Abstract
Investigators use in vitro joint simulations to invasively study the biomechanical behaviors of the anterior cruciate ligament. The aims of these simulations are to replicate physiologic conditions, but multiple mechanisms can be used to drive in vitro motions, which may influence biomechanical outcomes. The objective of this review was to examine, summarize, and compare biomechanical evidence related to anterior cruciate ligament function from in vitro simulations of knee motion. A systematic review was conducted (2004 to 2013) in Scopus, PubMed/Medline, and SPORTDiscus to identify peer-reviewed studies that reported kinematic and kinetic outcomes from in vitro simulations of physiologic or clinical tasks at the knee. Inclusion criteria for relevant studies were articles published in English that reported on whole-ligament anterior cruciate ligament mechanics during the in vitro simulation of physiologic or clinical motions on cadaveric knees that were unaltered outside of the anterior-cruciate-ligament-intact, -deficient, and -reconstructed conditions. A meta-analysis was performed to synthesize biomechanical differences between the anterior-cruciate-ligament-intact and reconstructed conditions. 77 studies met our inclusion/exclusion criteria and were reviewed. Combined joint rotations have the greatest impact on anterior cruciate ligament loads, but the magnitude by which individual kinematic degrees of freedom contribute to ligament loading during in vitro simulations is technique-dependent. Biomechanical data collected in prospective, longitudinal studies corresponds better with robotic-manipulator simulations than mechanical-impact simulations. Robotic simulation indicated that the ability to restore intact anterior cruciate ligament mechanics with anterior cruciate ligament reconstructions was dependent on loading condition and degree of freedom examined.
Keywords: anterior cruciate ligament reconstruction, knee kinetics and kinematics, knee injury prevention, joint motion simulation, robotic manipulation of joints, knee ligament mechanics
1. INTRODUCTION
Worldwide it is estimated that over 2 million anterior cruciate ligament (ACL) injuries occur annually.[1] These injuries are devastating to athletic careers and expensive to repair and rehabilitate, as conservative estimates place the cost of an ACL reconstruction (ACLR) surgery at $17,000 plus rehabilitation.[2] These surgeries are known to exhibit short-term promise in the restoration of knee function as up to 86% percent of ACLR patients have a negative pivot-shift score three years post-operative.[3] However, long-term outcomes are less desirable as up to 90% of ACLR patients continue to develop early onset osteoarthritis and knee degeneration within 20 years post-surgery.[4]
In order to optimize preventative and reparative strategies for injured ACLs, it is essential to establish the underlying mechanics that contribute to excessive ligament loads and lead to failure. Approximately 65% of ACL ruptures occur in noncontact situations, which indicate that the injuries are likely influenced by poor neuromuscular control and mechanics, rather than an external impact force delivered directly to the knee joint.[5] Therefore, prophylactic training protocols are effective in the enhancement of neuromuscular control and reduction of the incidence of ACL injuries.[6] In order to design effective training protocols, the biomechanical contributors to ACL forces and strain must be identified. An expanse of in vivo research has been directed at the mechanisms associated with ACL failure and has identified that factors such as excessive knee valgus, asymmetry, and poor trunk position are associated with increased injury risk.[7–9] Despite their contributions, in vivo studies are limited in that direct, invasive measurements of ACL mechanics are unethical to perform on living subjects and the presence of sensors would interrupt native function.
Unlike in vivo investigations, during in vitro studies investigators can apply invasive techniques that directly evaluate ACL mechanics relative to loads and stresses. In vitro studies have been used to reveal the relative contributions of anterior tibial force (ATF) force, [10] resistance to internal tibial torsion (ITT), [11] and muscular contributions to ACL strain.[12] Though valuable, many of these in vitro investigations have been used to examine maximal, uniaxial loading, rather than complex multi-planar scenarios that are likely more physiologic. Functional tissue engineering principles indicate that the evaluation of ligament biomechanics within kinematic ranges that mimic in vivo activity will likely provide greater clinical relevance than information obtained from non-physiologic methodologies.[13] Over the past 20 years, investigators have focused on in vitro approaches with methods designed to simulate in vivo loading conditions from daily activities or clinical settings.[14–22] Fundamental differences exist amongst these in vitro methodologies as some protocols drive motion with robotic manipulators that apply constant force and actively control limb position, while other protocols drive motion with a singular impulse force and have restraints passively regulate limb position. Though all in vitro methods aim to correlate with in vivo physiologic conditions, variation in the mechanisms used to drive motion simulations could lead to disparities in biomechanical outcomes. It is important to synthesize in vitro data gathered from these varied methods in order to derive optimal ACL injury prevention and treatment recommendations for the clinical environment.
In vitro investigations are particularly conducive to ACLR evaluation as investigators can injure and repair a specimen to make direct biomechanical comparisons between the native and grafted ligaments using repeated measures. ACLR is the primary method used to treat athletes intending to return to sport after ACL injury.[23, 24] Functionally, the ACL is the primary resistor to anterior tibial translation (ATT) and patients exhibit anterior-posterior instability at the knee following injury.[10, 25] Surgeons focus on the restoration of this instability during ACLR; however, up to 25% of ACLR patients suffer secondary injuries within two years of returning to sport.[26] This rate far exceeds that of primary injury and may indicate that knee mechanics are altered following repair.[2, 5, 24] In vitro methods can be used to identify altered intra-articular mechanics between native and reconstructed ACLs in order to help explain this disparity in injury incidence.
The objective of this systematic review and meta-analysis was to synthesize the current data and compare robotic and mechanical methods of in vitro knee simulation. Specifically, we aimed to investigate the functional behavior of the ACL and ACLR and to analyze differences observed between methodologies. It was hypothesized that the different control mechanisms applied during robotically-driven and mechanical-impact knee simulations would elicit variation in mechanical responses during similar simulated tasks. It was further hypothesized that ACLRs will restore native ATT, but will fail to restore the additional kinetic and kinematic responses relative to the intact ligament.
2. METHODS
2.1 Systematic Review
A literature search related to methods of knee simulation was performed in the PubMed/MEDLINE, SPORTDiscus, and Scopus databases in May 2013. The systematic review focus was to identify research articles published within the last decade (2004–2013) that investigated in vitro ACL biomechanics through knee motion simulation. Search terms were limited to ‘anterior cruciate ligament’ OR ‘ACL’ and was further limited with ‘robot’, ‘robotic’, ‘knee simulator’, OR ‘knee simulation.’ Additional articles were added through cross-referencing the identified studies. As this review focused on functional biomechanics, simulations were limited to physiologic (passive flexion, gait, and jump landing) or clinical (Lachman’s and pivot shift test) knee motions. Non-physiologic simulations, such as uniaxial force or torque loading to joint failure, were excluded. Knee conditions included in this review were ACL-intact, ACLD, ACLR, and ACL-only. Inclusion was also limited to whole-ligament biomechanics; thus, any studies that investigated specimens with arthroplasty or individual bundle mechanics were excluded. In order to focus the review to ACL biomechanical contributions in a normal knee, data collected after the selective alteration of additional passive restraint structures within the knee (including but not limited to tibial osteotomy, posterior cruciate ligament resection, or meniscus resection) were excluded. In vivo simulations, simulations on joints other than the knee, computer models, computational models, papers without kinematic or kinetic dependent variables, methodology papers, review papers, and non-English articles were also excluded. The initial search compiled 621 published papers, which were then reduced to 77 papers as documented in Figure 1. The included papers were divided into 3 classifications of robotic simulation (passive flexion, weight-bearing flexion, and kinematic reproduction) and one classification of mechanical-impact simulation.
Figure 1.
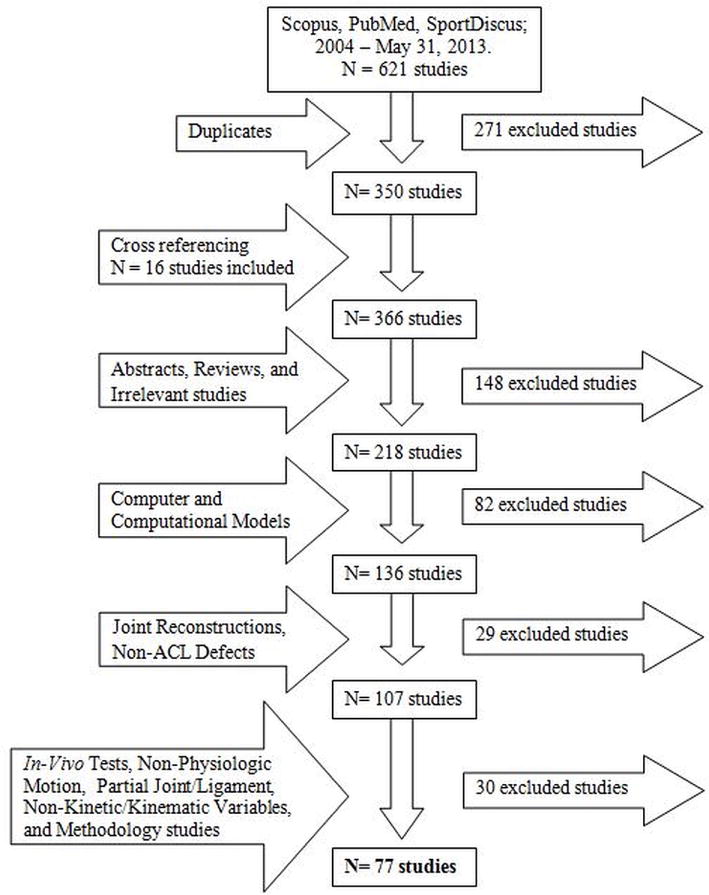
Flow chart of the literature review.
2.2 Meta-analysis
Following review, the passive flexion method of robotic simulation was selected for further meta-analysis due to its prevalence and congruity between studies. To reduce confounding factors, force applications were limited to 134 N ATF in the simulated Lachman’s test and 10 N*m abduction torque combined with 4–5 N*m internal rotation torque in the simulated pivot-shift test. Unless otherwise noted, literature that did not adhere to the prescribed loading protocols was excluded from the meta-analysis. Data and standard deviations from the literature were digitized at predetermined intervals (0°, 15°, 30°, 45°, 60°, 90°, 120° of flexion) and an average, weighted relative to the number of specimens in each qualified study, was determined. Standard deviations were used to calculate corresponding standard error of the means at each data point. This was repeated for the ACL-intact, ACLD, and ACLR conditions and the results were plotted (Figure 2 & 3). Two-sample t-tests (α = 0.05) determined the presence of statistical differences between each condition at each interval. ATT, internal tibial rotation (ITR), and ligament forces were tracked due to their consistently reported outcomes in passive flexion simulations during a Lachman’s assessment (Figure 1) and pivot-shift assessment (Figure 2). This method of analysis was then adapted to assess differences in abduction loading magnitude during pivot-shift tests (Figure 4), simulated muscle forces during Lachman’s tests (Figure 5), and ACL condition under simulated quadriceps force during Lachman’s tests (Figure 6).
