Abstract
Stimulus class formation is inferred when conditional discrimination training yields new (emergent) conditional relations between the training stimuli. The present experiments demonstrated two such relations in pigeons after successive matching-to-sample training. Experiment 1 showed that transitivity (AC matching) emerged after training on AB and BC arbitrary matching plus BB identity matching: Pigeons responded relatively more to the comparisons on AC test trials in which both the A samples and C comparisons were elements of reinforced arbitrary baseline relations involving the same nominal B stimulus. Experiment 2 showed the opposite effect (“anti-transitivity”) after training on the same arbitrary relations but with BB oddity instead: Pigeons responded relatively more to the comparisons on AC test trials in which the A sample was an element of a reinforced baseline relation and the C comparison was an element of a non-reinforced baseline relation, or vice versa. Experiment 2 also showed that AB and BC training alone generally does not yield an emergent effect. These findings extend the range of emergent phenomena observed in non-human animals and are consistent with predictions from Urcuioli's (2008) theory of pigeons’ stimulus class formation.
Keywords: transitivity, anti-transitivity, emergent relations, stimulus classes, successive matching, pigeons, key peck
This paper reports two experiments from a continuing line of research with pigeons investigating stimulus-class formation, a topic germane to categorization and concept formation (Lazareva & Wasserman, 2008; Zentall, Wasserman, & Urcuioli, 2014; see also Urcuioli, 2013) and other aspects of cognitive functioning (e.g., Jenkins & Palermo, 1964; Horne & Lowe, 1997; Maydak, Stromer, Mackay, & Stoddard, 1995; Sidman, 1971). The fact that non-human animals can, under certain conditions, also group together disparate stimuli shows that human language is not necessary for categorization (cf. Carr, Wilkinson, Blackman, & McIlvane, 2000) and that the reinforcement contingencies of training can be sufficient to generate novel forms of stimulus control (Sidman, 2000). For example, stimuli that occasion the same reinforced response, signal the same distinctive reinforcer, or have some other common association are often interchangeable with one another in new contexts (e.g., Edwards, Jagielo, Zentall, & Hogan, 1982; Honey & Hall, 1989; Johnson, Meleshkevich & Dube, 2014; Urcuioli, Zentall, Jackson-Smith, & Steirn, 1989; Vaughan, 1988; cf. Goldiamond, 1992) as would be expected if they were members of a stimulus class (Saunders & Green, 1992; Urcuioli, 2013).
An example are the transfer effects shown by pigeons and other animals after training on many-to-one or “comparison-as-node” matching-to sample (cf. Fields, Verhave & Fath, 1984; McDaniel, Neufeld, & Damico-Nettleton, 2001; Spradlin & Saunders, 1986). As the name suggests, this procedure involves reinforcing the same comparison choice response after more than one (separately presented) sample stimulus (Urcuioli et al., 1989; Wasserman, DeVolder, & Coppage 1992; see also Bovet & Vauclair, 1998; Hall, Mitchell, Graham, & Lavis, 2003; Smeets, Barnes, & Roche, 1997). Training can be designated as AB and CB matching where the first letter of each pair refers to a set of sample stimuli and the second letter of each pair refers to a set of reinforced comparison stimuli. The notation indicates that subjects learn to match the same B comparisons to two different sets of sample stimuli, A and C. Although such training contingencies might simply result in two independent sets of conditional relations (viz., “match An to Bn” and “match Cn to Bn”), another possibility is that A and C samples occasioning the same reinforced B-comparison choice become members of the same stimulus class. To find out, researchers then train subjects to match just the A samples to a new set of comparison stimuli (D), after which they observe whether or not subjects are now able to match the C samples to the D comparisons despite never having been explicitly reinforced to do so. In fact, subjects are able to immediately transfer their D comparison choices from the A to the C samples (Spradlin, Cotter, & Baxley, 1973; Urcuioli et al., 1989, Experiment 2; Wasserman et al., 1992). Thus, CD relations have emerged from the explicitly trained AB, CB, and AD relations, demonstrating the interchangeability of the A and C samples and indicating that they are members of the same stimulus class.
A second example is seen in the variety of emergent relations observed in humans who have learned other combinations of matching-to-sample tasks (e.g., AB and BC). Following such training, they typically exhibit symmetry in which they now match former comparisons to former samples (viz., BA and CB; the reverse of what was explicitly taught), transitivity in which they now match the A samples to C comparisons (viz., AC matching), and combined symmetry and transitivity (viz., CA matching). Along with an ability to match each stimulus to itself (reflexivity: AA, BB, and CC matching), these findings are evidence for stimulus equivalence/equivalence-class formation (Sidman & Tailby, 1982; Sidman, 1990; 2000).
Until recently and in contrast with humans, non-human animals have only rarely exhibited symmetry. Indeed, the many unsuccessful attempts to demonstrate this emergent relation (e.g., D'Amato, Salmon, Loukas, & Tomie, 1985; Dugdale & Lowe, 2000; Hogan & Zentall, 1977; Lionello-DeNolf & Urcuioli, 2002; Lipkens, Kop, & Matthijs, 1988; Sidman et al., 1982) led some to argue that language may be a prerequisite for symmetry and for equivalence more generally (see, for example, Devany, Hayes, & Nelson, 1986; Dugdale & Lowe, 1990; Horne & Lowe, 1996). However, the difficulty in finding evidence for symmetry in non-human animals is due more to methodology rather than to capability. Specifically, a symmetry test following arbitrary matching training in the typical n-alternative (choice) paradigm is not a valid one because it does not actually assess what the experimenter believes. The reason is that the functional matching stimuli for many animals include a spatial location component – in other words, each nominal stimulus is actually that-stimulus-at-a-particular-location (e.g., for pigeons, red-on-the-center key, a stimulus that is not the same as red-on-the-left/right-key (Lionello & Urcuioli, 1998; see also Iversen, 1997; Iversen, Sidman, & Carrigan, 1986). This is important because in the shift from training to testing, the matching stimuli appear in different locations, thus generating new stimuli for the subject. Because of this, the symmetry test does not assess the truly symmetrical versions of the training relations.
Successive or go/no-go matching (Wasserman, 1976) avoids this spatial location problem by arranging that the individually presented sample and comparison stimulus on each matching trial appear in the same location. Responding to a particular comparison is reinforced after a particular sample stimulus (“go” trials) but not after the alternative sample stimulus (“no-go” trials). Each comparison (like each sample) is presented for some extended period of time (e.g., 5 or 10 s), so rate of comparison responding (rather than percentage correct) is the dependent variable. Learning and accurate conditional discrimination performances are indexed by higher rates on reinforced than on non-reinforced trials. Importantly, Frank and Wasserman (2005) and Urcuioli (2008, Experiment 3) showed that pigeons concurrently trained to accurate levels of performance on AB, AA, and BB successive matching subsequently showed BA symmetry in testing. Specifically, they responded more to the comparisons on BA test trials that were the reverse of the reinforced AB training trials than on BA test trials that were the reverse of the non-reinforced AB training trials (see also Campos, Urcuioli, & Swisher, 2014).
Initially, the rationale for concurrently training AA and BB identity matching with AB arbitrary matching was to minimize generalization decrement from AB training to BA testing by insuring that pigeons saw each nominal stimulus both as a sample and as a comparison prior to testing. However, Urcuioli (2008, Experiment 4) and Urcuioli and Swisher (2012b) showed that if one of the concurrently trained tasks was oddity rather than identity, the opposite effect – termed “antisymmetry” – emerged in testing. In other words, pigeons responded relatively more to the comparisons on BA test trials that were the reverse of the non-reinforced (rather than reinforced) AB training trials. To take a specific example, if a red sample – triangle comparison combination was reinforced in training, but a red sample – horizontal-lines comparison combination was not, in testing pigeons responded relatively more to the red comparison after the horizontal sample, not after a triangle sample. Clearly, the tasks trained concurrently with AB successive matching did something more than to minimize generalization decrement.
The antisymmetry effect prompted Urcuioli (2008) to propose a theory of pigeons’ stimulus class formation based in large part on the assumption that each functional matching stimulus consists of its nominal properties plus its ordinal position within a trial (first or second – i.e., as a sample or as a comparison, respectively). Thus, a red sample is functionally red-in-the- first-ordinal-position (R1) whereas a red comparison is functionally red-in-the-second-ordinal-position (R2). (Note that the theory also recognizes a spatial location component, but that component can be safely ignored when all stimuli appear in the same location – cf. Swisher & Urcuioli, 2013). The theory assumes that successive matching contingencies are conducive to stimulus class formation because non-reinforced sample-comparison combinations occur equally as often as reinforced combinations throughout training (i.e., independent of the level of discriminative performance). This should promote segregation of the functional stimuli into different classes each of which is assumed to consist of the elements of a reinforced combination (cf. Sidman, 2000). For example, if a red sample – triangle comparison and a green sample – horizontal combination are reinforced, but a red sample – horizontal comparison and a green sample – triangle combination are not, this should yield a [R1, T2] class and a [G1, H2] class1. Urcuioli's theory also assumes that elements common to more than one class will cause their respective classes to merge (cf. Johnson et al., 2014; Sidman, Kirk, & Willson-Morris, 1985). For instance, a [R1, T2] class and a [T1, T2] class should merge via the common T2 element into a larger [R1, T1, T2] class. Finally, theory assumes that responding will occur more frequently to a comparison in the same class as its preceding sample. Both symmetry and antisymmetry can be derived from these theoretical assumptions (see Urcuioli, 2008), as can other emergent relations (e.g., Sweeney & Urcuioli, 2010).
