses to CoEnzyme Q10 vs. Placebo in Confirmed Myalgic Subjects
| Simvastatin + CoQ10 (n=20) | Simvastatin + Placebo (n=18) | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Age (yrs) | 58 ± 10 | ---- | 60 ± 10 | ---- |
| LDL-C (mg/dL) | 145 ± 31 | 99 ± 21* | 158 ± 33 | 103 ± 19* |
| CK (U/L) | 172 ± 123 | 173 ± 125 | 145 ± 74 | 186 ± 122 |
| Vit D (ng/mL) | 38 ± 12 | 35 ± 16 | 41 ± 18 | 38 ± 14 |
| VO2max (mL/kg/min) | 26 ± 8 | 25 ± 8 | 24 ± 5 | 24 ± 5 |
| RER | 0.85 ± 0.06 | 0.84 ± 0.07 | 0.86 ± 0.08 | 0.87 ± 0.06 |
| Handgrip (kg) | 34 ± 10 | 34 ± 12 | 33 ± 11 | 32 ± 12 |
| Isomet Knee ext1 | 136 ± 56 | 140 ± 57 | 150 ± 68 | 154 ± 61 |
| Isomet Knee flex | 59 ± 22 | 57 ± 25 | 66 ± 24 | 63 ± 24 |
| Isokin knee 60 ext | 115 ± 41 | 117 ± 43 | 118 ± 45 | 120 ± 45 |
| Isokin knee 60 flex | 51 ± 23 | 52 ± 22 | 56 ± 20 | 50 ± 17 |
| Isokin knee 180 ext | 70 ± 27 | 74 ± 30 | 75 ± 31 | 77 ± 29 |
| Isokin knee 180 flex | 35 ± 19 | 38 ± 17 | 37 ± 17 | 37 ± 13 |
| Isomet Elb Ext | 32 ± 13 | 33 ± 14 | 37 ± 16 | 35 ± 15 |
| Isomet Elb Flex | 40 ± 14 | 41 ± 14 | 41 ± 17 | 37 ± 16 |
| Isokin Elb 60 Ext | 33 ± 14 | 34 ± 13 | 35 ± 14 | 34 ± 13 |
| Isokin Elb 60 Flex | 35 ± 13 | 34 ± 14 | 36 ± 13 | 35 ± 13 |
| Isokin Elb 180 Ext | 26 ± 10 | 26 ± 10 | 27 ± 11 | 26 ± 9 |
| Isokin Elb 180 Flex | 29 ± 11 | 29 ± 13 | 27 ± 10 | 27 ± 9 |
| PSS (points) | 0.0 ± 0.0 | 2.2 ± 2.3* | 0.0 ± 0.0 | 1.7 ± 2.4* |
| PIS (points) | 0.0 ± 0.0 | 1.9 ± 2.4* | 0.0 ± 0.0 | 1.4 ± 2.5* |
Pre = before simvastatin + CoQ10 or placebo treatment; Post = after simvastatin + CoQ10 or placebo treatment; LDL-C = low density lipoprotein cholesterol; CK = creatine kinase; Vit D = Vitamin D; VO2max = maximal oxygen uptake; RER = respiratory exchange ratio; Handgrip = handgrip strength; Knee = Knee extensor or flexor strength variable; Isomet = isometric; Ext = Extension; Flex = flexion; Isokin = Isokinetic strength at either 60 or 180 degrees/sec; Elbow = Elbow extensor or flexor strength variable; PSS = pain severity score; PIS = pain intensity score.
All knee and elbow strength variables are reported as average peak torque in Newton-Meters.
denotes a significant change from baseline within a group at p < 0.05. There were no significant between-group differences in any variable.
Table 3.