Figure 2.
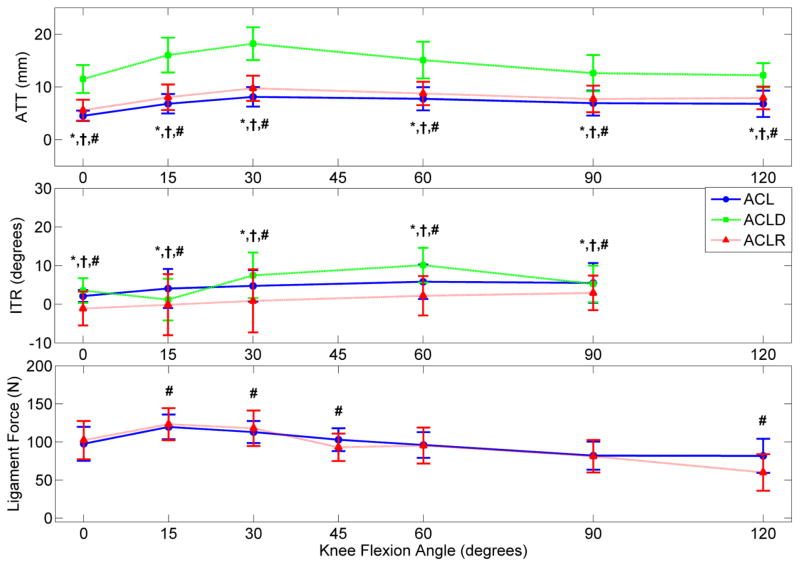
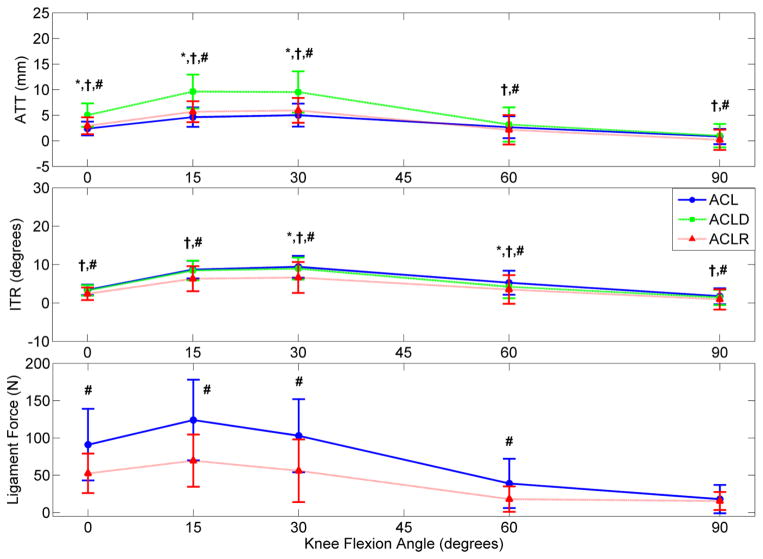
Displays the overall mean ATT, ITR, and ligament force for the ACL-intact, ACLD, and ACLR conditions in response to a Lachman’s test simulated with 134 N applied ATF. A 134 N stimulus was selected for clinical relevance as this value represents the force generated by 30-pound test on a KT1000 device. (* indicates significant difference between ACL and ACLD, † between ACLD and ACLR, and # between ACL and ACLR; n = 29 studies) [28, 31, 36–61]
Figure 3.
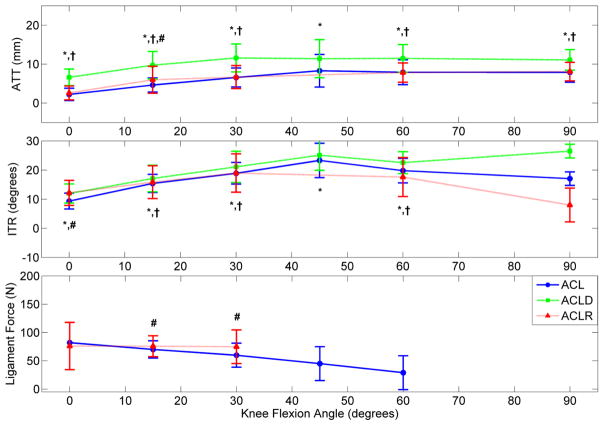
Displays the overall mean ATT, ITR, and ligament force for the ACL-intact, ACLD, and ACLR conditions in response to a Pivot-Shift test simulated with a combined 10 N*m abduction and 4–5 N*m internal torque. This loading condition was selected as it represented the most common Pivot-Shift scenario simulated in the non-weight-bearing passive flexion methodology. (* indicates significant difference between ACL and ACLD, † between ACLD and ACLR, and # between ACL and ACLR; n = 25 studies) [28, 31, 36–61]
Figure 4.
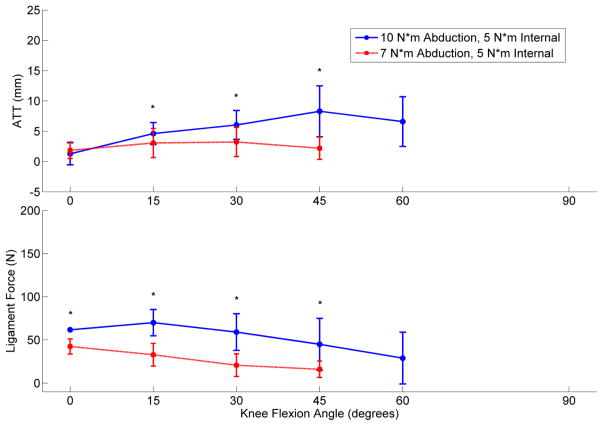
Displays the mean ATT and ligament force for the ACL-intact condition at two separate levels of Pivot-Shift loading. The applied torques were 10 N*m abduction with 5 N*m internal and 7 N*m abduction with 5 N*m internal. (* indicates a significant difference was present; n = 18 studies) [28, 36, 38, 42, 44–49, 55, 57, 58, 60, 63–66]
Figure 5.
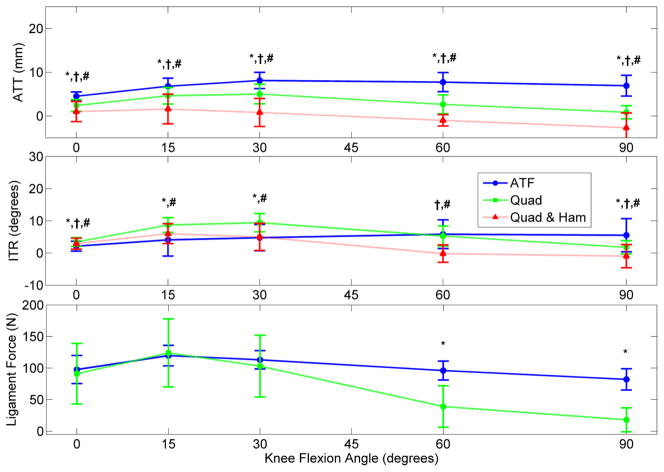
Displays the mean ATT, ITR, and ligament force for the ACL-intact conditions in response to a simulated 134 N ATF, 400 N quadriceps load, and a combined 400 N quadriceps with 200 N hamstrings load. These loads were simulated in the non-weight-bearing passive flexion methodology. (* indicates significant difference between ATF and quadriceps loading, † between ATF and combined loading, and # between quadriceps and combined loading; n = 5 studies) [39, 41–44]
Figure 6.
Displays the mean ATT, ITR, and ligament force throughout non-weight-bearing, passive flexion for the ACL-intact, ACLD, and ACLR condition in response to simulated muscle loads of 400 N quadriceps. (* indicates significant difference between ACL and ACLD, † between ACLD and ACLR, and # between ACL and ACLR; n = 5 studies) [7,9–12]
3. RESULTS
3.1 Methods of Robotic Simulation
In one method of robotic simulation, investigators have used a highly accurate and precise six-degree-of-freedom (6-DOF) robotic manipulator in conjunction with a universal force sensor (UFS) to articulate a specimen through passive flexion with minimal loading.[15, 16] Specimens were resected of soft tissue outside the knee joint, cemented into rigid fixtures, and affixed to the robotic end effector (tibia) and a static frame (femur). Local coordinate frames were identified by anatomical landmarks and were digitized relative to the robot’s global position, which allowed for tibial articulation relative to the femur. Flexion was recreated in 1.0° increments while the robotic/UFS zeroed loads at each position. Simulations of clinical Lachman’s test, through ATF, and pivot-shift tests, through combined abduction and internal torque, were executed at predetermined intervals. The initial path of passive flexion was recorded by the robot, which was able to reproduce it with high precision. Thus, the same motion was applied to the same specimen for the ACL-intact, ACL-deficient (ACLD), and ACLR conditions, which allowed superposition to determine relative force contributions.[17] Since its inception at the University of Pittsburgh, this methodology has been adopted at multiple research facilities including Harvard Medical Center, Wilhelms University, Kogakuin University, the Hospital for Special Surgery, and the United States Naval Academy.[27–32]
An upright knee simulator (UKS) was developed at the University of Tubingen to simulate knee motion for weight-bearing flexion conditions.[14] Similar to the passive flexion methodology, this device was used to simulate flexion at the knee, provide external tibial loads with a robotic/UFS, and calculate ACL forces through superposition. Unlike passive flexion, the weight-bearing flexion path was not controlled by the robotic/UFS. Rather, the proximal end of the potted femur was attached to a hip assembly with a vertically-oriented linear actuator and two rotational DOFs, while the potted tibia was attached to a vertically static ankle assembly that allowed for three rotational DOFs. Starting at 15°, the hip assembly actuator drove each specimen into knee flexion at a rate of 1° /second until 90° of flexion was reached. Linear actuators, attached to the quadriceps and hamstrings tendons via tension wires, were used to simulate up to 1000 N muscle forces to create constant weight-bearing forces of between 0–250 N at the ankle.[14, 18, 33] Similarly, investigators at the University of Waterloo developed a weight-bearing flexion simulator based on the vertical motion of a hip assembly and in vivo muscle forces.[18] This dynamic knee simulator (DKS) did not incorporate a robotic/UFS and was used to simulate flexion via regulated descent of its hip assembly, while the remaining DOFs were dictated by applied muscle forces.