Here, we test and confirm theoretically derived predictions for two other emergent relations, transitivity and its opposite – which we call “anti-transitivity” – in separate experiments. Each derivation along with its corresponding training relations is described more fully in the introduction to each experiment.
1. Experiment 1
In Experiment 1, pigeons were trained on two arbitrary successive matching tasks (AB and BC) in which the nominal comparisons for one task were the nominal samples for the other, along with identity matching with those common stimuli (BB). Concurrent AB, BC, and BB training should, according to Urcuioli (2008), yield emergent AC matching (transitivity) in testing. For this experiment, the A stimuli were red (R) and green (G) hues, the B stimuli were triangle (T) and horizontal-lines (H) forms, and the C stimuli were blue (B) and white (W) hues. Table 1 summarizes the successive matching training contingencies for Experiment 1 and the subsequent probe (transitivity test) trials following acquisition of the 3 sets of conditional relations. Figures 1-3 visually depict how the theory predicts transitivity under these conditions.
Table 1.
Successive Matching Training Contingencies (left three columns) and Probe Test Trials (right column) for the Pigeons in Experiment 1.
| Pigeons T1, T2, T5, and T7 | |||
|---|---|---|---|
| Hue-Form (AB) | Form-Hue (BC) | Form-Form (BB) Identity | Probe Trials (AC) |
| R → T - FI 5 s | T → B - FI 5 s | T → T - FI 5 s | R → B √ |
| R → H - EXT | T → W - EXT | T → H - EXT | R → W |
| G → T - EXT | H → B - EXT | H → T - EXT | G → B |
| G → H - FI 5 s | H → W - FI 5 s | H → H - FI 5 s | G → W √ |
| Pigeons T3, T4, T6, and T8 | |||
|---|---|---|---|
| Hue-Form (AB) | Form-Hue (BC) | Form-Form (BB) Identity | Probe Trials (AC) |
| R → T - EXT | T → B - FI 5 s | T → T - FI 5 s | R → B |
| R → H - FI 5 s | T → W - EXT | T → H - EXT | R → W √ |
| G → T - FI 5 s | H → B - EXT | H → T- EXT | G → B √ |
| G → H - EXT | H → W - FI 5 s | H → H - FI 5 s | G → W |
Note. R = red, G = green, T = triangle, H = horizontal, B = blue, W = white, FI = fixed interval schedule, EXT = non-reinforced, √ = probe-test trials predicted to generate higher comparison response rates. The first stimulus in the trial sequence (the sample) is shown to the left of the arrows, and the second stimulus (the comparison) is shown to the right. Note the counterbalancing of the hue-form (AB) matching contingencies.
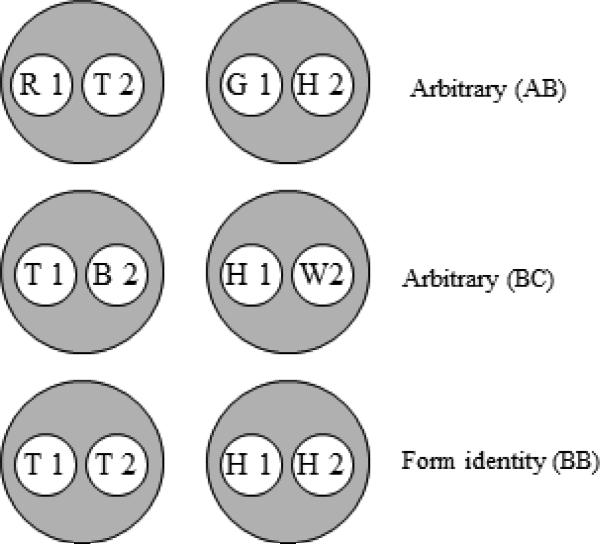
Figure 1.
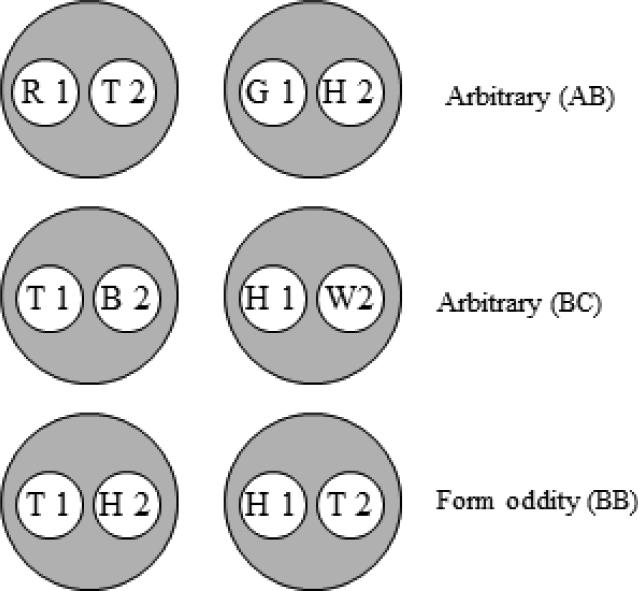
The six stimulus classes hypothesized to result from arbitrary (AB and BC) form identity (BB) successive matching training in Experiment 1. Letters denote the nominal stimuli (R = red, G = green, B = blue, W = white, T = triangle, H = horizontal) and numbers denote ordinal position in a trial (1 = first (sample), 2 = second (comparison)).
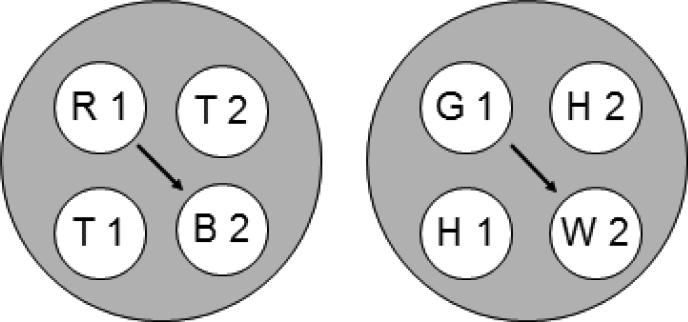
Figure 3.
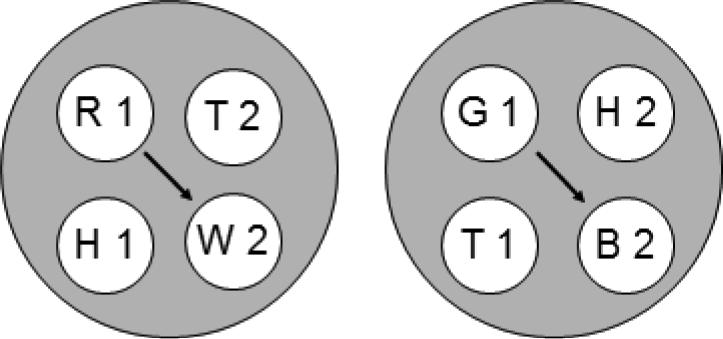
The two 4-member stimulus classes hypothesized to result from merging classes that contain common elements (cf. Figure 2). Letters denote the nominal stimuli (R = red, G = green, B = blue, W = white, T = triangle, H = horizontal) and numbers denote ordinal position in matching trial (1 = first (sample), 2 = second (comparison)). Arrows indicate predicted emergent transitive relations.
If, in AB training, pecking to the triangle comparison (T2) is reinforced after the red sample (R1) and pecking to the horizontal comparison (H2) is reinforced after the green sample (G1), then a [R1, T2] and a [G1, H2] class should form (top row of Figure 1). Likewise, if pecking to the blue comparison (B2) is reinforced after the triangle sample (T1) and pecking to the white comparison (W2) is reinforced after the horizontal sample (H1), a [T1, B2] and a [H1, W2] class should form (middle row of Figure 1). Finally, reinforcing pecking to a form comparison that is nominally identical to a form sample should yield a [T1, T2] and a [H1, H2] class (bottom row of Figure 1).
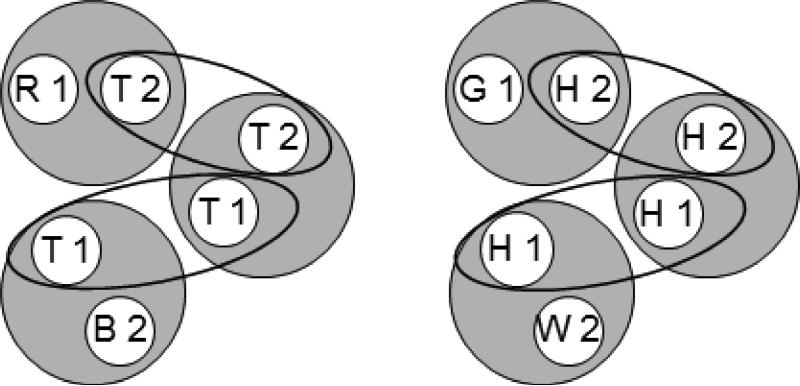
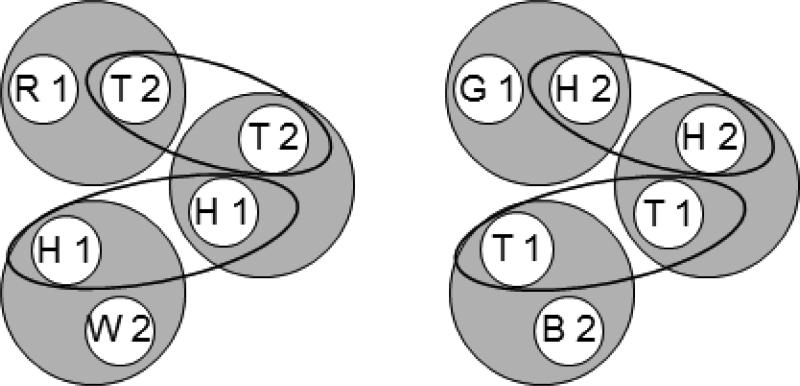
Each hypothesized class contains stimulus elements common to other classes (e.g., the triangle comparison (T2) is common to the [R1, T2] and [T1, T2] classes). Figure 2 rearranges the six classes shown in Figure 1 to highlight those common elements (connected via the ellipses). Assuming that these elements cause their respective classes to merge, the net result is the two 4-member classes shown in Figure 3. Each 4-member class contains both the elements of the reinforced baseline relations [e.g., red sample and triangle comparison (R1 and T2), triangle sample and blue comparison (T1 and B2), and triangle sample and triangle comparison (T1 and T2)] and the elements of an untrained, potentially emergent relation [e.g., red sample and blue comparison (R1 and B2)]. The arrows connect the latter elements which represent the predicted transitive relations. More specifically, Urcuioli's (2008) theory predicts that the baseline training contingencies will yield higher comparison response rates on the red sample – blue comparison (R1→B2) and green sample – white comparison (G1→W2) probe trials in testing.
