Abstract
Syntaxins are a family of transmembrane proteins that participate in SNARE complexes to mediate membrane fusion events including exocytosis. Different syntaxins are thought to participate in exocytosis in different compartments of the nervous system such as the axon, the soma/dendrites or astrocytes. It is well known that exocytosis of synaptic vesicles at axonal presynaptic terminals involves syntaxin 1 but distributions of syntaxins on neuronal somal and dendritic, postsynaptic or astroglial plasma membranes are less well characterized. Here, we use pre-embedding immunogold labeling to compare the distribution of two plasma membrane-enriched syntaxins (1 and 4) in dissociated rat hippocampal cultures as well as in perfusion-fixed mouse brains. Comparison of Western blots of neuronal cultures, consisting of a mixture of hippocampal neurons and glia, with glial cultures, consisting of mostly astrocytes, show that syntaxin 1 is enriched in neuronal cultures, whereas syntaxin 4 is enriched in glial cultures. EM-Immunogold labeling shows that syntaxin 1 is most abundant at the plasma membranes of axons and terminals, while syntaxin 4 is most abundant at astroglial plasma membranes. This differential distribution was evident even at close appositions of membranes at synapses, where syntaxin 1 was localized to the plasma membrane of the presynaptic terminal, including that at the active zone, while syntaxin 4 was localized to nearby peri-synaptic astroglial processes. These results show that syntaxin 4 is available to support exocytosis in astroglia.
Keywords: syntaxin 4, syntaxin 1, exocytosis, electron microscopy, synapse, astroglia
INTRODUCTION
The SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex is required for membrane fusion, including exocytosis (Chen and Scheller, 2001; Jahn and Scheller, 2006; Südhof and Rothman 2009). Syntaxins are a family of transmembrane proteins that are a part of the SNARE complex, with syntaxin 1, 2, 3, 4 being plasma membrane proteins (Teng et al., 2001). A familiar form of exocytosis in the nervous system is release of synaptic vesicles from the presynaptic terminal, where syntaxin 1 is one of the plasma membrane target-SNAREs (Rizo and Südhof, 2012). Syntaxin 1 is distributed throughout the plasma membrane of axons, not just the active zone membrane (Garcia et al., 1996; Tao-Cheng et al., 2000; Hagiwara et al., 2005), and thus, may be employed in the insertion of various axon-specific plasma membrane proteins.
Information about the roles of syntaxin in exocytosis at other neuronal compartments is scarce and equivocal. Syntaxin 4 has been proposed to be the target-SNARE involved in AMPA receptor exocytosis near synapses (Kennedy et al., 2010), while syntaxin 3 is involved with AMPA receptors associated with LTP (Jurado et al., 2013). Since AMPA receptors are exocytosed throughout the somatodendritic compartment of the neuron (Opazo and Choquet, 2011; Anggono and Huganir, 2012), the necessary syntaxins must be distributed throughout this compartment where they could support the insertion of other plasma membrane proteins trafficked to this compartment (Ovsepian and Dolly, 2011; Kennedy and Ehlers, 2012).
Which syntaxins are associated with astroglial plasma membranes is also uncertain. Syntaxin1A and syntaxin 3 were reported not to be associated with glia (Fournier and Robinson, 2006), but syntaxin 1 was reported to be associated with astroglia (Hepp et al., 1999; Montana et al., 2004; Schubert et al., 2011). Syntaxin 4, however, appears to be highly enriched in astrocytes (Fournier and Robinson, 2006; Paco et al., 2009).
Here we investigate the distributions of syntaxins 1 and 4 in hippocampal cultures as well as in intact brain by using pre-embedding immunogold EM with three different antibodies against syntaxin 1, and two different antibodies against syntaxin 4. A clear picture emerges that different syntaxin family members have differential distributions in neurons and astroglia.
EXPERIMENTAL PROCEDURES
1.1. Materials and antibodies
A mouse monoclonal antibody against syntaxin 1 (clone HPC-1, syntaxin 1 ab1; used at 1:100 for EM), a rabbit polyclonal antibody against syntaxin 4 (syntaxin 4 ab1, raised against aa 2-23 of rat; used at 1: 50 for Western and 1:100 for EM) and a mouse monoclonal antibody against GFAP (clone G-A-5; used at 1:10,000 for Western) were from Sigma (Saint Louis, MU). Mouse monoclonal antibodies against syntaxin 1 (clone 78.2, syntaxin 1 ab2; used at 1:100 for EM), syntaxin 1A (clone 78.3; a subfamily of syntaxin 1; used at 1:250 for Western and 1:100 for EM), and rabbit polyclonal antibodies against syntaxin 3 (raised against aa 1-260 of rat, affinity purified, this bleed is no longer available) and syntaxin 4 (syntaxin 4 ab2, raised against aa 1-273 of rat, affinity purified; used at 1: 80 for Western and 1:100 for EM) were from Synaptic Systems (Göttingen, Germany). Mouse monoclonal antibody against alpha CaMKII (clone 6G9; used at 1:125 for Western) is from Cayman Chemical (Ann Arbor, MI). The specificity of the primary antibodies was also tested by confirming that the three different syntaxin 1 antibodies and the two different syntaxin 4 antibodies produced consistent labeling patterns.
