Abstract
Converging evidence supports the hypothesis that the prefrontal cortex is critical for cognitive control. One prefrontal subregion, the anterior cingulate cortex, is hypothesized to be necessary to resolve response conflicts, disregard salient distractors and alter behavior in response to the generation of an error. These situations all involve goal-oriented monitoring of performance in order to effectively adjust cognitive processes. Several neuropsychological disorders, e.g., schizophrenia, attention deficit hyperactivity and obsessive compulsive disorder, are accompanied by morphological changes in the anterior cingulate cortex. These changes are hypothesized to underlie the impairments on tasks that require cognitive control found in these subjects. A novel conflict monitoring task was used to assess the effects on cognitive control of excitotoxic lesions to anterior cingulate cortex in rats. Prior to surgery all subjects showed improved accuracy on the second of two consecutive, incongruent trials. Lesions to the anterior cingulate cortex abolished this. Lesioned animals had difficulty in adjusting cognitive control on a trial-by-trial basis regardless of whether cognitive changes were increased or decreased. These results support a role for the anterior cingulate cortex in adjustments in cognitive control.
Keywords: executive function, Stroop, flanker, prefrontal cortex, conflict monitoring, distractibility
1. Introduction
One aspect of cognitive control is the ability to discriminate important information from inessential and then respond (Alexander & Brown, 2010; Kennerley, Walton, Behrens, Buckley, & Rushworth, 2006; Rudebeck et al., 2008; Walton, Croxson, Behrens, Kennerley, & Rushworth, 2007). This process requires the prefrontal cortex to integrate information from memory with current sensory input and respond in an appropriate manner (Alexander & Brown, 2010; Baddeley & Della Sala, 1996; Carter et al., 2000; Goldman-Rakic, 1996; Holroyd et al., 2004). When previously successful responses generate errors or fail to yield reinforcement, cognitive control is required to adapt to these changes in environmental contingencies (Brown & Braver, 2005; Carter et al., 1998; Garavan, Ross, Kaufman, & Stein, 2003; Hester, Foxe, Molholm, Shpaner, & Garavan, 2005; Holroyd et al., 2004; Kennerley et al., 2006; Kolling, Wittmann, & Rushworth, 2014; Krigolson & Holroyd, 2007; Ridderinkhof, Nieuwenhuis, & Bashore, 2003; Rudebeck et al., 2008; Walton, Croxson, et al., 2007). The anterior cingulate cortex (ACC) is one of the the subregions of prefrontal cortex that is active when cognitive control is required (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Brown & Braver, 2005; Cole & Schneider, 2007; Kerns et al., 2004; Lorist, Boksem, & Ridderinkhof, 2005; Magno, Foxe, Molholm, Robertson, & Garavan, 2006; Woodward, Metzak, Meier, & Holroyd, 2008).
Emitting an error or being in a situation where errors are likely correlates with activation of the ACC (Amiez, Joseph, & Procyk, 2005; Brown & Braver, 2005; Carter et al., 1998; Hester et al., 2005; Holroyd et al., 2004; Yeung, Botvinick, & Cohen, 2004). Kennerley, et al. (2006) found non-human primates with ACC lesions require more trials to reach asymptotic performance and generate more errors after correct responses than controls. In non-human primates and rats, the ACC is connected to the parietal cortex and dorsolateral prefrontal cortex or the rat homolog, the prelimbic cortex (Hoover & Vertes, 2007; Selemon & Goldman-Rakic, 1988). This connectivity may allow the ACC to recruit these areas following the detection of conflict to increase attentional control (Banich et al., 2000; Carter et al., 1998; Kerns et al., 2004; MacDonald, Cohen, Stenger, & Carter, 2000).
The ACC has been implicated in decision making where evaluating the utility of stimuli and responses is required (Bush et al., 2002; Kennerley et al., 2006; Lee, Rushworth, Walton, Watanabe, & Sakagami, 2007). Specifically, ACC is necessary to integrate error, conflict, and reinforcement information leading to the hypothesis that ACC is critical to effortful processing, e.g., in evaluating the utility of an effortful action based on potential reward (Kennerley et al., 2006; Lee et al., 2007). Different factors can devalue reinforcement such as a delayed reinforcement or an increased physical effort (climbing a barrier, increasing the number of responses required; Walton et al. 2006). Subjects will perform the more demanding task under conditions when this effort yields a larger reward (Kennerley et al., 2006; Walton, Kennerley, Bannerman, Phillips, & Rushworth, 2006). Lesions of the ACC impair calculations of the cost of a behavior to produce inefficient actions (Kolling et al., 2014; Walton, Bannerman, Alterescu, & Rushworth, 2003; Walton et al., 2009). These data support the hypothesis that the ACC is important for utilizing action-outcome history to optimize reward. One limitation of this prior work is that physical effort not cognitive effort was varied.
Functional imaging studies have shown the ACC is activated in the presence of conflicting stimuli or responses (Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; Botvinick et al., 2001; Chen, Wei, & Zhou, 2006; Kerns et al., 2004; Mitchell, 2006; van Veen, Cohen, Botvinick, Stenger, & Carter, 2001). Botvinick, et al.(1999) tested subjects in a task previously described by Gratton (1992) that requires the individual to attend to central directional cues, e.g., left arrow respond on left button. Some trials include flanker arrows that point in the same or opposite direction as the target. On congruent trials, flanker and central stimuli require the same response, but these stimuli provide conflicting information on incongruent trials. Response latencies are shorter on congruent trials than incongruent trials (Botvinick et al., 1999). Participants also respond faster on the second of two consecutively presented incongruent trials (Botvinick et al., 1999; Botvinick et al., 2001; Gratton, Coles, & Donchin, 1992; Kerns et al., 2004; Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004; Sheth et al., 2012). This speeded reaction is coincident with greater activation of the dorsal region of the ACC (Botvinick et al., 1999; Sheth et al., 2012). It has been hypothesized that the activation of the ACC increases top-down control and narrows the attentional focus so conflicting information is disregarded on the following trial (Botvinick et al., 1999).
While fMRI studies support a role for the ACC in conflict monitoring, focal lesion studies in humans do not necessarily show deficits on classic tests of conflict monitoring such as the Stroop task (Fellows & Farah, 2005; Glascher et al., 2012; Stuss, Floden, Alexander, Levine, & Katz, 2001). The functional sparing found in these studies may be because the test session did not interleave congruent and incongruent trials thus minimizing the need for trial-by-trial cognitive control. When congruent and incongruent trials are intermixed, participants with ACC lesions show slower reaction times than controls on both types of trials and fail to show reaction time improvements on the second of two consecutive incongruent trials (Alexander, Stuss, Picton, Shallice, & Gillingham, 2007; di Pellegrino, Ciaramelli, & Ládavas, 2007). Subjects with damage to the ACC have also been shown to be less accurate on incongruent trials than control subjects (Swick & Jovanovic, 2002).
