Abstract
Recombinant T-cell Receptor Ligand 1000 (RTL1000), a partial human major histocompatibility complex (MHC) molecule coupled to a human myelin peptide, reduces infarct size after experimental stroke in HLA-DRB1*1502 transgenic (DR2-Tg) mice. In this study, we characterized the therapeutic time window of opportunity for RTL1000; we explored the efficacy of single dose of RTL1000 administration and determined if RTL1000 affordslong-term neurobehavioral functional improvement after ischemic stroke. Male DR2-Tg mice underwent 60 min of intraluminal reversible middle cerebral artery occlusion (MCAO). RTL1000 or vehicle was injected 4, 6 or 8 h after MCAO, followed by 3 daily injections. In single dose study, one-time injection of RTL1000 was applied 4 h after MCAO. Cortical, striatal and hemispheric infarct sizes were measured 24 h or 96 h after stroke. Behavioral testing, including neuroscore evaluation, open field, paw preference and novel object recognition was performed up to 28 days after stroke. Our data showed RTL1000 significantly reduced infarct size 96 h after MCAO when first injection was given 4 and 6, but not 8 h after the onset of stroke. A single dose of 400 µg or 100 µg RTL1000 also significantly reduced infarct size 24 h after MCAO. Behavioral testing showed RTL1000 treatment used 4 h after MCAO improved long-term cognitive outcome 28 days after stroke. Taken together, RTL1000 protects against acute injury if applied within a 6-h time window and improves long-term functional recovery after experimental stroke in DR2-Tg mice.
Keywords: Ischemicstroke, Neurobehavioral evaluation, Immunotherapy, Recombinant T-cell receptor Ligand, HLA-DR2transgenic mice
Introduction
Ischemic stroke induces activation of the peripheral immune system and recruitment of inflammatory cells, including neutrophils, macrophages and lymphocytes [1–4]. These immune cells infiltrate the brain and exacerbate the developing infarction. T-cell and B-cell deficient animals have reduced infarct size, and modulation of the inflammatory response improves outcomes in experimental ischemic stroke [5–6]. However, the inflammatory response to cerebral ischemia is biphasic, characterized by an initial activation phase during the first 6–22 h [7], and followed by an immunosuppressive phase characterized by pronounced atrophy of spleen and thymus 96 h after stroke [8–9]. Therefore, non-specific immune-suppressive therapy could worsen outcome by increasing risk of fatal infection [10].
Recombinant T-cell receptor ligands (RTLs) are a class of partial major histocompatibility complex (MHC) class II molecules comprised of covalently linked α1 and β1 chains that are tethered to antigenic peptides which selectively modulate auto-aggressive CD4+ T cells by delivering partial agonist signals through the T cell receptor (TCR) [11–13]. CD4+ cells are believed to play a key role in the pathogenesis of stroke through the release of pro-inflammatory cytokines, including IL-2, IL-12, IFN-γ and TNF-α. Modulation of RTL on CD4+ T cells inhibits the accumulation of inflammatory cells, particularly macrophages/activated microglial cells, reducing infarct size and improving neurological deficit after experimental stroke [14].
Myelin oligodendrocyte glycoprotein (MOG), which leaks out of the brain with the breakdown of blood-brain barrier and is detected by the peripheral immune system after stroke, leads to MOG-specific T cells migrating into brain, mistaking brain antigens for foreign pathogens and attacking them. Therefore, RTL with a myelin antigenic peptide can selectively modulate brain-targeted immune response via TCR while leaving other portions of the immune system intact [14–15].
We have previously demonstrated that RTL551, a mouse MHC coupled to mouse myelin peptide, is effective in reducing infarct size in experimental stroke in C57BL/6 mice [15]. Further investigations confirmed that the mechanism of protection was indeed related to the modulation of inflammatory activity of brain myelin antigen reactive T lymphocytes. To determine if a similar strategy would work against human T cells, we determined the efficacy of humanized RTL1000, which contains a human MHC covalently linked to a human myelin peptidein experimental stroke in humanized DR2-Tg mice which expresses human TCR [14]. Furthermore, RTL1000 has been evaluated in a multicenter, double-blind, placebo-controlled Phase I dose-escalating clinical trial for treatment of Multiple Sclerosis (MS) [16]. To move this potential stroke therapy closer to the clinic, and in meeting the preclinical criteria set by the Stroke Therapy Academic Industry Roundtable (STAIR) [17–18], in the current study, we evaluated the therapeutic window of efficacy of RTL1000 in DR2-Tg mice; tested the efficacy of single dose of RTL1000 and examined its potential long-term neurobehavioral functional benefit.
Materials and Methods
Ethics Statement
Animal experiments were conducted in accordance with National Institutes of Health guidelines for the use of experimental animals, and protocols were approved by the Animal Care and Use Committee at Oregon Health & Science University and the Portland Veteran Affairs Medical Center.
Animals and experimental groups
Experiments were carried out on 164 male HLA-DRB1*1502 transgenic (DR2-Tg) mice (produced at the Portland VA Medical Center with foundation breeders provided by Dr. Chella David [19]) aged 8 to 12 weeks and weighing 20 to 27 grams. Twelve to fifteen mice per group were used for time window studies, 20 mice per group for long-term behavioral assessment and 5 mice per group for Sham MCAO groups. In single dose studies, 6 mice per group were used in 100 µg RTL1000 groups and 12 mice per group in 400 µg RTL1000 groups. Mice were randomly assigned to either RTL1000 or vehicle groups, and the surgeon and the examiner who performed and scored the behavior tests were blinded to treatment groups.
RTL 1000 production and purification
RTL molecules consist of the α1 and β1 domains of MHC II molecule expressed as a single polypeptide with or without antigenic amino terminal extensions [11, 20]. RTL1000 is a HLA-DRB1*1502 (DR2) molecule linked to human MOG−35–55 peptide (MEVGWYRPPFSRVVHLYRNGK) [14]. RTL1000 was constructed de novo or by sequential site-directed mutagenesis of previous constructs. Protein purification was performed with a 30-to 40-mg yield of purified protein per liter of bacterial cell culture.