1.2. Dissociated hippocampal glial and neuronal cultures
Rat hippocampal glial cultures were prepared from E20 fetuses and maintained in a 10% CO2 incubator at 35°C, with media containing 10% fetal bovine serum for two weeks. These conditions favor glial cell growth and the cultures consisted of almost exclusively of glial cells. Rat hippocampal neuronal cultures were then prepared from E20 fetuses and plated on the 2-week old glial culture. Both the neurons on top of glia (N+G) cultures and the parallel solo-glial (G) cultures were maintained in 5% horse serum and 2% fetal bovine serum in MEM containing Glutamax1 and N3 for 3-4 weeks. For Western blotting, both N+G and G cultures were harvested and homogenized by sonication in 20 mM HEPES buffer containing Halt phosphatase and protease inhibitor cocktails at 1:100 (Thermo Scientific, Waltham, MA).
1.3. Electrophoresis and immunoblotting
Equal amounts of protein (10 or 20 μg) were loaded into each lane for Western analysis. Samples were separated by SDS-PAGE on 4-12% gradient Bis-Tris gels from Life Technologies (Carlsbad, CA), and transferred to PVDF membranes, blocked, incubated with specified primary antibodies and then with horseradish peroxidase-conjugated secondary antibodies (1:50,000 dilution), and the signal was finally visualized by chemiluminescence (SuperSignal West Pico, Thermo Scientific, Waltham, MA).
1.4. Perfusion-fixed mouse brains
The animal protocol was approved by the NIH Animal Use and Care Committee and conforms to NIH guidelines. Perfusion fixation was performed as previously described in Tao-Cheng et al., 2007. Briefly, adult mice were deeply anesthetized with isoflurane, and intracardially perfusion fixed with 4% paraformaldehyde in PBS. Perfusion pressure was maintained at 150 mm Hg with a Perfusion-One System (MyNeurolab, Maryland Heights, MO). The perfusion-fixed brains were dissected and vibratomed into 100 μm thick coronal slices and stored in PBS at 4°C. Total fixation time was closely monitored to be 40-60 min from the flow of the fixative into the heart to the time the brain slices were vibratomed. Specific areas of the brains including the cerebral cortex, the hippocampus and the cerebellum were sampled.
1.5. Pre-embedding immunogold labeling and electron microscopy
Fixed samples [perfusion-fixed brain vibratomed into 100 μm slices, or immersion-fixed (4% paraformaldehyde in PBS for 30-45 min at room temperature) monolayer dissociated hippocampal neuronal cultures] werewashed, and then blocked and permeabilized with 5% normal goat serum and 0.1% saponin for 40-60 min. Samples were incubated with primary and secondary antibodies (Nanogold, at 1:200-250, Nanoprobes, Yaphand, NY) for 1-2 hr, fixed with 2% glutaraldehyde in PBS for 30 min, and sometimes stored in this fixative for a few days, silver enhanced (HQ kit, Nanoprobes), en block mordanted with 0.25-0.5% uranyl acetate in acetate buffer at pH 5.0 for 1 hr, treated with 0.2% osmium tetroxide in 0.1M phosphate buffer at pH 7.4 for 30 min, dehydrated in graded ethanols, and embedded in epoxy resin. The primary antibody was omitted in some experiments to control for nonspecific labeling by the secondary antibody.
1.6. Identification of cell types and morphometry
In dissociated hippocampal cultures, astrocytes formed a flat glial bed on the substrate, while the neuronal somas and processes were situated on top of the glial bed. Neuronal somas and dendrites were identified by the presence of synapses onto their plasma membrane, and sampling of dendrites included dendritic shafts as well as spines. Axons were identified by the presence of abundant synaptic vesicles, and sampling of axons included axonal processes and terminals. Synapses were identified by clusters of synaptic vesicles in the presynaptic axon terminal, rigidly apposed synaptic gap, and the postsynaptic density. Astrocytes were identified by their abundant intermediate filaments, glycogen granules and/or the gap junctions between plasma membranes. In perfusion-fixed brains, astrocytes were further identified by their peri-endothelial or peri-synaptic locations (Peters et al., 1991).