Studies in rats have shown that ACC inactivation or lesions impairs the ability of these subjects to disregard previously reinforced stimuli (Newman & McGaughy, 2011; Ragozzino & Rozman, 2007). Additionally, rats with ACC inactivation or lesions maintain ineffective response strategies longer than controls (Bussey, Everitt, & Robbins, 1997; Chudasama et al., 2003; Newman & McGaughy, 2011; Ragozzino & Rozman, 2007). Together these data support the hypothesis that ACC may be crucial for recognizing situations where the behavioral response is not effective and a shift in cognitive processing is required (Dias & Aggleton, 2000; Kennerley et al., 2006; Kolling et al., 2014; Lee et al., 2007).
Few studies have attempted to establish a translational model of conflict monitoring in rats (Haddon & Killcross, 2006; Kashtelyan, Tobia, Burton, Bryden, & Roesch, 2012; Marx et al., 2012). The current work is aimed at redressing possible shortcomings in these previous tasks as described in brief here. In some cases, animals were not reinforced for correct responses or not consistently reinforced during the simultaneous presentation of stimuli (Haddon & Killcross, 2006; Kashtelyan et al., 2012). Reinforcement is consistent in our task to prevent extinction. Previously, stimuli of different modalities were paired with different reinforcers (i.e. sucrose or food pellets) (Haddon & Killcross, 2006). Innate preferences for the different reinforcers may alter responding such that subjects are biased to attend to stimuli associated with the preferred reinforcer, thus decreasing the effects of conflicting information when the non-preferred reinforce is the target modality. As a result, we reinforced all stimuli in an equivalent manner. In some cases response latencies were not measured and nonstandard formulas were used to calculate accuracy (Haddon & Killcross, 2006). Finally, all of the prior studies failed to analyze inter-trial effects of congruent and incongruent trials (Haddon & Killcross, 2006; Kashtelyan et al., 2012; Marx et al., 2012). As previous focal lesion studies in humans suggest trial by trial adjustments to cognitive control are the aspect of cognition most sensitive to ACC damage, it is important to include these analyses as they may be critical to revealing impairments (Alexander et al., 2007; di Pellegrino et al., 2007). In the present study, dependent measures and trial-by-trial analyses were performed in a manner similar to those obtained in humans to facilitate comparison between species (Botvinick et al., 1999; Kerns et al., 2004; Roelofs, Turennout, & Coles, 2006; van Veen et al., 2001).
Currently, we describe the characterization of a novel, cognitive control task for rats with attentional demands similar to those in Gratton’s flanker task (Botvinick et al., 1999; Gratton et al., 1992) and determine the effects of excitotoxic lesions to the ACC on performance in this task. Accuracy and response latencies from neutral or congruent trials were compared to those on incongruent trials. In addition, trial-by-trial analyses were completed to determine if rats, like humans, show improved performance on the second of two consecutive incongruent trials and to determine if subjects were sensitive to decreases in cognitive demands, e.g., performed better on non-conflict trials subsequent to a conflict trial. After this characterization, half of the subjects received excitotoxic lesions to the ACC to determine the impact of this damage on the conflict monitoring task. We also assessed performance in a conditional discrimination task with distraction. In this task, subjects experienced the simultaneous presentation of a target stimuli and a novel, salient distractor. In contrast to the conflict monitoring task, the distracting stimuli in the conditional discrimination task were not associated with a response or prior reinforcement. We hypothesized that damage to the ACC would impair performance in the conflict monitoring task, but not the test of distractibility with a novel, but never before reinforced stimulus. Additionally, we hypothesized that ACC lesions would decrease the ability of subjects to respond to trial-by-trial changes in cognitive demands similar to impairments found in humans after excision of the dorsal ACC (Sheth et al., 2012)
2. Research Design and Methods
2.1. Apparatus and materials
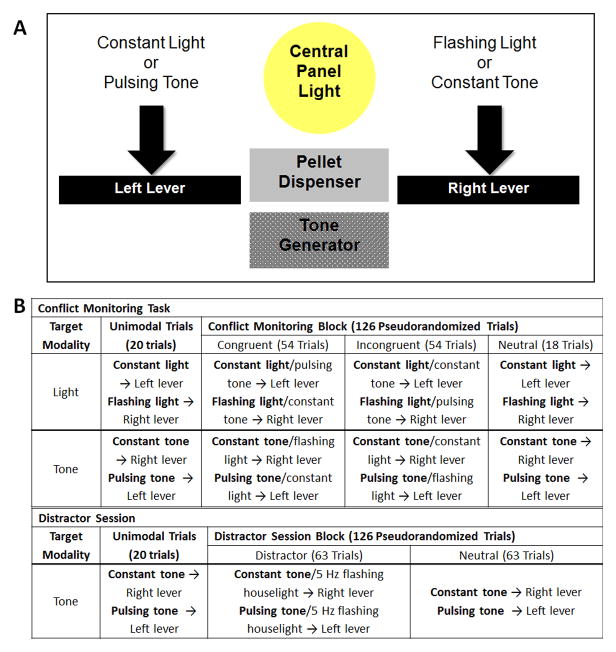
Operant chambers (Med Associates, St. Albans, VT) equipped with two retractable levers, a houselight (2.8 W), a 45 mg pellet dispenser, a 2900 Hz sonalert tone generator, and three panel lights (2.8 W) were used. The food dispenser, panel lights, tone generator, and retractable levers were all located on the same wall (see Figure 1A). The houselight was located on the opposite wall. Records of signal presentation, lever operation, and food pellet (Dustless Precision Pellets, 45 mg; Bio-serv, Frenchtown, NJ) delivery were maintained using a personal computer with Windows XP (Microsoft, Seattle, WA) and the Med-PC IV software (Med Associates).
Figure 1.
A. Stimulus response rules for both the conflict monitoring. The black bars represent the levers. Printed over the levers are the stimuli that are reinforced by pressing that lever. The flashing light and constant tone are presented at 20 Hz. Note the quality of the stimulus (constant vs. 20 Hz pulsing) is paired with the opposite lever across modality to prevent the animals from engaging in cross-modal matching where they learn to respond to the lever according to quality. Both the light and tone stimuli are located on the intelligence panel around the pellet dispenser. B. Conflict monitoring task and distractor session. The conflict monitoring block presents 54 congruent, 54 incongruent, and 18 neutral trials in a pseudorandomized order. The distractor session does not have congruent or incongruent trials but instead has 63 distractor trials and 63 neutral trials.
2.2. Behavioral training
20 male Long Evans rats were trained in an operant chamber on two sets of conditional discriminations (see Figure 1A). All animals were food restricted to maintain at least 90% of their free fed weight prior to training.
2.2.1 Conditional discrimination tasks
In the visual discrimination, rats were reinforced for pressing the left lever after the presentation of a constantly illuminated light and the right lever when presented with a flashing light at 20 Hz. In the auditory discrimination, the constant tone was paired with the right lever while the pulsing tone was paired with the left. The same quality stimuli, e.g. constant light and constant tone, require opposite lever responses and prohibit the rats from generalizing response rules across modality. Trials were presented in a pseudorandomized order with a fixed inter-trial interval (ITI) of 12 seconds. The order of training for these discriminations was counterbalanced across subjects. After achieving 65% accuracy, i.e., performance significantly above chance, in both the visual and auditory conditional discriminations, the animals were tested on the conflict monitoring task.
