Abstract
Objective
Dentin matrices release ICTP and CTX fragments during collagen degradation. ICTP fragments are known to be produced by MMPs. CTX fragments are thought to come from cathepsin K activity. The purpose of this study was to determine if quaternary methacrylates (QAMs) can inhibit matrix MMPs and cathepsins.
Methods
Dentin beams were demineralizated, and dried to constant weight. Beams were incubated with rh-cathepsin B, K, L or S for 24 h at pH 7.4 to identify which cathepsins release CTX at neutral pH. Beams were dipped in ATA, an antimicrobial QAM to determine if it can inhibit dentin matrix proteases. Other beams were dipped in another QAM (MDPB) to determine if it produced similar inhibition of dentin proteases.
Results
Only beams incubated with cathepsin K lost more dry mass than the controls and released CTX. Dentin beams dipped in ATA and incubated for 1 week at pH 7.4, showed a concentration-dependent reduction in weight-loss. There was no change in ICTP release from control values, meaning that ATA did not inhibit MMPs. Media concentrations of CTX fell significantly at 15 wt% ATA indicating that ATA inhibits capthesins.
Beams dipped in increasing concentrations of MDPB lost progressively less mass, showing that MDPB is a protease-inhibitor. ICTP released from controls or beams exposed to low concentrations were the same, while 5 or 10% MDPB significantly lowered ICTP production. CTX levels were strongly inhibited by 2.5–10% MDPB, indicating that MDPB is a potent inhibitor of both MMPs and cathepsin K.
Significance
CTX seems to be released from dentin matrix only by cathepsin K. MMPs and cathepsin K and B may all contribute to matrix degradation.
Keywords: degradation of collagen, CTX, ICTP, quaternary ammonium compounds, MMPs, cathepsins
1. Introduction
In order to bond tooth-colored resin composites to enamel and dentin, these hard tissues are acid-etched to increase their micro- and nanoporosity. After infiltrating resins into these porosities, the results is a new hard tissue that is called the hybrid layer [1].
Hybrid layers are formed when solvated comonomers are infiltrated into dentin surfaces that have been acid-etched with 37% phosphoric acid for 15 sec. Acid-etching uncovers the collagen matrix and activates the proforms of endogenous dentin proteases [2,3]. If resin does not replace all of the rinse water, portions of the hybrid layer will include water-filled, resin-poor, collagen fibrils containing activated proteases that can slowly destroy the very fibrils that anchor resin-composites to dental hard tissues. This causes a loss of retention of tooth colored restorations, requiring their replacement [4].
The cyclic loading of hybrid layers during mastication induces excessive strain [5] in the low modulus of elasticity water-filled zones, causing accelerated degradation. This degradation is thought to occur at pH 7, in contrast to the cyclic changes in pH encountered in carious lesions where intralesion pH’s swing from 7.4 to 5. As the optimum pH for cathepsin K is 5.0, its collagenolytic activity is very prominent in carious lesions [5]. However, that does not mean that cathepsin K has no collagenase activity at pH 7.4. Kometoni et al. [7] reported that rh cathepsin K enzyme activity in vitro at pH 5.5 was 91% of this maximal activity at pH 5.0; when the pH was adjusted to 6.5, the enzyme showed 85% of its residual activity. At pH 7.5, the enzyme still retained 11% of its activity.
Peripheral dentin is a cell-free, mineralized connective tissue that does not turn-over [8]. Hybrid layers are sequestered from saliva by bonded resin composites, and from pulpal fluids by resin tags that occlude the tubules. Thus, there is no source of replacement proteases in resin bonded dentin. Any degradation that occurs in the absence of carious bacteria, probably occurs at pH 7.4.
Since both active cathepsins and MMPs have been identified in peripheral dentin [9], and since both CTX [10] and ICTP telopeptide [10,11] have been identified in the incubation medium of demineralized dentin beams at pH 7.4, we assume that both classes of proteases contribute to collagen degradation of hybrid layers.
Because hybrid layers are only 1–10 μm thick, they do not release enough telopeptides to be detected by specific ELISAs, even after prolonged incubation. Thus, many investigators use a macrohybrid layer model [12] where dentin beam 0.3–1.0 mm thick are completely demineralized in 10–37% phosphoric acid for 12–16 hrs to uncover and activate the endogenous proteases of dentin. While this might seem extreme treatment that might inactivate endogenous proteases, Tezvergil-Mutluay et al. [10] compared the ICTP and CTX telopeptide release from dentin beams that were completely demineralized in 0.5 M EDTA pH 7.4 (controls) to EDTA-demineralized beams that were then exposed to 1, 10 or 37% phosphoric acid for up to 15 min. After rinsing, they were incubated in pH 7.4 buffer for 3 days. There were no changes in either ICTP or CTX release from control or experimental beams, indicating that phosphoric acids at pH 0.4–1.0 for 15 min had no influence of telopeptidase activity. The CTX release was only one-tenth that of ICTP, presumably because cathepsin K was operating at pH 7.4 instead of its optimum pH of 5.0. These results suggest that collagen-bound proteases are resistant to inactivation or denaturation by acids used in adhesive dentistry and confirm that cathepsin K can continue to function, albeit more slowly at pH 7.4 that is 2.5 pH units away from its optimum pH.
The nonspecific, cationic protease inhibitor chlorhexidine [13] has recently been shown to inhibit cysteine cathepsins [14], in addition to MMPs [13]. We have recently shown that cationic quaternary ammonium methacrylates (QAMs) can also inhibit MMPs [15].
The purpose of this study was to determine if the most effective QAMs screened for their anti-MMP activity [15], can also inhibit cathepsin K at pH 7.4 by measuring the release of ICTP from demineralized dentin beams as an indirect measure of MMP activity, and the release of CTX as an indirect measure of cathepsin K activity. The test null hypotheses were that 1, 5, 10, 15 wt% QAMs have no inhibitory activity on either endogenous dentin matrix MMPs or cathepsin K, at pH 7.4, the pH of hybrid layers.