Unlike the previous methods of robotic knee simulation, which simulated flexion motions relative to the geometry of each specimen, researchers at the University of Calgary developed a method of reproducing in vivo recorded kinematics.[19] Rigid markers were directly implanted on bony structures to record in vivo kinematics from ovine treadmill gait. The recorded limb was sacrificed, resected of soft tissues outside the joint capsule, and potted into a 6-DOF parallel robot. The limb was digitized relative to the robot using global, tibial, and femoral coordinate frames. The in vivo gait kinematics were then used as positional input for the robot to articulate the tibia about the femoral coordinate system. This method was adapted to a serial robotic/UFS manipulator by the University of Cincinnati.[20] Further adaptations were made to apply the mean ovine motion, as well as in vivo recorded human gait kinematics, onto cadaveric specimens.[34] Superposition again allowed investigators to quantify the biomechanical contributions of the ACL during kinematic-derived simulations.
Mechanical-impact simulations of knee motion have been based on driving forces equivalent in magnitude to in vivo ground reaction forces through in vitro specimens and allowing each specimen to determine its own path of articulation. Investigators designed the jump-landing simulator to deliver a drop-weight impact force through the tibia of a lower extremity specimen resected of all soft tissues outside the knee joint capsule.[21] The proximal end of the femur and distal end of the tibia were potted into assemblies that simulated the hip and ankle joints as sagittal plane hinges. Limbs were flexed 10–40° prior to impact. Actuator cables were drilled into the approximate quadriceps and hamstrings insertion sites on the patella and tibia and simulated muscle forces that stabilized the joint during impact. Impact forces were designed to fit within the magnitude (2–4 * bodyweight) and rise time (~0.1 sec) of in vivo ground reaction forces during landing. Similarly, Withrow and colleagues developed the knee testing apparatus to simulate jump landings.[22] Specimens were resected of soft tissue down to the joint capsule and muscle tendons then potted at the proximal femur and distal tibia. These fixtures were adjustable in order to manipulate the rotational alignment of the knee in both the sagittal (15–25° flexion) and frontal planes (0–15° abduction/adduction) prior to impact testing. The tibial assembly was locked to prevent translational movement during impact. Cables were connected between the quadriceps, hamstrings, and gastrocnemius tendons and independent tensioning mechanisms to mimic in vivo pre-landing muscle activations. Specimens were oriented vertically and impact was applied to the proximal end of the femur. Later iterations of the knee testing apparatus incorporated a torsional transformer that converted some of the vertical impact force into rotational torque, which simulated pivot landings.[35]
3.2 Passive Flexion
In response to 134 N ATF during passive flexion, ACLR knees were found to reduce mean ATT relative to ACLD, but fail to match the intact ACLs (Figure 2).[28, 31, 36–61] However, under combined torsional loading, the mean ATT for ACLRs relative to intact ACLs increased by 1.3 mm at 15° flexion (Figure 3).[28, 31, 36–61] During ATF, mean ITR was restricted in ACLRs compared to intact ACLs. However, under combined torsional loads, mean ITR between ACLRs and intact ACLs increased by 2.8° at full extension. No ITR differences (range 35°–41°) were expressed in either the intact, reconstructed or deficient ACL when 4 N*m of isolated ITT were simulated.[62] However, a 3 N*m increase in abduction torque during pivot-shift loading was responsible for increasing ensemble mean ATT and ensemble mean in situ ACL force in low flexion angles (Figure 4).[28, 36, 38, 42, 44–49, 55, 57, 58, 60, 63–66] These isolated abduction/adduction torques produced greater loads on intact ACLs (peak 41 N) than internal/external torques (peak 34 N).[32, 67] However, ATF increased in situ ACL force (peak 120 N) more than isolated abduction moments.[32, 68, 69] When under ATF or combined torsional loads at full extension, ACLRs were found to match the ligament forces seen in the intact ACLs. However, as the knee entered flexion, ligament force for the ACLRs was in excess of that in the intact ACL (Figure 2 & 3).
The application of a simulated 400 N quadriceps load to the intact knee induced ATT, ITR, and force on the ACL (Figure 5).[39, 41–44] On a knee flexed under 45°, the application of quadriceps loads created less ATT, greater ITR, and equivalent ligament forces relative to ATF. An applied hamstrings co-contraction of 200 N reduced ATT and ITR relative to isolated quadriceps values and created a net posterior tibial shift when knee flexion exceeded 60°.[39] Abduction rotation and medial translation were consistently greater under isolated quadriceps loading than under ATF, whereas the application of a hamstrings co-contraction also decreased the magnitude of valgus rotations.[40] Abduction knee rotation and medial translation under a quadriceps load were similar for an intact ACL and ACLR, but ACLD.[40, 44] The application of quadriceps load did not alter the relative relationships of ATT between the ACL-intact, ACLD, and ACLR knees, but did exhibit a relative reduction in ITR for the ACLD knee and ligament loading for the ACLR knee (Figure 6).[7,9–12]
3.3 Weight-bearing Flexion
The application of weight-bearing to passive flexion simulations altered knee kinematics relative to zero load flexion simulations. The application of 200 N axial compressive force to passively flexed knees increased ATT.[36, 70] Axial compression also generated slightly greater ACL forces than did isolated valgus or isolated internal torque; however, these forces were lower than those produced by combined torsional loading.[32, 36, 69]
In static positions of lower flexion, the addition of 1*body weight increased ACL force (Table 1).[71] The addition of weight-bearing in the UKS increased ITR, valgus rotation, and medial, anterior, and proximal tibial translation compared with passive flexion.[72] When compared with weight-bearing flexion, the application of ATF increased peak ACL force, while the application of ITT did not affect ACL force relative to weight-bearing alone.[14] Inclusion of ITT did increase ITR in both intact and ACLD specimens.[14, 73] When compared to weight-bearing alone, the addition of ATF and ITT each slightly increased ATT throughout flexion in both the ACL and ACLD condition.[14, 73] ATT was increased from the ACL-intact to ACLD condition in weight-bearing, in weight-bearing with ATF, and in weight-bearing with ITT. No ITR differences existed between ACL and ACLD specimens under any weight-bearing conditions.[73] Peak ACL strain for DKS weight-bearing was 4.3% with a peak rate of 120% /sec.[18] Compared to ACLDs, ACLRs tested in the UKS had less ATT during weight-bearing, less ATT during weight-bearing plus ATF, and had no ITR change during weight-bearing plus ITT.[74]
Table 1.
Summarization of findings from the literature where the UKS was used to apply weight-bearing flexion methods of knee simulation.[14, 71–74]
| Reference | Baseline Condition | Test Condition | Flexion Angle | Outcome Change |
|---|---|---|---|---|
| Hosseini, et al. 2011 | non-weight- bearing static flexion | 1*bodyweight | 15° | +131.4 N ACL force |
| 30° | +106.7 N ACL force | |||
| 45° | +34.6 N ACL force | |||
|
| ||||
| Muller, et al. 2009 | non-weight- bearing passive flexion | 100 N weight-bearing | full range | +16° ITR |
| +2° valgus rotation | ||||
| < +3 mm medial, anterior, and proximal translation | ||||
|
| ||||
| Lo, et al. 2008 | weight-bearing alone | 100 N weight-bearing | ||
| & 50 N ATF | 15–55° | +33 to 55 N ACL force | ||
| full range | > +2 mm ATT | |||
| & 5 N*m ITT | 15–55° | negligible ACL force | ||
| >20° | > +9° ITR | |||
| full range | > +2mm ATT | |||
|
| ||||
| Wunschel, et al. 2010 | ACL-intact specimens | ACLD, 100 N weight-bearing | 20–40° | > +2 mm ATT |
| & 50 N ATF | 15–65° | > +4 mm ATT | ||
| & 5 N*m ITT | 15–30° | > +2 mm ATT | ||
| All conditions | full range | no ITR change | ||
|
| ||||
| Wunschel, et al. 2011 | ACL-intact specimens | ACLD, 50 N weight-bearing | full range | +2.4 mm ATT |
| & 50 N ATF | +3.1 mm ATT | |||
| & 5 N*m ITT | no ITR change | |||
3.4 Kinematic Simulation
Knee simulations driven by in vivo kinematics were used to identify that porcine and ovine stifle joints are ACL-dependent structures during the stance phase of gait as they sustained peak ACL loads of up to 400 N.[20, 75] The ACL-dependency of the ovine and porcine joints has been used to suggest that they may be applied as acceptable surrogate models to human cadavers for the evaluation of ACLR biomechanics.[76] Application of these animal models has allowed investigators to study graft healing following ACLR, an impossibility in cadaveric models. Following hoof-strike, the mean ovine ACL load increased from 0–45° knee flexion and partial resection of the ACL decreased the ATF required to produce equivalent levels of ATT.[77] Compared to an intact specimen, investigators found that ACLD significantly reduced hoof-strike force in the anterior (44.3 vs. −5.9 N), medial (19.5 vs. −0.3 N), and compressive (70.8 vs. 33.6 N) directions and peak moments in flexion (9.3 vs. 0.6 N*m) and abduction (3.5 vs. 0.2 N*m) rotation during gait. Peak internal rotation moment during gait experienced no change between ACL-intact and ACLD knees.[76] The addition of up to 4 mm of ATT during simulated gait in the porcine knee was found to increase peak anterior knee force in a linear fashion (~40 N /2 mm).[20] Force response to tibial displacement and rotation during cadaveric gait simulations was used to indicate that the ACL was a primary restraint to ATT (peak force 135 N) and a secondary restraint to medial tibial translation (peak force 47.8 N), knee flexion rotation (peak torque 12.4 N*m), and knee abduction/adduction rotation (peak torque 18.1 N*m).[34] Rotational perturbations of −0.5 to 0.5° applied to the initial position of cadaveric specimens had no effect on ACL-intact or ACL-only knee kinetics during gait. Conversely, anterior and compressive perturbations moving from −0.5 mm to 0.5 mm increased forces in the anterior, medial, and compressive directions.[78] In the porcine model, a bone-patellar-tendon-bone ACLR was able to restore the anterior forces observed in the intact ACL, but unable to match its rate of force loss. Relative force contributions during stance phase of gait were not consistent between the intact ACL and ACLR. The intact ACL was the primary contributor to anterior force, medial/lateral force, and flexion/extension moments and secondary contributor to compressive force and abduction/adduction moments, while the bone-patellar-tendon-bone ACLR was the primary contributor to all forces and moments except internal/external rotation.[76] The ACLR force contributions relative to ACL-intact and ACLD conditions are summarized in Table 2.
Table 2.