RTL1000 Treatment
Mice were randomized to injections of 100 µL of either RTL1000 (1 µg/µL) or vehicle (5% dextrose in Tris-HCl, pH 8.5) by subcutaneous (S.C.) injection. In the time window study, animals were divided into 3 groups according to the time of first injection (4, 6 or 8 h after MCAO), which was followed by 3 injections of the same volume and concentration at 24, 48, and 72 h after MCAO. In single dose studies, animals were treated with a one-time of 400 µg or 100 µg RTL1000 4 h after MCAO. In behavioral testing animals, the first injection was given at 4 h after stroke, followed by 3 daily injections at 24, 48, and 72 h after MCAO. The reason that 4 h after MCAO was selected as the first injection time in behavioral study is because the greatest effect on infarct size was seen during that time window.
Reversible Middle Cerebral Artery Occlusion
Reversible middle cerebral artery occlusion (MCAO) was induced via the intraluminal filament technique as described previously with slight modifications [21]. Mice were anesthetized with isoflurane (5% induction; 1% maintenance) using a mask connected with a vaporizer (Isotec 4; Cyprane, England) throughout surgery and during 60 min vascular occlusion until filament withdrawal and initiation of reperfusion. Rectal temperature was monitored and maintained at 36.5 ± 0.5°C throughout surgery with a warm water pad and a heating lamp. Cortical blood flow (CBF) was monitored by Laser-Doppler flowmetry (LDF; Model DRT4, Moor Instruments Ltd., Oxford, England). The right lateral common carotid artery (CCA) was exposed and temporarily ligated. The right external carotid artery (ECA) was ligated and cauterized. Ipsilateral MCAO was accomplished by inserting a 6-0 nylon monofilament surgical suture (ETHICON, Inc., Somerville, NJ, USA) with a heat-rounded and silicone-coated (Xantopren comfort light, Heraeus, Germany) tip into the internal carotid artery (ICA) via the ECA stump till the tip of filament reach the beginning of middle cerebral artery (MCA). The successful occlusion of MCA was confirmed by abrupt drop and then sustained reduction in CBF. The filament was withdrawn and the CCA was released to allow for reperfusion at 60 min occlusion. The mice were then allowed to recover from anesthesia and survived for 96 h following onset of ischemia. In sham groups, the right CCA was exposed and temporarily ligated and then the right ECA was ligated and cauterized. No filament was placed or advanced (no MCAO), with the mice being anesthetized throughout surgery until CCA ligation released 60 min later. Animals were excluded if CBF did not drop below 30% of baseline during MCAO or due to subarachnoid hemorrhage (SAH).
Determination of Infarct Volume
Animals were euthanized and brains were harvested 96 h after MCAO. Infarct volumes were measured after staining brain slices with 1.2% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma, St. Louis, MO, USA). Four slices of 2-mm-thick coronal sections were incubated in 1.2% TTC for 15 min at 37°C, and then fixed in 10% formalin overnight. Both sides of each stained slice were photographed and evaluated by SigmaScan Pro 5.0 (Jandel, San G, Rarael, CA, USA). Infarct volume was expressed as a percentage of contralateral structure (cortex, striatum, and hemisphere). To account for the effect of edema, infarct volume was calculated by subtracting the ipsilateral non-infarct region from the total contralateral structure volume, and dividing the difference by the contralateral volume [22].
Neurobehavioral evaluations
We selected behavioral tests which have consistently provided reliable evaluations of outcome across experimental rodent ischemia studies [21, 23–24]. Mice in the following groups were randomized for behavioral testing: RTL1000 and vehicle treated MCAO (n=16 each group), and RTL1000 and vehicle treated sham mice (n=5 per group). All mice were singly housed in a 12/12-h light/dark cycle and all assessments were carried out during the first half of the light cycle (08:00–12:00 hours). All instruments were sterilized with a 10% ethanol solution between trials.
Locomotor activity in the open field
Spontaneous locomotor activity was measured by conducting the open field protocol [25]. Mice were placed individually into a 41 cm (W) ×41 cm (D) ×38 cm (H) plastic enclosure equipped with a video camera mounted above to record movement in four arenas simultaneously. The video was analyzed post-capture off-line using Noldus software (Ethovision 3.1, Noldus, Leesburg, VA, USA). Total distance traveled, mean velocity and total movement during a 30-minute open field test were assessed at baseline, one day prior to and 6 days after MCAO.
Neurodeficit Score
A neurodeficit score was conducted for each mouse on days 1 and 3 after surgery [26]. The score was calculated by using a graded scoring system with 0 indicating no deficit and the upper limit indicating the most impaired. The categories scored included consciousness (0–3), interaction (0–2), eye appearance (0–2), breathing (0–2), food and water intake (0–2), ability to grip wire bar (0–2), gait disturbance (0–5) and activity (0–2).
Novel Object Recognition
Mice were placed individually into a 41 cm (W) × 41 cm (D) × 38 cm (H) plastic enclosure and allowed to habituate to the arena before novel object recognition testing [27]. During the sample session at 27 days after MCAO (examining long term cognitive impairment), two identical objects were placed in opposite corners of the arena, approximately 2.5 cm from the wall. Time spent exploring each object during the sample session was hand scored with stop watches and each mouse was removed from the arena after 5 minutes. After a 24 h delay, mice were again placed in the arena for a test session on day 28 after MCAO, with one object replaced with a novel object. The location of the novel object was determined by replacing the object explored less during the sample session in efforts to reduce spatial bias. A 7-min test session was performed and the time exploring each object was recorded and hand scored with stopwatches. The final score was calculated as the percentage of time spent exploring the new object divided by total exploration time during the test session. All objects used in this study were characterized previously in our laboratory to ensure that mice prefer each object equally.