2. Materials and methods
2.1 Effects of rh cathepsins on mass loss (experiment 1)
Recombinant human cysteine cathepsins B, L and S (catalytic domain) were obtained from Athens Research & Technology (Athens, GA). Procathepsin K was obtained from EnzoLife Sciences (Plymouth Meeting, PA) and activated with 100 mM sodium acetate, pH 3.9, 10 mM DTT and 5 mM EDTA for 40 min at 25°C. Cathepsins B, L and S were already active when purchased. Dentin beams in all groups (2 × 1 × 6 mm) were prepared from 18–21 year old mid-coronal dentin of human third molars using an Isomet saw (Buehler Ltd., Lake Bluff, IL). The beams were completely demineralized in 10 wt% phosphoric acid for 18 hrs at 25°C with tumbling, and then rinsed free of acid in water. After obtaining constant dry masses, the individual beams (n = 5 beams/group) were incubated in 10 μg of one of the specific cathepsins, dissolved in 0.5 ml of simulated artificial saliva (SBF) [16], containing KCl 13 mM, KSCN 2 mM, Na2SO4 7.5 mM, HEPES 5 mM, NH4Cl 3 mM, CaCl2 1.5 mM, NaHCO3 7.5 mM, ZnCl2 0.02, PO4−3 4, and NaN3 0.02%, pH adjusted to pH 7.4. Another set of beams was incubated with 10 μg rh cathepsin K at pH 5.0 to demonstrate the maximum activity of the enzyme. After incubation in SBF (37°C with shaking) for 24 h, the beams were removed, rinsed with water to remove buffer salts, and re-dried to a constant weight in sealed containers of dry calcium sulfate. Fifty μL aliquots to media was analyzed for ICTP and CTX by ELISA kits (TSZ ELISA, Cat.#HU9655, Framingham, MA, and Serum CrossLaps ELISA, Immunodiagnotics Systems, Scottsdale, AZ, USA). The telopeptide results were expressed in ng ICTP or CTX/mg dry dentin/day and as ng/L. Loss of dry mass was expressed as % dry weight loss per 24 h. Statistical analyses were done using a one-way ANOVA and Tukey’s test at α = 0.05 for normally distributed data. The ICTP data were not normally distributed. They were analyzed using Kruskal-Wallis test, followed by Dunn’s multiple comparison with an α - 0.05.
2.2 Effects of QAMs on dry mass loss and ICTP/CTX production (experiments 2 and 3)
All demineralized beams were rinsed in water and dried to a constant weight to provide initial dry masses. Individual beams (n = 10 separate beams/group) were dipped for 30 sec in increasing concentrations of ATA in (1, 5, 10 or 15 wt% in experiment 2) or MDPB (0.1, 1.0, 2.5, 5.0 or 10 wt% in experiment 3) and then incubated separately in 500 μL of SBF (pH 7.4) in capped containers for one week at 37°C with shaking (60 Hz). At the end of one week, the beams were removed from the media, rinsed in water to remove buffer salts, and dried to a constant weight in anhydrous calcium sulfate to obtain the post-incubation dry mass. Fifty μL of media was analyzed (n = 10) for ICTP/CTX telopeptides by specific ELISA kits mentioned above, and expressed in ng telopeptides/mg dry dentin mass/unit time. Statistical analyses were done using one-way ANOVA seeking significance differences of α = 0.05. Multiple comparisons were done with Tukey’s test at α = 0.05. When data were not normally distributed, they were analyzed using Kruskel-Wallis, followed by Dunn’s multiple comparison. Regression analysis was used to test the correlations between the ICTP or CTX release rates at different ATA or MDPB concentrations versus loss of dry mass over one week incubation.
3. Results
3.1 Effects of exogenous cathepsins on dry mass loss and ICTP/CTX production (experiment 1)
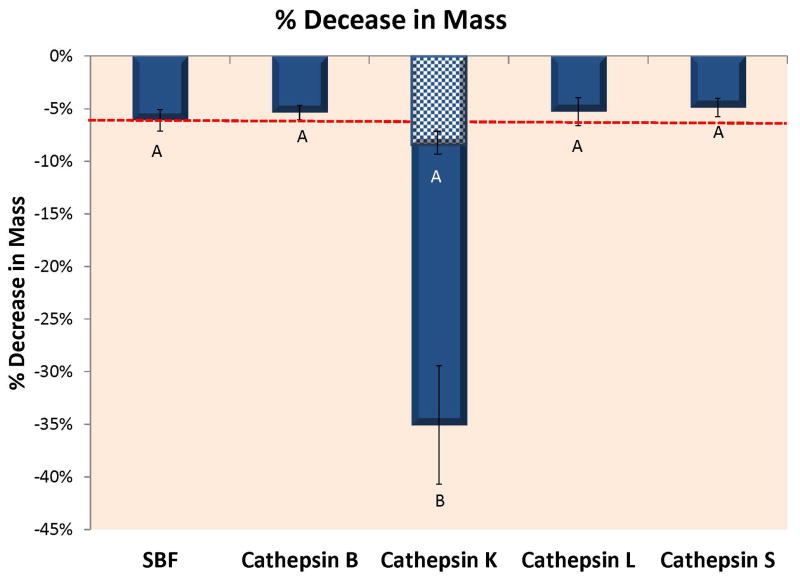
Figure 1A shows the loss of dry mass (in %) of completely demineralized individual dentin beams incubated in SBF containing 10 μg of rh cathepsins B, K, L or S at pH 7.4 (and at pH 5 for cathepsin K only). Control beams incubated in SBF lost 6.11 ± 1.01% (n=4) per 24 h. Beams incubated in rh cathepsin B lost 5.37 ± 0.69% of dry mass/24 h which was not significantly different from controls. Beams incubated in rh cathepsin K lost 35.05 ± 5.63%/24 h (p<0.01) at pH 5.5 but only 8.1 ± 2.1% at pH 7.4 (cross-hatched region). Beams incubated in rh cathepsin L lost 5.28 ± 1.33% of dry mass/24 h, while beams incubated in rh cathepsin S lost 4.89 ± 0.88% of dry mass/24 h. Clearly, the only significant increase in mass loss of dry weight was due to cathepsin K.