Forces and moments for the Native ACL (n = 11) and ACL-reconstructed (n = 6 each) knees at heelstrike (HS), midstance (MS), and toeoff (TO).
| Native ACL, ACL-D, and Grafted Knees - Forces (N (SEM)) and Moments (Nm (SEM))
| ||||||
|---|---|---|---|---|---|---|
| ACL-D | Native ACL | Hybrid | RTM | BPTB | ||
|
|
||||||
| Anterior Force | HS | −5.9 (1.9) * | 44.3 (7.7) † | 56.0 (7.4) † | 64.2 (7.3) † | 62.5 (4.2) † |
| MS | −5.0 (0.4) * | 34.3 (6.0) † | 32.0 (6.9) † | 33.1 (5.1) † | 36.7 (4.0) † | |
| TO | −7.7 (1.0) * | 34.3 (5.1) † | 46.5 (7.6) † | 47.9 (6.7) *† | 53.3 (2.5) *† | |
|
|
||||||
| Medial Force | HS | −0.3 (0.8) * | 19.5 (1.9) † | 33.8 (5.7 ) *† | 44.7 (4.1 ) *† | 40.5 (4.3) *† |
| MS | −2.1 (0.5) * | 10.4 (1.7) † | 15.4 (4.2) † | 20.7 (2.8) *† | 22.6 (5.7) † | |
| TO | −1.8 (0.7) * | 13.9 (1.4) † | 26.2 (5.4) *† | 33.6 (4.2) *† | 33.8 (3.3) *† | |
|
|
||||||
| Compression Force | HS | 33.6 (5.1) * | 70.8 (8.2) | 115.2 (15.7) *† | 103.0 (6.7) *† | 142.7 (6.9) *† |
| MS | −0.6 (1.5) | 7.4 (5.1) † | 37.3 (16.0) *† | 31.0 (8.0) *† | 29.0 (13.4) † | |
| TO | 33.3 (3.3) * | 75.4 (7.6) † | 110.8 (15.8) *† | 100.8 (11.3) *† | 129.2 (8.5) *† | |
|
|
||||||
| Abduction Moment | HS | 0.2 (0.4) * | 3.5 (0.5) † | 5.7 (0.7) † | 7.5 (0.5 ) *† | 7.6 (0.8) *† |
| MS | −0.5 (0.1) * | 1.3 (0.5) † | 2.1 (0.7) † | 3.3 (0.5) † | 3.0 (1.5) † | |
| TO | −0.8 (0.4) * | 1.9 (0.4) † | 3.8 (0.7) † | 5.2 (0.6) *† | 5.8 (0.7) *† | |
|
|
||||||
| Flexion Moment | HS | 0.6 (0.3) * | 9.3 (1.3) † | 10.7 (1.3) † | 11.6 (1.6) † | 11.1 (0.5) † |
| MS | −0.1 (0.1) * | 6.8 (0.8) † | 6.2 (1.2) † | 5.9 (1.3) † | 6.7 (0.7) † | |
| TO | 0.0 (0.2) * | 7.4 (0.9) † | 8.8 (1.3) † | 10.1 (1.6) † | 9.5 (0.4) † | |
|
|
||||||
| Internal Moment | HS | −0.2 (0.1) | −0.2 (0.1) | 0.2 (0.5) | −0.2 (0.1) | −0.3 (0.3) |
| MS | 0.1 (0.0) | 0.2 (0.1) | 0.6 (0.5) | 0.0 (0.0) | 0.2 (0.2) | |
| TO | 0.0 (0.1) | 0.0 (0.1) | 0.4 (0.5) | −0.1 (0.1) | −0.1 (0.2) | |
Significant differences from the ACL-intact condition are highlighted in bold (p < 0.05), with * indicating a significant difference from the intact ACL and † indicating a significant increase from ACLD. This table was adapted with permission from Boguszewski 2012.[76]
3.5 Mechanical Simulation
Investigators used the jump-landing simulator to create ACL ruptures through impulse forces with limited quadriceps tensioning (Table 3).[21] However, they could not rupture the ACL in any loading scenario where large quadriceps loads were applied. A positive correlation was identified between quadriceps pretension and static ACL strain, while a negative correlation was found between quadriceps pretension and the dynamic ACL strain that leads to rupture.[21, 79] Conversely, the knee testing apparatus was used to demonstrate that ACL strain directly correlated with increased quadriceps tension force.[22, 80] Further, the application of hamstrings tension in this apparatus was used to limit ACL strain by up to 70%.[81] Additional investigations performed with the knee testing apparatus were used to illustrate that ACL strain was not dependent on impact force, but did correspond with valgus knee rotation.[22, 80] Once a torsional transformer was used to incorporate ITT, the peak relative ACL strain and strain rate were increased.[82] The application of ITT was also found to increase ITR and ATT in both ACL-intact and ACLD specimens.[35] The combined application of ITT with varus or valgus knee torque was observed to create the highest peak relative ACL strain output from the knee testing apparatus.[83] Overall, it was reported that the ACL was a secondary contributor to ITT resistance and accounted for approximately 13% of ITT resistance during landing simulations.[35]
Table 3.
Summarization of review results from literature where mechanical simulation was the applied method of knee articulation.[21, 22, 35, 79–83]
| Reference | System | Method | Baseline Condition | Test Condition | Change Effected on Joint |
|---|---|---|---|---|---|
| Withrow, et al. 2006 Am J Sports Med |
knee testing apparatus | 1400 N mean impulse force, 25° knee flexion, constant force muscle pretensioning | N/A | Quadriceps tendon lengthening | Peak relative ACL strain 2.0–3.5%, peak quadriceps force 800–1500 N, r-squared = 0.74 |
| Withrow, et al. 2006 Clin Bio |
knee testing apparatus | 1610 N mean impulse force, 25° knee flexion, constant force muscle pretensioning | Neutral knee varus/valgus rotation | 15° knee varus/valgus rotation | Peak relative ACL strain 4.3%, increased relative ACL strain by 0.8%, increased ATT by 1.1 mm |
| Hashemi, et al. 2007 | jump- landing simulator | 1100–1400 N impulse force, fixed hip flexion, 10–40° knee flexion, 0–10° knee valgus, 50– 1500 N quadriceps tension | N/A | 165 N Quadriceps tendon tension at 20° knee flexion | ACL rupture occurred at 11.6% relative strain and strain rate of 261% /sec |
| Withrow, et al. 2008 | knee testing apparatus | 1700 N mean impulse force, 25° knee flexion, constant force muscle pretensioning | Constant hamstrings force | Hamstring tendon lengthening | Decreased relative ACL strain 1.2%, decreased ATT 1.2 mm, decreased knee valgus 0.7°, decreased ITR 0.9° |
| Hashemi, et al. 2010 | jump- landing simulator | Unspecified magnitude impulse force, 20° knee flexion | N/A | Varied quadriceps pre-activation force range: 25–700 N | ACL pre-activation strain 0.2–2.0%, r-squared with quads force = 0.67. Peak ACL landing strain 3.0–4.5%, r-squared with quad force = −0.24. |
| Oh, et al. 2011 | knee testing apparatus | 2*bodyweight mean impulse force, 15° knee flexion, constant force muscle pretensioning | No ITT applied to knee at impulse | 10–25 N*m ITT added to knee at impulse | Increased ACL-intact ITR from 1.6°–11.6°, and ACLD ITR from 2.5°–12.5°. Increased ACL-intact ATT by 2.6 mm and ACLD ATT by 3.8 mm |
| Oh, et al. 2012 J Ortho Res |
knee testing apparatus | 2*bodyweight mean impulse force, 15° knee flexion, constant force muscle pretensioning | No ITT applied to knee at impulse | 10–25 N*m ITT added to knee at impulse | Increased relative ACL strain from 3.0% to 5.4%, increased relative ACL strain rate from 184% to 252% |
| Oh, et al. 2012 Am J Sports Med |
knee testing apparatus | 2*bodyweight mean impulsive force, 15° knee flexion, constant force muscle pretensioning | No combined ITT or valgus torque | Combined 25 N*m ITT and 50 N*m valgus torque | Peak relative ACL strain 7.0%, increased relative ACL strain by 3.2%, increased ATT by 2.9 mm |
4. DISCUSSION
In vitro simulations of the knee have been used to evaluate how kinematics and kinetics contribute to ACL loading and injuries. The purpose of this review and meta-analysis was to compare robotic and mechanical methods of in vitro knee simulation in order to investigate the functional behavior of the ACL and ACLR and to analyze differences observed between these methodologies.
The ACL is the primary restraint to ATT in the tibiofemoral joint, resisting up to 87% of the ATF.[10] ATF was the most influential loading condition in all simulation methodologies. This behavior was evidenced in the ensemble mean ATT and ligament force data compiled from passive flexion (Figure 2). When the intact ACL was resected, the largest kinematic change occurred in ATT during both the Lachman’s and pivot-shift loading conditions (Figure 2 and 3). Also, the peak ligament forces that were generated from ATF and isolated quadriceps simulation were greater than the force from combined abduction and internal torsional loading. Throughout kinematic gait simulations, the largest force drops between the ACL and ACLD conditions occurred in the anterior direction and averaged above 40 N.[76] Further, from 0–30° flexion, the application of quadriceps force, which generates ATF due to its tendon insertion on the proximal anterior tibia, to passive flexion simulations increased ATT and equivalent ligament forces to those produced by ATF (Figure 5). These behaviors were a confirmation that robotic simulations maintained the integrity of the ACL as the primary restraint to ATT and ATF throughout motion. However, within mechanical impact simulations there was conflict over how ACL strain correlated with quadriceps force. The knee testing apparatus maintained the traditional convention of a direct relationship between increased quadriceps force and increased ACL strain, while the jump-landing simulator indicated an inverse relationship between quadriceps force and dynamic ACL strain.[21, 22, 79, 80] The jump-landing simulator claimed that the compressive forces generated by simulated quadriceps contraction protected the ACL during motion more than the generated ATF strained the ligament.[55,58] This finding is in direct contrast with the passive flexion and weight-bearing flexion techniques that reported combined ATF and axial compressive loads increased ACL forces relative to either isolated loading condition.[14, 36, 70] The quadriceps forces that were applied in both impact testing devices and the weight-bearing flexion simulations were in excess of 1000 N; therefore, dissimilarities between methods did not arise from a disparity in muscle force magnitude.[14, 18, 21, 22, 33, 80, 81]
Findings from robotic simulations demonstrated that abduction torque and rotation at the knee had greater impact on ACL forces and kinematics than internal torque. A slight increase in abduction torque throughout passive flexion increased ATT and in situ ACL force (Figure 4), which exemplified its impact on ACL mechanics. Isolated abduction torque produced peak ACL forces that were ~20% greater than isolated internal torque.[32] The application of weight-bearing altered ITR angle more than any other kinematic variable with 8 times greater change than was observed in knee abduction under the same loads.[72] These magnitude differences indicate that knee abduction was more influential to ACL mechanics than internal rotation and are congruent with kinematic simulations that depicted the ACL was a secondary restraint to abduction torque during gait.[76] Hence, significant knee abduction torque, but not ITT, differences were observed between ACL-intact and ACLD specimens.[76] During weight-bearing flexion, ITT did not increase ACL force throughout flexion.[14] A lack of mechanical resistance to ITR was echoed in passive flexion simulations as ACLD did not increase ITR during weight-bearing and isolated ITT produced no ITR differences between ACL and ACLD specimens.[62, 73] If the ACL were a functional restraint to ITT, then the intact ligament should have restricted the observed ITR relative to the ACLD condition.