Cylinder test
The cylinder test was performed to analyze forelimb use bias [23, 24]. Each mouse was placed in a transparent cylinder measuring 9 cm in diameter and 15 cm in height. The cylinder’s dimensions were designed to be wide enough for the mouse to move freely and yet small enough to encourage rearing behavior. Four separate video cameras were placed around the cylinder at 90° intervals in order to record rearing behavior from all angles. A maximum of one paw touch for each rearing event was recorded. A maximum of 20 and a minimum of 10 forelimb touches were recorded during the 15-min test. If the minimum number of touches was not reached during the first trial, additional trials were completed. The final score was calculated as the percentage of total touches that used the impaired forelimb. Baseline paw preference was assessed one day before MCAO with subsequent assessments made at 7 and 28 days after MCAO. The final score was calculated as the percentage of left paw touches (right-sided stroke) divided by the total number of touches.
Statistical Analysis
Data are presented as mean ± SEM. Differences in infarct size were determined by 2-way ANOVA, followed Holm-Sidak post hoc test, with the two factors being brain region and treatment group. Similarly, differences in neurobehavioral performance and body weight were also analyzed by 2-way ANOVA and the Holm-Sidak test, with the two factors being experimental group and time (days treated). Differences in novel object recognition scores among 4 groups were analyzed by 1-way ANOVA with post hoc Newman-Keuls test. Finally, Kruskal-Wallis ANOVA on ranks was conducted to evaluate differences among the four experimental groups (Sham + Vehicle, Sham + RTL1000, MCAO + Vehicle, MCAO + RTL1000) in median neurodeficit score, with a post-hoc Dunn’s test being used for all pairwise comparisons against the control (Sham + Vehicle) group following rank-based ANOVA as treatment group sizes were unequal. The multiple comparisons on ranks did not include an adjustment for ties. Statistical analysis was performed using SigmaStat3 statistical software (Systat Software, Inc., Chicago, IL, USA). Statistical significance was set at p<0.05.
Results
Exclusions and mortality
Surgery successful rate was 98.7%, with 2 mice being excluded due to subarachnoid hemorrhage (SAH) and the other one excluded because CBF did not drop 70% of baseline during MCAO. In the behavioral study, 3 mice were excluded due to severe neck tilt after MCAO surgery (which interfered with performance in behavioral tests). Overall mortality was 12.8% (12.3% vs. 13.2% in RTL1000 vs. vehicle treated groups).
RTL1000 reduced infarct size when administered no later than 6h after the onset of experimental ischemic stroke
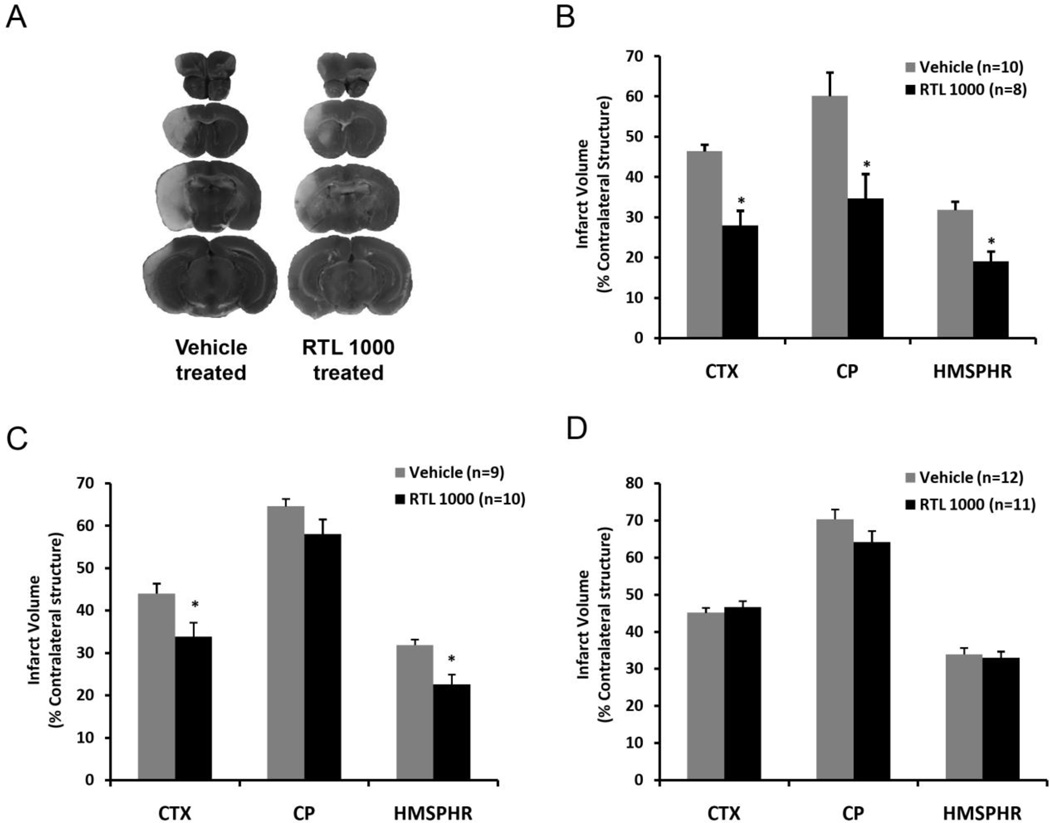
We first tested the effect of RTL1000 when it was administered at 4, 24, 48 and 72 h after MCAO. As shown in Figure 1A and 1B, male DR2-Tg mice treated with RTL1000 had smaller infarcts compared to vehicle treated mice. Quantitative analysis of TTC-stained brain slices 96 h after ischemia showed that infarct size was 28.0% ± 3.7%, 34.7% ± 6.1% and 19.1% ± 2.4% in cortex, striatum and total hemisphere respectively in RTL1000 treated mice, compared to 46.4% ± 1.7%, 60.1% ± 5.9% and 31.8% ± 2.1% in vehicle treated mice (p<0.05).