Figure 1.
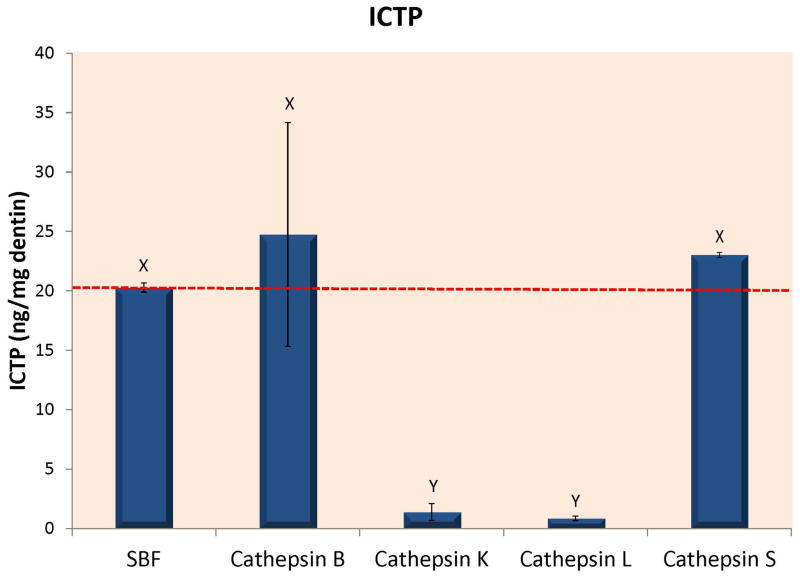
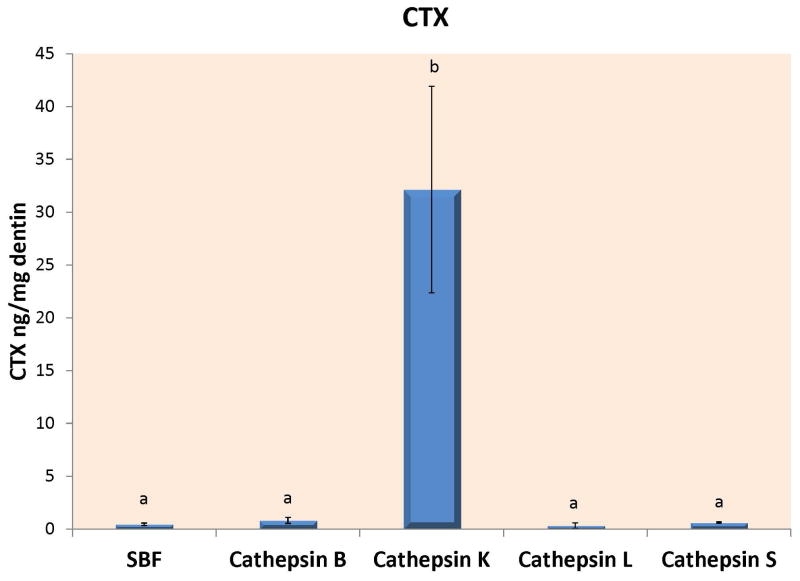
Figure 1A – Loss of dry mass of completely demineralized dentin beams incubated in a simulated body fluid (SBF) alone, or in SBF containing 10 μg of recombinant human cathepsin B, K, L or S after 24 h of incubation at 37°C in a simulated body fluid (SBF) at pH 7.4 even though that pH is not optimal for cathepsins. An additional group of beams were incubated in a SBF adjusted to pH 5.0 to ensure that the rh cathepsin K enzyme was active. Both cathepsin K groups are presented together. The lower mass loss was produced by rh cathepsin K at pH 7.4. The higher level was produced by cathepsin K at pH 5.0. Different letters indicate significant differences (p<0.01). N=10 specimens/group. Values are mean ± SD. 1B – When the media of the specimens shown in Fig. 1A were analyzed for ICTP telopeptide fragments, the controls (dotted line), and cathepsins B and S released about the same amount of ICTP, while there was little ICTP in the media of beams incubated with cathepsin K or L, indicating that these cathepsins destroyed the ICTP produced by endogenous dentin MMPs. Groups identified by different letters are significantly different (p<0.05). N=10 specimens/group. Values are mean ± SD. 1C – When the media of the specimens shown in Fig. 1A were analyzed for CTX by specific ELISAs, all media contained between 0.5–1 ng CTX/mg dry dentin/24 h, while the media from the cathepsin K specimens incubated at pH 5.0 contained 32 ng CTX/mg dry dentin/24 h. The specimens incubated at pH 7.4 released 5.1 ± 1.3 ng CTX/mg dry dentin/24 h. Groups identified by different letters were significantly different from controls (p<0.01). N=10 specimens/group. Values are mean ± SD.
When the media was analyzed for ICTP telopeptides, the control media released 20.27 ± 0.39 ng ICTP/mg dry dentin/24 h (Fig. 1B) (dotted line). The media from beams incubated with cathepsin B or S contained 24.7 ± 9.4 and 23.0 ± 0.21 ng ICTP/mg dry dentin/24 h, respectively, values that were not significantly different from controls. In contrast, the media from beams incubated with cathepsin K or L showed significantly less (p<0.01) ICTP; 1.4 ± 0.7 and 0.9 ± 0.2 ng ICTP/mg dry dentin/24 h, respectively.