Mechanical impact testing exhibited different ACL mechanics in response to rotational stimuli than robotic simulation methods. Though the knee testing apparatus showed that additional knee valgus at the time of impact corresponds with increased ACL strain, it also reported that isolated ITT had a potentially greater influence on increasing ACL strain during landing.[22, 80] The increase in ACL strain from additional valgus rotation at impact collaborates both with results from robotic simulations that indicate abduction is a significant antagonist to ACL forces and with literature that reported an 8° increase in knee abduction angle at initial contact of landing is associated with increased ACL injury risk.[84] However, the effect of ITT on ACL strain during mechanical simulation contrasts the findings of robotic simulations. Whereas ACL contributions to resist ITT could not be quantified in kinematic gait simulations,[76] the knee testing apparatus found the ACL to be a secondary resistor to ITT, much as it is to knee abduction.[35] Peak ITR with respect to isolated ITT in the knee testing apparatus was less than the peak ITR for passive flexion, yet had a more profound impact on ACL mechanics.[32]
All methods of simulation demonstrated that combined loading with coupled abduction and ITT had greater impact on ligament mechanics than either isolated condition. In mechanical testing, ACL strain was ~2% larger under combined loads than in either isolated condition.[22, 80, 82, 83] In robotic testing, ACL forces from coupled loading were ~40 N larger than isolated abduction torque and ~50 N larger than isolated ITT at full extension.[32] These mechanical behaviors are in agreement with literature that supports valgus collapse at the knee, defined as “the outward angulation of the distal segment of a bone or joint” due to “a pure abduction motion of the distal tibia relative to the femur or from transverse plane knee rotation motions”, [85] to be a primary mechanism of ACL injury and injury risk prediction.[84, 86] Therefore, though combined torsional loading had the greatest impact on ACL mechanics in all simulation methods, mechanical differences that were produced by isolated torques and quadriceps forces were in support of the primary hypothesis that the different control mechanisms applied during robotically-driven and mechanical-impact knee simulations would elicit variation in mechanical responses during similar simulated tasks.
Some of the biomechanical dissimilarities between robotic simulation and mechanical impact may have arisen from structural and motion-constraint limitations within each testing apparatus. Variability is naturally associated with human movement cycles as even passive flexion articulations of the knee exhibit pathway variance.[87, 88] In robotic simulations the natural variability associated with human movement cycles is excluded by the high-precision of robotic manipulators, which predicates that structures are being abnormally loaded through constant and repetitive force application. Unlike robotic simulations, where limb positions were dictated by either in vivo recorded kinematics or clinical exam procedures, motion pathways in mechanical impact simulations were not preset.[21, 22, 35, 79–83] Instead, specimens uniquely reacted to each force impulse while pre-tensioned muscles and artificial hip and ankle joints constrained movement. However, during in vivo motion, individual muscle force contributions are in flux throughout a landing as subjects adapt to changes in geometry and ground reaction forces.[89, 90] Conversely, muscle forces within the mechanical apparatus were constrained to either a constant level or lengthening determined by bone position.[21, 22, 35, 79–83] The inability to match the dynamic nature of in vivo structures implies that mechanical simulation constraints may have been physiologically inaccurate. This concern of non-physiologic response was augmented by data that indicated peak knee flexion range of motion was ~6° during mechanical simulation, [22, 83] whereas in vivo data from comparable-force landings has indicated that peak flexion range of motion exceeds 60°.[91] An order of magnitude difference in knee flexion angle suggests that the mechanical testing apparatus enacted potentially non-physiologic pathways of force distribution as compared to in vivo landings. The genesis of these knee flexion differences may be that mechanical-impact simulations deliver singular impulse loads to each specimen; whereas, during in vivo landings, ground reaction forces propagate through the leg for the duration of stance phase. The instantaneous versus continuous application of force could greatly influence knee biomechanics. Also, for a methodology dependent on tissue structures to constrain motion pathways, much of the natural anatomy was resected including the iliotibial band, muscle mass, skin, and the ankle and hip joints.[21, 22, 35, 79–83] Tissues were also resected in robotic simulations; however, in those models, the motion was prerecorded and constrained by the manipulator, not by soft-tissues within the model.[14–19] In robotic simulations, positional control provided by the robotic manipulator represented the muscles and other resected tissues that would have constrained in vivo joint motion.
Inconsistent restoration of ACL-intact kinematics across a wide spectrum of simulated, functional loading conditions partially rejected the hypothesis that ACLRs would restore ATT and ATF observed in the intact ACL, but fail to restore kinetics and kinematics in the other degrees of freedom. Though ATT increased at 15° flexion, ACLRs were able to restore ensemble mean ATT values to ACL-intact levels at 0°, 30°, 60°, and 90° of passive flexion under combined torsional loading (Figure 3). However, under ATF, ACLRs reduced ATT relative to the ACLD condition, but did not restore ATT to the ACL-intact condition (Figure 2). Under constant quadriceps loading, ACLRs produced significantly lower ligament forces and greater ATT when compared to ACL-intact knees at flexion below 60° (Figure 5). Thus, the hypothesis was supported under combined torsional loading, but rejected under ATF loading. Mean ITR following ACLR was comparable to intact kinematics during combined torsional loading, but over constrained during ATF and quadriceps loading (Figures 2, 3, and 6). This again provided mixed support of the hypothesis based on the loading condition. Over-constrained knee kinematics were also present in simulated gait as ACLR grafts became primary loading restraints in two additional DOFs and exhibited larger medial forces compared to the intact ACL.[76] In the remaining 5-DOFs, ACLRs exhibited an inconsistent ability to restore ACL-intact mechanics throughout a gait cycle. Compared to the intact knee, bone-patellar-tendon-bone ACL grafts restored anterior force at heel strike and mid-stance, but expressed greater force at toe-off; matched the medial and compressive forces at mid-stance, but exhibited greater forces at heel strike and toe-off; restored the flexion and internal rotation moments of the intact knee at all points during gait; and only restored abduction moments at mid-stance. With the exception of internal rotation, forces from bone-patellar-tendon-bone ACLRs were significantly increased in all DOFs at all points in the gait cycle relative to the ACLD condition.[76] Again, the inconsistency of these graft loading patterns both supported and rejected the hypothesis. It has been well documented that ACL injuries leads to early onset osteoarthritis, [4, 92] and ACLRs do not appear to greatly improve the long term prospectus for knee injuries as 75% of athletes still complain of knee degradation affecting their quality of life less than 15 years post surgery.[92, 93] It is possible that the inconsistent ability of the ACLR to restore intact kinematics across multiple loading scenarios may alter the normal mechanical conditions across the articulating surfaces of the knee and lead to joint degeneration.
Limitations to this systematic review include that it did not account for confounding variables that can impact the mechanical integrity of ACLRs. Variability in attributes such as anatomic versus non-anatomic tunnel placement, [94, 95] double versus single bundle grafts, [44] graft materials, [76] number of tunnels, [28] graft fixation method, [46] graft tension, [29] and graft length[96] has been shown to alter the mechanical response of an ACLR and could alter the ensemble mean. Potentially confounding factors within ACLR grafts were not accounted for in the exclusionary criteria because opinions on optimal surgical technique varies between orthopaedic surgeons, which leads to graft variability in ACLR populations that should not be artificially controlled in a meta-analysis. An additional limitation to the evaluation of ACLRs in in vitro specimens is that the grafts are being evaluated for integrity at the time of surgery. In vivo, grafts are provided time to heal, experience bony ingrowth, and potentially restructure fiber orientation relative to mechanical environment prior to return to sport. Due to the nature of in vitro specimens, natural processes cannot be reproduced, which may alter graft response to mechanical stimuli.[96]
For passive flexion simulations, this investigation was limited to Lachman’s tests of 134 N ATF, which corresponds to a 30-pound KT1000 test, and pivot-shift tests of 10 N*m valgus and 4–5 N*m internal torque. These loading magnitudes were restricted to constant values to improve comparability between results. Altered magnitudes in external loading could have confounded or biased the ensemble averages through increased variability. However, biomechanical tendencies in investigations with different loading magnitudes were often similar as ACLD increased ATT relative to ACL-intact knees throughout flexion under each Lachman’s and pivot-shift loads;[32, 62, 64, 66, 69, 96–101] ACLR restored ATT relative to ACLD under Lachman’s loads, but not necessarily to the level of ACL-intact knees;[62, 96, 99, 101] ACLR restored ITR relative to ACL-intact knees under rotational loads;[31, 41, 62] and ACLR graft forces were not consistent with ACL-intact ligament forces.[63–65, 99, 101] Therefore, normalized mechanical response of ACL, ACLD, and ACLR specimens generally remains constant across varied magnitudes of external loading, though absolute values may differ. Another limitation that was faced by this study was the definition of coordinate systems at the knee. The joint coordinate system, which was the system that was most commonly applied in the literature, does not match the anatomical axis of the joint. The use of different coordinate systems to analyze in vivo joint mechanics has been shown to alter the magnitude and rank order of joint kinetics.[102] Accordingly, it can be somewhat difficult to directly compare absolute magnitude results from investigations that employ separate coordinate systems.
Future studies should be used to explore the development of more dynamic motion pathways through robotically simulated motions. ACL failures are most commonly associated with the jump-landing and side-step cutting activities in basketball and soccer, not gait or clinical motions presented in robotic simulations.[103, 104] A combination of rigorous motion activities, such as the landing impact simulated in mechanical testing, combined with the precision of robotic manipulation should produce a wealth of biomechanical data that could be utilized to design more efficacious ACL injury prevention protocols and ACLR graft constructs. However, robotic systems require 6-DOF kinematic input to reproduce a motion and, though they are relatively accurate rotationally, 3D motion capture systems introduce large errors in the translational DOFs due to skin artifacts.[105, 106] In order to appropriately simulate dynamic, in vivo activities, investigators will either need to capture motion with bone-based markers, as was done in the development of gait simulations, [19, 34] or address the kinematic errors generated by skin-based markers.