Fig. 1. Therapeutic time window of RTL1000 for protection from stroke in DR2-Tg mice.
Male DR2-Tgmice were subjected to 60-min transient MCAO and treated with 100 µg RTL1000 or 100 µl vehicle given subcutaneously (S.C.) starting at 4 (A, B), 6 (C) or 8h (D) after MCAO, followed by injections at 24, 48 and 72 h after MCAO. Brains were harvested 96 h after MCAO, brain slices were stained with TTC and infarct volumes measured and expressed as a percentage of the contralateral region. Panel “A” shows the four 2-mm TTC-stained coronal brain sections, which cover the entire MCA territory of RTL1000- and vehicle-treated animals. *indicates p<0.05 compared to vehicle group. CTX: cortex; CP: striatum; HMSPHR: hemisphere.
When the first administration was delayed until 6 h after MCAO, RTL1000 significantly reduced infarct size only in cortex, but not in striatum (Fig. 1C). Cortical infarct size was 33.9 % ± 3.3% and hemispheric infarct was 22.6% ± 2.3% in RTL1000 treated mice compared to 44.1% ± 2.3% and 31.9% ± 1.3% in vehicle treated animals (p<0.05). Infarct size in striatum was 58.0% ± 3.5% and 64.6% ± 1.7% respectively in RTL1000 and vehicle treatment group (p=0.12).
RTL1000 had no effect on infarct size when first treatment was delayed until 8 h after MCAO (Fig. 1D). Infarct size was 46.7% ± 1.6%, 64.1% ± 3.0% and 33.0% ± 1.7% in cortex, striatum and hemisphere in RTL1000 treated mice, and 45.1% ± 1.3% (p=0.16), 70.4% ± 2.7% (p=0.08) and 33.9% ± 1.7% (p=0.72) in vehicle treated mice.
There were no significant differences in LDF before, during MCAO or after reperfusion and no difference in age or baseline body weight between RTL1000 and vehicle treated animals (data not shown).
Single dose of RTL1000 administration reduced infarct size 24 h after MCAO
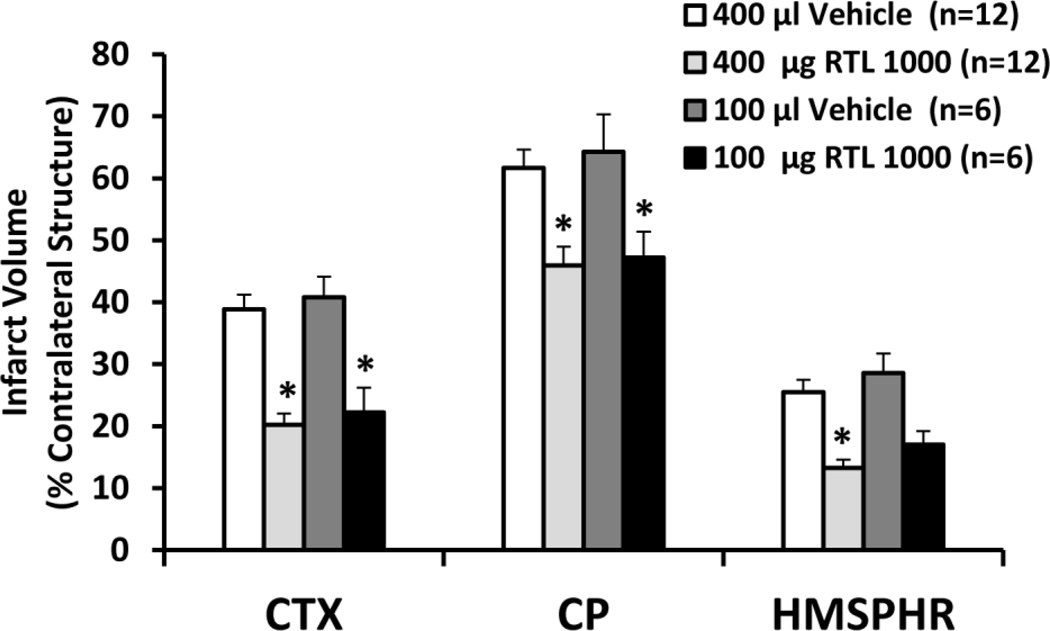
We explored if a single dose of RTL1000 treatment can reduce infarct size. Our data showed a single dose of 400 µg of RTL1000 given 4h after MCAO significantly reduced infarct size 24 h after stroke (Fig. 2). Infarct volumes were 20.2% ± 1.8%, 45.9% ± 3.0% and 13.3% ± 1.3% in cortex, striatum and hemisphere respectively in RTL1000 treated mice, compared to 38.9% ± 2.4%, 61.7% ± 3.1% and 25.5% ± 2.1% in vehicle treated mice (p<0.05). We further found that one-time 100 µg RTL100 injection also reduced infarct size in cortex and striatum 24 h after MCAO with infarct size being 22.2% ± 4.0% and 47.2% ± 4.2% respectively in RTL1000 treated mice, compared to 40.8% ± 3.3%, and 64.3% ± 6.0% (p<0.05. There was, however, no statistically significant difference in total hemispheric infarct between RTL1000 and vehicle-treated mice in the 100 µg group (28.6% ± 3.1% vs. 17.0% ± 2.2%). This is likely due to the small number of animals, and not related to efficacy differences between the two doses, since cortical and striatal infarcts were statistically significant in both groups. Furthermore, means of infarct size measurements were similar in animals treated with either 100 or 400 µg RTL1000, although the difference from vehicle was statistically significant only in the 400 µg group.
Fig. 2. Effect of a single injection of RTL1000 on infarct size after MCAO in DR2-Tg mice.