Changes From Pre Treatment to Post Treatment Wtih Simvastatin and CoQ10 or Placebo Treatment in Confirmed Statin Myalgic Subjects Who Completed Both Phases of the Crossover Study
| Simvastatin + CoQ10 | Simvastatin + Placebo | |
|---|---|---|
| Δ LDL-C (mg/dL) | -50 ± 25 | -49 ± 19 |
| Δ CK (U/L) | +22 ± 129 | +33 ± 106 |
| Δ Vit D (ng/mL) | -3 ± 8 | 0 ± 12 |
| Δ Total CoQ10 | +4 ± 2* | -1 ± 0 |
| Δ VO2max (mL/kg/min) | -1 ± 6 | 0 ± 2 |
| Δ RER | +0.02 ± 0.2 | +0.04 ± 0.2 |
| Δ Handgrip (kg) | +1 ± 6 | 0 ± 5 |
| Δ Isomet Knee ext1 | -5 ± 19 | +6 ± 28 |
| Δ Isomet Knee flex | -3 ± 9 | -1 ± 10 |
| Δ Isokin knee 60 ext | +1 ± 12 | +2 ± 10 |
| Δ Isokin knee 60 flex | -1 ± 10 | -4 ± 10 |
| Δ Isokin knee 180 ext | +3 ± 12 | +3 ± 11 |
| Δ Isokin knee 180 flex | +3 ± 11 | +2 ± 10 |
| Δ Isomet Elb Ext | +1 ± 4 | -1 ± 4 |
| Δ Isomet Elb Flex | +2 ± 8 | -1 ± 5 |
| Δ Isokin Elb 60 Ext | -1 ± 8 | 0 ± 5 |
| Δ Isokin Elb 60 Flex | -1 ± 7 | -1 ± 5 |
| Δ Isokin Elb 180 Ext | 0 ± 7 | 0 ± 5 |
| Δ Isokin Elb 180 Flex | +1 ± 7 | 0 ± 5 |
| Δ PSS (points) | +2 ± 2 | +2 ± 2 |
| Δ PIS (points) | +1 ± 2 | +2 ± 5 |
Δ = Change scores calculated as post simvastatin + CoQ10 or placebo treatment -pre treatment; LDL-C = low density lipoprotein cholesterol; CK = creatine kinase; Vit D = Vitamin D; VO2max = maximal oxygen uptake; RER = respiratory exchange ratio; Handgrip = handgrip strength; Knee = Knee extensor or flexor strength variable; Isomet = isometric; Ext = Extension; Flex = flexion; Isokin = Isokinetic strength at either 60 or 180 degrees/sec; Elbow = Elbow extensor or flexor strength variable; PSS = pain severity score; PIS = pain intensity score.
All knee and elbow strength variables are reported as average peak torque in Newton-Meters.
denotes a significant difference between groups at p < 0.05.
The Pain Severity Score (PSS) and the Pain Intensity Score (PIS) increased with statin therapy (both p < 0.01) in both groups with no difference between CoQ10 and placebo treatment (p = 0.53 for PSS and 0.56 for PIS for drug*time interaction). There were no changes in CK, muscle strength or aerobic performance with statin treatment in the CoQ10 and placebo treatment groups (Table 2). The number of subjects reporting muscle pain at the end of treatment was marginally higher with CoQ10 treatment:14 of 20 (70.0%) vs 7 of 18 patients (38.8%); χ2= 3.7, p = 0.05. Time to pain onset was similar during the CoQ10 and placebo plus statin treatments (3.0 ± 2.0 vs. 2.4 ± 2.1 wks; p = 0.55). Pain was analyzed further by comparing each subject’s PSS and PIS at the end of treatment to the score recorded at the end of the confirmation trial. There were again no differences in PSS or PIS values for subjects receiving CoQ10 or placebo with the statin (p = 0.26 and 0.41 for drug*time interaction, respectively).
In the 24 subjects who participated in both arms of the CoQ10 or placebo phase, there were no differences in muscle and exercise performance, PSS and PIS, or pain at the end of statin treatment. There was also no difference in the number of subjects reporting muscle pain (17 of 24 (70.8%) on CoQ10 vs 13 of 24 (54.2%) on placebo; χ2= 1.4, p=0.23), or time to pain onset (3.2 ± 1.9 weeks for CoQ10 vs. 3.2 ± 2.0 weeks for placebo; p=0.53 for comparison).
DISCUSSION
Seven previous trials have evaluated the impact of coenzyme Q10 on muscle symptoms with four showing benefit and three showing no effect (6,7,12-16). In addition, a recent meta-analysis of 5 of these studies with 253 participants found no of CoQ10 effect on muscle pain despite a trend toward a decrease (17). The discrepancy between trials is not readily explained by the dose of CoQ10 used, the patient population studied, the statins administered, or the duration of therapy. We designed a trial specifically for high interval validity to explore this area and generated some important findings. Unlike previous trials, subjects who reported previous statin myalgia in our trial were first put through a confirmation phase where they were randomized to simvastatin 20mg daily or placebo for 8 weeks and then crossed over to alternative therapy. Only 35.8% of patients experienced myalgia on simvastatin and did not experience it on placebo, what we term true or confirmed statin myalgia, and 17.5% of patients had no symptoms on simvastatin or placebo which could have been because the dose we selected was too low. However, 29.2% experienced pain on placebo but not on simvastatin and 17.5% experienced pain on both simvastatin and placebo during the confirmation phase. Therefore, it is likely that only 30-50% of subjects in previous CoQ10 studies actually had muscle pain due to statins and not from other causes. This plus the small sample sizes of these trials may have contributed to the varying results.