When the incubation media was analyzed for CTX telopeptides (Fig. 1C), control media released 0.45 ± 0.12 ng CTX/mg dry dentin/24h, while cathepsins B, L and S media contained 0.8 ± 0.3, 0.5 ± 0.1 and 0.6 ± 0.1 ng CTX/mg dry dentin/24h. None of these values were significantly different. However, media from the beams incubated with cathepsin K contained 32.1 ± 9.8 ng CTX/mg dry dentin/24 h when incubated at pH 5. This value was significantly higher (p<0.01) compared to the others (Fig. 1C). When rh cathepsin K was incubated with demineralized beams at pH 7.4, the medium contained 6.1 ± 1.3 ng CTX/mg dry dentin/24 h indicating that cathepsin K activity at pH 7.4 was 6.1/32.1 × 100 = 19.0% as much as was seen at pH 5.0
3.2 Effects of increasing concentrations of ATA on dry mass loss or ICTP/CTX telopeptide production (experiment 2)
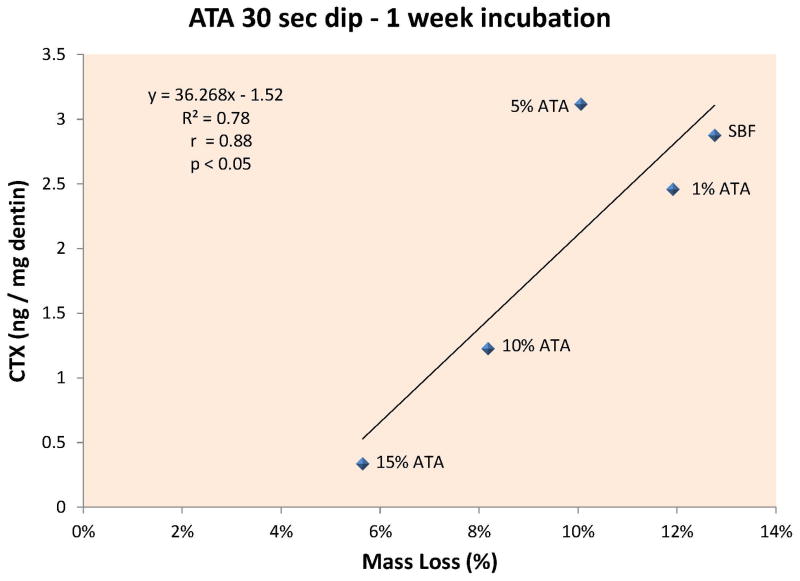
The results of experiment 2 are summarized in table 1. When completely demineralized dentin beams were incubated in control SBF for 1 week, their loss of dry mass was 12.8 ± 2.8% (Fig. 2A, dotted line). Experimental demineralized beams dipped in 1, 5, 10 or 15 wt% ATA made up in SBF (pH 7.4) for 30 sec showed a loss of dry mass 11.9 ± 1.9, 10.0 ± 2.8, 8.2 ± 2.8 and 5.7 ± 1.8%. Only the 15 wt% ATA result was significantly different (p<0.05) from the rest. Was this loss of dry mass due to MMP or cathepsin activity or both?
Table 1.
Comparison of the effects of ATA concentrations on the loss of dry mass and release of ICTP and CTX from dentin
| Release of telopeptides (ng/mg dry mass/wk) | |||
|---|---|---|---|
| wt% ATA | % loss of dry mass | ICTP | CTX |
| 0 | 12.8 ± 2.8 (10)a | 4.0 ± 0.7 (10)A (408 ± 70 ng/L) | 2.8 ± 0.4 (10)a (287 ± 22 ng/L) |
| 1 | 11.9 ± 1.9 (10)a | 3.7 ± 0.5 (10)A (379 ± 62 ng/L) | 2.5 ± 0.8 (10)a (251 ± 80 ng/L) |
| 5 | 10.0 ± 2.8 (10)a,b | 3.8 ± 0.8 (10)a (382 ± 62 ng/L) | 3.1 ± 0.8 (10)a (307 ± 73 ng/L) |
| 10 | 8.2 ± 2.8 (10)a,b | 4.0 ± 1.0 (10)a (400 ± 85 ng/L) | 1.2 ± 0.7 (10)a,b (124 ± 67 ng/L) |
| 15 | 5.7 ± 1.8 (10)b | 3.4 ± 0.7 (10)B (337 ± 70 ng/L) | 0.3 ± 0.2 (10)b (34 ± 23 ng/L) |
Values are means ± 1 SD (n = 10). Groups identified by different superscript letters are significantly different (p<0.05). Numbers in parentheses labeled ng/L are the same result as the previous value, but expressed in ng telopeptide/L rather than ng telopeptide/mg dry mass/wk.
Figure 2.
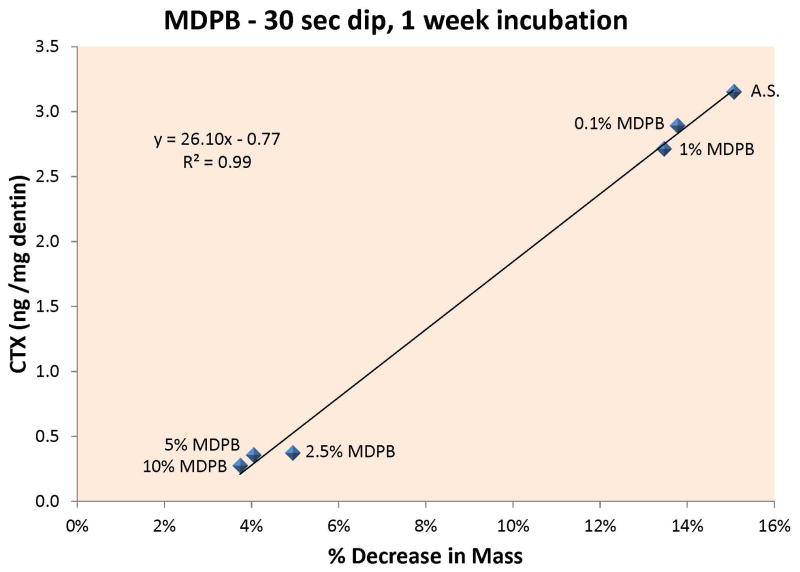
Figure 2A – When the rate of release of CTX from dentin beams incubated in increasing concentrations of ATA was plotted against the percent loss in dry mass, there was a highly significant (p<0.05) positive correlation between CTX release and loss of dry mass, indicating that cathepsin K can cause significant loss of dry mass at pH 7.4 and that the enzyme is inhibited by ATA concentrations ≥ 10 wt% at pH 7.4. 2B – When the rate of release of CTX from dentin beams incubated in increasing concentrations of MDPB were plotted against the % loss of dry mass of dentin, there was a highly significant (p<0.001) positive correlation, indicating that MDPB inhibited cathepsin K at concentrations ≥ 2.5 wt% MDPB, at pH 7.4.