Figure 2.
The six stimulus classes shown in Figure 1 rearranged to show common class elements (ellipses). Letters denote the nominal stimuli (R = red, G = green, B = blue, W = white, T = triangle, H = horizontal) and numbers denote ordinal position in a trial (1 = first (sample), 2 = second (comparison)).
2. Method
2.1. Subjects
Eight experimentally naïve White Carneau pigeons, 1-2 years old and obtained from Double “T” Farms (Glenwood, IA), participated in this experiment. All were maintained via food restriction at 80% of their free-feeding body weights which were determined within 2-3 weeks upon arrival in the laboratory. Pigeons obtained their daily food allotment of Purina ProGrains in the experimental sessions except on those days in which the experiment was not run. They were housed individually in stainless steel, wire mesh cages in a colony room on a 14h-10h light-dark schedule (lights on at 7 am). Water and grit were available at all times in their home cages.
2.2. Apparatus
Two BRS/LVE (Laurel, MD) pigeon chambers (Model PIP-016 panel inside a Model SEC-002 enclosure) were used in this experiment. Each panel was equipped with three horizontally aligned, 2.5-cm response keys spaced 5.7 cm apart (center-to-center) and 7.5 cm from the top. A BRS/LVE Model IC-901-IDD stimulus projector was mounted behind each key although only the center-key projector was used. This projector could display red (R), green (G), blue (B), and white (W) homogeneous fields, and three white horizontal lines (H) and a solid white inverted triangle (T) on black backgrounds (BRS/LVE Pattern No. 692). A rear-mounted food hopper located 13 cm below the center key was accessible via a 5.8-cm-square opening. When raised, the food hopper was illuminated by a small miniature bulb (ESB-28). A GE #1829 bulb located 7.6 cm above the center key in each chamber served as a house light, and a continuously running blower fan provided ventilation and masking noise. An IBM-compatible computer controlled the experimental events in both chambers. Four pigeons each were randomly assigned to be run in each experimental chamber.
2.3 Procedure
2.3.1. Preliminary training
After training to eat quickly and reliably from a raised food hopper and shaping by the method of successive approximations to peck a lit center key, three 60-trial preliminary training sessions were run. In each session, two stimuli that would later appear in successive matching were presented equally often in randomized order on the center key: triangle and horizontal (first session), blue and white (second session), and red and green (third session). A single peck to each center-key stimulus turned off that stimulus and produced 2-6 s access to grain. Reinforcement duration was constant within a session but was adjusted between subjects and across sessions in a manner that maintained body weights at 80% of their free-feeding values. Stimulus presentations were separated by a 15-s intertrial interval (ITI), and house light remained on throughout these sessions.
Next, pecking each stimulus was reinforced on gradually increasing fixed-interval (FI) schedules using the blue and white stimuli first, red and green stimuli second, and triangle and horizontal stimuli third. Each pair of stimuli appeared in five successive sessions with a FI 2 s schedule in effect for the first session, a FI 3 s schedule for the second session, and a FI 5 s schedule for the third, fourth, and fifth sessions. The first peck after the FI duration had elapsed turned off the center-key stimulus and produced food with the exception of the last session in which only 50% of the trials ended in reinforcement. Stimulus presentations were randomized within sessions and were separated by a 15-s ITI, the first 14 s of which were spent in darkness. The house light was turned on for the last 1 s of the ITI and remained on until the end of the trial.
2.3.2. Successive matching acquisition
Next, pigeons were concurrently trained on three successive matching tasks: hue-form (AB) arbitrary matching, form-hue (BC) arbitrary matching and form-form (BB) identity matching (see Table 1).
For half of the pigeons, pecking the triangle comparison after the red sample (R→T) and pecking the horizontal comparison after the green sample (G→H) ended in reinforcement in the AB task, whereas the remaining sample-comparison combinations (R→H and G→T) ended without reinforcement (top half of Table 1). For the other half of the pigeons, the opposite contingencies were in effect (bottom half of Table 1).
The successive matching contingencies for the other two tasks were identical for all pigeons. Specifically, in the BC task, pecking the blue comparison after the triangle sample (T→B) and pecking the white comparison after the horizontal sample (H→W) ended in reinforcement, whereas the remaining combinations (T→W and H→B) ended without reinforcement. In the BB identity task, pecking the triangle comparison after the triangle sample (T→T) and pecking the horizontal comparison after the horizontal sample (H→H) ended in reinforcement, whereas the non-matching combinations (T→H and H→T) ended without reinforcement.
Each matching trial began with the presentation of a sample stimulus on the center key. The first sample key peck initiated a FI 5-s schedule. The first peck after 5 s turned off the sample stimulus and produced a blank 1-s interval after which a single comparison stimulus appeared on the same key. On reinforced trials, the first comparison peck began a 5-s interval after which a single peck turned off the comparison and produced food. On non-reinforced trials, the comparison stimulus went off automatically 5 s after comparison onset. A 15-s ITI, the first 14 s of which the house light was off, followed food presentation (reinforced trials) or comparison offset (non-reinforced trials).
Each 96-trial training session contained 32 trials each of the AB, BC, and BB identity relations. Every possible sample-comparison combination was presented eight times per session in pseudorandom order with the constraint that the same combination could not appear more than twice in a row. Baseline acquisition for each pigeon was achieved when it exhibited at least a .80 discrimination ratio (DR) for five of six consecutive sessions on all three tasks. The DR for each task was calculated by dividing the total number of pecks to the comparison stimuli on reinforced trials by the total number of pecks to the comparison stimuli on both reinforced and non-reinforced trials. (Only pecks within 5 s of comparison onset were recorded.) After meeting the acquisition criterion, pigeons received a minimum of 10 additional (overtraining) sessions which ended when a .80 or greater DR was achieved on all three tasks for five of six consecutive overtraining sessions.
2.3.3. Successive matching testing
In testing, infrequent non-reinforced AC probe trials (see Table 1) were presented among the baseline trials from all three tasks. Each test session contained 96 baseline trials and eight non-reinforced probes divided equally among the four possible probe trial types (viz., R→B, R→W, G→B and G→W). Probe trials ended automatically 5 s after comparison stimulus onset. The first probe in a session occurred after each baseline trial type was presented at least once; subsequent probe trials were separated by at least five baseline trials. A total of eight test sessions were conducted, organized in 2-session blocks separated by at least five baseline sessions at criterion levels of performance.
Transitivity was assessed by comparing the number of probe-trial comparison pecks/s on “positive” trials (see check-marked relations in Table 1) with the number of probe-trial comparison pecks/s on “negative” trials. “Positive” was operationally defined as probes resulting from the combination of the reinforced AB and BC baseline trials that shared the same nominal stimulus. An example is R→B (see top half of Table 1) which combines the sample from the reinforced R→T sample-comparison sequence with the comparison from the reinforced T→B sample-comparison sequence. “Negative” was operationally defined as probes resulting from a combination of a reinforced AB and a non-reinforced BC baseline trial (or vice versa), that likewise shared the same nominal stimulus. An example is R→W (see top half of Table 1) which combines the sample from the reinforced R→T sample-comparison sequence with the comparison from the non-reinforced T→W sample-comparison sequence. All other procedural details for testing were the same as those for acquisition.
2.4. Statistical analyses
Analyses of variance (ANOVA) were conducted on various baseline data (viz., sessions-to-criterion and terminal DRs) and on the differences in peck rates on positive versus negative probe trials. Observed F ratios were compared to the tabled F values reported by Rodger (1975), which control Type I error rate on a per-decision basis. Type I error rate was set at 0.05.
3. Results and Discussion
3.1. Acquisition
In general, pigeons acquired the AB (hue-form) successive matching task to criterion levels of performance in fewer sessions than they acquired the BC (form-hue) and BB (form-form) tasks. The average number of sessions to reach criterion were 23.9, 36.0, and 35.5 for the AB, BC, and BB tasks, respectively, F (2, 14) = 8.47. Sessions-to-criterion was lower for the AB task than for either BC and BB which did not differ from one another, Fs (2, 14) = 8.45 and 0.02, respectively. More importantly, average DRs for the three tasks over the last five overtraining (baseline) sessions preceding testing did not differ significantly from one another: .91 (AB) versus .90 (BC) versus .89 (BB), F (2, 14) = 1.72.