We only allowed subjects with confirmed statin myalgia to enter the intervention phase where they were again placed on simvastatin 20mg daily (in order to exactly replicate the regiment that evoked myalgia in the lead-in phase) plus either CoQ10 or placebo for 8 weeks. CoQ10 supplementation had no effect on the incidence and severity of myalgia, time to onset of pain, muscle strength, or aerobic performance. These negative results occurred in the presence of elevated serum concentrations of CoQ10 in the treatment group (as opposed to reductions in the placebo group) and no group differences in the LDL-lowering effects of simvastatin. This demonstrates that CoQ10 does not reduce the lipid-lowering effect of statins but also does not reduce muscle symptoms.
We did not detect any reductions in muscle strength or aerobic performance when statins were combined with placebo in our confirmed statin myalgics. There are observational reports of decreased muscle strength and aerobic performance in statin myalgics (2,18), but few rigorous assessments of muscle strength and aerobic performance with statins in general and particularly in patients with confirmed myalgia. We examined muscle strength and exercise performance in subjects meeting a study definition of statin myalgia in our previous Effect of STatins On Skeletal Muscle Performance or STOMP trial which randomized healthy, statin-naïve patients to atorvastatin 80 mg daily or placebo for 6 months (3). There were no significant differences in the two groups in muscle and exercise performance in STOMP. The statin plus placebo intervention phase of our present study examined subjects with confirmed statin myalgia and also failed to find reductions in muscle strength, resting and aerobic performance.
We were surprised by the low number of subjects with a history of statin myalgia who tested positive with our confirmation process, although a recent small study using an n-of-1 double-blind crossover method of statin vs. placebo administration to confirm statin myalgia in individual patients with muscle complaints also found no difference between muscle pain and myalgia scores in both treatment conditions (19). Collectively, these findings emphasize the need to develop more objective tools to diagnose statin myalgia for both clinical and research use. While there was no discernable pattern of location or type of muscle pain in subjects with or without confirmed myalgia that would distinguish the groups clinically, the time to pain onset in patients with confirmed statin myalgia was significantly shorter (1.7 ± 1.4 weeks vs. 3.0 ± 1.8 weeks) than in those patients who did not test positive for myalgia but still experienced muscle pain on simvastatin (i.e., the subset of patients who experienced muscle pain on both simvastatin and placebo). We have reported similar findings regarding a shorter time to pain onset with statin therapy in myagics on atorvastatin vs. placebo (3) and thus the time course of pain could be used clinically to more accurately assess reports of statin myalgia. We also identified three additional factors that were different in those with confirmed statin myalgia. Subjects with confirmed myalgia had higher baseline CK values which suggests that some pre-statin muscle process predisposes these patients to statin myalgia. This is to our knowledge the first time that differences in CK have been identified as a possible marker of statin myalgia. Baseline BMI was also higher in those with confirmed statin myalgia. Subjects with confirmed statin myalgia were also more likely to have received statin during the first phase of the confirmation trial. It is possible that muscle discomfort during subsequent placebo treatment could be compared with earlier, possibly more severe, complaints during statin treatment and permitted subjects to differentiate correctly between statin-associated and non-specific muscle complaints. None of these factors could be used clinically to predict which individuals will or will not develop confirmed statin myalgia.
There are limitations to the study. We chose to use simvastatin because it is widely used, may evoke more adverse muscle effects than other statins at similar LDL-lowering doses (20,21), and was generically available at study onset. We chose 20mg daily because we were concerned that with higher doses, patients would not participate or would not tolerate treatment long enough to detect beneficial effects of CoQ10 during the intervention phase. Higher simvastatin doses may have increased the number of patients developing myalgia with statins in the confirmation phase thereby increasing the sample for the intervention phase. Limiting treatment duration in the confirmation and treatment phrases to 8 weeks of therapy may have limited our ability to detect confirmed statin myalgic patients. We chose 600 mg/d of the reduced form of CoQ10, ubiquinol, which is more effectively absorbed by older adults because previous studies using CoQ10 doses between 100 and 200 mg/d of CoQ10 have been criticized for using too low a dose (6,7). Neurocognitive studies have used 1200-2400 mg/d of CoQ10 doses (22), but 600mg/d increased CoQ10 serum concentrations above baseline and prevented the reduction in CoQ10 concentrations with statin therapy observed in the placebo group. We did not, however, measure intramuscular CoQ10 concentrations so it is possible that we did not elevate muscle CoQ10, but oral administration of CoQ10 in animal models increases both plasma and skeletal muscle CoQ10 concentrations (23,24), suggesting that the absence of a CoQ10 effect in the current study is not due to failure of CoQ10 to enter the muscle. Finally, we were only able to collect complete data on 38 patients into the initial study design and 24 patients into the cross-over treatment design because we were able to confirm statin myalgia in only 36% of recruited subjects with a history of myalgia. Consequently, we did not randomize 50 subjects to each treatment group as planned and had to alter our design to include a subject cross-over in the treatment phase. Nevertheless, the present study is one of the largest to test CoQ10 treatment in statin myalgia and the only study using only confirmed statin myalgic subjects. Furthermore, muscle pain and myalgia increased marginally in the CoQ10 group, and we estimate from our results that approximately 360 subjects per group would have been required to prove that CoQ10 does indeed reduce statin myalgia.