Analyzing the media from the beams for ICTP telopeptides revealed that control media contained 4.0 ± 0.7 ng ICTP/mg dry dentin/week (Table 1). Beams dipped in 1, 5, 10 or 15% ATA released 3.7 ± 0.5, 3.8 ± 0.8, 4.0 ± 1.0 and 3.4 ± 0.7 ng ICTP/mg dry dentin/week (Table 1). There were no significant differences among those values (p>0.05). Regression analysis of ICTP release at increasing ATA concentrations versus loss of dry mass failed to disclose any significant correlations.
Analyzing the media from the beams for CTX telopeptides showed that the controls released 2.8 ± 0.4 ng CTX/mg dry dentin/week. Beams dipped in 1, 5, 10 or 15% ATA released 2.5 ± 0.8, 3.1 ± 0.8, 1.2 ± 0.7 and 0.3 ± 0.2 ng CTX/mg dry dentin/week (Table 1). The last value was significantly (p<0.05) lower than all other values. When the rate of release of CTX from dentin beams incubated in increasing concentrations of ATA was plotted against the percent loss of dry mass, there was a significant (p<0.05) positive correlation (Fig. 2A) between CTX release and loss of dry mass indicating that ATA inhibited cathepsin K.
3.3 Effects of increasing concentration of MDPB on dry mass loss and ICTP/CTX telopeptide production (experiment 3)
The results of experiment three are summarized in Table 2. When completely demineralized dentin beams were dipped in water (control) or 0.1, 1.0, 2.5, 5.0 or 10.0 wt% MDPB for 30 sec prior to incubation in SBF for one week, the beams exhibited the following loss of dry mass: controls lost 15.0 ± 2.6% of their dry mass, while beams treated with 0.1 and 1.0 wt% MDPB lost 13.8 ± 1.6 and 13.5 ± 2.6% of their dry mass, respectively (p>0.05). Beams treated with 2.5, 5.0 or 10 wt% MDPB lost 5.0 ± 1.2, 4.1 ± 0.8% and 3.7 ± 1.8% of their dry mass loss in one week (Table 2). The last three values are significantly lower (p<0.05) than the first three values (Table 2). Was this loss of dry mass due to MMPs or cathepsins or both?
Table 2.
Comparison of the effects of MDPB concentrations on the loss of dry mass and release of telopeptides from dentin
| Release of telopeptides (ng/mg dry mass/wk) | |||
|---|---|---|---|
| wt% MDPB | % loss of dry mass | ICTP | CTX |
| 0 | 15.0 ± 2.6 (10)a | 1.1 ± 0.2 (10)A (356 ± 27 ng/L) | 3.2 ± 0.3 (10)a (305 ± 6 ng/L) |
| 0.1 | 13.8 ± 1.6 (10)a | 0.8 ± 0.1 (10)A (326 ± 4 ng/L) | 2.9 ± 0.4 (10)a,b (303 ± 9 ng/L) |
| 1.0 | 13.5 ± 2.6 (10)a | 0.8 ± 0.2 (10)A (320 ± 19 ng/L) | 2.7 ± 0.2 (10)b (284 ± 22 ng/L) |
| 2.5 | 5.0 ± 1.2 (10)b | 0.9 ± 0.3 (10)A (339 ± 37 ng/L) | 0.4 ± 0.1 (10)c (39 ± 13 ng/L) |
| 5.0 | 4.1 ± 0.8 (10)b | 0.4 ± 0.2 (10)B (281 ± 25 ng/L) | 0.4 ± 0.1 (10)c (36 ± 10 ng/L) |
| 10.0 | 3.1 ± 1.8 (10)b | 0.3 ± 0.02 (10)B (267 ± 4 ng/L) | 0.3 ± 0.1 (10)c (29 ± 6 ng/L) |
Values are means ± 1 SD (n = 10). Groups identified by different superscript letters are significantly different (p<0.05). Numbers in parentheses labeled ng/L are the same results as the previous value, but expressed in ng/L instead of ng/mg dry wt/wk.
When the incubation medium was analyzed for ICTP telopeptides, the controls released 1.1 ± 0.2 ng ICTP/mg dry dentin/week (Table 2). Experimental beams dipped in 0.1, 1.0, 2.5, 5.0 or 10.0 wt% MDPB in SBF, released 0.8 ± 0.1, 0.8 ± 0.2, 0.9 ± 0.3, 0.4 ± 0.2 and 0.3 ± 0.02 ng ICTP/mg dry dentin/week. The last value was significantly lower (p<0.05) than the other values.
When the incubation media was analyzed for CTX telopeptides, the controls release 3.2 ± 0.3 ng/mg dry dentin/week, while the beams dipped in 0.1 wt% MDPB lost 2.9 ± 0.4 ng CTX/mg dry dentin/week. Beams treated with 1.0, 2.5, 5.0 or 10.0 wt% MDPB released 2.7 ± 0.2, 0.4 ± 0.1, 0.4 ± 0.1, 0.3 ± 0.1 ng CTX/mg dry dentin/week (Table 2). The last three values were significantly lower (p<0.05) than the first three values. When the CTX release rate was plotted against the loss of dry mass, a highly significant positive correlation was found (Fig. 2B), p<0.001.
