Abstract
According to the World Health Organization (WHO), more than 80% of worldwide diabetes (DM)-related deaths presently occur in low- and middle- income countries (LMIC), and left unchecked these DM-related deaths will likely double over the next 20 years. Cardiovascular disease (CVD) is the most prevalent and detrimental complication of DM: doubling the risk of CVD events (including stroke) and accounting for up to 80% of DM-related deaths. Given the aforementioned, interventions targeted at reducing CVD risk among people with DM are integral to limiting DM-related morbidity and mortality in LMIC, a majority of which are located in Sub-Saharan Africa (SSA). However, SSA is contextually unique: socioeconomic obstacles, cultural barriers, under-diagnosis, uncoordinated care, and shortage of physicians currently limit the capacity of SSA countries to implement CVD prevention among people with DM in a timely and sustainable manner. This article proposes a theory-based framework for conceptualizing integrated protocol-driven risk factor patient self-management interventions that could be adopted or adapted in future studies among hospitalized stroke patients with DM encountered in SSA. These interventions include systematic health education at hospital discharge, use of post-discharge trained community lay navigators, implementation of nurse-led group clinics and administration of health technology (personalized phone text messaging and home tele-monitoring), all aimed at increasing patient self-efficacy and intrinsic motivation for sustained adherence to therapies proven to reduce CVD event risk.
Keywords: Stroke, Diabetes, mobile health, navigators, Africa, global health
BURDEN OF DIABETES AND STROKE IN SUB_SAHARAN AFRICA (SSA)
As of 2012, roughly 14 million Africans (4.8%) had Diabetes (DM), and approximately 81% were undiagnosed (vs. 50% worldwide), making Africa the continent with the highest proportion of people with undiagnosed DM. 1 Projections for Sub-Saharan Africa (SSA) indicate the number of diabetics will rise by 71% to 23.9 million by 2030 (predicted global increase is 37%).1 Pre-DM in SSA is expected to rise by 75.8%, from 26.9 million in 2010 to 47.3 million in 2030. 1 DM accounts for 6.1% of deaths from all causes in SSA with absolute and relative mortality rates highest in 20 to 39 year olds, i.e., the most economically productive range of population.2
Major CVD events (including stroke) cause about 80% of the total mortality in people with DM.3 World Health Organization (WHO) estimates indicate stroke deaths in LMIC account for 85.5% of stroke deaths worldwide. 4 The disability-adjusted life years lost in these countries was almost seven times those lost in high-income countries (HIC). 4 Beyond the personal toll, costs (e.g. direct expenditures and lost productivity) related to stroke are prohibitive.5 Data from SSA suggest an annual stroke incidence rate up to 316 per 100,000, a prevalence rate up to 315 per 100,000 and 3-year fatality rate up to 84%.6 Stroke is the leading cause of adult medical admissions and comas.7,8 Among survivors, a major source of subsequent mortality and functional decline is recurrent stroke and myocardial infarction (MI). 9–13
Of note, stroke risk is substantially higher in people with DM.14 Optimal management of DM is likely an important recurrent CVD prevention activity. 15–17 Moreover, while people with a known date of pre-DM onset progress to DM in <3 years,16 pre-DM is itself independently linked with CVD events. 15–17 A meta-analysis of prospective cohort studies suggested that pre-DM is independently associated with stroke events.17 Also analysis of nationally representative US data showed that 3.7% stroke survivors had undiagnosed DM, 32.3% had undiagnosed pre-DM, and prevalence of undiagnosed DM and pre-DM were highest in racial-ethnic minorities.18 Similar data are not available for stroke survivors in SSA, but it’s likely that under-diagnosis in SSA is much worse than the US.