Male DR2-Tgmice were subjected to 60-min transient MCAO and treated with 400 or 100 µg RTL1000 given S.C. at 4 h after MCAO. Corresponding volumes of vehicle (400 or 100 µL) were used in control groups. Brains were harvested 24h after MCAO and infarct volumes were measured by TTC and expressed as a percentage of the contralateral region. *indicates p<0.05 compared with corresponding vehicle group. CTX: cortex; CP: striatum; HMSPHR: hemisphere.
Neurobehavioral testing
The open field test was performed the day before MCAO or sham surgery and on day 6 after surgery when motor function has recovered, in order to avoid the potential confounding effect of motor activities differences on cognitive testing. No significant differences in total distance travelled, velocity or total movement were observed during a 30-minute open field test, regardless of surgery or treatment (data not shown), therefore no mice were excluded due to inability of performing this task.
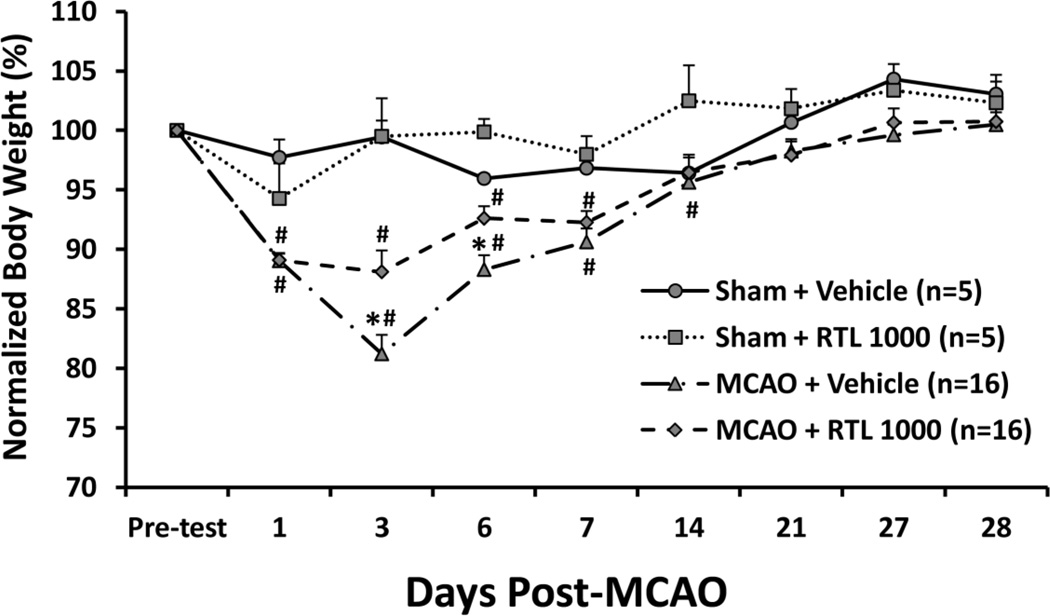
Body weights were measured 1 day before MCAO (pre-test) and on days 1, 3, 6, 7, 14, 21, 27 and 28 after MCAO. Both vehicle- and RTL1000-treated animals displayed a significant weight loss beginning on day 1 after MCAO, and all animals returned to their baseline body weight by 28 days after surgery. Compared to vehicle-treated animals, RTL1000-treated mice lost less weight on days 3 and 6 (p < 0.05). Furthermore, we found that RTL1000-treated mice recovered from body weight loss as early as 14 days after stroke, while vehicle-treated mice did not return to their baseline body weight until day 21 after MCAO. Both sham MCAO animals in RTL1000 and vehicle treated group didn’t lose their body weight compared to baseline (Fig. 3).
Fig. 3. RTL1000 alleviates weight loss after MCAO in DR2-Tg mice.
Male DR2-Tgmice were subjected to 60-min transient MCAO or sham surgery, and were treated with 100 µg RTL1000 or 100 µL vehicle given S.C. at 4, 24, 48 and 72 h after MCAO. Body weights were measured 1 day before MCAO (pre-test) and on days 1, 3, 6, 7, 14, 21, 27 and 28. Changes in each animal’s body weight were normalized to its own baseline. * indicates p < 0.05 between RTL1000 and corresponding vehicle group; # indicates p < 0.05 compared to pre-test baseline and day 28 within each group.
Distribution of neurodeficit scores within the Sham and MCAO groups regardless of treatment at each time point would suggest that MCAO had an impact on increasing, and thus worsening, neurodeficit score over time compared to Sham (Table 1). Distribution of neurodeficit scores within the Sham + Vehicle and Sham + RTL1000 groups were the same at each time point evaluated (Table 1). Distribution of neurodeficit scores within the MCAO + Vehicle and MCAO + RTL1000 groups at each time point would suggest that RTL had an impact on decreasing, and thus improving, neurodeficit score over time compared to Vehicle (Table 1). Differences in the median neurodeficit scores among the four experimental groups (Sham + Vehicle, Sham + RTL1000, MCAO + Vehicle, MCAO + RTL1000) were greater than would be expected by chance at 1 (P<0.05) and 3 (P<0.05) days following MCAO (Table 1). A significant difference (P<0.05) was observed between Sham and MCAO groups regardless of treatment at 1 and 3 days following MCAO (Table 1). However, no differences were seen between Sham + Vehicle and Sham + RTL1000 groups and between MCAO + Vehicle and MCAO + RTL1000 groups at 1 and 3 days following MCAO.
Table 1.