2.2.2. Conflict monitoring task
The conflict monitoring task assesses the effects of concurrent presentation of a tone and light stimulus (see Figure 1B). This session began with 20 unimodal trials to cue subjects which modality was reinforced (i.e. the target modality). After the priming block, subjects received a pseudo-randomized sequence of three types of trials, incongruent (54 trials), congruent (54 trials) or neutral (18 trials). It is important to note that neutral trials are identical to the unimodal priming blocks, but are dubbed neutral trials because they are interleaved with other trial types in the test of conflict monitoring. Subjects were not reinforced if they responded according to the non-target modality response rules. In the following example, boldface font indicates the target modality. On incongruent trials, the two stimuli presented were associated with conflicting response rules (e.g. constant light → left lever press and constant tone → right lever). The correct response shown in bold was determined by the target modality. On congruent trials, both stimuli were associated with the same response (e.g. flashing light and constant tone → right lever press). During neutral trials one stimulus from the target modality was presented (neutral trials; e.g. constant light → left lever press). On the day after a conflict monitoring session, animals were trained in unimodal sessions of the previously non-target modality. After the animal reached 65% accuracy on the interceding training days the conflict monitoring task was administered on the subsequent day using this modality as the target. Once an animal achieved > 65% accuracy on congruent trials in the light conflict monitoring task and the tone conflict monitoring task, they were considered eligible for surgery.
Response latencies from the onset of the stimulus to the lever press were measured as well as accuracy on congruent, incongruent and neutral trials. Accuracy to the target modality on congruent, incongruent and neutral trials was defined as correct/(correct + error) * 100. Accuracy and latencies for congruent and incongruent trials were also assessed based on what type of trial preceded it to determine if there was a facilitation of performance when the same trial type was presented on successive trials.
The sessions prior to tests of conflict monitoring and the neutral trials within the conflict monitoring sessions were assessed to determine the ability of the animals to perform the conditional discriminations. Because we hypothesized that the trial-to-trial cognitive demands would impact performance on these conditional discriminations, we compared performance on the first twenty trials of the task, the priming block, separately to that on neutral trials embedded within the conflict monitoring task. The same behavioral comparisons were made before and after surgery.
2.2.3. Susceptibility to distraction
In order to determine whether the ACC is critical for responding to a salient, distracting stimulus never paired with a lever response or reinforcement, the effects of a novel, visual stimulus was tested during auditory discrimination. In this session, the houselight was flashed at a rate of 5 Hz contemporaneously with the tone (Figure 1B). The timing of simultaneous stimulus presentation was similar to the conflict monitoring task, but these sessions differed in two critical ways. First, the flashing houselight had never been associated with a lever response or reinforcement and so was irrelevant to performance in the task. Second, the position of the light differed from that used in the visual discrimination so subjects were unlikely to confuse this houselight with the associative stimuli used in prior training. This session followed a similar design to the conflict monitoring sessions, but the number of neutral trials was increased in order to compensate for the loss of congruent trials. The test session now consisted of 20 unimodal tone trials followed by a block of 126 distractor trials (tone and 5 Hz houselight, 63 trials) and neutral trials (tone only, 63 trials) presented in a pseudorandomized order. Accuracy and response latencies were compared on trials where the flashing houselight is present and on trials from a session where the houselight remains constantly illuminated.
2.3. Surgery
Animals were matched by accuracy performance and one from each pair was randomly assigned to either receive the excitotoxin, ibotenic acid (0.06 M), or its vehicle, 0.1 M phosphate buffer, by infusion into the ACC. Rats were anesthetized using ketamine and xylazine (85 mg/kg ketamine and 8.5 mg/kg xylazine). Infusions were made into the ACC via a 10 μl, 26 gauge beveled tip microsyringe mounted on a stereotaxic frame. For each infusion the beveled tip was angled so the opening was pointing towards the midline. A small volume of the toxin or vehicle (0.2 μl/site) was infused bilaterally at a rate of 125 nl per minute using an electronic infusion pump (Micro 4, Microsyringe Pump Controller, World Precision Instruments, Sarasota, FL). The needle was left in place for 4 minutes prior to and subsequent to infusion to minimize bleeding and undesired diffusion of the toxin. The following coordinates were used: AP: +2.7, ML: ±0.6, DV: −2.4; AP: +2.2, ML: ±0.6, DV: −2.2 with anteroposterior (AP) measurement relative to Bregma and dorsoventral (DV) measurement relative to the skull and the toothbar at −3.0 mm (modified from Chudasama et al, 2003). Animals received 4 days of ad libitum food and water before food restriction was reinstated. An additional 3 days of food restriction (~18 g/day) were established before postoperative training commenced. After recovery the animals were re-trained on the conditional discriminations. Once the animals achieved two days criterion performance (65%) on both tone and light discriminations, they were tested on the conflict monitoring task (see Figure 1B). The order of the target modality in the conflict monitoring task was counterbalanced.
2.4. Histology
After completion of testing, animals were given an overdose of Euthasol (1.5 mL/kg). The rats were then transcardially perfused with 0.9% saline, followed by 4% paraformaldehyde in phosphate buffer. Brains were removed and placed in a 30% sucrose solution until they sunk and then sliced into 40 μm coronal sections. Sections were thionin stained for Nissl bodies after they were mounted on gelatin-coated slides. Histological assessment of cell loss was made on an Olympus Optical BX51 microscope (Optical Analysis Corporation, Nashua, NH) using the 4X objective. Images of the sections were photographed using a SPOT Insight digital camera (Diagnostic Imaging, Sterling Heights, MI). Images were collected using Image Pro Plus version 6.0 software (Media Cybernetics, Silver Springs, MD), and the area of damage was outlined using ImageJ version 1.41o (National Institutes of Health, Bethesda, MD, USA). The percentage of anterior cingulate cortex loss was then calculated at bregma +3.7, +2.7, and +1.7 mm (Paxinos & Watson, 2005). The amount of damage to the surrounding areas was also noted.
2.5. Statistical analyses
All statistical analyses were performed with SPSS v. 20.0 (SPSS, Chicago, IL). Pre-surgical data were analyzed using separate repeated measures ANOVAs. All post-surgical dependent measures were analyzed using separate mixed-factor ANOVAs with Lesion as a between subjects factor. The extent of the lesions was characterized with a repeated measures ANOVA with Hemisphere (2 levels) and Rostrocaudal position (3 levels) as within-subject factors.