AMELIORATING THE BURDEN OF DIABETES AND STROKE IN SSA
Fortunately, effective interventions exist to prevent progression of pre-DM to DM. Consensus guidelines recommend that persons with pre-DM be informed of their increased risk, counseled about effective strategies to lower risks, have CVD risk factors treated, and be regularly monitored for DM.19 Prevention of future CVD events is critical to reducing the morbidity/mortality of patients with stroke, since the risk is highest within 3 months of the index stroke.20,21 Longitudinal studies in HIC have identified modifiable risk factors including hypertension, DM, and dyslipidemia, 22 which if controlled, could substantially lessen CVD burden. Yet, use of evidence-based therapies for CVD prevention among stroke patients receiving conventional care in LMIC is extremely low.23 Scaling up interventions to prevent primary and secondary CVD in LMIC could meet a global goal of reducing chronic disease death rates by an additional 2% per year, with only a moderate rise in health expenditure.24 However, Africans in general, do not use health services unless they are very sick or there is a specific need,25 and even in times of infirmity, self-medication and use of traditional medicine are usually the first line of action. 26 A study at the University College Hospital Ibadan, Nigeria, found that glycemic control was achieved in only one third of encountered DM patients, due to poor adherence with prescribed drug regimen, and practice of self-management.27
Beyond barriers to optimal DM control at the patient level, in SSA countries, systems-level barriers seem to have an even more adverse impact on health care in general, particularly among those with chronic diseases. 28,29 Inadequate workforces are perhaps the most serious challenges. Poor working conditions and low salaries have triggered a wave of migration of health professionals from SSA to western countries.30,31 Successful and sustainable strategies for incrementally overcoming impediments to mitigating the burgeoning DM-related CVD epidemic in SSA will need to include: 1) an initial focus on high-risk individuals motivated to improve their health and likely to command the attention of policy-makers,24 2) multidisciplinary care coordination initiatives with clinical decision support for management of CVD via easy-to-follow algorithms that can be incorporated into existing hospital-based systems, 32 3) evidence-based interventions tailored for cultural relevance, 4) task shifting of select chronic care management duties from physicians to nurses and other health providers, 33 5) involvement of lay community navigators to facilitate patient access to medical and social resources, 34–36 6) use of novel patient-accessible tools that are relatively inexpensive,37 7) a multi-level approach that includes individual- and system- level components. 38–41
GUIDING THEORETICAL FRAMEWORKS TO IMPROVE OUTCOMES IN SSA
Given the myriad of barriers to management of DM and stroke at various levels in SSA, and evidence from HIC that chronic care interventions are most successful when they simultaneously target several levels in a multidimensional manner,38–41 it is anticipated that an effective intervention to improve CVD outcomes among DM patients in SSA will require a multimodal intervention based in solid theoretical constructs tailored to the unique health care situation. Furthermore, for any innovation to be implemented successfully, it is necessary to identify potential interacting determining factors derived from different theories that need to be tested for their combined influence on change.42 Indeed, a lack of theoretical development has been proposed as a major contributor to failure of complex interventions in preventive care after stroke to demonstrate efficacy.43 Table 1 depicts key theoretical frameworks that underlie possibly successful intervention(s) to enhance outcomes for hospitalized stroke patients with diabetes or pre-diabetes in SSA.
Table 1.
Guiding theoretical frameworks/models to support strategies to improve outcomes among stroke patients with or at risk of diabetes in Sub-Saharan Africa (SSA)
| Model/Theory | Description | Justification |
|---|---|---|
| Transtheoretical |
|
|
| Health Care Navigator Model |
|
|
| Chronic Care Model (CCM) |
|
|
POTENTIAL INTERVENTION STRATEGIES TO IMPROVE OUTCOMES IN SSA
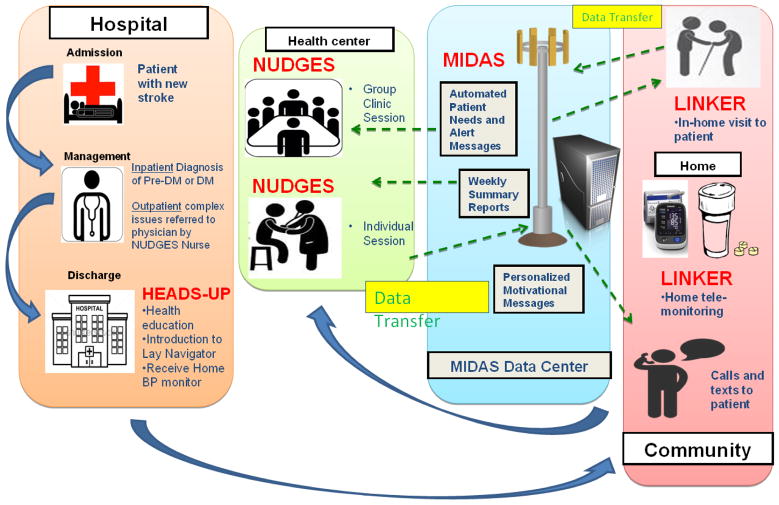
A review of evidence regarding effectiveness of patient, provider, and health system interventions to improve DM care among socially disadvantaged populations revealed that intervention features with consistently positive effects included cultural tailoring, community educators or lay people leading the intervention, one-on-one interventions with individualized assessment and reassessment, incorporating treatment algorithms, focusing on behavior-related tasks, providing feedback, and high-intensity interventions (>10 contact times) delivered over a long duration (>or=6 months). 44 Table 2 provides a detailed breakdown of various promising strategies based on a literature review and the justification for their consideration. While any of these interventions could individually be tested in a clinical trial, multi-level strategies that include individual- and system- level components, have been consistently been shown to have better efficacy than individual interventions. 38–41 Table 3 proposes four study interventions that may individually enhance outcomes among stroke patients with or at risk of diabetes in Sub-Saharan Africa (SSA). However, with evidence that multi-level strategies may work best, 38–41 Figure 1 depicts the flow within a bundled multimodal approach for reducing CVD risk among hospitalized stroke patients in SSA with DM or Pre-DM that incorporates all the proposed individual interventions mentioned in Table 3.