Neurodeficit score distribution and median scores at various reperfusion time points following 1 hour of middle cerebral artery occlusion in male HLA-DRB1*1502 (DR2-Tg) mice
| Experimental Groups | Distribution Of Neurodeficit Scores |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Day | 3 Days | |||||||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Sham + Vehicle (n=5) | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sham + RTL1000 (n=5) | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MCAO + Vehicle (n=16) | 0 | 1 | 1 | 6 | 2 | 4 | 0 | 1 | 1 | 0 | 1 | 4 | 3 | 3 | 3 | 0 | 1 | 1 |
| MCAO + RTL1000 (n=16) | 0 | 1 | 5 | 8 | 0 | 2 | 0 | 0 | 0 | 1 | 3 | 4 | 6 | 1 | 1 | 0 | 0 | 0 |
| Experimental Groups | Median Neurodeficit Scores | |||||||||||||||||
|
1 Day (P<0.0001) |
3 Days (P<0.0001) |
|||||||||||||||||
| Sham + Vehicle (n=5) | 0 | 0 | ||||||||||||||||
| Sham + RTL1000 (n=5) | 0 | 0 | ||||||||||||||||
| MCAO + Vehicle (n=16) | 3.5#,* | 3.5#,* | ||||||||||||||||
| MCAO + RTL1000 (n=16) | 3#,* | 2.5#,* | ||||||||||||||||
P < 0.05 compared to Sham + Vehicle;
P < 0.05 compared to Sham + RTL1000.
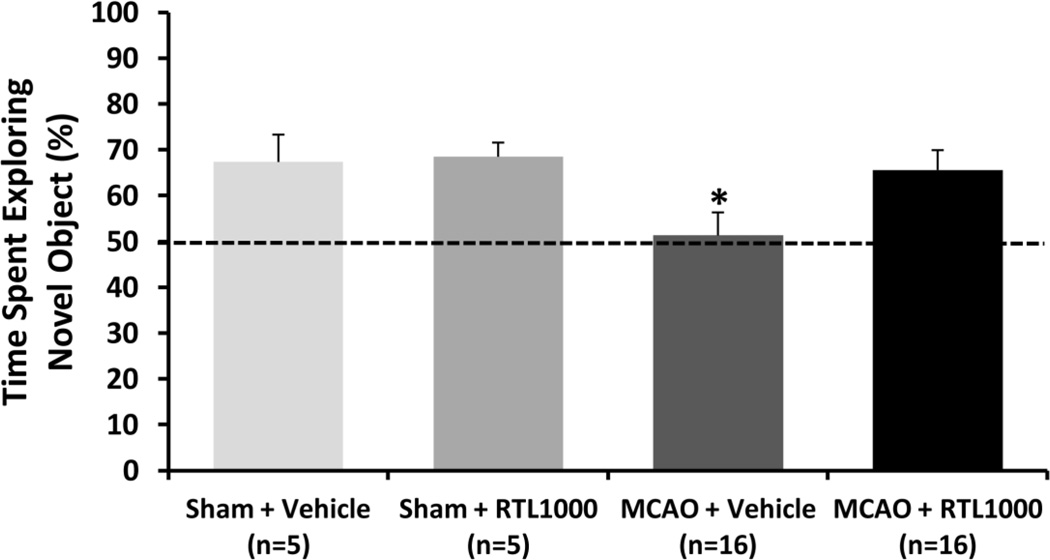
The novel object recognition test was performed on day 28 after surgery (Fig. 4). Vehicle treated MCAO mice showed a deficit in novel object preference (51.3% ± 5%) compared to all other groups indicating impaired memory retention 4 weeks after MCAO (p<0.01). The RTL1000 treated MCAO mice explored the novel object 65.6% ± 4.2%, which was similar to RTL1000 treated sham (68.5% ± 3%) and vehicle treated sham mice (67.3% ± 3.0%).
Fig. 4. RTL1000 improves cognitive function after MCAO in DR2-Tg mice.
Male DR2-Tg mice were subjected to 60-min transient MCAO or sham surgery, and were treated with 100 µg RTL1000 or 100 µL vehicle given S.C. at 4, 24, 48 and 72 h after MCAO. In the novel object recognition memory test, time spent exploring each object was scored on day 28 post-MCAO. The final score was calculated as the percentage of time spent exploring the new object divided by the total exploration time during the test session. The dotted line indicates the percentage of time spent exploring the novel object when an animal fails to recognize the object as novel (50% of total time; i.e., equal time spent on novel and familiar objects). * indicates p<0.05
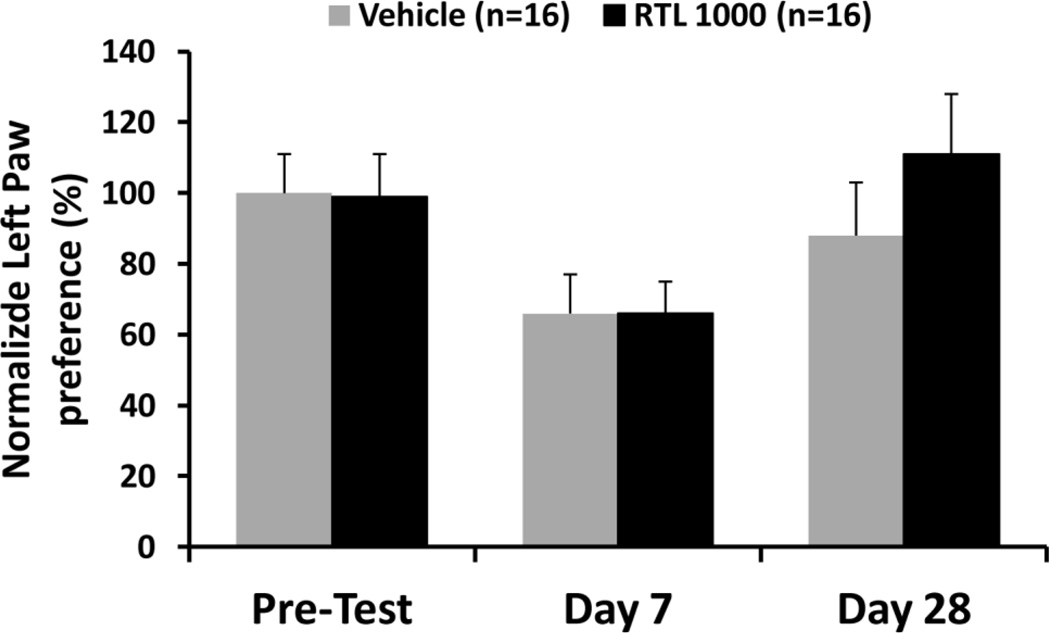
Motor dysfunction was assessed via the cylinder test on the day before surgery and again on days 7 and 28 after surgery by measuring the use of the left paw, the contralateral side of the MCAO. No differences were found between RTL1000 and vehicle treated MCAO mice on day 7 and day 28 (Fig. 5). There were trends of impaired left paw use on day 7 and recovered left paw motor function on day 28, both in RTL1000- and vehicle-treated mice. However, these trends did not reach statistical significance.