2.5.1 Task validation: Pre-surgical assessment
Using a repeated measures ANOVA accuracy on neutral, congruent and incongruent trials was compared with Modality (2 levels, light vs. tone), and Trial Type (3 levels, neutral, congruent or incongruent). A separate ANOVA was used to analyze correct response latencies using the same mixed factors analyses. The small number of errors generated on neutral trials, i.e. ~4, precluded analyses of errors on these trials so the incorrect latency analyses were limited to incongruent and congruent trials. Performance on the block of unimodal priming trials that preceded the conflict monitoring task were compared to the neutral trials interleaved with congruent and incongruent trials in terms of accuracy, correct latencies. Evaluation of the previous trial type on accuracy and latencies of incongruent trials was done using a repeated measures ANOVA of Modality (2 levels) and Trial (3 levels, incongruent/incongruent vs. congruent/incongruent vs. neutral/incongruent; II vs. CI vs. NI). Similar analyses were performed for congruent trials. Finally in order to assess error processing, a repeated measures ANOVA with Previous Trial Outcome (2 levels, correct vs. error) by Modality (2 levels, light vs. tone) was used for accuracy. The analyses of latencies also included the factor Current Trial outcome (2 levels: correct vs. incorrect).
2.5.2. Post-surgical assessment
Post-surgical performance was assessed using the ANOVAs described for pre-surgical performance with a few exceptions. All ANOVAs now became mixed factors because the between-subjects factor of lesion was now included. Additionally, there were no congruent or incongruent trials in the test of distractibility to the effects of the 5 Hz houselight distractor on performance of the auditory, conditional discrimination.
3. Results
3.1. Task characterization: Pre-surgical results
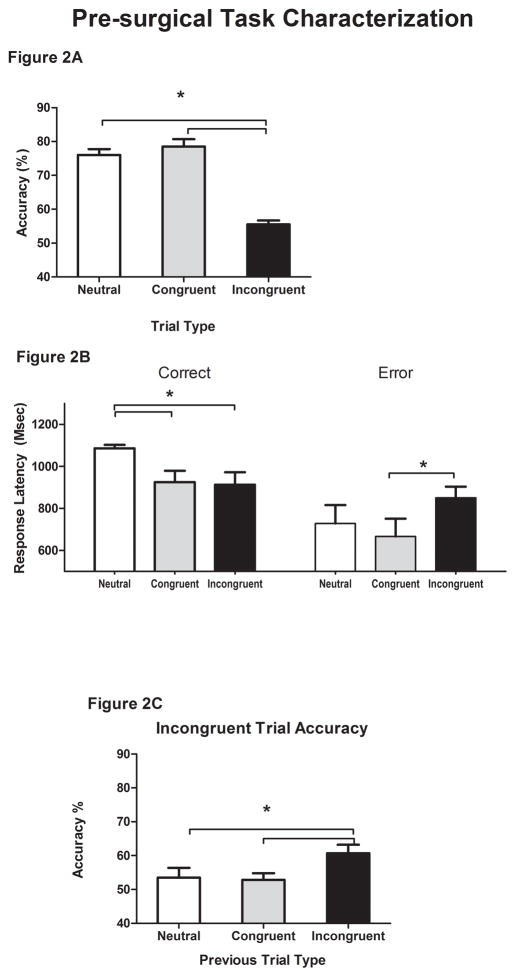
All rats were more accurate on neutral and congruent trials than incongruent trials (Figure 2A; F2,38 =77.07, p<0.001; Neutral: 76.04 ± 1.78, Congruent: 78.5% ± 2.2%, Incongruent: 55.5% ± 1.2%; Neutral vs. Incongruent: t19=9.98, Congruent vs. Incongruent: t19=10.28, both p<0.001). There was a small but significant effect of modality on accuracy such that animals were more accurate when tone was the target modality than when light was the target modality (F1,19 = 5.46, p < 0.05; Tone: 71.6 ± 1.7%, Light: 68. 1 ± 1.5 %), but this modality based difference did not impact trial type performance (F2,38 = 0.39; p > 0.05). Correct response latencies also differed by trial type (F2,38 = 5.27, p<0.05) with animals requiring more time to emit a correct response on neutral trials (Figure 2B; Neutral: 1086.1 ± 106.7 msec) than on congruent (925.2 ± 54.6 msec, t19=2.78, p<0.05) or incongruent trials (912.7 ± 60.4 msec, t19=2.45, p<0.05). All animals had significantly longer error latencies on incongruent than congruent trials (Figure 2B; F1,19 = 11.65, p<0.05; Incongruent: 850.7 ± 53.9 msec, Congruent: 666.8 ± 84.1 msec). The performance on neutral trials within the conflict monitoring task was identical to performance during the priming block for all dependent measures (all p > 0.05). There was no evidence for post error slowing or a change in accuracy after errors (all p > 0.05).
Figure 2.
Pre-surgical Conflict Monitoring. A. All animals were significantly more accurate (y-axis) on neutral (white) and congruent (gray) than incongruent (black) trials p<0.05). B. Correct latencies (y-axis in msec) were longer on neutral trials (white bars) than on congruent (gray bars) or incongruent (black bars) trials. Error latencies that occurred on incongruent trials (black bars) were longer than error latencies on congruent trials (gray bars) or neutral trials (white bars). C. Successive presentation of incongruent trials facilitated performance, i.e., accuracy was higher on incongruent trials preceded by an incongruent trial (Black bars) than incongruent trials preceded by a congruent (gray bars) or neutral trial (white bars) (p <0.05).
3.2. Trial by trial analyses
Accuracy on incongruent trials improved when two incongruent trials were presented consecutively (Figure 2C; F2,38 = 4.17, p<0.05; II: 60.7% ± 2.5%). Performance on these consecutive incongruent trials was better than on incongruent trials preceded by a congruent trial (t19 = 2.73; p < 0.05; CI: 51.9% ± 1.9%) or by a neutral trial (t19 = 2.05; p = 0.05; NI: 54.1% ± 2.7%). The previous trial type did not have a significant effect on the correct or error latencies emitted during incongruent trials. In contrast to performance on incongruent trials, there was no effect previous trial type on the accuracy or response latencies of the subsequent congruent trial (all p > 0.05). There were no significant differences between the assigned groups in terms of accuracy or response latency (p>0.1) prior to surgery.