4. Discussion
The results of the first part of this study conclusively show that after the addition of exogenous cathepsins B, K, L or S to demineralized dentin beams, cathepsin K was the only cathepsin tested that released more (p<0.05) CTX from demineralized dentin at pH 7.4 than did controls. The previous work on CTX production from bone powder was also done at pH 5.5 [17,18]. This requires rejection of the first null hypothesis that cathepsin K is not the only cathepsin that can form CTX telopeptides. Control dentin beams released 0.45 ng CTX/mg dry dentin/24 h, while beams incubated with 10 μg of rh cathepsin K released 32 ng CTX/mg dry dentin/24 h at pH 5.0. When we ran the same reaction at pH 7.4, cathepsin K released 6.1 ± 1.3 ng CTX/mg dry dentin/24 h indicating that although the optimum pH for cathepsin K is 5.0, it is still active, albeit at a low level at pH 7.4 [7]. When the media was analyzed for ICTP telopeptides, the control beams released 20 ng ICTP/mg dry dentin/24 h. However, dentin beams incubated with 10 μg of rh cathepsin K or L had almost no ICTP in their media. If these beams released the same amount to ICTP from endogenous MMP activity, the ICTP levels should have been at the level of the dotted line. Since they were much lower, it suggests that both cathepsin K and L can degrade ICTP produced by endogenous dentin MMPs at pH 7.4. More research using specific cathepsin inhibitors and measuring ICTP telopeptide levels before and after incubation with cathepsins K and L at pH 5.5 vs. 7.4 are required to prove that cathepsin L can destroy ICTP. Sassi et al. [18] was the first to report that cathepsin K could degrade ICTP at pH 5.5. To our knowledge, no one has reported that cathepsin L can destroy ICTP telopeptides at pH 7.4.
In addition, the low rate of release of CTX in control beams (Fig. 1C) indicates that the previously suggested telopeptidase activity of non-collagenolytic cysteine cathepsins [19,20] may not be true, at least at pH 7.4.
The slight increase of ICTP release by rh cathepsin B and S may be related to their ability to remove proteoglycans (PGs) and their glycosaminoglycan (GAG) side chains from the collagenous matrix. Interactions of collagen microfibrils within fibrils depend on PG-GAG interfibrillar bridges [21], and these PGs are also essential in collagen fibrillogenesis. Non-collagenolytic cathepsins, including cathepsin B and S, can destabilize the insoluble type I collagen structure by removing PGs [19], which may allow increased MMP-2 and -9 telopetidase activity [22] by exposing cleavage sites and thus resulting with increase in ICTP values. Alternatively, cathepsin B and S may activate PG-proMMP-9 complexes by removing PG from the complex. In other tissues, PG-proMMP-9 heteromers bind type I collagen as well as gelatin in vivo, while monomeric MMP-9 has much greater affinity to gelatin [22]. Removal of PG from the complex activates MMP-9 [21]. Since MMP-2 has been suggested to be the main MMP within dentin matrix [9] and MMP-9 may be present in considerably lower amounts, the increase of ICTP with cathepsin B and L observed in this study may be due to increased MMP-9 activity after PG removal.
Karsdal et al. [23] reported that the presence of CTX in body fluids or incubation media confirms that there is active cathepsin K in the tissue. Thus, one can unequivocally state the presence of CTX in media confirms that the tissue bathed by that medium contains active cathepsin K. Although many experts do not expect cathepsin K to be active at pH 7.4, it apparently was active at that pH in the current experiments. Kometoni et al. [7] reported that human cathepsin K activity at pH 5.5 was 91% of its maximum activity at pH 5.5, 85% of its maximum at pH 6.5 and 11% of its maximum at pH 7.5. Cathepsin K collagenolytic activity is regulated by complex formation with GAGs; if the complex formation is inhibited, cathepsin K retains its proteoglycan degradation ability but loses collagenolytic capacity [24]. Incubation of insoluble type I collagen with exogenous monomeric cathepsin K is believed to first release GAGs from the collagen fiber surface fibrils, allowing GAG-cathepsin K complex formation. This would result in collagenolysis, allowing access to cryptic PGs within the fibril core, with the unfolding of fibril and subsequent release of more GAGs and further increase in collagenolysis [21]. This kind of collagen fibril unfolding was seen in demineralized dentin surface after 24h and 90 days in SBF and after 250 days when SBF contained proteases that reduced dentin collagenolytic activity by 75% [25]. Therefore, it is possible that the cathepsin K-related collagenolytic sequence proposed by Panwar et al. [26] also occurs in demineralized dentin.
The ability of high concentrations (15%) of ATA to lower the loss of dry mass from demineralized dentin, combined with its ability to lower the rate of release of CTX by 90%, whereas it only lowered ICTP release rate by 18%, indicates that ATA inhibits the endogenous cathepsin K in dentin matrices more than it inhibits total MMP activity. It also suggests that endogenous matrix cathepsin K causes significant degradation of dentin collagen.
In contrast, the ability of 5% MDPB to lower dry mass loss, combined with its ability to lower the rate of release of CTX by 87% and ICTP by 73%, indicates that MDPB inhibits both degradation pathways equally well. These results require rejection of the second test null hypothesis that ATA and MDPB cannot inhibit both degradation pathways of dentin collagen.
Both of the quaternary ammonium methacrylates (ATA and MDPB) were used as pretreatments of demineralized dentin. That is, the beams were dipped into the appropriate concentration for 30 s, the excess blotted off, and then the beams were dropped into 0.5 ml of SBF. The carry-over of ATA or MDPB would only be 1.4% of the original concentration if all of the QAMs diffused into the incubation medium. These two QAMs seem to bind to demineralized dentin very well. How well they inhibit MMPs and cathepsins after resin infiltration and photopolymerization remains to be determined.
To date, no one has extracted cathepsin K from demineralized dentin and then isolated it using SDS-PAGE and shown its zymographic activity. Indeed, Sulkala et al. [3] were able to detect MMP-8 but not cathepsin K in demineralized dentin with Western blot, indicating that the cathepsin K levels in relation to MMPs level are truly low. Alternatively, cathepsin K may be difficult to renature after extraction and SDS-PAGE separation.
By extracting total RNA from mature human odontoblasts and pulp tissue, Tersariol et al. [27] reported gene expression of cathepsins B, C, D, K, L, L2, O and S in the dentin-pulp complex. Immunostaining of dentin matrix with cathepsin B-specific antibody revealed the presence of cathepsin B, and the use of the fluorogenic substrate Z-FR-MCA (Sigma), revealed significant cathepsin B activity in dentin [27,28]. That activity was strongly inhibited by E-64, a cathepsin inhibitor, but not with a serine protease inhibitor, MMP inhibitor or aspartyl protease inhibitors. However, no one has published zymograms showing gelatinolytic bands at 28–37 kDa that are positively identified by Western blots as antibody-specific cathepsins.