Fig. 5. RTL1000 treatment did not improve motor function recovery in DR2-Tg mice.
Male DR2-Tg mice were subjected to 60-min transient MCAO and treated with 100 µg RTL1000 or100 µl vehicle given S.C. at 4, 24, 48 and 72 h after MCAO. The paw preference test (cylinder test) was performed to analyze forelimb use bias. A maximum of one paw touch for each rearing event was recorded. Baseline paw preference was assessed one day before MCAO with subsequent assessments at 7 and 28 days after MCAO. The final score was calculated as the percentage of left paw touches (right-sided stroke) divided by the total number of touches.
Discussion
We have previously demonstrated that RTL1000 reduced infarct size 96 h after MCAO [14]. In this study, we further characterized the therapeutic time window, the effect of single dose and the long-term functional benefit of RTL1000 in humanized DR2-Tg male mice.
A clinically applicable therapeutic time window is a significant factor that will have a major impact on success of pilot clinical trials [17]. Our previous data showed that RTL1000 significantly reduced infarct size when administrated 1 or 3 h after the onset of ischemia and followed by 3 daily injections [14, 28]. However, the timing of treatment is not practical for clinical trials because very few patients can get treatment within 3 h after stroke onset. We therefore tested the effect of first RTL1000 administration given at 4 h following ischemia. Our data showed that RTL1000 significantly reduced infarct size at 96 h after MCAO. We then tried to extend the therapeutic time window to 6 and 8 h after occlusion. However, there was no therapeutic benefit when RTL1000 was first given 8 h after ischemia, but there was some benefit, at least in cortex, when RTL1000 was administered 6 h after MCAO. We conclude according to these results that RTL1000 treatment protects against ischemic stroke when RTL1000 first administration is given within 6 h following ischemia and the protection is more robust when it is applied at 4 h after MCAO.
Clinically, single dose therapy is much more convenient than multiple administrations. We therefore tested if RTL1000 is still effective when a one-time injection applied 4 h after MCAO. We demonstrated that both 400 µg and 100 µg of RTL1000 reduce infarct size 24 h after stroke. However, further characterization, including long-term functional assessment is still needed for the single-dose administration.
Difficulty in translating animal experimental results into clinical trials has led to guidelines recommending evaluation of clinically-relevant long-term neurobehavioral functional outcomes for the preclinical validation of acute ischemic stroke therapies [17, 18]. We performed several behavioral tasks to assess functional outcome up to 28 days following ischemia. Our data indicate that RTL1000 treatment improves body weight recovery, which is related to the animals’ overall health state. However, there was no difference in median neurodeficit score between RTL1000- and vehicle-treated on either day 1 or 3. Thus, health status did not affect the results of motor activity and cognitive testing. The novel object recognition memory test showed that RTL1000 treated mice performed cognitively better than vehicle treated mice 4 weeks after stroke. However, in the cylinder test, no asymmetries were observed between vehicle and RTL1000 treated mice. Taken together, these findings indicate that RTL1000 improve long-term neurocognitive function, but has no effect on sensorimotor deficit recovery.
Mechanistically, RTL1000 bind to and modulate expression of the MHC class II invariant chain, CD74, the cellular receptor for macrophage migration inhibitory factor, which reduces the transmigration of inflammatory cells from the periphery into the central nervous system (CNS) infarcted region [15, 29]. Furthermore, blockade of migration inhibitory factor activity reduces the release of inflammatory cytokines by T-cells, including myelin oligodendrocyte glycoprotein (MOG)-specific cells that have been implicated previously as contributors to stroke severity [30], and inhibits activation of microglial cells in the CNS [31]. These effects coupled with neuroprotective activity of RTLs observed in other CNS disease models provide a strong rationale supporting the use of RTL1000 in treatment of MCAO [32].
Conclusions
In summary, we demonstrated that RTL1000 protects against acute ischemic brain injury. The therapeutic time window is within 6 h after the onset of stroke, and the best effect is seen at 4 h after stroke. Single dose treatment may be used clinically. RTL1000 improves long-term neurocognitive functional recovery, but there is no effect in sensorimotor function recovery.
Acknowledgements
This work was supported by NIH Grants #NS076013 (STTR) and by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development.
Footnotes
Conflict Of Interest
Dr. Offner, Dr. Alkayed and OHSU have a significant financial interest in Artielle Immuno Therapeutics, Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by the OHSU and VAMC Conflict of Interest in Research Committees.
Wenbin Zhu declares that he has no conflict of interest. Amanda Casper declares that she has no conflict of interest. Nicole L. Libal declares that she has no conflict of interest. Stephanie J. Murphy declares that she has no conflict of interest. Sheetal Bodhankar declares that she has no conflict of interest. All institutional and national guidelines for the care and use of laboratory animals were followed. This article does not contain any studies with human subjects.