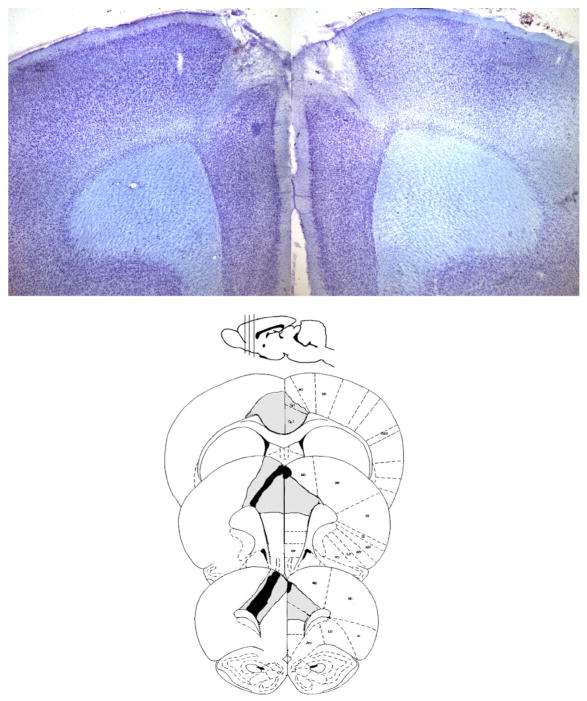
3.3. Histology
Upon assessment of the ACC lesions, 1 animal was found to insufficient lesion and was excluded. One Sham-Lx animal did not complete all stages of the post-surgical training and was also excluded. Then final n’s were ACC-Lx = 9 and Sham-Lx animals = 9. Damage to the ACC was greater in rostral than caudal regions (Rostrocaudal: F2,16 = 10.09, p<0.01; percent loss at 3.7: 69.68% ± 8.78%, percent loss at 2.7: 61.93% ± 6.94%, percent loss at 1.7: 25.06% ± 11.4%; 3.7 vs. 1.7: t8=3.28, p<0.017, 2.7 vs. 1.7: t8=3.27, p<0.017; Figure 3A). Figure 3B shows the smallest (black region) bilateral lesion included in these analyses while the gray area delineates the largest lesion. Though the smallest lesion shown in Figure 3 is from a subject with variable damage between hemispheres, other animals sustained bilateral damage and there was no statistically significant difference between hemispheres for the group(p> 0.1).
Figure 3.
ACC Histology. A) A representative image of the damage to the ACC at 2.7 mm anterior from bregma. The coronal section is 40 μm thick and stained with thionin. Damage was centered on rostral portions of the ACC and restricted to ACC. B) A diagram of the smallest (black) and largest lesions (gray) shows the regional selectivity of damage produced in the current study. All subjects included in the results sustained bilateral damage to the ACC.
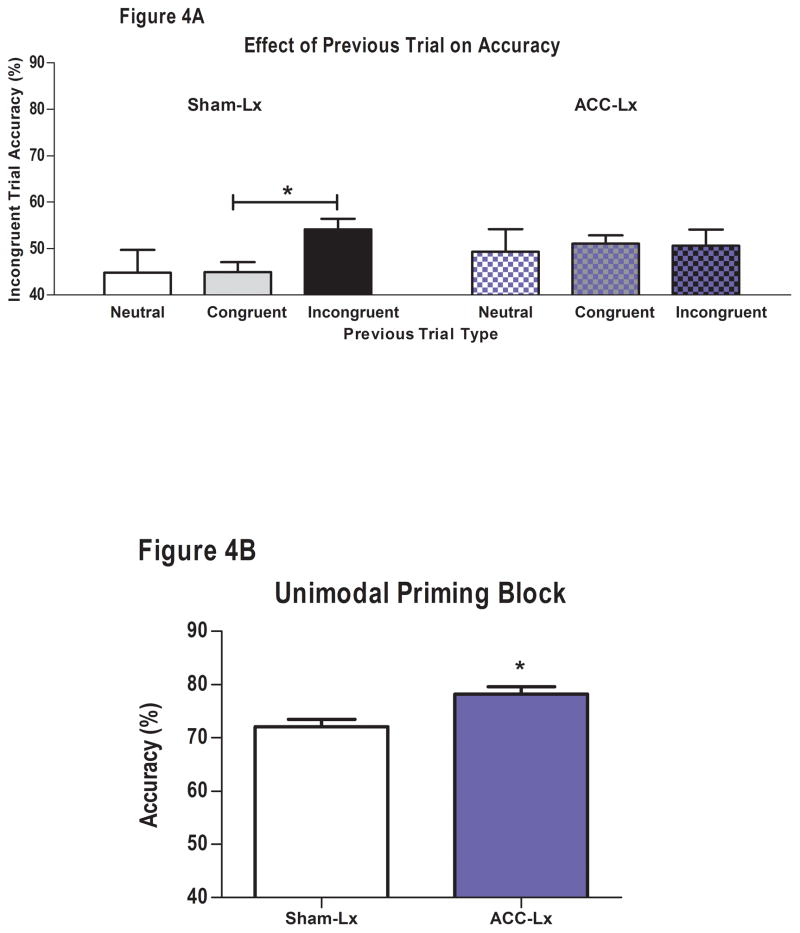
3.4. Post-surgical performance trial by trial analyses
Lesions to the ACC produced specific impairments in the ability of rats to increase cognitive control on a trial-by-trial basis. Planned comparisons showed that ACC-Lx rats no longer benefitted from the successive presentations of incongruent trials (ACC-Lx: II vs. CI p>0.7; Figure 4A), but Sham-Lx rats remained more accurate on these trials than incongruent trials preceded by a congruent trial or a neutral trial (Sham-Lx: II vs. CI: t8=3.40, p<0.01; II vs. NI: t8=1.95; p > 0.05).
Figure 4.
Effects of ACC lesions on conflict monitoring. A. ACC-Lx rats (blue bars) were more accurate on the unimodal priming trials of the conflict monitoring task than Sham-Lx rats (white bars). B. Sham-Lx rats were more accurate on incongruent trials that were preceded by an incongruent trial (black bars) than those preceded by a congruent (gray) or neutral (white) trial. ACC-Lx rats no longer showed facilitated performance on the second of two incongruent trials (checkerboard bars). C. Sham-Lx but not ACC-Lx rats were more accurate on neutral trials that occurred after an incongruent trial than those that occurred after a neutral trial (Sham-Lx p<0.01; ACC-Lx p>0.3) in the tone conflict monitoring task.
3.4.1. Post-surgical overall task performance
Similar to the pre-surgical assessment, all rats were more accurate on congruent and neutral than incongruent trials during the conflict monitoring task (F2,32= 166.34, p<0.001 Neutral: 75.1 ± 1.6%, Congruent: 82.3% ± 1.7%, Incongruent: 49.6% ± 0.9%; Congruent vs. Incongruent: t17=15.12, p < 0.001, Neutral vs. Incongruent: : t17=12.47, p < 0.001). In contrast to pre-surgical performance, accuracy on congruent trials was higher than on neutral trials: Congruent vs. Neutral: t17=5.58, p < 0.001, There was no effect of lesion on accuracy, correct or error latencies in the overall analyses of trial type. Correct response latencies on neutral trials were slower than incongruent trials (F2,32=15.65, p<0.001) similar to what was seen pre-surgically. All rats were slower in making an error on an incongruent trial than a congruent trial (F1,16=18.66, p<0.001). ACC-Lx rats were more accurate than Sham-Lx rats on the first 20 unimodal trials of the conflict monitoring task (F1,16=10.05, p<0.01; Sham-Lx: 72.1% ± 1.4%, ACC-Lx: 78.2% ± 1.4%; Figure 4B). Correct response latencies were slower on neutral trials than unimodal trials post-surgery in both Sham-Lx and ACC-Lx rats (F1,16=4.61, p<0.05; Unimodal:1092.7 ± 128.0 msec, Neutral 1185.8± 115.8 msec). There were no differences on accuracy or latency if the previous trial was an error (p > 0.05).