Recently, Vidal et al. [6] using fluorescent antibodies of cathepsin K, identified the presence of both cathepsin B and cathepsin K in both intact and caries-affected dentin being 6-7-fold high in caries-affected dentin. This is the first time the presence of cathepsin K has been demonstrated in dentin.
The presence of cathepsin K is important because this mammalian cathepsin attacks type I collagen in both helical and N and C-terminal portions. It acts much like Clostrodium histolyticum collagenase does, with multiple cleavage sites, including CTX, rather than one-quarter/three quarter fragments [29].
There appear to be differences in inhibition of MMPs and cathepsins by ATA and MDPB even though both are QAMs. Tezvergil-Mutluay et al. [15] speculated that the catalytic site of MMPs contains cysteine-rich repeats that are necessary for QAM binding. The repeats include a glutamic acid residue adjacent to a histidine residue, both of which are necessary for its catalytic activity [30]. The free carboxylic acid group would give a fixed negative charge at pH 7.4. The cationic QAM may electrostatitically bind to such a negative charge, sterically blocking the active site, thereby inhibiting MMP activity. The active sites of cysteine cathepsins contain cysteine, histidine and aspartane residues. The cysteine forms a catalytic thiolate-imidazolium ion pair [31]. The cysteine thiolate acts as a nucleophile for attack on the carbonyl carbon atom of the scissle peptide bond [31]. Scaffa et al. [14] performed computational modeling of the docking of chlorhexidine (CHX), another cationic protease inhibitor, to various cathepsins. They identified the docking of CHX to the cysteine 29 and histidine 199 portions of the active site(s) and Tyr 75, Pro 76 and Glu 245 at subsite 52 binding sites. It is likely that cationic QAMs like ATA and MDPB would show similar electrostatic attractions that would block the active sites of MMPs and cathepsins. In addition, MDPB, with its long (12 methylene groups) tail may also interact with more hydrophobic sites that would allow it to inhibit both MMPs and cathepsins. Future docking studies may shed additional insight on QAM-protease interactions. ATA is a relatively short acrylate with a cationic charge on its trimethylammonium termination. Its small size may allow it to penetrate around collagen to reach matrix-bound proteases.
We speculate that the ratio of the rate of production of CTX to the rate of production of ICTP telopeptides per unit time, provides quantitative information about the relative contribution of cathepsin K to the total degradation activity of dentin matrix even at pH 7.4. Although dentin matrix may contain cathepsins B, L and S, in addition to cathepsin K, these cathepsins do not release CTX or ICTP telopeptides from dentin. Further experiments will examine the potential release of N-terminal cross-linked telopeptides from dentin matrices.
As there seem to be more different MMPs in dentin (MMP-2, -8 and -9) [2,3,9] that can attack collagen than there are collagenolytic cathepsins, what may be more important than their number is their specific activity. That is, the actual enzymatic activity of these proteases per μg of enzyme protein. Also, the sequences of activation and inhibition of are of interest. Clearly, more research is needed before we truly understand all the details of the endogenous proteases of dentin and their regulation.
Under in vitro conditions that simulate degrading hybrid layers in vivo, MMPs and cathepsin K seem to be responsible for most of the degradation of dentin matrices medium at pH 7.4, even though cathepsin K cannot be operating at its optimum pH of 5.5. We are coming closer to the goal of understanding how MMPs vs. cathepsin K function in the degradation of dentin hybrid layers.
Acknowledgments
This work was supported, in part, by grant R01 DE015306 from the NIDCR to DHP (PI) and by grants from the Academy of Finland and the Finnish Dental Society Apollonia to AT-M and LT (PIs). Dr. Pashley is a visiting scholar at the King Abdulaziz University, School of Dentistry, Jeddah, Saudi Arabia. The authors are grateful to Mrs. Michelle Barnes for her secretarial support.
Footnotes
None of the authors have any conflict of interests regarding the products or procedures used in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakabayashi N, Pashley DH. Hybridization of Dental Hard Tissues. Quintessence Publishing Co; Chicago: 1998. [Google Scholar]
- 2.Mazzoni A, Mannello F, Tay FR, Tonti GAM, Papa S, Mazzotti G, Di Lenarda R, Pashley DH, Breschi L. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. J Dent Res. 2007;86:436–440. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- 3.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. 2007;52:121–127. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Simecck JW, Diefenderfer KE, Cohen ME. An evaluation of replacement rates for posterior resin-based composites and amalgam restorations in US Navy and Marine Corps recruits. J Am Dent Assoc. 2009;140:200–209. doi: 10.14219/jada.archive.2009.0134. [DOI] [PubMed] [Google Scholar]
- 5.Wood J, Sobolewski P, Brougher JA, Thakur V, Arola D, Nazari A, Tay FR, Pashley DH. Measurement of micro-strain across resin-dentin interfaces using microscopic moire interferometry. Dent Mater. 2008;24:859–866. doi: 10.1016/j.dental.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vital CMP, Tjäderhane L, Scaffa PM, Tersariol IL, Pashley DH, Nader HB, Nascimento FD, Carrilho MR. Abundance of MMPs and cysteine cathepsin in caries-affected dentin. J Dent Res. 2014;93:269–274. doi: 10.1177/0022034513516979. [DOI] [PubMed] [Google Scholar]
- 7.Kometoni M, Nonomura K, Tomoo T, Nina S. Hurdles in the drug discovery of cathepsin K inhibitors. Curr Topics in Med Chem. 2010;10:734–744. doi: 10.2174/156802610791113478. [DOI] [PubMed] [Google Scholar]
- 8.Tjäderhane L, Carrilho MR, Breschi L, Tay FR, Pashley DH. Dentin basic structure and composition – an overview. Endod Topics. 2012;20:3–29. [Google Scholar]