Comparisons of labeling intensity among astrocytes, axons or dendrites were carried out in dissociated cultures because the penetration of reagents was relatively even in these monolayers in contrast to that in perfusion-fixed brains where the depth of penetration is only ~ 1-2 μm so that labeling intensity may vary widely.
Since syntaxins are transmembrane proteins, gold particles located in the cytoplasm that are not associated with membranes were attributed to non-specific background signals. One characteristic of the pre-embedding immunogold method, distinct from the post-embedding method, is that both the primary and secondary antibodies, once bound to the antigen located on one side of the membrane, will stay limited by the membrane. The epitopes of all syntaxin antibodies used here are located on the cytoplasmic side of the membrane, and indeed, very little signal was detected on the opposite side of the membrane.
Labeling intensity was measured by first tracing the plasma membranes of identified entities, and then counting all associated gold particles within 20 nm of the correct side of the plasma membrane. Labeling intensity was calculated by dividing the number of gold particles by the length of the plasma membrane, expressed as label/μm plasma membrane.
At least five grid openings randomly chosen from each thin section were examined. Every identifiable element was photographed at 10,000 magnification on a JEOL 1200 EXII transmission electron microscope with a bottom-mounted digital CCD camera which collects 2.6 K × 2.6 K pixels (AMT XR-100, Danvers, MA, USA).
RESULTS
2.1. Syntaxin 4 but not syntaxin 1 is enriched in glial cultures
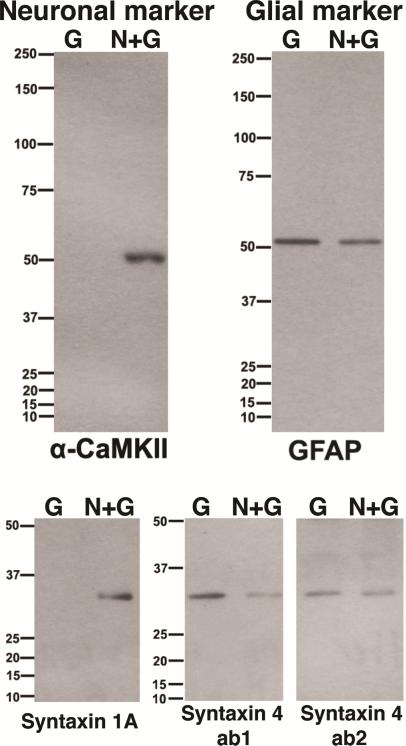
Dissociated hippocampal glial cultures (G) and neuronal cultures containing glia cells (N+G) were homogenized, electrophoresed and processed for Western immunoblot analysis. Alpha CaMKII and GFAP were used as neuronal and glial markers, respectively. Syntaxin 1A appeared in neuronal cultures, while syntaxin 4 was enriched in glial cultures (Fig. 1)
Fig. 1. Different distribution of syntaxin 1 and 4 in neurons and glia with Western immunoblotting.
G - from glial cultures consisting of astrocytes essentially, and N+G - from dissociated hippocampal neurons grown on top of glial cells. Equal amounts of protein (10 μg) were loaded into each lane. (top left,) Calcium calmodulin dependent kinase II (α-CaMKII) is a neuronal marker present exclusively in N+G cultures but not in G. (top right,) Glial fribrillary acidic protein (GFAP) is an astroglial marker present more in G than in N+G cultures. (lower left,) Syntaxin 1A was exclusively in N+G cultures. (lower right,) Syntaxin 4 (two different antibodies, ab1 and ab2) is enriched in glia (more in G than in N+G cultures).
2.2. Label for Syntaxin 1 is abundant on axonal but not on dendritic or astroglial plasma membranes
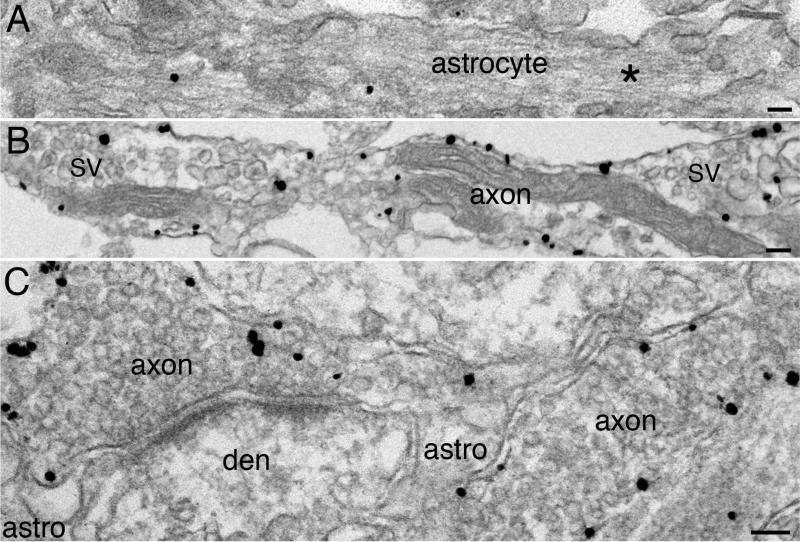
Immunogold labeling with three different syntaxin 1 antibodies showed plasma membrane label tracking axons (Fig. 2B and C), consistent with previous reports (Garcia et al., 1996; Tao-Cheng et al., 2000; Hagiwara et al., 2005). Very little, if any, syntaxin 1 label was detected on astroglial plasma membrane (Fig. 2A and C) or dendritic membrane (Fig. 2C). Intensity of label for syntaxin 1 at plasma membrane of axons was significantly higher than that of dendrites and astrocytes (Fig. 3).