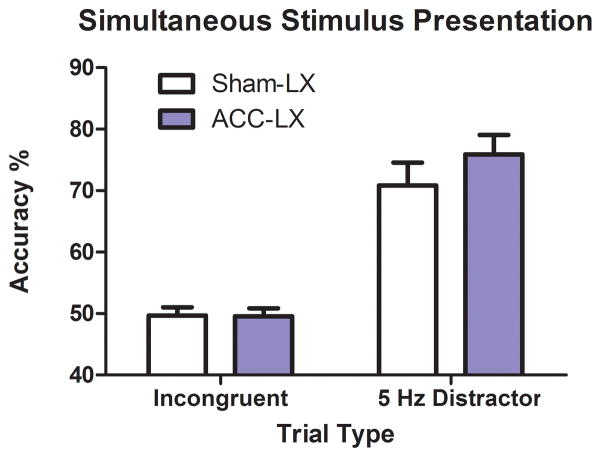
3.4.2 Post-surgical distractor session
All rats were more accurate on the houselight distractor trials than incongruent trials in the conflict monitoring task (Figure 5). It is important to note that distractor trials did not affect accuracy or latency on the following trial for either group (p>0.2). This is in contrast to the presentation of consecutive incongruent trials in the conflict monitoring task which improved accuracy (Figure 2B & 4B).
Figure 5.
Sham-Lx (white bars) and ACC-Lx rats (blue bars) were more accurate on trials where a 5 Hz houselight distractor was presented during the tone conditional discrimination than incongruent trials during the conflict monitoring task.
4. Discussion
4.1 Task characterization
The results of this experiment support this paradigm as a valid test of conflict monitoring in rats. As shown in Figure 2A, rats performed better on congruent than incongruent trials similar to humans (Botvinick et al., 1999; Gratton et al., 1992; Kerns et al., 2004; Yeung et al., 2004). The accuracy difference found here was greater than previously found in humans (20–30% in the present study vs. 2–7% in humans), but this comparison is often omitted if the overall error rate is low (<3%; Botvinick et al., 1999; Gratton et al., 1992; Kerns et al., 2004; Yeung et al., 2004). The novel task described here also demonstrates that rats and humans have similar trial-by-trial cognitive control. Prior work by Consecutive presentation of two incongruent trials facilitates performance in both humans (~8% accuracy improvement and 10–50 msec latency improvement) (Botvinick et al., 1999; Gratton et al., 1992; di Pellegrino, Ciaramelli, & Ládavas, 2007)) and rats (Figure 2B; 8–10% accuracy improvement) to a similar degree. This facilitation has been hypothesized to depend upon activity in the ACC (Botvinick et al., 1999) with recent evidence supporting the hypothesis that the neural mechanisms underlying this control are the same for rats and humans (Narayanan et al., 2013). When the ACC is intact, i.e., all animals prior to surgery and in the Sham-Lx animals post-surgery, the improved accuracy on the second incongruent trial is hypothesized to result from increased attentional focus that optimizes reward-seeking efforts (Kennerley et al., 2006; Lee et al., 2007; Newman & McGaughy, 2011; Rudebeck et al., 2008; Walton et al., 2003; Walton, Croxson, et al., 2007; Walton, Devlin, & Rushworth, 2004; Walton et al., 2006; Walton, Rudebeck, Bannerman, & Rushworth, 2007).
4.2. The effects of lesions to the ACC
Lesions to the ACC in rats abolished the benefit to accuracy found in controls by successive presentation of incongruent trials (Figure 4B). Humans subjects show a similar abolition of trial-by-trial modulation of performance after circumscribed lesions to either the right rostral ACC (di Pellegrino, Ciaramelli, & Ládavas, 2007) or left dACC (Sheth et al., 2012). Because ACC-Lx rats cannot exert the same attentional effort, performance on the next trial is not improved when attentional demands remain high such as found on the successive presentation of incongruent trials. This difference as a result of damage to the ACC cannot be explained by an impairment in the conditional discrimination as lesioned rats are more, not less, accurate than controls on the priming trials prior to the conflict monitoring task (Figure 4A). Moreover lesioned rats were as accurate as Sham-Lx rats on congruent and neutral trials within the conflict monitoring task. Though performance on the unimodal priming blocks was similar to performance on the neutral trials, all subjects required more time to emit a correct response on neutral trials than unimodal trials. We hypothesize that embedding the neutral trials among congruent and incongruent trials increased the cognitive demands on these trials resulting in a speed-accuracy trade-off on neutral trials. In similar studies with humans, neutral trials have been shown to cause greater activation of the ACC than congruent trials which suggests a neutral trial should improve performance on a subsequent incongruent trial (Roelofs et al., 2006). The present study found no evidence to support this hypothesis. This difference may be due to infrequency of neutral trials in the present task relative to the task used by Roelefs and colleagues. Future studies will be aimed at determining how the proportion of neutral trials impacts performance in intact and ACC-Lx rats.
These data support the hypothesis that ACC-Lx rats are specifically impaired in ability to rapidly increase cognitive control that allows one to evaluate the outcome of the previous trial and utilize this information to respond effectively on a subsequent trial. The role of the ACC in trial-by-trial adjustments to maximize efficiency in situations where stimuli are conflicting has been documented in prior work with humans, non-human primates and rats (Botvinick et al., 1999; Kennerley et al., 2006; Lapish, Durstewitz, Chandler, & Seamans, 2008; Sheth et al., 2012). Other studies have also demonstrated the ACC is necessary in evaluating decisions when task difficulty increases and reward opportunity changes (Sallet et al., 2007; Walton, Croxson, et al., 2007; Walton et al., 2004; Walton, Rudebeck, et al., 2007). The current study did not find evidence for a role for the ACC in error processing as previously shown (Amiez et al., 2005; Brown & Braver, 2005; Carter et al., 1998; Hester et al., 2005; Holroyd et al., 2004; Yeung et al., 2004) as errors did not change the accuracy or latency of the next trial.
In contrast to the conflict monitoring sessions, ACC-Lx animals performed on the distractor session as well as Sham-Lx animals. In the conflict monitoring session the non-target modality has previously been associated with a reinforced response. In the distractor session, the 5 Hz houselight has never been associated with a reinforced response. The ability of ACC-Lx animals to perform the distractor session in contrast to the conflict monitoring sessions supports the hypothesis that the previous associations of the incongruent stimuli with reinforcement contribute to these impairments. This finding is consonant with prior data from our lab showing that rats with lesions to the ACC are not generally distractible and do not show gross attentional impairments. They are distracted only by stimuli that have previously been associated with reinforcement and maintain the ability to perform conditional discriminations (Newman & McGaughy, 2011).