- 9.Mazzoni A, Breschi L, Carrilho M, Nascimento FD, Orsini G, Ruggeri A, Gobbi P, Mazoli L, Tay FR, Pashley DH, Tjäderhane L. A review of the nature, role and function of dentin non-collagenous proteins. Part II: enzymes, serum proteins, and growth factors. Endod Topics. 2012;21:19–40. [Google Scholar]
- 10.Tezvergil-Mutluay A, Mutluay M, Sesogullari-Dirihan R, Agee KA, Key WO, Scheffel DLS, Breschi L, Mazzoni A, Tjäderhane L, Nishitani Y, Tay FR, Pashley DH. Effects of phosphoric acid on the degradation of human dentin matrix. J Dent Res. 2013;92:87–91. doi: 10.1177/0022034512466264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osorio R, Yamauti M, Osorio E, Ruiz-Requena ME, Pashley DH, Tay FR, Toledano M. Effect of dentin etching and chlorhexidine application on metalloproteinase-mediated collagen degradation. Eur J Oral Sci. 2011;119:79–85. doi: 10.1111/j.1600-0722.2010.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, Donnelly A, Garcia-Godoy F. From dry bonding to wet bonding to ethanol-wet bonding: A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent. 2007;20:7–20. [PubMed] [Google Scholar]
- 13.Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of matrix metalloproteinases 2, 8 and 9 by chlorhexidine. Clin Diagn Lab Immunol. 1999;6:437–439. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scaffa PMC, Vidal CMP, Barros N, Gesteira TF, Carmona AK, Breschi L, Pashley DH, Tjäderhane L, Terariol ILS, Nascimento FD, Carrilho MR. Chlorhexidine inhibits the activity of dental cysteine cathepsins. J Dent Res. 2012;91:420–425. doi: 10.1177/0022034511435329. [DOI] [PubMed] [Google Scholar]
- 15.Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay M, Cadenaro M, Breschi L, Nishitani Y, Tay FR, Pashley DH. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J Dent Res. 2011;90:535–540. doi: 10.1177/0022034510389472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung VW-H, Darvell BW. Artificial salivas for in vitro studies of dental materials. J Dent. 1997;25:475–484. doi: 10.1016/s0300-5712(96)00068-1. [DOI] [PubMed] [Google Scholar]
- 17.Garnero P, Ferreras M, Karsdal MA, Nicamhlaoibh R, Ristelli J, Borel O, Qvist P, Delmas PD, Foged NT, Delaisse JM. The type I collagen fragment ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J Bone & Min Res. 2003;18:859–867. doi: 10.1359/jbmr.2003.18.5.859. [DOI] [PubMed] [Google Scholar]
- 18.Sassi ML, Eriksen H, Risteli L, Niemi S, Mansell J, Gowen M, Risteli J. Immunological characterization of assay for carboxyterminal telopeptide of human type I collagen: loss of antigenicity by treatment with cathepsin K. Bone. 2000;26:367–373. doi: 10.1016/S8756-3282(00)00235-0. [DOI] [PubMed] [Google Scholar]
- 19.Burleigh MC, Barrett AJ, Lazarus GS. Cathepsin B1. A lysosomal enzyme that degrades native collagen. Biochem J. 1974;137:387–398. doi: 10.1042/bj1370387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirschke H, Kembhavi AA, Bohley P, Barrett AJ. Action of rat liver cathepsin L on collagen and other substrates. Biochem J. 1982;201:367–372. doi: 10.1042/bj2010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winberg JO, Berg E, Kolset SO, Uhlin-Hansen L. Calcium-induced activation and truncation of promatrix metalloproteinase-9 linked to the core protein of chondroitin sulfate proteoglycans. European Journal of Biochemistry. 2003;270:3996–4007. doi: 10.1046/j.1432-1033.2003.03788.x. [DOI] [PubMed] [Google Scholar]
- 22.Malla N, Berg E, Uhlin-Hansen L, Winberg JO. Interaction of pro-matrix metalloproteinase-9/proteoglycan heteromer with gelatin and collagen. Journal of Biological Chemistry. 2008;283:13652–65. doi: 10.1074/jbc.M709140200. [DOI] [PubMed] [Google Scholar]
- 23.Karsdal MA, Woodworth T, Henriksen K, Maksymowych W, Genant H, Vergnand P, Christiansen C, Schubert T, Qvist P, Schett G, Platt A, Bay-Jensen A-C. Biochemical markers of ongoing joint damage in rheumatoid arthritis – current and future applications, limitations and opportunities. Arthritis Res & Therapy. 2011;13:215–233. doi: 10.1186/ar3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lecaille F, Brömme D, Lalmanach G. Biochemical properties and regulation of cathepsin K activity. Biochimie. 2008;90:208–226. doi: 10.1016/j.biochi.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. College degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–221. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 26.Panwar P, Du X, Sharma V, Lamour G, Castro M, Li H, Brömme D. Effects of cysteine proteases on the structural and mechanical properties of collagen fibrils. J Biol Chem. 2013;288:5940–5950. doi: 10.1074/jbc.M112.419689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tersariol M, Geraldeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins MT, Carrilho MR, Pashley DH, Tay FR, Salo T, Tjäderhane L. Cysteine cathepsins in human dentin-pulp complex. J Endod. 2010;36:475–81. doi: 10.1016/j.joen.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 28.Nascimento FD, Minciotti CL, Geraldeli S, Carrilho MR, Pashley DH, Tay FR, Nader HR, Salo T, Tjäderhane L, Tersariol LS. Cysteine cathepsins in human carious dentin. J Endod. 2011;40:506–511. doi: 10.1177/0022034510391906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novinec M, Lenarćić B. Cathepsin K: a unique collagenolytic cysteine peptidase. Biol Chem. 2013;394:1163–1179. doi: 10.1515/hsz-2013-0134. [DOI] [PubMed] [Google Scholar]
- 30.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases. Structure, function and biochemistry. Cir Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 31.Mellor GW, Thomas EW, Topham CM, Brocklehurst K. Ionization characteristics of the cyst 25/his-129 interactive system and of the modulatory group of papain: resolution of ambiguity by electronic perturbation of the quasi-mercaptopyridine leaving group in a new pyridimyl disulphide reactivity probe. Biochem J. 1993;290:289–296. doi: 10.1042/bj2900289. [DOI] [PMC free article] [PubMed] [Google Scholar]