CONCLUSIONS
CoQ10 supplementation is widely prescribed for statin-associated muscle side effects but previous trials were small and yielded varying results. We used a verification lead-in phase to confirm statin myalgia and entered only those subjects who had myalgia on simvastatin but not on placebo into the CoQ10 or placebo intervention phase. Only a third of patients with a history of statin myalgia had myalgia with simvastatin 20mg but not with placebo during the confirmation protocol demonstrating the difficulty of diagnosing and evaluating possible therapies in statin myalgia. Our results demonstrate that Co Q10 does not reduce statin myalgia in subjects with confirmed statin myalgia.
Highlights.
Approximately 1/3 of patients who reported statin myalgia in the past had verified statin myalgia through the double-blind run-in confirmation phase, emphasizing the difficulty for clinicians to diagnose and treat statin-intolerant patients
CoQ10 supplementation had no beneficial effect on muscle pain, muscle strength, or aerobic exercise capacity
A possible reason for discordant findings in previous trials is because they likely only had 30-50% of patients with true statin myalgia
The low number of confirmed myalgics and lack of conclusive predictors from the current study support the need to develop more objective tools to diagnose and differentiate statin myalgia for both clinical and research use
Acknowledgments
Coenzyme Q10 in Statin Myopathy study is funded by NCCAM grant 1RC1AT005836 (P. Thompson). All authors made substantial contributions to conception or design of the work and/or acquisition, analysis, or interpretation of data for the work as well as drafting, revising and reviewing the manuscript. In addition, Drs. Taylor and Thompson had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors are grateful for the support of the Data Safety Monitoring Board: Ira Ockene, M.D., JoAnne Foody, M.D., and Pamela Hartigan, Ph.D.
ABBREVIATIONS
- CoQ10
Coenzyme Q10
- HMG-CoA
Hydroxy-methyl-glutaryl-coenzyme A
- CK
Creatine Kinase
- VO2max
Maximal oxygen uptake
- BPI
Brief Pain Inventory
- PSS
Pain Severity Score
- PIS
Pain Interference Score
Footnotes
Financial Disclosures:
Dr. Thompson has received research support from Genomas, Roche, Sanolfi, Regeneron, Esperion, Amarin and Pfizer; has served as a consultant for Amgen, Regeneron, Merck, Genomas, Runners World, Sanolfi, Esperion, and Amarin; has received speaker honoraria from Merck, Astra Zenica, Kowa, and Amarin; owns stock in Abbvie, Abbott Labs, General Electric, J&J; and has provided expert legal testimony on exercise-related cardiac events and statin myopathy. Dr. Taylor served on the Pharmacovigilance Monitoring Board for Amgen, Inc.