Fig. 2. Syntaxin 1 is concentrated on axolemma but not detected on plasma membrane of astrocytes or dendrites.
(A & B) are from dissociated hippocampal cultures, (C) is from perfusion-fixed mouse cerebral cortex. Plasma membrane of astrocytes was not labeled by syntaxin 1A antibody (A). Asterisk (* in A) mark the characteristic intermediate filaments in astrocytes, and a few labels in the astroglial cytoplasm represent background noise. Label for syntaxin 1 was detected on the plasma membrane along axons (B, ab2), characterized by clusters of synaptic vesicles (SV). Label for syntaxin 1A outlined the axonal plasma membranes (C), but not the plasma membrane of dendrites (den) or the peri-synaptic astrocyte processes (astro). Scale bar = 0.1 μm.
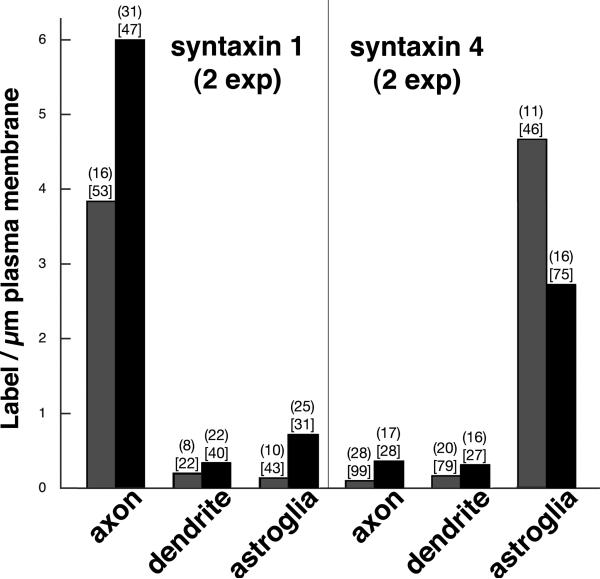
Fig. 3. Differential labeling intensity for syntaxin 1 and syntaxin 4 on plasma membranes of axons, dendrites and astroglia.
For syntaxin 1, exp 1 is with syntaxin 1 ab1, exp 2 is with syntaxin 1A antibody. Statistical significance is at P<0.0001 between axon and dendrite or astroglia for both exp (ANOVA with Tukey's post test). For syntaxin 4, exp 1 is with ab1, exp 2 is with ab2. Statistical significance is at P<0.0001 between astroglia and axon or dendrite for both exp. The first number above each bar is the sample size (n) of identified entities. The second number in [bracket] is the total length of plasma membrane measured in μm. Although it could not be ruled out that low levels of syntaxin 1 may be present in plasma membranes of dendrites and astrocytes, and that low levels of syntaxin 4 may be present in plasma membranes of axons and dendrites, we attribute most of this low level labeling to non-specific background.
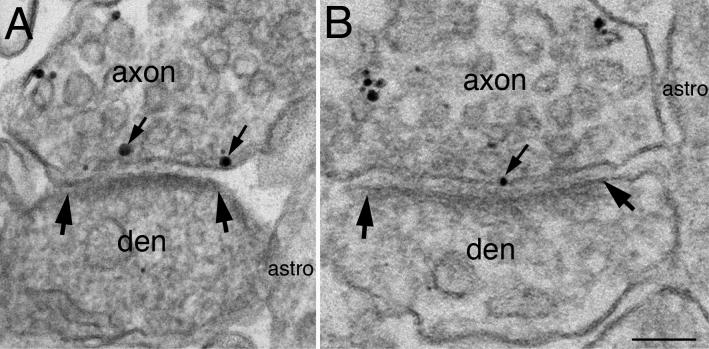
At synapses, the plasma membrane of the axon terminals was labeled for syntaxin 1, including the active zone facing the postsynaptic density (Fig. 4). Both peri-synaptic astroglial and dendritic plasma membranes at synapses were typically negative for syntaxin 1.