5. CONCLUSIONS
Investigators have used in vitro simulations of knee motion to attribute ATT as the primary mechanical antagonist to the ACL and indicate that combined torsional loading has a greater biomechanical influence than uniaxial moments. Abduction rotation was found to have a greater mechanical influence in robotic simulations, while ITR may have had a greater influence during the mechanical-impact simulations reviewed in this study. Limitations were identified in both methodologies, but greater concerns were raised with the ability of impact simulations to accurately recreate physiologic motions and in vivo data currently better supports the behaviors observed during robotically-driven simulations. Robotic simulations have been used to discover that ACLRs constrain ACLD knees, but their ability to restore ACL-intact mechanics is dependent on the DOF observed and loading conditions applied.
Acknowledgments
This work was supported by NIH grants R01-AR049735, R01-AR055563, R01-AR056259 and R03-057551. The authors thank the clinical research staffs at The Ohio State University’s Sports Health and Performance Institute and the Cincinnati Children’s Hospital Sports Medicine Biodynamics Center.
Footnotes
CONFLICT OF INTEREST
There were no conflicts of interest in the preparation of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Renstrom PA. Eight clinical conundrums relating to anterior cruciate ligament (ACL) injury in sport: recent evidence and a personal reflection. Br J Sports Med. 2013 Apr;47(6):367–72. doi: 10.1136/bjsports-2012-091623. [DOI] [PubMed] [Google Scholar]
- 2.Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am J Sports Med. 1999 Nov-Dec;27(6):699–706. doi: 10.1177/03635465990270060301. [DOI] [PubMed] [Google Scholar]
- 3.Beynnon BD, Johnson RJ, Fleming BC, Kannus P, Kaplan M, Samani J, et al. Anterior cruciate ligament replacement: comparison of bone-patellar tendon-bone grafts with two-strand hamstring grafts. A prospective, randomized study. Journal of Bone and Joint Surgery. 2002;84(9):1503–13. doi: 10.2106/00004623-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007 Oct;35(10):1756–69. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 5.Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009 Nov;12(6):622–7. doi: 10.1016/j.jsams.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Sugimoto D, Myer GD, McKeon JM, Hewett TE. Evaluation of the effectiveness of neuromuscular training to reduce anterior cruciate ligament injury in female athletes: a critical review of relative risk reduction and numbers-needed-to-treat analyses. Br J Sports Med. 2012 Nov;46(14):979–88. doi: 10.1136/bjsports-2011-090895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin LY, Agel J, Albohm MJ, Arendt EA, Dick RW, Garrett WE, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000 May-Jun;8(3):141–50. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Griffin LY, Albohm MJ, Arendt EA, Bahr R, Beynnon BD, Demaio M, et al. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006 Sep;34(9):1512–32. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 9.Pappas E, Zampeli F, Xergia SA, Georgoulis AD. Lessons learned from the last 20 years of ACL-related in vivo-biomechanics research of the knee joint. Knee Surg Sports Traumatol Arthrosc. 2013 Apr;21(4):755–66. doi: 10.1007/s00167-012-1955-0. [DOI] [PubMed] [Google Scholar]
- 10.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J Bone Joint Surg Am. 1980 Mar;62(2):259–70. [PubMed] [Google Scholar]
- 11.Meyer EG, Haut RC. Anterior cruciate ligament injury induced by internal tibial torsion or tibiofemoral compression. J Biomech. 2008 Dec 5;41(16):3377–83. doi: 10.1016/j.jbiomech.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Renstrom P, Arms SW, Stanwyck TS, Johnson RJ, Pope MH. Strain within the anterior cruciate ligament during hamstring and quadriceps activity. Am J Sports Med. 1986 Jan-Feb;14(1):83–7. doi: 10.1177/036354658601400114. [DOI] [PubMed] [Google Scholar]
- 13.Butler DL, Goldstein SA, Guilak F. Functional Tissue Engineering: The Role of Biomechanics. J Biomech Eng. 2000;122(6):570–5. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 14.Lo J, Muller O, Wunschel M, Bauer S, Wulker N. Forces in anterior cruciate ligament during simulated weight-bearing flexion with anterior and internal rotational tibial load. J Biomech. 2008;41(9):1855–61. doi: 10.1016/j.jbiomech.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Fujie H, Livesay GA, Woo SL-Y, Kashiwaguchi S, Blomstrom G. The use of a universal force moment sensor to determine in situ forces in ligaments: A new methodology. J Biomech Eng. 1995;117(1):1–7. doi: 10.1115/1.2792266. [DOI] [PubMed] [Google Scholar]
- 16.Fujie H, Mabuchi K, Woo SL, Livesay GA, Arai S, Tsukamoto Y. The use of robotics technology to study human joint kinematics: A new methodology. J Biomech Eng. 1993;115(3):211–7. doi: 10.1115/1.2895477. [DOI] [PubMed] [Google Scholar]
- 17.Rudy TW, Livesay GA, Woo SL, Fu FH. A combined robotic/universal force sensor approach to determine in situ forces of knee ligaments. J Biomech. 1996;29(10):1357–60. doi: 10.1016/0021-9290(96)00056-5. [DOI] [PubMed] [Google Scholar]
- 18.Cassidy K. Combined In Vivo/In Vitro Method to Study Anteriomedial Bundle Strain in the Anterior Cruciate Ligament Using a Dynamic Knee Simulator. J Biomech Eng. 2013;135(3):035001. doi: 10.1115/1.4023520. [DOI] [PubMed] [Google Scholar]
- 19.Howard RA, Rosvold JM, Darcy SP, Corr DT, Shrive NG, Tapper JE, et al. Reproduction of in vivo motion using a parallel robot. J Biomech Eng. 2007 Oct;129(5):743–9. doi: 10.1115/1.2768983. [DOI] [PubMed] [Google Scholar]
- 20.Boguszewski DV, Shearn JT, Wagner CT, Butler DL. Investigating the effects of anterior tibial translation on anterior knee force in the porcine model: is the porcine knee ACL dependent? J Orthop Res. 2011 May;29(5):641–6. doi: 10.1002/jor.21298. [DOI] [PubMed] [Google Scholar]
- 21.Hashemi J, Chandrashekar N, Jang T, Karpat F, Oseto M, Ekwaro-Osire S. An Alternative Mechanism of Non-contact Anterior Cruciate Ligament Injury During Jump-landing: In-vitro Simulation. Experimental Mechanics. 2007;47(3):347–54. [Google Scholar]
- 22.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The relationship between quadriceps muscle force, knee flexion, and anterior cruciate ligament strain in an in vitro simulated jump landing. Am J Sports Med. 2006 Feb;34(2):269–74. doi: 10.1177/0363546505280906. [DOI] [PubMed] [Google Scholar]
- 23.Murray MM. Current status and potential of primary ACL repair. Clin Sports Med. 2009 Jan;28(1):51–61. doi: 10.1016/j.csm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007 Oct;26(4):661–81. doi: 10.1016/j.csm.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Sernert N, Kartus J, Kohler K, Stener S, Larsson J, Eriksson BI, et al. Analysis of subjective, objective and functional examination tests after anterior cruciate ligament reconstruction. A follow-up of 527 patients. Knee Surg Sports Traumatol Arthrosc. 1999;7(3):160–5. doi: 10.1007/s001670050141. [DOI] [PubMed] [Google Scholar]
- 26.Paterno MV, Schmitt LC, Ford KR, Rauh MJ, Myer GD, Huang B, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010 Oct;38(10):1968–78. doi: 10.1177/0363546510376053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G, Zayontz S, Most E, DeFrate LE, Suggs JF, Rubash HE. In situ forces of the anterior cruciate and posterior cruciate ligaments in high knee flexion: an in vitro investigation. J Orthop Res. 2004;22:293–7. doi: 10.1016/S0736-0266(03)00179-7. [DOI] [PubMed] [Google Scholar]
- 28.Petersen W, Tretow H, Weimann A, Herbort M, Fu FH, Raschke M, et al. Biomechanical evaluation of two techniques for double-bundle anterior cruciate ligament reconstruction: one tibial tunnel versus two tibial tunnels. Am J Sports Med. 2007 Feb;35(2):228–34. doi: 10.1177/0363546506294468. [DOI] [PubMed] [Google Scholar]
- 29.Mae T, Shino K, Nakata K, Toritsuka Y, Otsubo H, Fujie H. Optimization of graft fixation at the time of anterior cruciate ligament reconstruction. Part I: effect of initial tension. Am J Sports Med. 2008 Jun;36(6):1087–93. doi: 10.1177/0363546508314433. [DOI] [PubMed] [Google Scholar]
- 30.Mae T, Shino K, Nakata K, Toritsuka Y, Otsubo H, Fujie H. Optimization of graft fixation at the time of anterior cruciate ligament reconstruction. Part II: effect of knee flexion angle. Am J Sports Med. 2008 Jun;36(6):1094–100. doi: 10.1177/0363546508317412. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy MM, Tucker S, Nguyen JT, Green DW, Imhauser CW, Cordasco FA. Contact Stress and Kinematic Analysis of All-Epiphyseal and Over-the-Top Pediatric Reconstruction Techniques for the Anterior Cruciate Ligament. Am J Sports Med. 2013 Jun;41(6):1330–9. doi: 10.1177/0363546513483269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Battaglia MJ, 2nd, Lenhoff MW, Ehteshami JR, Lyman S, Provencher MT, Wickiewicz TL, et al. Medial collateral ligament injuries and subsequent load on the anterior cruciate ligament: a biomechanical evaluation in a cadaveric model. Am J Sports Med. 2009 Feb;37(2):305–11. doi: 10.1177/0363546508324969. [DOI] [PubMed] [Google Scholar]
- 33.Wunschel M, Wulker N, Muller O. Gender differences in tibio-femoral kinematics and quadriceps muscle force during weight-bearing knee flexion in vitro. Knee Surg Sports Traumatol Arthrosc. 2012 Jun 14; doi: 10.1007/s00167-012-2082-7. [DOI] [PubMed] [Google Scholar]
- 34.Herfat ST, Boguszewski DV, Shearn JT. Applying simulated in vivo motions to measure human knee and ACL kinetics. Ann Biomed Eng. 2012 Jul;40(7):1545–53. doi: 10.1007/s10439-011-0500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh YK, Kreinbrink JL, Ashton-Miller JA, Wojtys EM. Effect of ACL Transection on Internal Tibial Rotation in an in Vitro Simulated Pivot Landing. J Bone Joint Surg Am. 2011 Feb;93(4):372–80. doi: 10.2106/JBJS.J.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giffin JR. Effects of Increasing Tibial Slope on the Biomechanics of the Knee. Am J Sports Med. 2004;32(2):376–82. doi: 10.1177/0363546503258880. [DOI] [PubMed] [Google Scholar]