Conflict of Interest: Mrs. Lorson, and Dr. White do not have any conflict of interest or financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 2.Phillips PS, Haas RH, Bannykh S, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581–5. doi: 10.7326/0003-4819-137-7-200210010-00009. [DOI] [PubMed] [Google Scholar]
- 3.Parker BA, Capizzi JA, Grimaldi AS, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127:96–103. doi: 10.1161/CIRCULATIONAHA.112.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–14. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 5.Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol. 2007;49:2231–7. doi: 10.1016/j.jacc.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 6.Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol. 2007;99:1409–12. doi: 10.1016/j.amjcard.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 7.Young JM, Florkowski CM, Molyneux SL, et al. Effect of coenzyme Q(10) supplementation on simvastatin-induced myalgia. Am J Cardiol. 2007;100:1400–3. doi: 10.1016/j.amjcard.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Parker BA, Gregory SM, Lorson L, Polk D, White CM, Thompson PD. A randomized trial of coenzyme Q10 in patients with statin myopathy: rationale and study design. J Clin Lipidol. 2013;7:187–93. doi: 10.1016/j.jacl.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang PH, Miles MV, DeGrauw A, Hershey A, Pesce A. HPLC analysis of reduced and oxidized coenzyme Q(10) in human plasma. Clin Chem. 2001;47:256–65. [PubMed] [Google Scholar]
- 10.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–7. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Thompson PD, Parker BA, Clarkson PM, et al. A randomized clinical trial to assess the effect of statins on skeletal muscle function and performance: rationale and study design. Prev Cardiol. 2010;13:104–11. doi: 10.1111/j.1751-7141.2009.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogsrud MP, Langslet G, Ose L, et al. No effect of combined coenzyme Q10 and selenium supplementation on atorvastatin-induced myopathy. Scand Cardiovasc J. 2013;47:80–7. doi: 10.3109/14017431.2012.756119. [DOI] [PubMed] [Google Scholar]
- 13.Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:526–9. doi: 10.1016/j.amjcard.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 14.Fedacko J, Pella D, Fedackova P, et al. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can J Physiol Pharmacol. 2013;91:165–70. doi: 10.1139/cjpp-2012-0118. [DOI] [PubMed] [Google Scholar]
- 15.Deichmann RE, Lavie CJ, Dornelles AC. Impact of coenzyme Q-10 on parameters of cardiorespiratory fitness and muscle performance in older athletes taking statins. Phys Sportsmed. 2012;40:88–95. doi: 10.3810/psm.2012.11.1991. [DOI] [PubMed] [Google Scholar]
- 16.Skarlovnik A, Janić M, Lunder M, Turk M, Sabovič M. Coenzyme Q10 Supplementation Decreases Statin-Related Mild-to-Moderate Muscle Symptoms: A Randomized Clinical Study. Med Sci Monit. 2014;20:2183–8. doi: 10.12659/MSM.890777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banach M, Serban C, Sahebkar A, Ursoniu S, Rysz J, Muntner P, Toth PP, Jones SR, Rizzo M, Glasser SP, Lip GY, Dragan S, Mikhailidis DP Lipid and Blood Pressure Meta-analysis Collaboration Group. Effects of Coenzyme Q10 on Statin-Induced Myopathy: A Meta-analysis of Randomized Controlled Trials. Mayo Clin Proc. 2014 Nov 14; doi: 10.1016/j.mayocp.2014.08.021. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Phillips PS, Phillips CT, Sullivan MJ, Naviaux RK, Haas RH. Statin myotoxicity is associated with changes in the cardiopulmonary function. Atherosclerosis. 2004;177:183–8. doi: 10.1016/j.atherosclerosis.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Joy TR, Monjed A, Zou GY, Hegele RA, McDonald CG, Mahon JL. N-of-1 (single-patient) trials for statin-related myalgia. Ann Intern Med. 2014;160:301–10. doi: 10.7326/M13-1921. [DOI] [PubMed] [Google Scholar]; Sinzinger H, O’Grady J. Professional athletes suffering from familial hypercholesterolaemia rarely tolerate statin treatment because of muscular problems. Br J Clin Pharmacol. 2004;57:525–8. doi: 10.1111/j.1365-2125.2004.02044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman KB, Kraus C, Dimbil M, Golomb BA. A survey of the FDA’s AERS database regarding muscle and tendon adverse events linked to the statin drug class. PLoS One. 2012;7:e42866. doi: 10.1371/journal.pone.0042866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cham S, Evans MA, Denenberg JO, Golomb BA. Statin-associated muscle-related adverse effects: a case series of 354 patients. Pharmacotherapy. 2010;30:541–53. doi: 10.1592/phco.30.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beal MF, Oakes D, Shoulson I, et al. A Randomized Clinical Trial of High-Dosage Coenzyme Q10 in Early Parkinson Disease: No Evidence of Benefit. JAMA Neurol. 2014;71:543–52. doi: 10.1001/jamaneurol.2014.131. [DOI] [PubMed] [Google Scholar]
- 23.Kon M, Kimura F, Akimoto T, et al. Effect of Coenzyme Q10 supplementation on exercise-induced muscular injury of rats. Exerc Immunol Rev. 2007;13:76–88. [PubMed] [Google Scholar]
- 24.Kamzalov S, Sumien N, Forster MJ, Sohal RS. Coenzyme Q intake elevates the mitochondrial and tissue levels of Coenzyme Q and alpha-tocopherol in young mice. J Nutr. 2003;133:3175–80. doi: 10.1093/jn/133.10.3175. [DOI] [PubMed] [Google Scholar]