Fig. 4. Syntaxin 1 is present at the active zone of the presynaptic terminal.
(A) is from dissociated hippocampal cultures labeled for syntaxin 1A, and (B) is from CA1 region of hippocampus from perfusion-fixed mouse labeled for syntaxin 1 (ab2). Label for both antibodies were present on the plasma membrane of axons, but not dendrites (den) or peri-synaptic astrocytes (astro). Small arrows point to label at the active zone, which apposes the postsynaptic density, the area between two large arrows.
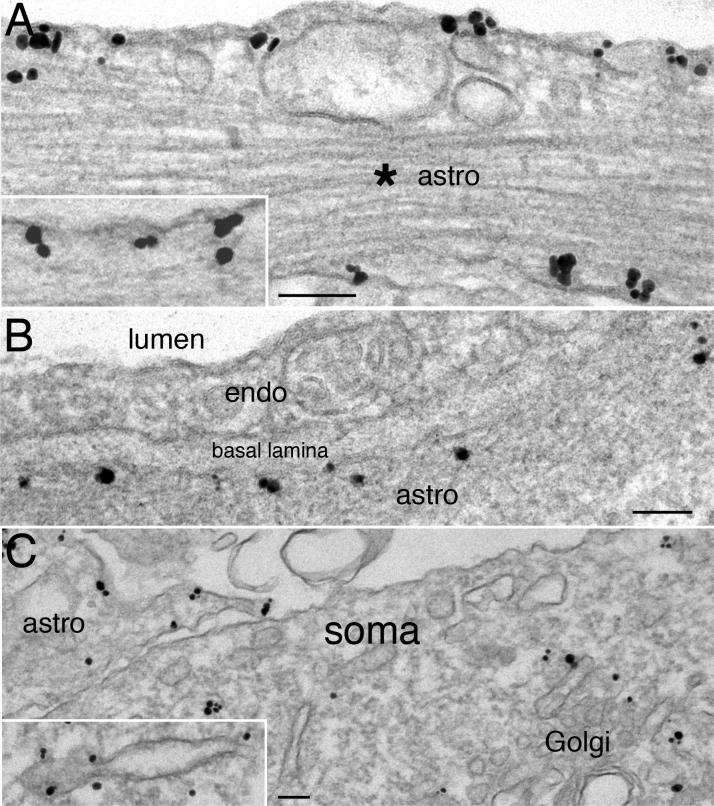
2.3. Syntaxin 4 is concentrated on the plasma membrane of astrocytes
Results with two different syntaxin 4 antibodies agreed that both antibodies intensely labeled the astroglial plasma membrane (Fig. 5A). In Fig. 5B, the identification of the syntaxin 4-labeled astrocytic process was by its periendothelial location and the characteristic basal lamina (Peters et al., 1991). Plasma membrane of neuronal soma (Fig. 5C) or dendrites (not shown) was typically not labeled for syntaxin 4. Intensity of label for syntaxin 4 at plasma membrane of astroglia was significantly higher than that of the axons or dendrites (Fig. 3), which were indistinguishable from the non-specific background noise.
Fig. 5. Syntaxin 4 is concentrated on plasma membrane of astrocytes but not hippocampal neurons.
(A & C) are from rat hippocampal cultures. (A) Plasma membrane of astrocytes was labeled with syntaxin 4 (ab1 in A, ab2 in inset of A). Asterisk (* ) marks astroglial intermediate filaments. (B) is from the CA1 region of perfusion-fixed mouse hippocampus. Label for syntaxin 4 (ab1) outlined the peri-endothelial astroglial (astro) plasma membrane, but not the brain endothelial cell (endo) that lined the lumen of a blood vessel. A characteristic basal lamina between the brain endothelial cell and the astroglial process further unequivocally identified these cell types. (C) Plasma membrane of a neuron was unlabeled, while that of an astroglial process (astro on upper left) clearly labeled for syntaxin 4 (ab1). The Golgi complex and some tubulovesicular structures in neurons (inset of C, ab2) were labeled for syntaxin 4. Scale bar = 0.1 μm.
Since membrane proteins pass through endoplasmic reticulum (ER) and Golgi complexes during their biosynthesis (Rothman and Wieland, 1996), it is not surprising to see label for syntaxin 4 in these organelles in astrocytes (data not shown). However, it is interesting that the Golgi complex, ER and some tubulovesicular membranous structures in the neuronal soma labeled for syntaxin 4. Such labeling patterns occurred in dissociated neurons (Fig. 5C) as well as in perfusion-fixed brains (data not shown). These observations indicate that while syntaxin 4 is not readily detectable at neuronal plasma membranes, it is present in intracellular organelles in neurons, perhaps involved in vesicular fusion events.