4.3. Limitations of the current work
Though many aspects of performance of rats in the current conflict monitoring task are similar to humans, there are limitations to the our work. In humans, consecutive trials of similar cognitive demand reduces response time and is without effect on accuracy (Sheth et al., 2012). In the current study, the consecutive presentation of incongruent trials improved accuracy but was without effect on latencies. The lack of effect of accuracy in humans is likely due to a ceiling effect on this dependent measure. Moreover, the accuracy impairments on the incongruent trials are greater in rats than those in humans. This poorer baseline performance allows the detection of improvements in this measure. These differences likely reflect limitations in top-down control of rats relative to humans as has previously been shown (Demeter et al., 2008). This limited top-down control is also hypothesized to underlie the latency costs found in rats performing neutral trials. Prior work in humans has shown that response latencies are highest on trials with the highest level of conflict In contrast, we find the highest cost occurring on the least frequent trial type which also has the lowest cognitive demand. We hypothesized that increasing the frequency of neutral trials would mitigate this cost and will test this hypothesis in future studies. Finally, the findings regarding the role of the ACC in the current task may also be limited by the small lesions produced, i.e., 60% of the rostral ACC. The extent of the lesions was intentionally restricted to prevent damage to other regions of the medial prefrontal cortex that may have impeded interpretation of the data. While clear effects were found, the small lesions may leave residual ACC function that leads to an underestimation of the role of ACC in this task.
The current data show that conflict monitoring can be adapted for testing with rats and shows functional homology between rat ACC and human dACC. As many neuropsychiatric disorders are hypothesized to have a developmental origin, translation to subjects with more rapid ontogeny should aid in elucidating the neuroanatomical and neurophysiological abnormalities that underlie these disorders. The results also suggest the role of the ACC is dissociable from the prelimbic cortex in rats. Previous work in our lab has shown that neurochemical and regionally specific damage to the prelimbic cortex does produce a general increase in distractibility that is not limited to previously reinforced stimuli as found after ACC damage (Newman & McGaughy, 2008). Several studies suggest the prelimbic cortex (dorsolateral prefrontal cortex in primates) has also been shown to play a substantial role in conflict monitoring (Banich et al., 2000; Chen et al., 2006; Erickson et al., 2004; Milham, Banich, Claus, & Cohen, 2003). Future studies will be aimed at determining the effects of larger lesions to the ACC, as well as lesions to the prelimbic cortex on performance in this task to further dissociate the functions of these prefrontal subregions.
Highlights.
The current study characterizes a novel test of cognitive control for rats.
Both rats and humans show better performance on congruent than incongruent trials.
Both species show improved accuracy on the second of two incongruent trials.
Lesions to anterior cingulate cortex disrupt this improvement in accuracy.
Damage to the anterior cingulate cortex impedes trial-by-trial cognitive control.
Acknowledgments
The authors would like to thank Emily Carter, Michelle Wasserman, Rebecca Misra, Colin White, Sandra Woodman, and Matt Gerding for their excellent technical assistance. This work was supported in part by a University of New Hampshire Dissertation Year Fellowship to Lori Newman.
This work was supported in part by a University of New Hampshire Dissertation Year Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of References
- Alexander MP, Stuss DT, Picton T, Shallice T, Gillingham S. Regional frontal injuries cause distinct impairments in cognitive control. Neurology. 2007;68:1515–1523. doi: 10.1212/01.wnl.0000261482.99569.fb. [DOI] [PubMed] [Google Scholar]
- Alexander WH, Brown JW. Computational Models of Performance Monitoring and Cognitive Control. Topics in Cognitive Science. 2010;2:658–677. doi: 10.1111/j.1756-8765.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez Cl, Joseph JP, Procyk E. Anterior cingulate error-related activity is modulated by predicted reward. European Journal of Neuroscience. 2005;21:3447–3452. doi: 10.1111/j.1460-9568.2005.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Della Sala S. Working memory and executive control. Philosophical Transactions Of The Royal Society Of London Series B, Biological Sciences. 1996;351:1397. doi: 10.1098/rstb.1996.0123. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, Brown C. Prefrontal regions play a predominant role in imposing an attentional ‘set’: evidence from fMRI. Cognitive Brain Research. 2000;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned Predictions of Error Likelihood in the Anterior Cingulate Cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Everitt BJ, Robbins TW. Dissociable effects of cingulate and medial frontal cortex lesions on stimulus-reward learning using a novel Pavlovian autoshaping procedure for the rat: Implications for the neurobiology of emotion. Behavioral Neuroscience. 1997;111:908–919. doi: 10.1037//0735-7044.111.5.908. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: Strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Wei P, Zhou X. Distinct neural correlates for resolving stroop conflict at inhibited and noninhibited locations in inhibition of return. Journal of Cognitive Neuroscience. 2006;18:1937–1946. doi: 10.1162/jocn.2006.18.11.1937. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SEV, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behavioural Brain Research. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Demeter E, Sarter M, Lustig C. Rats and humans paying attention: cross-species task development for translational research. Neuropsychology. 2008;22:787–799. doi: 10.1037/a0013712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G, Ciaramelli E, Ládavas E. The Regulation of Cognitive Control following Rostral Anterior Cingulate Cortex Lesion in Humans. Journal of Cognitive Neuroscience. 2007;19:275–286. doi: 10.1162/jocn.2007.19.2.275. [DOI] [PubMed] [Google Scholar]
- Dias R, Aggleton JP. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioural flexibility. European Journal of Neuroscience. 2000;12:4457–4466. doi: 10.1046/j.0953-816x.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Milham MP, Colcombe SJ, Kramer AF, Banich MT, Webb A, Cohen NJ. Behavioral conflict, anterior cingulate cortex, and experiment duration: Implications of diverging data. Human Brain Mapping. 2004;21:98–107. doi: 10.1002/hbm.10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Is anterior cingulate cortex necessary for cognitive control? Brain: A Journal Of Neurology. 2005;128:788–796. doi: 10.1093/brain/awh405. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. NeuroImage. 2003;20:1132–1139. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- Glascher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, Tranel D. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proceedings of the National Academy of Sciences. 2012;109:14681–14686. doi: 10.1073/pnas.1206608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philosophical Transactions Of The Royal Society Of London Series B, Biological Sciences. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. Optimizing the use of information: Strategic control of activation of responses. Journal of Experimental Psychology: General. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Haddon JE, Killcross S. Prefrontal cortex lesions disrupt the contextual control of response conflict. Journal of Neuroscience. 2006;26:2933–2940. doi: 10.1523/JNEUROSCI.3243-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Foxe JJ, Molholm S, Shpaner M, Garavan H. Neural mechanisms involved in error processing: A comparison of errors made with and without awareness. NeuroImage. 