Table 2.
Promising strategies to consider for the improvement of outcomes among stroke patients with or at risk of diabetes in Sub-Saharan Africa (SSA)
| Strategy | Rationale |
|---|---|
| Lay health workers | |
| Task shifting |
|
| Nurse-led Risk Reduction | |
| Group clinics |
|
| Mobile Phone Technology |
|
| Home tele-monitoring |
|
| Multimodal Risk Modification |
|
Table 3.
Proposed individual study interventions that may enhance outcomes among stroke patients with or at risk of diabetes in Sub-Saharan Africa (SSA)
| Intervention | Description |
|---|---|
| HEADS-UP (Health Educator Advice for Diabetes and Stroke to Upgrade Prognoses) |
|
| LINKER (Lay Indigenous Navigator Know-how for Employing Resources) |
|
| NUDGES (Nurse Ushered Diabetes Group Encounters after Stroke) |
|
| MIDAS: (M-health Implementation in Diabetics After Stroke) |
|
Figure 1.
Flow within a bundled multimodal strategy for reducing cardiovascular risk among hospitalized stroke patients in Sub-Saharan Africa with diabetes or pre-diabetes that incorporates several individual interweaving interventions
Prior to conducting any study the components of the intervention(s) will need to be refined in iterative fashion using mixed methods research. Objectives that should serve as a boundary for such pre-trial investigation are: a) To explore help-seeking attitudes and beliefs among stroke patients with pre-DM/DM and their understanding about symptoms and risks; b) To integrate suggestions for enhancing and implementing the intervention; c) To examine patients and caregivers knowledge about clinical trial research design and purpose and how this may affect a subject’s decision to enroll in a study. Also after completion of the clinical trial, an examination of utilization/cost of the intervention(s) for the healthcare system will be useful to convince policy makers that the intervention(s) is economically worthwhile. Moreover, to ensure scalability and sustainability of a potentially successful intervention the early and continued involvement of local government agencies, private sectors, non-government organizations, academic institutions and communities will be imperative.
CONCLUSIONS
Very few countries in SSA can afford to broadly screen and treat the various complications of DM in a general population.45 A focused incremental approach built upon existing infrastructure with proof-of-concept among high risk persons may first be needed. 28 While there have been a few modestly successful strategies to improve care for DM patients in SSA, these have generally focused on uni-modal interventions aimed at primary care populations, evaluating effects on care processes, patient satisfaction, or glycemic control. Patients hospitalized with a recent stroke who have DM or Pre-DM represent a high risk population that could be targeted for theory-driven, evidence-based intervention(s) to lower major DM-related CVD complications. By starting during the inpatient encounter and extending well into the post-discharge/community period, the bundled multi-level intervention proposed in this article (figure 1) uniquely traverses the stroke care continuum and explores various care transition issues in SSA that could be vitally important for reducing recurrent vascular events.
A clinically-effective bundled strategy, if scaled up, could have the potential to reduce DM-related CVD morbidity, mortality in SSA and might be applicable to CVD risk reduction for pre-DM and DM patients with other major CVD entities like coronary artery disease, congestive heart failure and chronic kidney disease. It would facilitate the routine screening for pre-DM and DM when these patients are hospitalized, an issue of major significance given the level of under-diagnosis of both these conditions in SSA, and the opportunity to prevent progression of pre-DM to DM in high CVD risk patients. A cost-effective multimodal intervention could be highly appealing to policy makers in this under-resourced region, and would be impetus for broader implementation. 46 In fact, the proposed task-shifting strategy, which uses nurses to deliver CVD risk reduction, could potentially mitigate the critical shortage of healthcare workers in the region, and leveraging the high (and rising) mobile phone penetration in the region could ameliorate mHealth logistics challenges, integrate care systems, and improve patient access.
Highlights.
Article proposes a theory-based framework for stroke and diabetes management
Discussion centers on scalable strategies for patients in Sub-Saharan Africa
Proposed interventions include health education and mobile health technology
Proposed personnel include trained community lay navigators and nurse practitioners
Acknowledgments
Bruce Ovbiagele was supported by U01 NS079179 from the National Institutes of Health.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diabetes in the Africa region: 2012 update for IDF. 2012 Accessed at http://www.idf.org/sites/default/files/5E_IDFAtlasPoster_2012_EN.pdf.