Baseline performances during testing (see below) were generally well-maintained. Across all eight pigeons, three tasks and eight test sessions, only 21 of the 192 baseline DRs fell below .80 in a session. Eleven of those occurred on the form-form (BB) identity task and only two of the 21 fell below .70.
3.2. Testing
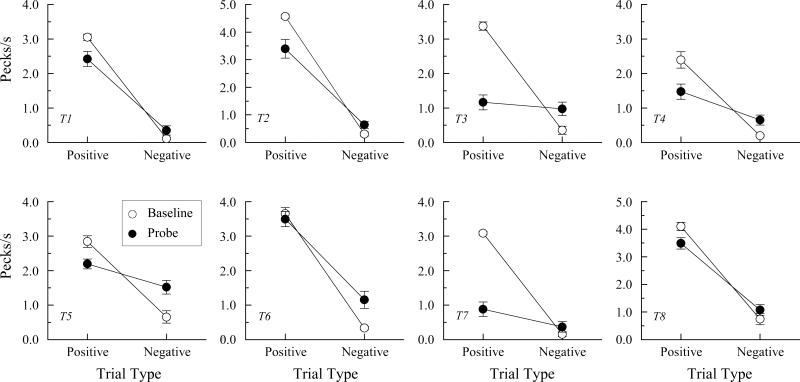
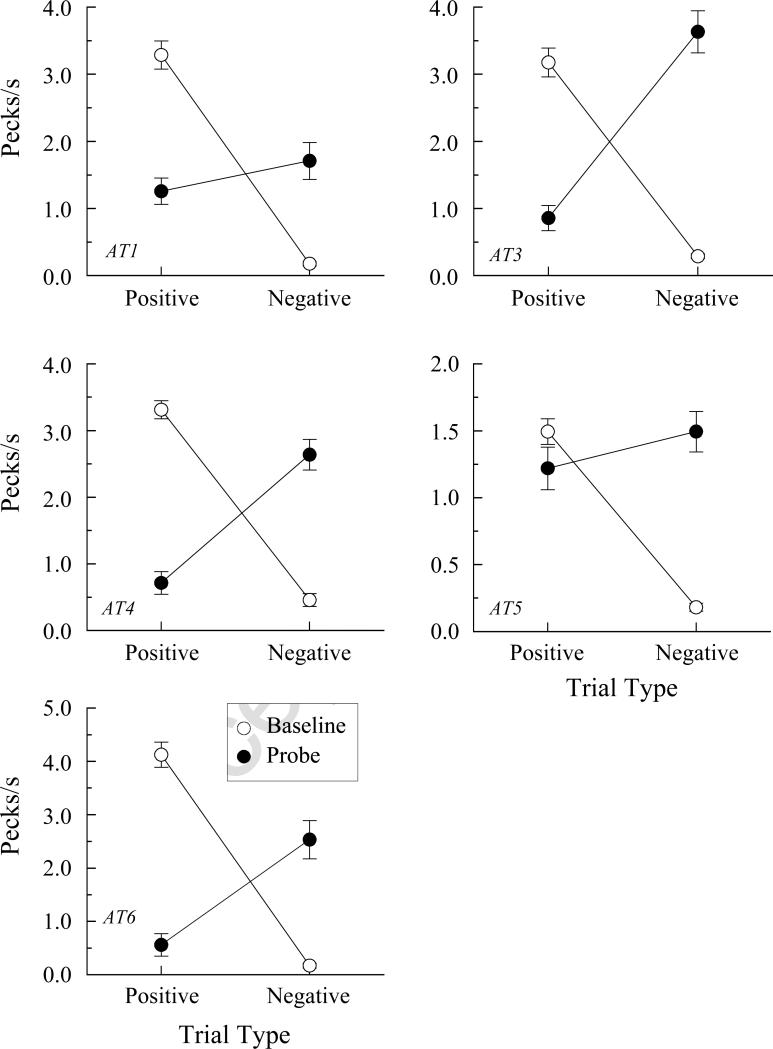
Figure 4 shows the individual AC probe-trial performances (filled symbols) averaged over all eight test sessions and individual AB baseline performances (open symbols) for those same sessions. The baseline data are averages of two randomly selected trials of each reinforced AB combination and two randomly selected trials of each non-reinforced AB combination from each test session (total of 32 reinforced and 32 non-reinforced AB trials). This was done to equate the number of data points included in the positive baseline and probe averages and in the negative and baseline probe averages.
Figure 4.
Comparison response rates in pecks/s (± 1 SEM) for the pigeons in Experiment 1 on arbitrary matching (AB) baseline trials (open circles) and the non-reinforced AC transitivity probe trials (filled circles) averaged over the eight test sessions. Positive = reinforced baseline trials and probe trials consisting of samples from reinforced AB baseline relations and comparisons from reinforced BC baseline relations. Negative = non-reinforced baseline trials and probe trials consisting of samples from reinforced AB baseline relations and comparisons from non-reinforced BC baseline relations or vice versa.
Not surprisingly, each pigeon exhibited much higher comparison-response rates on positive than on negative baseline trials. In other words, their reinforced baseline discrimination performances were well maintained throughout testing. More important were their response rates on the (non-reinforced) positive versus negative probe trials. Every pigeon pecked more often to the comparisons on the positive probes than on the negative probes. The differences for seven of the eight pigeons were statistically significant in ANOVA: Fs (1, 62) = 63.75, 58.80, 9.85, 7.67, 49.28, 3.90, and 74.50 for pigeons T1, T2, T4, T5, T6, T7, and T8, respectively. Not surprisingly, an overall ANOVA on the probe-trial performances for all pigeons showed a significant positive versus negative probe-trial difference in response rate, F (1, 7) = 16.66.
Clearly, these results demonstrate emergent transitive (AC) relations following successive matching training on AB, BC, and BB-identity relations and they confirm the prediction derived from Urcuioli's (2008) theory of stimulus class formation. The results also extend some previous experiments by Urcuioli and Swisher (2012a) in which pigeons concurrently trained on AB, BA, and BB-identity successive matching later exhibited emergent AA matching in testing. Although that finding can be viewed as an instance of reflexivity (Sweeney & Urcuioli, 2010), it can also be viewed as an instance of transitivity given the common nominal stimulus shared by the AB and BA baseline relations that were part of the training contingencies (although see Urcuioli & Swisher, 2012a, Experiment 3 for conflicting results). Here, there is no ambiguity in labeling the emergent relations seen in testing because the sample and comparison stimuli comprising the probe trials were not physically identical to one another as they were in Urcuioli and Swisher (2012a) and related studies (Sweeney & Urcuioli, 2010; Urcuioli, 2011).
The next experiment examined two other theoretically derived predictions from Urcuioli's (2008) theory, one which anticipates an emergent relation in which the pattern of probe-trial responding is the opposite of that observed in this experiment and another which anticipates no emergent effect.
4. Experiment 2
Experiment 2 was designed primarily to see if the opposite pattern of test-trial responding (viz., higher comparison response rates on negative than on positive AC probes) would be obtained by training pigeons on BB-oddity, rather than BB-identity, concurrently with AB and BC arbitrary successive matching (see top half of Table 2). Stated otherwise, would pigeons actually respond less on probe trials consisting of a sample and a comparison from two different reinforced arbitrary baseline trials that shared a common, nominal stimulus? Such a finding would provide a noteworthy and important parallel to the anti-symmetry results reported by Urcuioli (2008, Experiment 4) when concurrently training one oddity task produced a pattern of probe-trial responding opposite of that obtained with concurrent identity training (viz., symmetry – Urcuioli, 2008, Experiment 4). In short, we looked to see if “anti-transitivity”, rather than transitivity, would occur after similar concurrent training.
Table 2.
Successive Matching Training Contingencies (left columns) and Probe Test Trials (right column) for the Anti-Transitivity (AT) and Control Groups in Experiment 2.
| Group AT | |||
|---|---|---|---|
| Hue-Form (AB) | Form-Hue (BC) | Form-Form (BB) Oddity | Probe Trials (AC) |
| R → T - FI 5 s | T → B - FI 5 s | T → T - EXT | R → B |
| R → H - EXT | T → W - EXT | T → H - FI 5 s | R → W √ |
| G → T - EXT | H → B - EXT | H → T - FI 5 s | G → B √ |
| G → H - FI 5 s | H → W - FI 5 s | H → H - EXT | G → W |
| Group Control | ||
|---|---|---|
| Hue-Form (AB) | Form-Hue (BC) | Probe Trials (AC) |
| R → T - FI 5 s | T → B - FI 5 s | R → B |
| R → H - EXT | T → W - EXT | R → W |
| G → T - EXT | H → B - EXT | G → B |
| G → H - FI 5 s | H → W - FI 5 s | G → W |
Note. R = red, G = green, T = triangle, H = horizontal, B = blue, W = white, FI = fixed interval schedule, EXT = non-reinforced, √ = probe-test trials predicted to generate higher comparison response rates. The first stimulus in the trial sequence (the sample) is shown to the left of the arrows, and the second stimulus (the comparison) is shown to the right. Counterbalancing of the hue-form (AB) matching contingencies has been omitted.
The left three columns in the top half of Table 2 show the concurrently trained baseline tasks for this group. Note that the AB and BC successive matching tasks were identical to those in Experiment 1 (cf. Table 1). Unlike Experiment 1, however, the BB (form-form) task was oddity: Responding to the form comparison that did not match the preceding form sample was reinforced. This modification was predicted to yield higher comparison response rates on negative AC probe trials in subsequent testing (indicated by the check marks). In other words, pigeons were predicted to peck more on probe trials that consisted of a sample from a reinforced AB relation and a comparison from a non-reinforced BC relation, or vice versa. For example, the reinforced R→T relation in AB matching plus a non-reinforced T→W relation in BC matching should generate relatively high comparison response rates to a R→W probe as opposed to, say, a R→B probe which consists of a sample and a comparison from two reinforced arbitrary matching baseline trials (viz., R→T and T→B).