- 37.Gabriel MT, Wong EK, Woo SLY, Yagi M, Debski RE. Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. J Orthop Res. 2004;22(1):85–9. doi: 10.1016/S0736-0266(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto Y. Knee Stability and Graft Function After Anterior Cruciate Ligament Reconstruction: A Comparison of a Lateral and an Anatomical Femoral Tunnel Placement. Am J Sports Med. 2004;32(8):1825–32. doi: 10.1177/0363546504263947. [DOI] [PubMed] [Google Scholar]
- 39.Yoo JD. The Effect of Anterior Cruciate Ligament Reconstruction on Knee Joint Kinematics Under Simulated Muscle Loads. Am J Sports Med. 2005;33(2):240–6. doi: 10.1177/0363546504267806. [DOI] [PubMed] [Google Scholar]
- 40.Li G, Papannagari R, DeFrate LE, Yoo JD, Park SE, Gill TJ. The effects of ACL deficiency on mediolateral translation and varus-valgus rotation. Acta Orthop. 2007 Jun;78(3):355–60. doi: 10.1080/17453670710013924. [DOI] [PubMed] [Google Scholar]
- 41.Gadikota HR, Seon JK, Kozanek M, Oh LS, Gill TJ, Montgomery KD, et al. Biomechanical comparison of single-tunnel-double-bundle and single-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2009 May;37(5):962–9. doi: 10.1177/0363546508330145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gadikota HR, Wu JL, Seon JK, Sutton K, Gill TJ, Li G. Single-tunnel double-bundle anterior cruciate ligament reconstruction with anatomical placement of hamstring tendon graft: can it restore normal knee joint kinematics? Am J Sports Med. 2010 Apr;38(4):713–20. doi: 10.1177/0363546509353406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seon JK, Gadikota HR, Kozanek M, Oh LS, Gill TJ, Li G. The effect of anterior cruciate ligament reconstruction on kinematics of the knee with combined anterior cruciate ligament injury and subtotal medial meniscectomy: an in vitro robotic investigation. Arthroscopy. 2009 Feb;25(2):123–30. doi: 10.1016/j.arthro.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seon JK, Gadikota HR, Wu JL, Sutton K, Gill TJ, Li G. Comparison of single- and double-bundle anterior cruciate ligament reconstructions in restoration of knee kinematics and anterior cruciate ligament forces. Am J Sports Med. 2010 Jul;38(7):1359–67. doi: 10.1177/0363546510361494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kilger RH, Stehle J, Fisk JA, Thomas M, Miura K, Woo SL. Anatomical double-bundle anterior cruciate ligament reconstruction after valgus high tibial osteotomy: a biomechanical study. Am J Sports Med. 2006 Jun;34(6):961–7. doi: 10.1177/0363546505283269. [DOI] [PubMed] [Google Scholar]
- 46.Kilger RH, Thomas M, Hanford S, Alaseirlis DA, Paessler HH, Woo SL. The effectiveness of reconstruction of the anterior cruciate ligament using the novel knot/press-fit technique: a cadaveric study. Am J Sports Med. 2005 Jun;33(6):856–63. doi: 10.1177/0363546504271745. [DOI] [PubMed] [Google Scholar]
- 47.Musahl V, Plakseychuk A, VanScyoc A, Sasaki T, Debski RE, McMahon PJ, et al. Varying femoral tunnels between the anatomical footprint and isometric positions: effect on kinematics of the anterior cruciate ligament-reconstructed knee. Am J Sports Med. 2005 May;33(5):712–8. doi: 10.1177/0363546504271747. [DOI] [PubMed] [Google Scholar]
- 48.Miura K, Woo SL, Brinkley R, Fu YC, Noorani S. Effects of knee flexion angles for graft fixation on force distribution in double-bundle anterior cruciate ligament grafts. Am J Sports Med. 2006 Apr;34(4):577–85. doi: 10.1177/0363546505281814. [DOI] [PubMed] [Google Scholar]
- 49.Hsu WH, Fisk JA, Yamamoto Y, Debski RE, Woo SL. Differences in torsional joint stiffness of the knee between genders: a human cadaveric study. Am J Sports Med. 2006 May;34(5):765–70. doi: 10.1177/0363546505282623. [DOI] [PubMed] [Google Scholar]
- 50.Zantop T, Herbort M, Raschke MJ, Fu FH, Petersen W. The role of the anteromedial and posterolateral bundles of the anterior cruciate ligament in anterior tibial translation and internal rotation. Am J Sports Med. 2007 Feb;35(2):223–7. doi: 10.1177/0363546506294571. [DOI] [PubMed] [Google Scholar]
- 51.Zantop T, Schumacher T, Diermann N, Schanz S, Raschke MJ, Petersen W. Anterolateral rotational knee instability: role of posterolateral structures. Winner of the AGA-DonJoy Award 2006. Arch Orthop Trauma Surg. 2007 Nov;127(9):743–52. doi: 10.1007/s00402-006-0241-3. [DOI] [PubMed] [Google Scholar]
- 52.Zantop T, Schumacher T, Schanz S, Raschke M, Petersen W. Double-bundle reconstruction cannot restore intact knee kinematics in the ACL/LCL-deficient knee. Arch Orthop Trauma Surg. 2010;130:1019–26. doi: 10.1007/s00402-010-1081-8. [DOI] [PubMed] [Google Scholar]
- 53.Zantop T, Diermann N, Schumacher T, Schanz S, Fu FH, Petersen W. Anatomical and nonanatomical double-bundle anterior cruciate ligament reconstruction: importance of femoral tunnel location on knee kinematics. Am J Sports Med. 2008 Apr;36(4):678–85. doi: 10.1177/0363546508314414. [DOI] [PubMed] [Google Scholar]
- 54.Diermann N, Schumacher T, Schanz S, Raschke MJ, Petersen W, Zantop T. Rotational instability of the knee: internal tibial rotation under a simulated pivot shift test. Arch Orthop Trauma Surg. 2009 Mar;129(3):353–8. doi: 10.1007/s00402-008-0681-z. [DOI] [PubMed] [Google Scholar]
- 55.Wu C, Noorani S, Vercillo F, Woo SL. Tension patterns of the anteromedial and posterolateral grafts in a double-bundle anterior cruciate ligament reconstruction. J Orthop Res. 2009 Jul;27(7):879–84. doi: 10.1002/jor.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herbort M, Lenschow S, Fu FH, Petersen W, Zantop T. ACL mismatch reconstructions: influence of different tunnel placement strategies in single-bundle ACL reconstructions on the knee kinematics. Knee Surg Sports Traumatol Arthrosc. 2010 Nov;18(11):1551–8. doi: 10.1007/s00167-010-1163-8. [DOI] [PubMed] [Google Scholar]
- 57.Zamarra G, Fisher MB, Woo SL, Cerulli G. Biomechanical evaluation of using one hamstrings tendon for ACL reconstruction: a human cadaveric study. Knee Surg Sports Traumatol Arthrosc. 2010 Jan;18(1):11–9. doi: 10.1007/s00167-009-0911-0. [DOI] [PubMed] [Google Scholar]
- 58.Sim JA, Gadikota HR, Li JS, Li G, Gill TJ. Biomechanical evaluation of knee joint laxities and graft forces after anterior cruciate ligament reconstruction by anteromedial portal, outside-in, and transtibial techniques. Am J Sports Med. 2011 Dec;39(12):2604–10. doi: 10.1177/0363546511420810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imhauser C, Mauro C, Choi D, Rosenberg E, Mathew S, Nguyen J, et al. Abnormal tibiofemoral contact stress and its association with altered kinematics after center-center anterior cruciate ligament reconstruction: an in vitro study. Am J Sports Med. 2013 Apr;41(4):815–25. doi: 10.1177/0363546512475205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto Y, Hsu WH, Fisk JA, Van Scyoc AH, Miura K, Woo SL. Effect of the iliotibial band on knee biomechanics during a simulated pivot shift test. J Orthop Res. 2006 May;24(5):967–73. doi: 10.1002/jor.20122. [DOI] [PubMed] [Google Scholar]
- 61.Li G, Papannagari R, DeFrate LE, Yoo JD, Park SE, Gill TJ. Comparison of the ACL and ACL graft forces before and after ACL reconstruction: an in-vitro robotic investigation. Acta Orthop. 2006 Apr;77(2):267–74. doi: 10.1080/17453670610046019. [DOI] [PubMed] [Google Scholar]
- 62.Keklikci K, Yapici C, Kim D, Linde-Rosen M, Smolinski P, Fu FH. The effect of notchplasty in anterior cruciate ligament reconstruction: a biomechanical study in the porcine knee. Knee Surg Sports Traumatol Arthrosc. 2012 Dec 16; doi: 10.1007/s00167-012-2343-5. [DOI] [PubMed] [Google Scholar]
- 63.Kato Y, Maeyama A, Lertwanich P, Wang JH, Ingham SJ, Kramer S, et al. Biomechanical comparison of different graft positions for single-bundle anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2013 Apr;21(4):816–23. doi: 10.1007/s00167-012-1951-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lertwanich P, Kato Y, Martins CA, Maeyama A, Ingham SJ, Kramer S, et al. A Biomechanical Comparison of 2 Femoral Fixation Techniques for Anterior Cruciate Ligament Reconstruction in Skeletally Immature Patients: Over-the-Top Fixation Versus Transphyseal Technique. Arthroscopy. 2011 May;27(5):672–80. doi: 10.1016/j.arthro.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Xu Y, Liu J, Kramer S, Martins C, Kato Y, Linde-Rosen M, et al. Comparison of in situ forces and knee kinematics in anteromedial and high anteromedial bundle augmentation for partially ruptured anterior cruciate ligament. Am J Sports Med. 2011 Feb;39(2):272–8. doi: 10.1177/0363546510383479. [DOI] [PubMed] [Google Scholar]
- 66.Kato Y, Ingham SJ, Maeyama A, Lertwanich P, Wang JH, Mifune Y, et al. Biomechanics of the human triple-bundle anterior cruciate ligament. Arthroscopy. 2012 Feb;28(2):247–54. doi: 10.1016/j.arthro.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 67.Gadikota HR, Seon JK, Wu JL, Gill TJ, Li G. The effect of isolated popliteus tendon complex injury on graft force in anterior cruciate ligament reconstructed knees. Int Orthop. 2011 Sep;35(9):1403–8. doi: 10.1007/s00264-010-1118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Darcy SP, Kilger RH, Woo SL, Debski RE. Estimation of ACL forces by reproducing knee kinematics between sets of knees: A novel non-invasive methodology. J Biomech. 2006;39(13):2371–7. doi: 10.1016/j.jbiomech.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 69.Fisher MB, Jung HJ, McMahon PJ, Woo SL. Evaluation of bone tunnel placement for suture augmentation of an injured anterior cruciate ligament: effects on joint stability in a goat model. J Orthop Res. 2010 Oct;28(10):1373–9. doi: 10.1002/jor.21141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fisher MB, Jung HJ, McMahon PJ, Woo SL. Suture augmentation following ACL injury to restore the function of the ACL, MCL, and medial meniscus in the goat stifle joint. J Biomech. 2011 May 17;44(8):1530–5. doi: 10.1016/j.jbiomech.2011.02.141. [DOI] [PubMed] [Google Scholar]