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Coccheri S. Approaches to prevention of cardiovascular complications and events in diabetes mellitus. Drugs. 2007;67:997–1026. doi: 10.2165/00003495-200767070-00005. [DOI] [PubMed] [Google Scholar]
- 4.Feigin VL. Stroke epidemiology in the developing world. Lancet. 2005;365:2160–1. doi: 10.1016/S0140-6736(05)66755-4. [DOI] [PubMed] [Google Scholar]
- 5.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 6.BeLue R, Okoror TA, Iwelunmor J, et al. An overview of cardiovascular risk factor burden in sub-Saharan African countries: a socio-cultural perspective. Global Health. 2009;5:10. doi: 10.1186/1744-8603-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obiako OR, Oparah S, Ogunniyi A. Causes of medical coma in adult patients at the university college hospital, ibadan Nigeria. Niger Postgrad Med J. 18:1–7. [PubMed] [Google Scholar]
- 8.Ekenze OS, Onwuekwe IO, Ezeala Adikaibe BA. Profile of neurological admissions at the University of Nigeria Teaching Hospital Enugu. Niger J Med. 19:419–22. doi: 10.4314/njm.v19i4.61967. [DOI] [PubMed] [Google Scholar]
- 9.Bronnum-Hansen H, Davidsen M, Thorvaldsen P Group ftDMS. Long-term survival and cause of death after stroke. Stroke. 2001;32:2131–6. doi: 10.1161/hs0901.094253. [DOI] [PubMed] [Google Scholar]
- 10.Kernan WN, Viscoli CM, Brass LM, et al. The stroke prognosis instrument II (SPI-II): A clinical prediction instrument for patients with transient ischemic and non-disabling ischemic stroke. Stroke. 2000;31:456–62. doi: 10.1161/01.str.31.2.456. [DOI] [PubMed] [Google Scholar]
- 11.Petty GW, Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Survival and recurrence after first cerebral infarction. A population -based study in Rochester, Minnesota 1975–1989. Neurology. 1998;50:208–16. doi: 10.1212/wnl.50.1.208. [DOI] [PubMed] [Google Scholar]
- 12.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. Estrogen replacement after ischemic stroke: report of the Women’s Estrogen for Stroke Trial (WEST) The New England Journal of Medicine. 2001;345:1243–9. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 13.Vernino S, Brown RD, Sejvar JJ, Sicks JD, Petty GW, O’Fallon M. Cause-specific mortality after cerebral infarction. A population-based study. Stroke. 2003;34:1828–32. doi: 10.1161/01.STR.0000080534.98416.A0. [DOI] [PubMed] [Google Scholar]
- 14.Bejot Y, Giroud M. Stroke in diabetic patients. Diabetes Metab. 2010;36 (Suppl 3):S84–7. doi: 10.1016/S1262-3636(10)70472-9. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59:635–43. doi: 10.1016/j.jacc.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 16.Nichols GA, Hillier TA, Brown JB. Progression from newly acquired impaired fasting glusose to type 2 diabetes. Diabetes Care. 2007;30:228–33. doi: 10.2337/dc06-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee M, Saver JL, Hong KS, Song S, Chang KH, Ovbiagele B. Effect of pre-diabetes on future risk of stroke: meta-analysis. BMJ. 2012;344:e3564. doi: 10.1136/bmj.e3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Towfighi A, Markovic D, Ovbiagele B. Undiagnosed Diabetes and Pre-Diabetes among Stroke Survivors in the United States. Stroke. 2013;44:ATMP45. [Google Scholar]
- 19.Standards of medical care in diabetes--2013. Diabetes Care. 2013;36 (Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coull A, Lovett JK, Rothwell PM. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. Bmj. 2004;328:326. doi: 10.1136/bmj.37991.635266.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleindorfer D, Panagos P, Pancioli A, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36:720–3. doi: 10.1161/01.STR.0000158917.59233.b7. [DOI] [PubMed] [Google Scholar]
- 22.Sacco RL, Boden-Albala B, Gan R, et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. American Journal of Epidemiology. 1998;147:259–68. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 23.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 42:227–76. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 24.Lim SS, Gaziano TA, Gakidou E, et al. Prevention of cardiovascular disease in high-risk individuals in low-income and middle-income countries: health effects and costs. Lancet. 2007;370:2054–62. doi: 10.1016/S0140-6736(07)61699-7. [DOI] [PubMed] [Google Scholar]
- 25.Criel B. Studies in Health Services Organization and Policy 10. Antwerp: Prince Leopold Institute of Tropical Medicine; 1998. District-Based Health Insurance in Sub-Saharan Africa. Part 2: Case-Studies. [Google Scholar]
- 26.Amira CO, Okubadejo NU. Factors influencing non-compliance with anti-hypertensive drug therapy in Nigerians. Niger Postgrad Med J. 2007;14:325–9. [PubMed] [Google Scholar]