Figures 5 and 6 provide a visual depiction of how this prediction was derived. Figure 5 shows the six 2-member classes hypothesized to develop from baseline training. The top two rows of Figure 5 shows the classes corresponding to AB and BC arbitrary matching; these are identical to those shown in the top two rows of Figure 1 for Experiment 1. The bottom row of Figure 5 shows the 2-member classes hypothesized to result from BB-oddity. These differ from those shown for BB-identity in Experiment 1 (cf. bottom row of Figure 1) because each contains nominally different form stimuli (e.g., T(riangle) and H(orizontal)). Nevertheless, these six classes also share elements in common with each other, as indicated by the ellipses shown in Figure 6. Those common elements, by hypothesis, should merge their respective classes together yielding the two 4-member classes shown in Figure 7. The arrows indicate the anti-transitive relations predicted to emerge from the baseline relations. To reiterate, although responding to the triangle comparison after the red sample (R1→T2) is reinforced in AB matching and responding to the blue comparison after the triangle sample (T1→B2) is reinforced in BC matching, the theory predicts higher comparison response rates to the white (not the blue) comparison after the red sample (viz., R1→W2) in testing.
Figure 5.
The six stimulus classes hypothesized to result from arbitrary (AB and BC) form oddity (BB) successive matching training in Experiment 2. Letters denote the nominal stimuli (R = red, G = green, B = blue, W = white, T = triangle, H = horizontal) and numbers denote ordinal position in a trial (1 = first (sample), 2 = second (comparison)).
Figure 6.
The six stimulus classes shown in Figure 6 rearranged to show common class elements (ellipses). Letters denote the nominal stimuli (R = red, G = green, B = blue, W = white, T = triangle, H = horizontal) and numbers denote ordinal position in a trial (1 = first (sample), 2 = second (comparison)).
Figure 7.
The two 4-member stimulus classes hypothesized to result from merging classes that contain common elements (cf. Figure 3). Letters denote the nominal stimuli (R = red, G = green, B = blue, W = white, T = triangle, H = horizontal) and numbers denote ordinal position in matching trial (1 = first (sample), 2 = second (comparison)). Arrows indicate predicted emergent transitive relations.
Experiment 2 also included a control group appropriate to the group just described and to the group run in Experiment 1. Group Control (see bottom half of Table 2) was trained only on AB and BC successive matching. Urcuioli's (2008) theory predicts such training will be insufficient to yield emergent AC performances of any kind in testing. The reason can be appreciated by looking at just the top two rows of Figure 5 (or at just the top two rows of Figure 1) which show the hypothesized classes resulting from AB and BC training. Note the lack of common elements across just these classes. Without common elements, class merger is not possible and without merged (enlarged) classes, there is no basis for AC responding. In other words, unless both the red sample (R1) and the blue comparison (B2) are members of the same stimulus class (and likewise for the green sample (G1) and the white comparison (W2)), pigeons should respond non-differentially on the AC probe trials.
5. Method
5.1. Subjects and Apparatus
Twelve experimentally naïve White Carneau pigeons, 1-2 years old and obtained from Double “T” Farms (Glenwood, IA), participated in this experiment. They were housed and maintained in the same manner as described for Experiment 1. Prior to the experiment, they were randomly assigned to two groups (Group AT and Group Control) with 3 pigeons from each group assigned to each experimental chamber. One pigeon in Group AT was removed from the experiment for failure to achieve and maintain the required baseline performances.
The apparatuses and control equipment were identical to those in Experiment 1.
5.2. Procedure
5.2.1. Preliminary training
This was identical in all respects to preliminary training in Experiment 1.
5.2.2. Successive matching acquisition
Following completion of preliminary training, pigeons in Group AT were concurrently trained on three successive matching tasks: hue-form (AB) arbitrary matching, form-hue (BC) arbitrary matching and form-form (BB) oddity matching (see top half of Table 2). These tasks were structured in the same way as they were in Experiment 1 except for the BB trials. On those trials, pecking the horizontal comparison after the triangle sample (T→H) and pecking the triangle comparison after the horizontal sample (H→T) ended in reinforcement, whereas the matching sample-comparison combinations (T→T and H→H) ended without reinforcement – i.e., oddity contingencies were in effect.
Pigeons in Group Control (see bottom half of Table 2) were trained on just the AB (hue-form) and BC (form-hue) arbitrary tasks. Training sessions for both groups consisted of 32 AB trials, 32 BC trials, and (for Group AT only) 32 BB-oddity trials. Counterbalancing of the reinforced and non-reinforced AB relations (not shown in Table 2) and all other details were identical to those described in Experiment 1. As before, baseline training for each pigeon continued until it achieved at least a .80 DR for five of six consecutive sessions on each task on which it was trained. It then received a minimum of 10 additional (overtraining) sessions which ended when a .80 or greater DR was met on both (Group Control) or all (Group AT) tasks for five of six consecutive overtraining sessions.
5.2.3. Successive matching testing
Following acquisition, eight test sessions organized into four 2-session blocks that were separated by baseline training at criterion levels of performances were run. Testing again involved periodic presentations of non-reinforced AC probe trials (see Table 2) among each pigeon's baseline trials to assess possible emergent AC relations. Each test session and each probe trial (viz., R→B, R→W, G→B and G→W) was structured as they were in Experiment 1.
If “positive” versus “negative” test trials are defined in the same way as before (viz., “positive” = a combination of reinforced AB and BC baseline trials sharing the same nominal stimulus; “negative” = a combination of a reinforced AB and a non-reinforced BC baseline trial sharing the same nominal stimulus, or vice versa), Urcuioli's (2008) theory predicts that comparison response rates in Group AT will be higher on negative AC probes (check-marked in Table 2) than on positive AC probes. Stated otherwise, even though the R→T and T→B sample-comparison sequences were both reinforced during training, comparison-response rates on R→B (“positive”) probe trials which combine the sample from the former with the comparison from the latter should be lower than on R→W (“negative”) probe trials which combine the sample from the reinforced R→T sequence with the comparison from the non-reinforced T→W sequence.
For Group Control, the prediction is that pigeons will respond non-differentially on the AC probes; consequently, the bottom half of Table 2 shows no check mark beside any probe.
6. Results and Discussion
6.1. Acquisition
Pigeons in Group AT acquired their two arbitrary matching tasks (AB and BC) to criterion in fewer sessions on average than their BB-oddity task. The average sessions-to-criterion were 55.6, 66.2, and 106.2, respectively, for AB, BC, and BB-oddity. The differences were not statistically significant, F (2, 4) = 3.67, because of large variability across subjects. Pigeons in Group Control acquired their AB and BC tasks at roughly comparable rates: Average sessions-to-criterion were 35.2 and 49.0, respectively, F (1, 5) = 1.25.
Average DRs in Group AT over each pigeon's last five overtraining sessions before testing were .91, .87, and .85 for the AB, BC, and BB-oddity tasks. The significant between-task difference, F (2, 8) = 3.73, was largely attributable to a higher DR in AB successive matching than in BB-oddity but, as can be seen, the DRs were uniformly high and the differences between them small. The corresponding average DRs for Group Control were .91 and .90 for AB and BC matching, F (1, 5) = 1.0.
As in Experiment 1, baseline performances during testing were mostly maintained at or above criterion levels. Across all pigeons, tasks and test sessions, just 25 of the 216 baseline DRs fell below .80 and only four of those 25 were below .70.
6.2. Testing
Figures 8 and 9 show the average AC probe-trial performances (filled symbols) and average AB baseline performances (open symbols) for each pigeon in Group AT and for each pigeon in Group Control, respectively. The baseline data are averages of two randomly selected trials of each reinforced AB combination and two randomly selected trials of each non-reinforced AB combination from each test session (total of 32 reinforced and 32 non-reinforced AB trials). Again, this was done to equate the number of data points included in the positive baseline and probe averages and in the negative baseline and probe averages.
Figure 8.
Comparison response rates in pecks/s (± 1 SEM) for pigeons in the anti-transitivity (AT) group of Experiment 2 on arbitrary matching (AB) baseline trials (open circles) and the non-reinforced AC probe trials (filled circles) averaged over the eight test sessions. Positive = reinforced baseline trials and probe trials consisting of samples from reinforced AB baseline relations and comparisons from reinforced BC baseline relations. Negative = non-reinforced baseline trials and probe trials consisting of samples from reinforced AB baseline relations and comparisons from non-reinforced BC baseline relations or vice versa.
Figure 9.