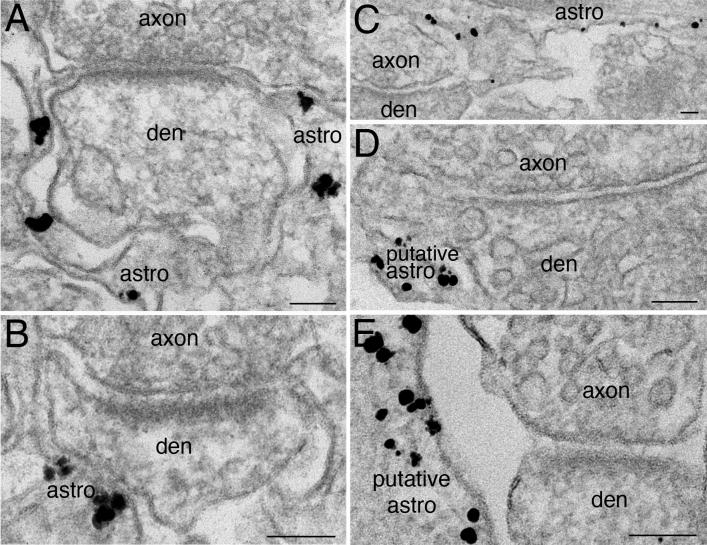
At synaptic locations neither presynaptic axon terminals nor postsynaptic dendritic elements preferentially labeled for syntaxin 4, but nearby processes did label for syntaxin 4 (Fig. 6). In perfusion-fixed brains, label for syntaxin 4 was prominently located on peri-synaptic astroglial processes apposed to spines (Fig. 6A and B). In dissociated cultures, putative astroglial processes, which could be situated very close to synapses, labeled for syntaxin 4, though the synapses themselves did not (Fig. 6C, D, E).
Fig. 6. Syntaxin 4 is concentrated on plasma membrane of astroglial processes at peri-synaptic locations but not the axonal or dendritic elements of the synapse.
(A) is from perfusion-fixed mouse cerebellum labeled with syntaxin 4 ab2, (B) is from mouse CA1 region of perfusion-fixed hippocampus labeled with syntaxin 4 ab1, and (C, D, E) are from dissociated hippocampal cultures labeled with syntaxin 4 ab1. In brain tissues (A, B), astrocytic processes can be unequivocally identified by their peri-synaptic locations (Peters et a., 1991). In dissociated cultures (C, D, E), astroglial identification cannot rely on peri-synaptic location alone. The syntaxin 4-labeled process in (C) is identified as astroglia due to the abundant intermediate filaments, but the processes in D and E can only be considered putative. Nevertheless, all axonal and dendritic elements nearby were clearly negative for syntaxin 4. Scale bar = 0.1 μm.
DISCUSSION
The present study documents the localization of two plasma membrane syntaxins, syntaxin 1 and syntaxin 4, using Western blot and immunogold electron microscopy. In mouse cerebral cortex, hippocampus and cerebellum as well as in rat dissociated hippocampal cultures, syntaxin 1 was abundant on axonal plasma membrane, and syntaxin 4 on astroglial plasma membrane. These results confirm previous reports that syntaxin 1 is an axonal target-SNARE, and suggests that syntaxin 4 is an astroglial target-SNARE.
Syntaxin 4 has been localized to horizontal cell processes of mammalian retina (Sherry et al., 2006; Hirano et al., 2007). However, we could not detect prominent neuronal plasma membrane labeling in hippocampus, cerebral and cerebellar cortex. In these brain regions, our EM observations showed strong preferential localization of syntaxin 4 to the plasma membrane of astrocytes, including the peri-synaptic astroglial plasma membrane but not the membranes of the immediately adjacent dendritic spine. These observations are inconsistent with a previous report that syntaxin 4 occupies a domain used for activity-dependent exocytosis at dendritic spines of the hippocampus (Kennedy et al., 2010), but consistent with the observation that syntaxin 3, but not syntaxin 1 or syntaxin 4, is required for delivery of AMPA receptor during LTP (Jurado et al., 2013).