- 27.Yusuff KB, Obe O, Joseph BY. Adherence to anti-diabetic drug therapy and self management practices among type-2 diabetics in Nigeria. Pharm World Sci. 2008;30:876–83. doi: 10.1007/s11096-008-9243-2. [DOI] [PubMed] [Google Scholar]
- 28.Whiting DR, Hayes L, Unwin NC. Diabetes in Africa. Challenges to health care for diabetes in Africa. J Cardiovasc Risk. 2003;10:103–10. doi: 10.1097/01.hjr.0000060846.48106.6d. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. World health report 2000. Health systems: improving performance. Geneva: 2000. [Google Scholar]
- 30.Dovlo D. The brain drain and retention of health professionals in Africa. Case studies prepared for regional training conference: Improving tertiary education in sub-Saharan Africa: things that work! Accra; 23–25 September 2003; Available at: http://www.worldbank.org/afr/teia/conf_0903/dela_dovlo.pd. [Google Scholar]
- 31.Chen L, Evans T, Anand S, et al. Human resources for health: overcoming the crisis. Lancet. 2004;364:1984–90. doi: 10.1016/S0140-6736(04)17482-5. [DOI] [PubMed] [Google Scholar]
- 32.Tshiananga JK, Kocher S, Weber C, Erny-Albrecht K, Berndt K, Neeser K. The effect of nurse-led diabetes self-management education on glycosylated hemoglobin and cardiovascular risk factors: a meta-analysis. The Diabetes educator. 2012;38:108–23. doi: 10.1177/0145721711423978. [DOI] [PubMed] [Google Scholar]
- 33.Mendis S, Johnston SC, Fan W, Oladapo O, Cameron A, Faramawi MF. Cardiovascular risk management and its impact on hypertension control in primary care in low-resource settings: a cluster-randomized trial. Bull World Health Organ. 2010;88:412–9. doi: 10.2471/BLT.08.062364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher EB, Boothroyd RI, Coufal MM, et al. Peer support for self-management of diabetes improved outcomes in international settings. Health affairs. 2012;31:130–9. doi: 10.1377/hlthaff.2011.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boothroyd RI, Fisher EB. Peers for progress: promoting peer support for health around the world. Family practice. 2010;27 (Suppl 1):i62–8. doi: 10.1093/fampra/cmq017. [DOI] [PubMed] [Google Scholar]
- 36.Fisher EB, Earp JA, Maman S, Zolotor A. Cross-cultural and international adaptation of peer support for diabetes management. Family practice. 2010;27 (Suppl 1):i6–16. doi: 10.1093/fampra/cmp013. [DOI] [PubMed] [Google Scholar]
- 37.Okoro EO, Sholagberu HO, Kolo PM. Mobile phone ownership among Nigerians with diabetes. Afr Health Sci. 10:183–6. [PMC free article] [PubMed] [Google Scholar]
- 38.Renders CM, Valk GD, Griffin SJ, Wagner EH, Eijk Van JT, Assendelft WJ. Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review. Diabetes Care. 2001;24:1821–33. doi: 10.2337/diacare.24.10.1821. [DOI] [PubMed] [Google Scholar]
- 39.Pare G, Jaana M, Sicotte C. Systematic review of home telemonitoring for chronic diseases: the evidence base. J Am Med Inform Assoc. 2007;14:269–77. doi: 10.1197/jamia.M2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11:478–88. [PMC free article] [PubMed] [Google Scholar]
- 41.Weingarten SR, Henning JM, Badamgarav E, et al. Interventions used in disease management programmes for patients with chronic illness-which ones work? Meta-analysis of published reports. BMJ. 2002;325:925. doi: 10.1136/bmj.325.7370.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grol RP, Bosch MC, Hulscher ME, Eccles MP, Wensing M. Planning and studying improvement in patient care: the use of theoretical perspectives. The Milbank quarterly. 2007;85:93–138. doi: 10.1111/j.1468-0009.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Redfern J, McKevitt C, Wolfe CD. Development of complex interventions in stroke care: a systematic review. Stroke; a journal of cerebral circulation. 2006;37:2410–9. doi: 10.1161/01.STR.0000237097.00342.a9. [DOI] [PubMed] [Google Scholar]
- 44.Glazier RH, Bajcar J, Kennie NR, Willson K. A systematic review of interventions to improve diabetes care in socially disadvantaged populations. Diabetes Care. 2006;29:1675–88. doi: 10.2337/dc05-1942. [DOI] [PubMed] [Google Scholar]
- 45.Dagogo-Jack S. DCCT results and diabetes care in developing countries. Diabetes Care. 1995;18:416–7. doi: 10.2337/diacare.18.3.416. [DOI] [PubMed] [Google Scholar]
- 46.The Diabetes Declaration and Strategy for Africa. A Call to Action and Plan of Action to Prevent and Control Diabetes and related Chronic Disease. Initiated and developed by the International Diabetes Federation-Africa, World Health Organization-AFRO, and the African Union. 2006 Accessed at http://www.idf.org/webdata/docs/DiabetesDeclaration&StrategyforAfrica_full.pdf.