- 71.Hosseini A, Gill TJ, Van de Velde SK, Li G. Estimation of in vivo ACL force changes in response to increased weightbearing. J Biomech Eng. 2011 May;133(5):051004. doi: 10.1115/1.4003780. [DOI] [PubMed] [Google Scholar]
- 72.Muller O, Lo J, Wunschel M, Obloh C, Wulker N. Simulation of force loaded knee movement in a newly developed in vitro knee simulator. Biomed Tech (Berl) 2009 Jun;54(3):142–9. doi: 10.1515/BMT.2009.015. [DOI] [PubMed] [Google Scholar]
- 73.Wunschel M, Muller O, Lo J, Obloh C, Wulker N. The anterior cruciate ligament provides resistance to externally applied anterior tibial force but not to internal rotational torque during simulated weight-bearing flexion. Arthroscopy. 2010 Nov;26(11):1520–7. doi: 10.1016/j.arthro.2010.04.069. [DOI] [PubMed] [Google Scholar]
- 74.Wunschel M, Treffler F, Ketelsen D, Lo J, Muller O, Suckel A. An implant-free double-bundle reconstruction of the anterior cruciate ligament: operative technique and influence on tibiofemoral kinematics. Clin Biomech. 2011 Aug;26(7):754–9. doi: 10.1016/j.clinbiomech.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 75.Atarod M, Rosvold JM, Frank CB, Shrive NG. Functional Activity of the Anterior and Posterior Cruciate Ligaments Under In Vivo Gait and Static Physiological Loads. Ann Biomed Eng. 2013 doi: 10.1007/s10439-013-0826-2. in-press. [DOI] [PubMed] [Google Scholar]
- 76.Boguszewski DV. PhD dissertation. Cincinnati, OH, USA: University of Cincinnati; 2012. Characterizing the porcine knee as a biomechanical surrogate model of the human knee to study the anterior cruciate ligament. [Google Scholar]
- 77.Atarod Pilambaraei M, O’Brien EJ, Frank CB, Shrive NG. There is significant load sharing and physical interaction between the anteromedial and posterolateral bundles of the ovine ACL under anterior tibial loads. Knee. 2012 Dec;19(6):797–803. doi: 10.1016/j.knee.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 78.Herfat ST, Boguszewski DV, Nesbitt RJ, Shearn JT. Effect of perturbing a simulated motion on knee and anterior cruciate ligament kinetics. J Biomech Eng. 2012 Oct;134(10):104504. doi: 10.1115/1.4007626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hashemi J, Breighner R, Jang TH, Chandrashekar N, Ekwaro-Osire S, Slauterbeck JR. Increasing pre-activation of the quadriceps muscle protects the anterior cruciate ligament during the landing phase of a jump: an in vitro simulation. Knee. 2010 Jun;17(3):235–41. doi: 10.1016/j.knee.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 80.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The effect of an impulsive knee valgus moment on in vitro relative ACL strain during a simulated jump landing. Clin Biomech. 2006 Nov;21(9):977–83. doi: 10.1016/j.clinbiomech.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 81.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. Effect of varying hamstring tension on anterior cruciate ligament strain during in vitro impulsive knee flexion and compression loading. J Bone Joint Surg Am. 2008 Apr;90(4):815–23. doi: 10.2106/JBJS.F.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oh YK, Kreinbrink JL, Wojtys EM, Ashton-Miller JA. Effect of axial tibial torque direction on ACL relative strain and strain rate in an in vitro simulated pivot landing. J Orthop Res. 2012 Apr;30(4):528–34. doi: 10.1002/jor.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oh YK, Lipps DB, Ashton-Miller JA, Wojtys EM. What strains the anterior cruciate ligament during a pivot landing? Am J Sports Med. 2012 Mar;40(3):574–83. doi: 10.1177/0363546511432544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hewett TE, Myer GD, Ford KR, Heidt RS, Jr, Colosimo AJ, McLean SG, et al. Biomechanical Measures of Neuromuscular Control and Valgus Loading of the Knee Predict Anterior Cruciate Ligament Injury Risk in Female Athletes: A Prospective Study. Am J Sports Med. 2005 Feb 8;33(4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 85.Quatman CE, Hewett TE. The anterior cruciate ligament injury controversy: is “valgus collapse” a sex-specific mechanism? Br J Sports Med. 2009 May;43(5):328–35. doi: 10.1136/bjsm.2009.059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ford KR, Myer GD, Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Med Sci Sports Exerc. 2003 Oct;35(10):1745–50. doi: 10.1249/01.MSS.0000089346.85744.D9. [DOI] [PubMed] [Google Scholar]
- 87.Stergiou N, Decker LM. Human movement variability, nonlinear dynamics, and pathology: is there a connection? Hum Mov Sci. 2011 Oct;30(5):869–88. doi: 10.1016/j.humov.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Darcy SP, Rosvold JM, Beveridge JE, Corr DT, Brown JJ, Sutherland CA, et al. A comparison of passive flexion-extension to normal gait in the ovine stifle joint. J Biomech. 2008;41(4):854–60. doi: 10.1016/j.jbiomech.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 89.Kar J, Quesada PM. A musculoskeletal modeling approach for estimating anterior cruciate ligament strains and knee anterior-posterior shear forces in stop-jumps performed by young recreational female athletes. Ann Biomed Eng. 2013 Feb;41(2):338–48. doi: 10.1007/s10439-012-0644-y. [DOI] [PubMed] [Google Scholar]
- 90.Kar J, Quesada PM. A numerical simulation approach to studying anterior cruciate ligament strains and internal forces among young recreational women performing valgus inducing stop-jump activities. Ann Biomed Eng. 2012 Aug;40(8):1679–91. doi: 10.1007/s10439-012-0572-x. [DOI] [PubMed] [Google Scholar]
- 91.Bates NA, Ford KR, Myer GD, Hewett TE. Between Landing Kinetic and Kinematic Differences in a Drop Vertical Jump. Submitted for Presentation to the American Society of Biomechanics Annual Meeting; 2012; Gainesville, Florida. [Google Scholar]
- 92.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004 Oct;50(10):3145–52. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 93.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004 Mar;63(3):269–73. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iriuchishima T, Tajima G, Ingham SJ, Shen W, Horaguchi T, Saito A, et al. Intercondylar roof impingement pressure after anterior cruciate ligament reconstruction in a porcine model. Knee Surg Sports Traumatol Arthrosc. 2009 Jun;17(6):590–4. doi: 10.1007/s00167-008-0691-y. [DOI] [PubMed] [Google Scholar]
- 95.Iriuchishima T, Tajima G, Ingham SJ, Shirakura K, Fu FH. PCL to graft impingement pressure after anatomical or non-anatomical single-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2012 May;20(5):964–9. doi: 10.1007/s00167-011-1680-0. [DOI] [PubMed] [Google Scholar]
- 96.Zantop T, Ferretti M, Bell KM, Brucker PU, Gilbertson L, Fu FH. Effect of tunnel-graft length on the biomechanics of anterior cruciate ligament-reconstructed knees: intra-articular study in a goat model. Am J Sports Med. 2008 Nov;36(11):2158–66. doi: 10.1177/0363546508320572. [DOI] [PubMed] [Google Scholar]
- 97.Tischer T, Ronga M, Tsai A, Ingham SJ, Ekdahl M, Smolinski P, et al. Biomechanics of the goat three bundle anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2009 Aug;17(8):935–40. doi: 10.1007/s00167-009-0784-2. [DOI] [PubMed] [Google Scholar]
- 98.Fujie H, Otsubo H, Fukano S, Suzuki T, Suzuki D, Mae T, et al. Mechanical functions of the three bundles consisting of the human anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2011 May 4; doi: 10.1007/s00167-011-1513-1. [DOI] [PubMed] [Google Scholar]
- 99.Kato Y, Ingham SJ, Kramer S, Smolinski P, Saito A, Fu FH. Effect of tunnel position for anatomic single-bundle ACL reconstruction on knee biomechanics in a porcine model. Knee Surg Sports Traumatol Arthrosc. 2010 Jan;18(1):2–10. doi: 10.1007/s00167-009-0916-8. [DOI] [PubMed] [Google Scholar]
- 100.Kato Y, Ingham SJ, Linde-Rosen M, Smolinski P, Horaguchi T, Fu FH. Biomechanics of the porcine triple bundle anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2010 Jan;18(1):20–5. doi: 10.1007/s00167-009-0893-y. [DOI] [PubMed] [Google Scholar]
- 101.Debandi A, Maeyama A, Lu S, Hume C, Asai S, Goto B, et al. Biomechanical comparison of three anatomic ACL reconstructions in a porcine model. Knee Surg Sports Traumatol Arthrosc. 2011 May;19(5):728–35. doi: 10.1007/s00167-010-1338-3. [DOI] [PubMed] [Google Scholar]
- 102.Kristianslund E, Krosshaug T, Mok KM, McLean S, van den Bogert AJ. Expressing the joint moments of drop jumps and sidestep cutting in different reference frames--does it matter? J Biomech. 2014 Jan 3;47(1):193–9. doi: 10.1016/j.jbiomech.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 103.Powell JW, Barber-Foss KD. Sex-related injury patterns among selected high school sports. Am J Sports Med. 2000;28(3):385–91. doi: 10.1177/03635465000280031801. [DOI] [PubMed] [Google Scholar]
- 104.Boden BP, Dean GS, Feagin JA, Garrett WE. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–8. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 105.Benoit DL, Ramsey DK, Lamontagne M, Xu L, Wretenberg P, Renstrom P. Effect of skin movement artifact on knee kinematics during gait and cutting motions measured in vivo. Gait Posture. 2006 Oct;24(2):152–64. doi: 10.1016/j.gaitpost.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 106.Miranda DL, Rainbow MJ, Crisco JJ, Fleming BC. Kinematic differences between optical motion capture and biplanar videoradiography during a jump-cut maneuver. J Biomech. 2013 Feb 1;46(3):567–73. doi: 10.1016/j.jbiomech.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]