References
- 1.Dirnagl U, Klehmet J, Braun JS, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–773. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- 2.Muir KW, Tyrrell P, Sattar N, et al. Inflammation and ischemic stroke. CurrOpin Neurol. 2007;20:334–342. doi: 10.1097/WCO.0b013e32813ba151. [DOI] [PubMed] [Google Scholar]
- 3.Gee JM, Kalil A, Shea C, et al. Lymphocytes: potential mediators of postischemic injury and neuroprotection. Stroke. 2007;38(2 Suppl):783–788. doi: 10.1161/01.STR.0000248425.59176.7b. [DOI] [PubMed] [Google Scholar]
- 4.NilupulPerera M, Ma HK, Arakawa S, et al. Inflammation following stroke. J ClinNeurosci. 2006;13(1):1–8. doi: 10.1016/j.jocn.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Hurn PD, Subramanian S, Parker SM, et al. T- and B-cell deficient mice with experimental stroke has reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yilmza G, Arumugan TV, Stokes KY, et al. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 7.Offner H, Subramanian S, Parker SM, et al. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 8.Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience. 2009;158:1098–1111. doi: 10.1016/j.neuroscience.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Offner H, Subramanian S, Parker SM, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 10.Prass K, Meisel C, Hoflich C, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is medicated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burrows GG, Chang JW, Bachinger HP, et al. Design, engineering and production of functional single-chain T cell receptor ligands. Protein Eng. 1999;12:771–778. doi: 10.1093/protein/12.9.771. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Mooney JL, Meza-Romero R, et al. Recombinant TCR ligand induces early TCT signaling and a unique pattern of downstream activation. J Immunol. 2003;171:1934–1940. doi: 10.4049/jimmunol.171.4.1934. [DOI] [PubMed] [Google Scholar]
- 13.Vandenbark AA, Rich C, Mooney J, et al. Recombinant TCR ligand induces tolerance to myelin oligodendrocyte glycoprotein 35–55 peptide and reverses clinical and histological signs of chronic experimental autoimmune encephalomyelitis in DR2-TG transgenic mice. J Immunol. 2003;171:127–133. doi: 10.4049/jimmunol.171.1.127. [DOI] [PubMed] [Google Scholar]
- 14.Subramanian S, Zhang B, Kosaka Y, et al. Recombinant T cell receptor ligand treats experimental stroke. Stroke. 2009;40:2539–2545. doi: 10.1161/STROKEAHA.108.543991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dziennis S, Mader S, Akiyoshi K, et al. Therapy with recombinant T-cell receptor ligand reduces infarct size and infiltrating inflammatory cells in brain after middle cerebral artery occlusion in mice. Metab Brain Dis. 2011;26:123–133. doi: 10.1007/s11011-011-9241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yadav V, Dennis NB, Bowen JD, et al. Recombinant T-Cell receptor Ligand (RTL) for treatment of multiple sclerosis: A double-Blind, placebo-controlled, phase I, dose-escalation study. Autoimmune Diseases. 2012:954739. doi: 10.1155/2012/954739. Article ID. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher M, Feuerstein G, David WH, et al. Update of stroke therapy academic industry rountable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroke therapy academic industry roundtable. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Gay MA, Zanelli E, Khare SD, et al. Human leukocyte antigen-DRB1*1502 (DR2-TGDW12) transgene reduces incidence and severity of arthritis in mice. Hum Immunol. 1996;50:54–60. doi: 10.1016/0198-8859(96)00123-1. [DOI] [PubMed] [Google Scholar]
- 20.Huan JY, Meza-Romero R, Mooney JL, et al. Rationally designed mutations convert complexes of human recombinant T cell receptor ligands into monomers that retain biological activity. J ChemTechnolBiotechnol. 2005;80:2–12. doi: 10.1002/jctb.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu W, Wang L, Zhang L, et al. Isoflurane preconditioning neuroprotection in experimental focal stroke is androgen-dependent in male mice. Neuroscience. 2010;169:758–769. doi: 10.1016/j.neuroscience.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Davis CM, Edin ML, et al. Role of endothelial soluble epoxide hydrolase in cerebrovascular function and ischemic injury. PLos One. 2013;8(4):e61244. doi: 10.1371/journal.pone.0061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchida M, Palmateer JM, Herson PS, et al. Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. J Cereb Blood Flow Metab. 2009;29:1454–1462. doi: 10.1038/jcbfm.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schallert T, Leasure JL, Kolb B. Experience-associated structural events, subependymalcelluar proliferative activity, and functional recovery after injury to the central nervous system. J Cereb Blood Flow Metab. 2000;20:1513–1528. doi: 10.1097/00004647-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Craft TK, Zhang N, Glasper ER, et al. Neonatal factors influence adult stroke outcome. Psychoneuroendocrinology. 2006;31:601–613. doi: 10.1016/j.psyneuen.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Blizzard KK, Zhu Z, et al. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Akiyoshi K, Dziennis S, Palmateer J, et al. T Cell receptor ligands improve outcome after experimental cerebral ischemia. Transl Stroke Res. 2011;2:404–410. doi: 10.1007/s12975-011-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vandenbark AA, Meza-Romero R, Benedek G, et al. A novel regulatory pathway for autoimmune disease: Binding of partial MHC class II constructs to monocytes reduces CD74 expression and induces both specific and bystander T-cell tolerance. J Autoimm. 2013;40C:96–110. doi: 10.1016/j.jaut.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren X, Akiyoshi K, Grafe MR, et al. Myelin specific cells infiltrate MCAO lesions and exacerbate stroke severity. Metabolic Brain Dis. 2012;27:7–15. doi: 10.1007/s11011-011-9267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benedek G, Meza-Romero R, Andrew S, et al. Partial MHC class II constructs inhibit MIF/CD74 binding and downstream effects. Eur J Immunol. 2013;43:1309–1321. doi: 10.1002/eji.201243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang C, Gold BG, Kaler LJ, et al. Antigen-specific therapy promotes repair of myelin and axonal damage in established EAE. J Neurochem. 2006;98:1817–1827. doi: 10.1111/j.1471-4159.2006.04081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]