- 47.Funnell MM, Anderson RM, Arnold MS, et al. Empowerment: an idea whose time has come in diabetes education. Diabetes Educ. 1991;17:37–41. doi: 10.1177/014572179101700108. [DOI] [PubMed] [Google Scholar]
- 48.Maldonato A, Bloise D, Ceci M, Fraticelli E, Fallucca F. Diabetes mellitus: lessons from patient education. Patient Educ Couns. 1995;26:57–66. doi: 10.1016/0738-3991(95)00736-j. [DOI] [PubMed] [Google Scholar]
- 49.Zuvekas A, Nolan L, Tumaylle C, Griffin L. Impact of community health workers on access, use of services, and patient knowledge and behavior. Journal of Ambulatory Care Management. 1999;22:33–44. doi: 10.1097/00004479-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Prevention CfDCa. Community health advisors (Vols 1,2) Atlanta: Centers for Disease Control and Prevention; 1994. [Google Scholar]
- 51.Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underservied. Cancer Practices. 1995;3:19. [PubMed] [Google Scholar]
- 52.Fisher EB, Brownson CA, O’Toole ML, Shetty G, Anwuri VV, Glasgow RE. Ecological approaches to self-management: the case of diabetes. Am J Public Health. 2005;95:1523–35. doi: 10.2105/AJPH.2005.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allen JK, Scott LB. Alternative models in the delivery of primary and secondary prevention programs. J Cardiovasc Nurs. 2003;18:150–6. doi: 10.1097/00005082-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 54.Two Feathers J, Kieffer EC, Palmisano G, et al. Racial and ethnic approaches to community health (REACH): improving diabetes-related outcomes among African-American and Latino adults. American Journal of Public Health. 2005;95:1552–60. doi: 10.2105/AJPH.2005.066134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarfaty M, Turner CH, Damotta BA. Use of a patient assistant to facilitate visits for Latino patients with low health literacy. Journal of Community Health. 2005;30:299–307. doi: 10.1007/s10900-005-3707-2. [DOI] [PubMed] [Google Scholar]
- 56.Medicine Io. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington D.C: National Academy Press; 2002. [PMC free article] [PubMed] [Google Scholar]
- 57.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA. 2002;288:1909–14. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 58.Sperl-Hillen JM, Solberg LI, Hroscikoski MC, Crain AL, Engebretson KI, O’Connor PJ. Do all components of the chronic care model contribute equally to quality improvement? Jt Comm J Qual Saf. 2004;30:303–9. doi: 10.1016/s1549-3741(04)30034-1. [DOI] [PubMed] [Google Scholar]
- 59.Katon W, Russo J, Von Korff M, et al. Long-term effects of a collaborative care intervention in persistently depressed primary care patients. J Gen Intern Med. 2002;17:741–8. doi: 10.1046/j.1525-1497.2002.11051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schillinger D, Bibbins-Domingo K, Vranizan K, Bacchetti P, Luce JM, Bindman AB. Effects of primary care coordination on public hospital patients. J Gen Intern Med. 2000;15:329–36. doi: 10.1046/j.1525-1497.2000.07010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–45. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 62.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469–75. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 63.Warsi A, Wang PS, LaValley MP, Avorn J, Solomon DH. Self-management education programs in chronic disease: a systematic review and methodological critique of the literature. Arch Intern Med. 2004;164:1641–9. doi: 10.1001/archinte.164.15.1641. [DOI] [PubMed] [Google Scholar]
- 64.Zwarenstein M, Schoeman JH, Vundule C, Lombard CJ, Tatley M. A randomised controlled trial of lay health workers as direct observers for treatment of tuberculosis. Int J Tuberc Lung Dis. 2000;4:550–4. [PubMed] [Google Scholar]
- 65.Kironde S, Kahirimbanyi M. Community participation in primary health care (PHC) programmes: lessons from tuberculosis treatment delivery in South Africa. Afr Health Sci. 2002;2:16–23. [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson BA, Khanna SK. Community health workers and home-based care programs for HIV clients. J Natl Med Assoc. 2004;96:496–502. [PMC free article] [PubMed] [Google Scholar]
- 67.Clarke M, Dick J, Zwarenstein M, Lombard CJ, Diwan VK. Lay health worker intervention with choice of DOT superior to standard TB care for farm dwellers in South Africa: a cluster randomised control trial. Int J Tuberc Lung Dis. 2005;9:673–9. [PubMed] [Google Scholar]