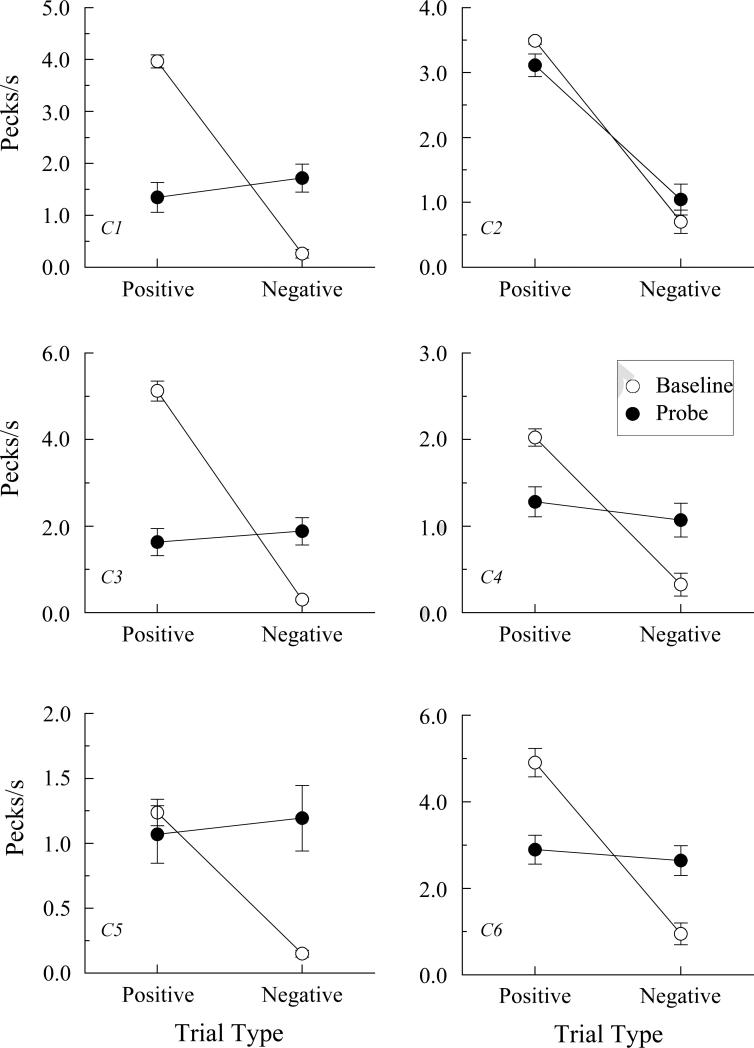
Comparison response rates in pecks/s (± 1 SEM) for the pigeons in the control group of Experiment 2 on arbitrary matching (AB) baseline trials (open circles) and the non-reinforced AC probe trials (filled circles) averaged over the eight test sessions. Positive = reinforced baseline trials and probe trials consisting of samples from reinforced AB baseline relations and comparisons from reinforced BC baseline relations. Negative = non-reinforced baseline trials and probe trials consisting of samples from reinforced AB baseline relations and comparisons from non-reinforced BC baseline relations or vice versa.
Pigeons in both groups continued to respond at much higher rates to the comparisons on positive (reinforced) than on negative (non-reinforced) baseline trials. In contrast, on the AC probe trials, every pigeon in Group AT responded at higher rates to the comparisons on negative than on positive probes, although the difference was statistically significant across the eight test sessions for only three of the five pigeons: AT3, AT4, and AT6, Fs (1, 62) = 57.68, 44.77, and 22.83, respectively. Pigeon AT5's response-rate difference was statistically significant over the first two test sessions, F (1, 14) = 4.98, and over the first six test sessions, F (1, 46) = 5.04. An overall ANOVA on the positive versus negative probe-trial rates for all five pigeons across all eight test sessions showed a significantly higher rate on the negative probe trials, F (1, 4) = 9.43.
The pattern of test results was entirely different in Group Control. Five of the six pigeons (C1, C3, C4, C5, and C6) responded at roughly the same rates to the comparisons on each type of probe trial; statistical analyses of their individual rates confirmed this observation, largest F (1, 62) = 0.91. Pigeon C2, however, responded at much higher rates on positive than on negative AC probe trials, F (1, 62) = 49.20. An ANOVA over all six pigeons showed (not surprisingly) no significant difference in positive versus negative probe-trial rates, F (1, 5) = .62, although this overall result obscures the substantial difference in Pigeon C2's performance.
There are three notable findings in this experiment. First, when BB-oddity rather than BB-identity is trained concurrently with AB and BC arbitrary successive matching, emergent anti-transitive AC relations are observed in testing. In other words, AC relations that combined a sample from a reinforced AB trial and a comparison from a reinforced BC trial yielded lower comparison-response rates than AC relations that combined a sample from a reinforced AB trial and a comparison from a non-reinforced BC trial (or vice versa). This finding is consistent with the prediction derived from Urcuioli's (2008) theory of stimulus class formation.
Second, simply training AB and BC successive matching is largely insufficient to produce emergent AC relations. This, too, is consistent with theoretical predictions.
Third, contrary to theoretical prediction, one Control group pigeon (C2) showed a clear AC transitivity effect following its training on AB and BC successive matching. Specifically, it responded at much higher comparison-response rates on AC probes that combined an A sample from a reinforced AB relation with a C comparison from a reinforced BC relation. At a minimum, its data indicate that other mechanisms besides those suggested by Urcuioli (2008) can produce emergent (untrained) relations in pigeons following conditional discrimination training.
7. General Discussion
The present experiments demonstrate that training pigeons on AB and BC arbitrary successive matching plus either BB-identity matching (Experiment 1) or BB-oddity (Experiment 2) yields emergent transitive and anti-transitive AC relations, respectively. Specifically, 7 of 8 pigeons in Experiment 1 responded significantly more in testing on positive AC probe trials (viz., trials involving A samples and C comparisons from reinforced AB and reinforced BC baseline relations) than on negative AC probe trials (viz., trials involving A samples from reinforced AB baseline relations and C comparisons from non-reinforced BC baseline relations, or vice versa.) In Experiment 2, the opposite pattern was observed: 3 of 5 pigeons responded significantly more on negative than on positive AC probe trials. In addition, Experiment 2 showed that training only AB and BC matching was mostly insufficient to yield any type of emergent effect in testing.
Comparing test results across the experiments might seem to suggest that transitive AC relations are more readily obtained than anti-transitive relations. But there are two reasons to be cautious about drawing such a conclusion. First, 3 fewer pigeons were run in Group AT in Experiment 2 than were used in Experiment 1. Consequently, we cannot be certain if the proportion of pigeons showing anti-transitivity would have been greater if more pigeons had been run (viz., 8). Second, one of the Group AT pigeons (AT5) that did not show a significant anti-transitivity effect over all eight test sessions did show a significant effect during its initial two test sessions (and, also, over its first six). Comparing results across the initial test sessions, 4 of the 5 AT pigeons demonstrated an anti-transitivity effect in Experiment 2 compared to 5 of 8 pigeons demonstrating a transitivity effect in Experiment 1. (Using a measure encompassing 6 test sessions, the corresponding proportions were 4 of 5 showing anti-transitivity versus 7 of 8 showing transitivity.) In any event, the more important point, in our estimation, is that these derived relations add to a growing list of emergent effects demonstrable in non-human animals generally and pigeons specifically. Moreover, the data mostly confirm the predictions of Urcuioli's (2008) theory of stimulus-class formation, again reinforcing the principal assumption that the functional matching stimuli for pigeons in these types of tasks are the nominal stimuli plus their ordinal position within a trial (and, of course, their spatial location – cf. Lionello & Urcuioli, 1998; Swisher & Urcuioli, 2013).
The results are also noteworthy in the context of many past failures to observe AC transitivity in pigeons after AB, BC training in the n-alternative matching paradigm. For example, Lipkens et al. (1988) found that pigeons responded at chance (50%) levels of accuracy on an AC transitivity test after AB, BC training with colors, key locations, and line orientations as the A, B, and C stimuli, respectively. Lionello-DeNolf (2001, Experiment 3) also found no evidence of transitivity in pigeons trained on AB, BC, and DA two-choice matching tasks and then tested on reinforced AC and DB relations in a manner that was either consistent or inconsistent with transitivity. Overall, accuracy in the transitive-consistent test condition averaged 55% versus 52% in the transitive-inconsistent test condition. Likewise, using a within-subjects reinforced test manipulation, D'Amato (1985, Experiment 3) also found that pigeons averaged 55% versus 52% correct, respectively, in transitive-consistent versus transitive-inconsistent tests. Two exceptions to this pattern of findings (viz., Kendall, 1983; Kuno et al., 1994) are difficult to interpret because of the absence of a necessary, within-test-learning control condition (Kendall, 1983) and the possibility of stimulus generalization between the A and B samples used during AB and BC training (Kuno et al., 1994).
Note that transitivity in the two-choice paradigm does not involve changing either the spatial location or the ordinal positions of the matching stimuli in testing vis-à-vis training. Specifically, the A samples continue to appear in the same spatial location as in the AB task, and the C comparisons continue to appear in the same spatial location as in the BC task. Likewise, the A stimuli continue to appear first (as samples) and the C stimuli continue to appear second (as comparisons) on each test trial. Consequently, the negative findings from prior studies cannot be attributed to altering functional stimuli in the shift from training to testing. It seems increasingly likely that n-alternative procedures are not conducive to pigeons’ stimulus class formation because with increasing baseline accuracies during training, pigeons encounter (by definition) fewer and fewer non-reinforced sample-comparison experiences. By contrast, the proportion of explicitly non-reinforced to explicitly reinforced sample-comparison trials (“experiences”) in successive matching remains constant throughout training (cf. Urcuioli, 2010). That continual juxtaposition of explicitly non-reinforced with explicitly reinforced trials may promote class formation by engendering both sample/S- as well sample/S+ stimulus control (see Carr et al., 2000; McIlvane, Withstandley, & Stoddard, 1984).
Data from other species, however, show that equating exposure to non-reinforced and reinforced combinations during training is not necessary to observe transitivity in testing. For example, Schusterman and Kastak (1993) trained a California sea lion on a variety of AB and BC relations in choice matching-to-sample and subsequently observed very high levels of accuracy (viz., greater than 95% correct) on the initial exposures to (reinforced) AC transitivity test trials (see also Lindemann-Biolsi & Reichmuth, 2014). Similarly, D'Amato et al. (1988, Experiment 2) reported average accuracies across four Cebus apella monkeys of 92% versus 22% correct on transitive-consistent versus transitive-inconsistent test trials after training on AB and BC two-choice matching-to-sample.