Two other plasma membrane syntaxins, syntaxin 2 and 3, were explored in the present study. Since EM immunogold labeling requires particular fixation and processing conditions, antibodies that are suitable for other assays may not be compatible with the present EM technique. Two different syntaxin 2 antibodies and ten different syntaxin 3 antibodies were tested, but we were only able to detect prominent labeling with one putative syntaxin 3 antibody on neuronal somatodendritic plasma membranes, including those of spines (Tao-Cheng et al., 2012 Neuroscience abstract). This labeling pattern is compatible with the requirement that the target-SNARE for AMPA receptor exocytosis should be present throughout the entire neuronal somatodendritic compartment (Opazo and Choquet, 2011; Anggono and Huganir, 2012). However, Western blot analysis showed that the one working syntaxin 3 antibody (rabbit polyclonal, Synaptic Systems, Göttingen, Germany) does not recognize the canonical form of syntaxin 3 at 33 kDa. Although it is possible that this antibody recognizes an isoform of syntaxin 3 at 28 kDa, antibodies to test this idea are no longer available, so this study on syntaxin 3 could not be completed.
Label for syntaxin 4 was detected in some intracellular vesicles in neuronal somas, suggesting that syntaxin 4 may be involved in some intracellular membrane fusion events in neurons. Alternatively, these syntaxin 4-positive vesicles may represent selective endocytosis to remove syntaxin 4 from the neuronal somatodendritic compartment. This type of selective removal is one of the mechanisms used by cells to maintain distinctive domains in polarized cells (Lasiecka and Winckler, 2011). Although syntaxin 4 was not found in the clathrin-coated vesicle fraction from HeLa cells (Borner et al., 2006), this finding does not exclude the possibility that syntaxin 4 is endocytosed via non-clathrin mediated route in neurons, or that neurons use syntaxin 4 in a way not used by HeLa cells.
Regulated exocytosis of glutamate as well as other substances in astrocyte has been intensely studied (Montana et al., 2006; Bergersen and Gundersen 2009; Martineau et al., 2013). These vesicular exocytosis events presumably require formation of SNARE complexes at the astroglial plasma membrane. Additionally, many other astroglial plasma membrane proteins need to be inserted into the membrane, which presumably requires SNARE complexes at the plasma membrane. For example, glutamate transporter (Melone et al., 2011), potassium channel Kir4.1 (Derouiche et al., 2012), and the water channel aquaporine 4 (Nielsen et al., 1997) are located on astroglial plasma membrane to uptake excess extracellular glutamate, regulate potassium gradient, and adjust water contents. Although syntaxin 1 has been shown in astrocytes (Hepp et al., 1999; Montana et al., 2004) by biochemical means, syntaxin 4 or other plasma membrane syntaxins were not tested in the same studies. Thus, the relative abundance of these syntaxins cannot be determined. Furthermore, the detection of syntaxin 1 in astrocytes by Western analysis can only show its presence but not its specific location within the cell.
The present study clearly shows via immunogold electron microscopy that syntaxin 4 is highly concentrated at astroglial plasma membrane and supports the idea that syntaxin 4 is a target-SNARE involved in the insertion of astroglial-specific plasma membrane proteins.
Syntaxin 4 is enriched in glial and concentrated on plasma membrane of astrocytes.
Syntaxin 4 is available to support exocytosis in astroglia.
Syntaxin 1 is concentrated on axonal but not astroglial or dendritic plasma membrane.
Acknowledgements
We thank Virginia Crocker and Rita Azzam for expert EM technical support, and Dr. Ayse Dosemeci for helpful discussions. Supported by the Intramural Research Program of the NIH, NINDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012;22:461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen LH, Gundersen V. Morphological evidence for vesicular glutamate release from astrocytes. Neuroscience. 2009;158:260–265. doi: 10.1016/j.neuroscience.2008.03.074. [DOI] [PubMed] [Google Scholar]
- Borner GH, Harbour M, Hester S, Lilley KS, Robinson MS. Comparative proteomics of clathrin-coated vesicles. J Cell Biol. 2006;175:571–578. doi: 10.1083/jcb.200607164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- Derouiche A, Pannicke T, Haseleu J, Blaess S, Grosche J, Reichenbach A. Beyond polarity: functional membrane domains in astrocytes and Müller cells. Neurochem Res. 2012;37:2513–2523. doi: 10.1007/s11064-012-0824-z. [DOI] [PubMed] [Google Scholar]