- 68.Dick J, Clarke M, van Zyl H, Daniels K. Primary health care nurses implement and evaluate a community outreach approach to health care in the South African agricultural sector. Int Nurs Rev. 2007;54:383–90. doi: 10.1111/j.1466-7657.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 69.Schneider H, Lehmann U. Lay health workers and HIV programmes: implications for health systems. AIDS Care. 2010;22 (Suppl 1):60–7. doi: 10.1080/09540120903483042. [DOI] [PubMed] [Google Scholar]
- 70.Fatti G, Meintjes G, Shea J, Eley B, Grimwood A. Improved survival and antiretroviral treatment outcomes in adults receiving community-based adherence support: 5-year results from a multicentre cohort study in South Africa. J Acquir Immune Defic Syndr. 2012;61:e50–8. doi: 10.1097/QAI.0b013e31826a6aee. [DOI] [PubMed] [Google Scholar]
- 71.Nkonki L, Cliff J, Sanders D. Lay health worker attrition: important but often ignored. Bull World Health Organ. 2011;89:919–23. doi: 10.2471/BLT.11.087825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Labhardt ND, Balo JR, Ndam M, Grimm JJ, Manga E. Task shifting to non-physician clinicians for integrated management of hypertension and diabetes in rural Cameroon: a programme assessment at two years. BMC Health Serv Res. 2010;10:339. doi: 10.1186/1472-6963-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mendis S, Lindholm LH, Mancia G, et al. World Health Organization (WHO) and International Society of Hypertension (ISH) risk prediction charts: assessment of cardiovascular risk for prevention and control of cardiovascular disease in low and middle-income countries. J Hypertens. 2007;25:1578–82. doi: 10.1097/HJH.0b013e3282861fd3. [DOI] [PubMed] [Google Scholar]
- 74.Gill GV, Price C, Shandu D, Dedicoat M, Wilkinson D. An effective system of nurse-led diabetes care in rural Africa. Diabet Med. 2008;25:606–11. doi: 10.1111/j.1464-5491.2008.02421.x. [DOI] [PubMed] [Google Scholar]
- 75.Kengne AP, Fezeu L, Sobngwi E, et al. Type 2 diabetes management in nurse-led primary healthcare settings in urban and rural Cameroon. Prim Care Diabetes. 2009;3:181–8. doi: 10.1016/j.pcd.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 76.Stilwell B, Wilson A, McCaffery J. Non-physician clinicians in sub-Saharan Africa. Lancet. 2008;371:1578. doi: 10.1016/S0140-6736(08)60687-X. [DOI] [PubMed] [Google Scholar]
- 77.Katz I, Schneider H, Shezi Z, et al. Managing type 2 diabetes in Soweto-The South African Chronic Disease Outreach Program experience. Prim Care Diabetes. 2009;3:157–64. doi: 10.1016/j.pcd.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 78.Sadur CN, Moline N, Costa M, et al. Diabetes management in a health maintenance organization. Efficacy of care management using cluster visits Diabetes Care. 1999;22:2011–7. doi: 10.2337/diacare.22.12.2011. [DOI] [PubMed] [Google Scholar]
- 79.Coleman EA, Grothaus LC, Sandhu N, Wagner EH. Chronic care clinics: a randomized controlled trial of a new model of primary care for frail older adults. J Am Geriatr Soc. 1999;47:775–83. doi: 10.1111/j.1532-5415.1999.tb03832.x. [DOI] [PubMed] [Google Scholar]
- 80.Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2013;185:E635–44. doi: 10.1503/cmaj.130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brennan J, Hwang D, Phelps K. Group Visits and Chronic Disease Management in Adults: A Review. Am J Lifestyle Med. 2011;5:69–84. [Google Scholar]
- 82.Trento M, Passera P, Tomalino M, et al. Group visits improve metabolic control in type 2 diabetes: a 2-year follow-up. Diabetes Care. 2001;24:995–1000. doi: 10.2337/diacare.24.6.995. [DOI] [PubMed] [Google Scholar]
- 83.Scott JC, Conner DA, Venohr I, et al. Effectiveness of a group outpatient visit model for chronically ill older health maintenance organization members: a 2-year randomized trial of the cooperative health care clinic. J Am Geriatr Soc. 2004;52:1463–70. doi: 10.1111/j.1532-5415.2004.52408.x. [DOI] [PubMed] [Google Scholar]
- 84.Coleman EA, Eilertsen TB, Kramer AM, Magid DJ, Beck A, Conner D. Reducing emergency visits in older adults with chronic illness. A randomized, controlled trial of group visits. Eff Clin Pract. 2001;4:49–57. [PubMed] [Google Scholar]