From the theoretical perspective (Urcuioli, 2008) that prompted our experiments, however, a more noteworthy finding is that Pigeon C2 in Experiment 2 exhibited transitivity in testing after training on just AB and BC successive matching. Its results clearly disconfirm the prediction that AB and BC training alone will not yield emergent AC performances in testing. Interestingly, when this pigeon was subsequently retrained on AB, BC, and BB-oddity successive matching (i.e., trained like the Group AT pigeons in Experiment 2), it did not show an anti-transitivity effect during subsequent retesting (also predicted by the theory) but, instead, continued to exhibit transitivity (data not shown). Perhaps, then, ordinal position was not a component of the functional matching stimuli for this pigeon, meaning that with spatial location held constant, the B nodal stimulus mediated transfer of the trained AB and BC performances to the observed AC performances in testing (Fields et al., 1984).
Interestingly, Strasser, Ehrlinger, and Bingman (2004) also reported emergent AC relations in hippocampal-lesioned and control-lesioned homing pigeons after training just AB and BC relations in a modified version of successive matching. In their procedure, seven pecks within 10 s produced food on reinforced sample-comparison trials versus a 5-s time-out period on non-reinforced trials. Failure to complete seven pecks to the comparison on any trial simply ended the trial and initiated the inter-trial interval. By the end of training, the time to complete the fixed-ratio (FR) comparison-response requirement was considerably shorter on reinforced than on non-reinforced trials. More important, the time to complete the FR 7 on “positive” transitivity probes was also significantly shorter than on “negative” transitivity probes. These data, too, imply stimulus class formation in which class members are simply the nominal matching stimuli themselves. If so, a similar time-to-completion difference would be expected if CA probes had also been tested.
It appears, then, that even for pigeons, multiple processes may be involved in transitivity (see also Steirn, Jackson-Smith, & Zentall, 1991). That said, it is important to recognize that the anti-transitive emergent relations observed in Experiment 2 can only be explained in terms of Urcuioli's (2008) theory.
Finally, it is worth noting that successive-matching-like procedures have also been used in studies of human equivalence-class formation (e.g., Layng & Chase, 2001; Takahashi, Yamamoto, & Noro, 2011; see also Fields, Doran, & Marroquin, 2009). In this literature, the precursor to the Relational Evaluation Procedure (pREP – e.g., Cullinan, Barnes, & Smeets, 1998; Leader & Barnes-Holmes, 2001; Smeets, van Wijngaarden, Barnes-Holmes, & Cullinan, 2004) represents the closest approximation to successive matching procedures used with pigeons. pREP trials consist of two successively presented stimuli followed by a response period during which no stimulus is present. Responding during the post-stimuli blank period is reinforced after certain (“positive”) sample-comparison sequences. The contingencies arranged for the remaining (“negative”) sequences vary across experiments and studies but, interestingly, it appears that the ones most like those used in standard successive matching with pigeons (i.e., no reinforcement for responding after negative sequences) are most successful in yielding emergent effects indicative of class formation (Smeets, Barnes-Holmes, & Striefel, 2006). These findings may reflect, once again, behavioral processes shared by human and other species in the animal kingdom (cf. Hughes & Barnes-Holmes, 2014).
Pigeons concurrently trained on AB, BC, and BB-identity successive matching show emergent AC matching (transitivity) in testing.
Pigeons concurrently trained on AB, BC, and BB-oddity successive matching show the opposite of transitivity (emergent anti-transitivity) in testing.
Most pigeons do not show any emergent relations after AB and BC training alone.
Transitive and anti-transitive emergent relations are predicted by a theory of stimulus-class formation (Urcuioli, 2008) that also accounts for other emergent effects recently observed in pigeons.
Acknowledgments
This research was supported by NICHD Grant R01 HD061322 to Peter J. Urcuioli. The results of these experiments were reported at the 21st International Conference on Comparative Cognition, Melbourne Beach, FL, March 2014. The authors thank Blake Polak and Heloisa Campos for their assistance in conducting this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The notation used in reference to Urcuioli's (2008) theory of stimulus class formation differs from that typically used in the stimulus equivalence literature. Here, the numerals 1 and 2 designate a stimulus’ ordinal position within a matching trial, not the hypothesized class to which it belongs. Likewise, the letter before each numeral (e.g., R, G, T, etc.) designates a specific matching stimulus (like red, green, triangle, etc) rather than a set of stimuli. In the stimulus equivalence literature, the letters “A”, “B”, and “C” are used to denote sets of stimuli which we do here as well but only when describing baseline or test relations and never in combination with a numeral. The notation differences may pose a challene for some readers, but we think it's important to maintain our notation to be consistent with Urcuioli's (2008) theory and with the experiments that followed it.
References
- Bovet D, Vauclair J. Functional categorization of objects and of their pictures in baboons (Papio anubis). Learning and Motivation. 1998;29:309–322. [Google Scholar]
- Campos HC, Urcuioli PJ, Swisher M. Concurrent identity training is not necessary for associative symmetry in successive matching. Journal of the Experimental Analysis of Behavior. 2014;101:10–25. doi: 10.1002/jeab.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr D, Wilkinson KM, Blackman D, McIlvane WJ. Equivalence classes in individuals with minimal verbal repertoires. Journal of the Experimental Analysis of Behavior. 2000;74:101–114. doi: 10.1901/jeab.2000.74-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan VA, Barnes D, Smeets PM. A precursor to the relational evaluation procedure: Analyzing stimulus equivalence. The Psychological Record. 1998;48:121–145. [Google Scholar]
- D'Amato MR, Salmon DP, Loukas E, Tomie A. Symmetry and transitivity in the conditional relations in monkeys (Cebus apella) and pigeons (Columba livia). Journal of the Experimental Analysis of Behavior. 1985;44:35–47. doi: 10.1901/jeab.1985.44-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devany JM, Hayes SC, Nelson RO. Equivalence class formation in languageable and language-disabled children. Journal of the Experimental Analysis of Behavior. 1986;46:243–257. doi: 10.1901/jeab.1986.46-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugdale N, Lowe CF. Naming and stimulus equivalence. In: Blackman DE, Lejeune H, editors. Behavior analysis in theory and practice: Contributions and controversies. Erlbaum; Hillsdale, NJ: 1990. pp. 115–138. [Google Scholar]
- Dugdale N, Lowe CF. Testing for symmetry in the conditional discriminations of language-trained chimpanzees. Journal of the Experimental Analysis of Behavior. 2000;73:5–22. doi: 10.1901/jeab.2000.73-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CA, Jagielo JA, Zentall TR, Hogan DE. Acquired equivalence and distinctiveness in matching to sample by pigeons: Mediation by reinforcer-specific expectancies. Journal of Experimental Psychology: Animal Behavior Processes. 1982;8:244–259. [Google Scholar]
- Fields L, Doran E, Marroquin M. Equivalence class formation in a trace stimulus pairing two-response format: Effects of response labels and prior programmed transitivity induction. Journal of the Experimental Analysis of Behavior. 2009;92:57–84. doi: 10.1901/jeab.2009.92-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields L, Verhave T, Fath S. Stimulus equivalence and transitive associations: A methodological analysis. Journal of the Experimental Analysis of Behavior. 1984;48:317–322. doi: 10.1901/jeab.1984.42-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank AJ, Wasserman EA. Associative symmetry in the pigeon after successive matching-to-sample training. Journal of the Experimental Analysis of Behavior. 2005;84:147–165. doi: 10.1901/jeab.2005.115-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldiamond I. Perception. In: Bachrach AJ, editor. Experimental foundations of clinical psychology. Basic Books; NY: 1962. pp. 280–340. [Google Scholar]
- Hall G, Mitchell C, Graham S, Lavis Y. Acquired equivalence and distinctiveness in human discrimination learning: Evidence for associative mediation. Journal of Experimental Psychology: General. 2003;132:266–276. doi: 10.1037/0096-3445.132.2.266. [DOI] [PubMed] [Google Scholar]
- Hogan DE, Zentall TR. Backward association in the pigeon. American Journal of Psychology. 1977;90:3–15. [Google Scholar]
- Honey RC, Hall G. The acquired equivalence and distinctiveness of cues. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:338–346. [PubMed] [Google Scholar]
- Horne PJ, Lowe CF. On the origins of naming and other symbolic behavior. Journal of the Experimental Analysis of Behavior. 1996;65:185–241. doi: 10.1901/jeab.1996.65-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne PJ, Lowe CF. Toward a theory of verbal behavior. Journal of the Experimental Analysis of Behavior. 1997;68:271–296. doi: 10.1901/jeab.1997.68-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S, Barnes-Holmes D. Associative concept learning, stimulus equivalence, and Relational Frame Theory: Working out the similarities and differences between human and nonhuman behavior. Journal of the Experimental Analysis of Behavior. 2014;101:156–160. doi: 10.1002/jeab.60. [DOI] [PubMed] [Google Scholar]