- Fournier KM, Robinson MB. A dominant-negative variant of SNAP-23 decreases the cell surface expression of the neuronal glutamate transporter EAAC1 by slowing constitutive delivery. Neurochem Int. 2006;48:596–603. doi: 10.1016/j.neuint.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Garcia EP, McPherson PS, Chilcote TJ, Takei K, De Camilli P. rbSec1A and B colocalize with syntaxin 1 and SNAP-25 throughout the axon, but are not in a stable complex with syntaxin. J Cell Biol. 1995;129:105–120. doi: 10.1083/jcb.129.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara A, Fukazawa Y, Deguchi-Tawarada M, Ohtsuka T, Shigemoto R. Differential distribution of release-related proteins in the hippocampal CA3 area as revealed by freeze-fracture replica labeling. J Comp Neurol. 2005;489:195–216. doi: 10.1002/cne.20633. [DOI] [PubMed] [Google Scholar]
- Hepp R, Perraut M, Chasserot-Golaz S, Galli T, Aunis D, Langley K, Grant NJ. Cultured glial cells express the SNAP-25 analogue SNAP-23. Glia. 1999;27:181–187. doi: 10.1002/(sici)1098-1136(199908)27:2<181::aid-glia8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Hirano AA, Brandstätter JH, Vila A, Brecha NC. Robust syntaxin-4 immunoreactivity in mammalian horizontal cell processes. Vis Neurosci. 2007;24:489–502. doi: 10.1017/S0952523807070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Jurado S, Goswami D, Zhang Y, Molina AJ, Südhof TC, Malenka RC. LTP requires a unique postsynaptic SNARE fusion machinery. Neuron. 2013;77:542–558. doi: 10.1016/j.neuron.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Davison IG, Robinson CG, Ehlers MD. Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell. 2010;141:524–535. doi: 10.1016/j.cell.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Ehlers MD. Mechanisms and function of dendritic exocytosis. Neuron. 2012;69:856–875. doi: 10.1016/j.neuron.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasiecka ZM, Winckler B. Mechanisms of polarized membrane trafficking in neurons -- focusing in on endosomes. Mol Cell Neurosci. 2011;48:278–287. doi: 10.1016/j.mcn.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau M, Shi T, Puyal J, Knolhoff AM, Dulong J, Gasnier B, Klingauf J, Sweedler JV, Jahn R, Mothet JP. Storage and uptake of D-serine into astrocytic synaptic-like vesicles specify gliotransmission. J Neurosci. 2013;33:3413–3423. doi: 10.1523/JNEUROSCI.3497-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melone M, Bellesi M, Ducati A, Iacoangeli M, Conti F. Cellular and Synaptic Localization of EAAT2a in Human Cerebral Cortex. Front. Neuroanat. 2011;4:151. doi: 10.3389/fnana.2010.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia. 2006;54:700–715. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P, Choquet D. A three-step model for the synaptic recruitment of AMPA receptors. Mol Cell Neurosci. 2011;46:1–8. doi: 10.1016/j.mcn.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Ovsepian SV, Dolly JO. Dendritic SNAREs add a new twist to the old neuron theory. Proc Natl Acad Sci U S A. 2011;108:19113–19120. doi: 10.1073/pnas.1017235108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paco S, Margelí MA, Olkkonen VM, Imai A, Blasi J, Fischer-Colbrie R, Aguado F. Regulation of exocytotic protein expression and Ca2+-dependent peptide secretion in astrocytes. J Neurochem. 2009;110:143–156. doi: 10.1111/j.1471-4159.2009.06116.x. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HdF. The fine structure of the nervous system. Oxford University Press; New York: 1991. [Google Scholar]
- Rizo J, Südhof TC. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices--guilty as charged? Annu Rev Cell Dev Biol. 2012;28:279–308. doi: 10.1146/annurev-cellbio-101011-155818. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Schubert V, Bouvier D, Volterra A. SNARE protein expression in synaptic terminals and astrocytes in the adult hippocampus: a comparative analysis. Glia. 2011;59:1472–1488. doi: 10.1002/glia.21190. [DOI] [PubMed] [Google Scholar]
- Sherry DM, Mitchell R, Standifer KM, du Plessis B. Distribution of plasma membrane-associated syntaxins 1 through 4 indicates distinct trafficking functions in the synaptic layers of the mouse retina. BMC Neurosci. 2006;7:54. doi: 10.1186/1471-2202-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH, Du J, McBain CJ. SNAP-25 is polarized to axons and abundant along the axolemma: an immunogold study of intact neurons. J Neurocytol. 2000;29:67–77. doi: 10.1023/a:1007168231323. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng J-H, Gallant PE, Brightman MW, Dosemeci A, Reese TS. Structural changes at synapses after delayed perfusion fixation in different regions of the mouse brain. J Comp Neurol. 2007;501:731–740. doi: 10.1002/cne.21276. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH, Dosemeci A, Gallant PE, Yang Y, Winters CA, Reese TS. Neuroscience Meeting Planner. Society for Neuroscience; New Orleans, LA: 2012. Differential distribution of syntaxin 3 and syntaxin 4 between hippocampal neurons and astrocytes. Program No. 47.03. 2012 2012. Online. [Google Scholar]
- Teng FY, Wang Y, Tang BL. The syntaxins. Genome Biol. 2001;2:REVIEWS3012. doi: 10.1186/gb-2001-2-11-reviews3012. [DOI] [PMC free article] [PubMed] [Google Scholar]