- 85.Arora S, Peters AL, Burner E, Lam CN, Menchine M. Trial to Examine Text Message-Based mHealth in Emergency Department Patients With Diabetes (TExT-MED): A Randomized Controlled Trial. Ann Emerg Med. 2013 doi: 10.1016/j.annemergmed.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 86.Buis LR, Hirzel L, Turske SA, Des Jardins TR, Yarandi H, Bondurant P. Use of a Text Message Program to Raise Type 2 Diabetes Risk Awareness and Promote Health Behavior Change (Part II): Assessment of Participants’ Perceptions on Efficacy. J Med Internet Res. 2013;15:e282. doi: 10.2196/jmir.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quinn CC, Shardell MD, Terrin ML, Barr EA, Ballew SH, Gruber-Baldini AL. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes care. 2011;34:1934–42. doi: 10.2337/dc11-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bosworth HB, Powers BJ, Olsen MK, et al. Home blood pressure management and improved blood pressure control: results from a randomized controlled trial. Arch Intern Med. 2011;171:1173–80. doi: 10.1001/archinternmed.2011.276. [DOI] [PubMed] [Google Scholar]
- 89.Blank E, Tuikong N, Misoi L, et al. Usability of implementing a tablet-based decision support and integrated record- keeping (DESIRE) tool in the nurse management of hypertension in rural Kenya. Stud Health Technol Inform. 2013;192:1002. [PMC free article] [PubMed] [Google Scholar]
- 90.Pezzin LE, Feldman PH, Mongoven JM, McDonald MV, Gerber LM, Peng TR. Improving blood pressure control: results of home-based post-acute care interventions. J Gen Intern Med. 2011;26:280–6. doi: 10.1007/s11606-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Joubert J, Reid C, Barton D, et al. Integrated care improves risk-factor modification after stroke: initial results of the Integrated Care for the Reduction of Secondary Stroke model. Journal of neurology, neurosurgery, and psychiatry. 2009;80:279–84. doi: 10.1136/jnnp.2008.148122. [DOI] [PubMed] [Google Scholar]
- 92.Hackam DG, Spence JD. Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke. 2007;38:1881–5. doi: 10.1161/STROKEAHA.106.475525. [DOI] [PubMed] [Google Scholar]
- 93.Allen JK, Dennison-Himmelfarb CR, Szanton SL, et al. Community Outreach and Cardiovascular Health (COACH) Trial: a randomized, controlled trial of nurse practitioner/community health worker cardiovascular disease risk reduction in urban community health centers. Circulation Cardiovascular quality and outcomes. 2011;4:595–602. doi: 10.1161/CIRCOUTCOMES.111.961573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Allen JK, Dennison Himmelfarb CR, Szanton SL, Frick KD. Cost-effectiveness of Nurse Practitioner/Community Health Worker Care to Reduce Cardiovascular Health Disparities. The Journal of cardiovascular nursing. 2013 doi: 10.1097/JCN.0b013e3182945243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Samsa GP, Bian J, Lipscomb J, Matchar DB. Epidemiology of recurrent cerebral infarction: a medicare claims-based comparison of first and recurrent strokes on 2-year survival and cost. Stroke. 1999;30:338–49. doi: 10.1161/01.str.30.2.338. [DOI] [PubMed] [Google Scholar]
- 96.Ovbiagele B, Saver JL, Fredieu A, et al. In-hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow-up. Stroke; a journal of cerebral circulation. 2004;35:2879–83. doi: 10.1161/01.STR.0000147967.49567.d6. [DOI] [PubMed] [Google Scholar]
- 97.Ovbiagele B, Kidwell CS, Selco S, Razinia T, Saver JL. Treatment adherence rates one year after initiation of a systematic hospital-based stroke prevention program. Cerebrovascular diseases. 2005;20:280–2. doi: 10.1159/000087711. [DOI] [PubMed] [Google Scholar]
- 98.Rahiman A, Saver JL, Porter V, et al. In-hospital initiation of secondary prevention is associated with improved vascular outcomes at 3 months. J Stroke Cerebrovasc Dis. 2008;17:5–8. doi: 10.1016/j.jstrokecerebrovasdis.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 99.Ovbiagele B. SWEET-FIX: a quality improvement initiative for hospitalized stroke patients with undiagnosed diabetes or prediabetes. Crit Pathw Cardiol. 2010;9:185–91. doi: 10.1097/HPC.0b013e3181f31af3. [DOI] [PubMed] [Google Scholar]
- 100.Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Archives of internal medicine. 2006;166:1822–8. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 101.Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The care span: The importance of transitional care in achieving health reform. Health affairs. 2011;30:746–54. doi: 10.1377/hlthaff.2011.0041. [DOI] [PubMed] [Google Scholar]
- 102.McGillicuddy JW, Gregoski MJ, Weiland AK, et al. Mobile Health Medication Adherence and Blood Pressure Control in Renal Transplant Recipients: A Proof-of-Concept Randomized Controlled Trial. JMIR research protocols. 2013;2:e32. doi: 10.2196/resprot.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sieverdes JC, Treiber F, Jenkins C. Improving diabetes management with mobile health technology. Am J Med Sci. 2013;345:289–95. doi: 10.1097/MAJ.0b013e3182896cee. [DOI] [PubMed] [Google Scholar]