- Iversen I. Matching-to-sample performance in rats: A case of mistaken identity? Journal of the Experimental Analysis of Behavior. 1997;68:27–47. doi: 10.1901/jeab.1997.68-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen I, Sidman M, Carrigan P. Stimulus definition in conditional discriminations. Journal of the Experimental Analysis of Behavior. 1986;45:297–304. doi: 10.1901/jeab.1986.45-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins JJ, Palermo DS. Bellugi U, Brown R, editors. Mediation processes and the acquisition of linguistic structure. The acquisition of language. Monographs of the Society for Research in Child Development. 1964;29:141–191. [PubMed] [Google Scholar]
- Johnson C, Meleshkevich O, Dube WV. Merging separately established stimulus classes with outcome-specific reinforcement. Journal of the Experimental Analysis of Behavior. 2014;101:38–50. doi: 10.1002/jeab.61. [DOI] [PubMed] [Google Scholar]
- Kendall SB. Tests for mediated transfer in pigeons. The Psychological Record. 1983;33:245–256. [Google Scholar]
- Kuno H, Kitadate T, Iwamoto T. Formation of transitivity in conditional matching to sample by pigeons. Journal of the Experimental Analysis of Behavior. 1994;62:399–408. doi: 10.1901/jeab.1994.62-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layng MP, Chase PN. Stimulus-stimulus pairing, matching-to-sample testing, and emergent relations. The Psychological Record. 2001;51:605–628. [Google Scholar]
- Lazareva OF, Wasserman EA. Categories and concepts in animals. In: Byrne J, editor. Learning theory and behavior – A comprehensive reference. Vol. 1. Elsevier; Oxford: 2008. pp. 197–226. [Google Scholar]
- Leader G, Barnes D, Smeets PM. Establishing equivalence relations using a respondent-type training procedure. The Psychological Record. 1996;46:685–706. [Google Scholar]
- Lindemann-Biolsi KL, Reichmuth C. Cross-modal transitivity in a California sea lion (Zalophus californianus). Animal Cognition. 2014;17:879–890. doi: 10.1007/s10071-013-0721-0. [DOI] [PubMed] [Google Scholar]
- Lionello KM, Urcuioli PJ. Control by sample location in pigeons’ matching to sample. Journal of the Experimental Analysis of Behavior. 1998;70:235–251. doi: 10.1901/jeab.1998.70-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-DeNolf KM. Retrieved from ProQuest Dissertations & Theses. 2001. Stimulus control topographies and tests for of symmetry in pigeons. p. 3043752. [Google Scholar]
- Lionello-DeNolf KM, Urcuioli PJ. Stimulus control topographies and tests of symmetry in pigeons. Journal of the Experimental Analysis of Behavior. 2002;78:467–495. doi: 10.1901/jeab.2002.78-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkens R, Kop PFM, Matthijs W. A test of symmetry and transitivity in the conditional discrimination performances of pigeons. Journal of the Experimental Analysis of Behavior. 1988;49:395–409. doi: 10.1901/jeab.1988.49-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maydak M, Stromer R, Mackay HA, Stoddard LT. Stimulus classes in matching to sample and sequence production: The emergence of numeric relations. Research in Developmental Disabilities. 1995;16:179–204. doi: 10.1016/0891-4222(95)00008-b. [DOI] [PubMed] [Google Scholar]
- McIlvane WJ, Withstandley JK, Stoddard LT. Positive and negative stimulus relations in severely retarded individuals’ conditional discrimination. Analysis and Intervention in Developmental Disabilities. 1984;4:235–251. [Google Scholar]
- McDaniel MA, Neufeld KH, Damico-Nettleton S. Many-to-one and one-to-many associative learning a naturalistic task. Journal of Experimental Psychology: Applied. 2001;7:182–194. [PubMed] [Google Scholar]
- Rodger RS. The number of non-zero, post hoc contrasts from ANOVA and error rate. I. British Journal of Mathematical and Statistical Psychology. 1975;28:71–78. [Google Scholar]
- Saunders RR, Green G. The nonequivalence of behavioral and mathematical equivalence. Journal of the Experimental Analysis of Behavior. 1992;57:227–241. doi: 10.1901/jeab.1992.57-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schusterman RJ, Kastak D. A California sea lion (Zalophus Californianus) is capable of forming equivalence relations. The Psychological Record. 1993;43:823–839. [Google Scholar]
- Sidman M. Reading and auditory-visual equivalences. Journal of Speech and Hearing Research. 1971;14:5–13. doi: 10.1044/jshr.1401.05. [DOI] [PubMed] [Google Scholar]
- Sidman M. Equivalence relations: Where do they come from? In: Lejeune H, Blackman D, editors. Behavior analysis in theory and practice: Contributions and controversies. Erlbaum; Hillsdale, NJ: 1990. pp. 93–114. [Google Scholar]
- Sidman M. Equivalence relations and the reinforcement contingency. Journal of the Experimental Analysis of Behavior. 2000;74:127–146. doi: 10.1901/jeab.2000.74-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Kirk B, Willson-Morris M. Six-member stimulus classes generated by conditional discrimination procedures. Journal of the Experimental Analysis of Behavior. 1985;43:21–42. doi: 10.1901/jeab.1985.43-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Rauzin R, Lazar R, Cunningham S, Tailby W, Carrigan P. A search for symmetry in the conditional discrimination of rhesus monkeys, baboons, and children. Journal of the Experimental Analysis of Behavior. 1982;37:23–44. doi: 10.1901/jeab.1982.37-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Tailby W. Conditional discrimination vs. matching to sample: An expansion of the testing paradigm. Journal of the Experimental Analysis of Behavior. 1982;37:5–22. doi: 10.1901/jeab.1982.37-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets PM, Barnes D, Roche B. Functional equivalence in children: Derived stimulus-response and stimulus-stimulus relations. Journal of Experimental Child Psychology. 1997;66:1–17. doi: 10.1006/jecp.1997.2378. [DOI] [PubMed] [Google Scholar]
- Smeets PM, Barnes-Holmes D, Striefel S. Establishing and reversing equivalence relations with a precursor to the relational evaluation procedure. The Psychological Record. 2006;56:267–286. [Google Scholar]
- Smeets PM, van Wijngaarden M, Barnes-Holmes D, Cullinan V. Assessing stimulus equivalence with a precursor to the relational evaluation procedure. Behavioural Processes. 2004;65:241–251. doi: 10.1016/j.beproc.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Spradlin JE, Cotter VW, Baxley N. Establishing a conditional discrimination without direct training: A study of transfer with retarded adolescents. American Journal of Mental Deficiency. 1973;77:556–566. [PubMed] [Google Scholar]
- Spradlin JE, Saunders RR. The development of stimulus classes using match-to-sample procedures: Sample classification versus comparison classification. Analysis and Intervention in Developmental Disabilities. 1986;6:41–48. [Google Scholar]
- Steirn JN, Jackson-Smith P, Zentall TR. Mediational use of internal representations of food and no-food events by pigeons. Learning and Motivation. 1991;22:353–365. [Google Scholar]
- Strasser R, Ehrlinger JM, Bingman VP. Transitive behavior in hippocampallesioned pigeons. Brain, Behavior, and Evolution. 2004;63:181–188. doi: 10.1159/000076442. [DOI] [PubMed] [Google Scholar]
- Sweeney MM, Urcuioli PJ. A reflexivity effect in pigeons. Journal of the Experimental Analysis of Behavior. 2010;94:267–282. doi: 10.1901/jeab.2010.94-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisher M, Urcuioli PJ. Symmetry in the pigeon with sample and comparison stimuli in different locations. Journal of the Experimental Analysis of Behavior. 2013;100:49–60. doi: 10.1002/jeab.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamamoto J, Noro F. Stimulus pairing training in children with autism spectrum disorder. Research in Autism Spectrum Disorders. 2011;5:547–552. [Google Scholar]
- Urcuioli PJ. Associative symmetry, antisymmetry, and a theory of pigeons’ equivalence-class formation. Journal of the Experimental Analysis of Behavior. 2008;90:257–282. doi: 10.1901/jeab.2008.90-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ. Associative symmetry and stimulus-class formation by pigeons: The role of non-reinforced baseline relations. Behavioural Processes. 2010;85:226–235. doi: 10.1016/j.beproc.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ. Emergent identity matching after successive matching training: Reflexivity or generalized identity? Journal of the Experimental Analysis of Behavior. 2011;96:329–341. doi: 10.1901/jeab.2011.96-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ. Stimulus control and stimulus class formation. In: Madden GJ, Dube WV, Hackenberg TD, Hanley GP, Lattal KA, editors. APA Handbook of Behavior Analysis. Vol. 1. American Psychological Association; Washington, DC: 2013. pp. 361–386. [Google Scholar]
- Urcuioli PJ, Swisher M. Emergent identity matching after successive matching training. II: Reflexivity or transitivity? Journal of the Experimental Analysis of Behavior. 2012a;97:5–27. doi: 10.1901/jeab.2012.97-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ, Swisher M. A replication and extension of the antisymmetry effect in pigeons. Journal of the Experimental Analysis of Behavior. 2012b;98:283–293. doi: 10.1901/jeab.2012.98-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli PJ, Zentall TR, Jackson-Smith P, Steirn JN. Evidence for common coding in many-to-one matching: Retention, intertrial interference, and transfer. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:264–273. [Google Scholar]
- Vaughan W., Jr. Formation of equivalence sets in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:36–42. [Google Scholar]
- Wasserman EA. Successive matching-to-sample in the pigeon: Variation on a theme by Konorski. Behavior Research Methods & Instrumentation. 1976;8:278–282. [Google Scholar]
- Wasserman EA, DeVolder CL, Coppage DJ. Nonsimilarity-based conceptualization in pigeons. Psychological Science. 1992;3:374–379. [Google Scholar]
- Zentall TR, Wasserman EA, Urcuioli PJ. Associative concept learning in animals. Journal of the Experimental Analysis of Behavior. 2014;101:130–151. doi: 10.1002/jeab.55. [DOI] [PMC free article] [PubMed] [Google Scholar]