2005;27:602–608. doi: 10.1016/j.neuroimage.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Nystrom L, Mars RB, Coles MGH, Cohen JD. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nature Neuroscience. 2004;7:497–498. doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure & Function. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Kashtelyan V, Tobia SC, Burton AC, Bryden DW, Roesch MR. Basolateral amygdala encodes upcoming errors but not response conflict. European Journal of Neuroscience. 2012;35:952–959. doi: 10.1111/j.1460-9568.2012.08022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Walton ME, Behrens TEJ, Buckley MJ, Rushworth MFS. Optimal decision making and the anterior cingulate cortex. Nature Neuroscience. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kolling N, Wittmann M, Rushworth MFS. Multiple neural mechanisms of decision making and their competition under changing risk pressure. Neuron. 2014;81:1190–1202. doi: 10.1016/j.neuron.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krigolson OE, Holroyd CB. Hierarchical error processing: Different errors, different systems. Brain Research. 2007;1155:70–80. doi: 10.1016/j.brainres.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Lapish CC, Durstewitz D, Chandler LJ, Seamans JK. Successful Choice Behavior Is Associated with Distinct and Coherent Network States in Anterior Cingulate Cortex. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11963–11968. doi: 10.1073/pnas.0804045105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Rushworth MFS, Walton ME, Watanabe M, Sakagami M. Functional specialization of the primate frontal cortex during decision making. Journal of Neuroscience. 2007;27:8170–8173. doi: 10.1523/JNEUROSCI.1561-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorist MM, Boksem MAS, Ridderinkhof KR. Impaired cognitive control and reduced cingulate activity during mental fatigue. Cognitive Brain Research. 2005;24:199–205. doi: 10.1016/j.cogbrainres.2005.01.018. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the Role of the Dorsolateral Prefrontal and Anterior Cingulate Cortex in Cognitive Control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Magno E, Foxe JJ, Molholm S, Robertson IH, Garavan H. The Anterior Cingulate and Error Avoidance. J Neurosci. 2006;26:4769–4773. doi: 10.1523/JNEUROSCI.0369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx C, Lex B, Calaminus C, Hauber W, Backes H, Neumaier B, Endepols H. Conflict processing in the rat brain: behavioral analysis and functional μPET imaging using [18F]fluorodeoxyglucose. Frontiers in Behavioral Neuroscience. 2012;6 doi: 10.3389/fnbeh.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus ED, Cohen NJ. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. NeuroImage. 2003;18:483–493. doi: 10.1016/s1053-8119(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Mitchell RLC. Anterior Cingulate Activity and Level of Cognitive Conflict: Explicit Comparisons. Behavioral Neuroscience. 2006;120:1395–1401. doi: 10.1037/0735-7044.120.6.1395. [DOI] [PubMed] [Google Scholar]
- Newman LA, McGaughy J. Cholinergic deafferentation of prefrontal cortex increases sensitivity to cross-modal distractors during a sustained attention task. Journal of Neuroscience. 2008;28:2642–2650. doi: 10.1523/JNEUROSCI.5112-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, McGaughy J. Attentional effects of lesions to the anterior cingulate cortex: How prior reinforcement influences distractibility. Behavioral Neuroscience. 2011;125:360–371. doi: 10.1037/a0023250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayaran NS, Cavanagh JF, Frank MJ, Laubach M. Common medial frontal mechanisms of adaptive control in humans and rats. Nature Neuroscience. 2013;16:1888–1895. doi: 10.1038/nn.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates: the new coronal set. 5. Oxford: Elsevier Academic Press; 2005. [Google Scholar]
- Ragozzino ME, Rozman S. The effect of rat anterior cingulate inactivation on cognitive flexibility. Behavioral Neuroscience. 2007;121:698–706. doi: 10.1037/0735-7044.121.4.698. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Nieuwenhuis S, Bashore TR. Errors are foreshadowed in brain potentials associated with action monitoring in cingulate cortex in humans. Neuroscience Letters. 2003;348:1–4. doi: 10.1016/s0304-3940(03)00566-4. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Roelofs A, Turennout Mv, Coles MGH. Anterior Cingulate Cortex Activity Can Be Independent of Response Conflict in Stroop-Like Tasks. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13884–13889. doi: 10.1073/pnas.0606265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ, Walton ME, Rushworth MFS. Frontal Cortex Subregions Play Distinct Roles in Choices between Actions and Stimuli. J Neurosci. 2008;28:13775–13785. doi: 10.1523/JNEUROSCI.3541-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallet J, Quilodran R, Rothe M, Vezoli J, Joseph JP, Procyk E. Expectations, gains, and losses in the anterior cingulate cortex. Cognitive, Affective & Behavioral Neuroscience. 2007;7:327–336. doi: 10.3758/cabn.7.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon L, Goldman-Rakic P. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, Eskander EN. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioral adaptation. Nature. 2012;488:218–221. doi: 10.1038/nature11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Floden D, Alexander MP, Levine B, Katz D. Stroop performance in focal lesion patients: dissociation of processes and frontal lobe lesion location. Neuropsychologia. 2001;39:771–786. doi: 10.1016/s0028-3932(01)00013-6. [DOI] [PubMed] [Google Scholar]
- Swick D, Jovanovic J. Anterior cingulate cortex and the Stroop task: Neuropsychological evidence for topographic specificity. Neuropsychologia. 2002;40:1240–1253. doi: 10.1016/s0028-3932(01)00226-3. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior Cingulate Cortex, Conflict Monitoring, and Levels of Processing. NeuroImage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K, Rushworth MFS. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. Journal of Neuroscience. 2003;23:6475–6479. doi: 10.1523/JNEUROSCI.23-16-06475.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Croxson PL, Behrens TEJ, Kennerley SW, Rushworth MFS. Adaptive decision making and value in the anterior cingulate cortex. NeuroImage. 2007;36:T142–T154. doi: 10.1016/j.neuroimage.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MFS. Interactions between decision making and performance monitoring within prefrontal cortex. Nature Neuroscience. 2004;7:1259–1265. doi: 10.1038/nn1339. [DOI] [PubMed] [Google Scholar]
- Walton ME, Groves J, Jennings KA, Croxson PL, Sharp T, Rushworth MFS, Bannerman DM. Comparing the role of the anterior cingulate cortex and 6-hydroxydopamine nucleus accumbens lesions on operant effort-based decision making. European Journal of Neuroscience. 2009;29:1678–1691. doi: 10.1111/j.1460-9568.2009.06726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Kennerley SW, Bannerman DM, Phillips PEM, Rushworth MFS. Weighing up the benefits of work: Behavioral and neural analyses of effort-related decision making. Neural Networks. 2006;19:1302–1314. doi: 10.1016/j.neunet.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Rudebeck PH, Bannerman DM, Rushworth MFS. Calculating the cost of acting in frontal cortex. Annals of the New York Academy of Sciences. 2007;1104:340–356. doi: 10.1196/annals.1390.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward TS, Metzak PD, Meier B, Holroyd CB. Anterior cingulate cortex signals the requirement to break inertia when switching tasks: A study of the bivalency effect. NeuroImage. 2008;40:1311–1318. doi: 10.1016/j.neuroimage.2007.12.049. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- Yeung N, Nieuwenhuis S. Dissociating response conflict and error likelihood in anterior cingulate cortex. The Journal of Neuroscience. 2009;29:14506–14510. doi: 10.1523/JNEUROSCI.3615-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]