Abstract
Survival has increased dramatically for patients with chronic phase chronic myeloid leukemia (CP-CML) using BCR-ABL targeted tyrosine kinase inhibitors, such that life expectancy is expected to approximate that of patients without CP-CML. Randomized controlled trials (RCTs) and observational studies provide valuable insights into the management of chronic diseases such as CP-CML. RCTs are undoubtedly the backbone of clinical research, and the ‘gold standard’ for evaluating the efficacy and safety of new therapies. However, many questions surrounding the optimal management of patients with CML remain unanswered, and it is widely accepted that these questions will be best answered by evaluating the use of available therapies in clinical practice. Observational studies can extend the knowledge base beyond the clinical trial setting and thus capture a more accurate picture of everyday clinical practice, particularly patients’ experiences of long-term CML treatment. There is therefore growing interest in and appreciation of the value of observational research. This review article will examine the relative merits of RCTs and observational studies in the setting of CML, highlighting those factors – such as the advancing age of the CML patient population and growing importance of patient-reported outcomes – that mean that observational studies should play an important role in shaping clinical practice. This article also provides an overview of what observational studies have told us thus far about the optimal management of patients with CML, outlines some of the key remaining unanswered clinical questions in CML, and summarizes ongoing observational studies designed to provide answers to these key questions.
Keywords: chronic myeloid leukemia, dasatinib, imatinib, nilotinib, observational study
Introduction
Chronic myeloid leukemia (CML) accounts for approximately 12% of all cases of leukemia in the United States (US) [Xie et al. 2003] and 7−20% of all leukemia cases worldwide [Redaelli et al. 2004]. The worldwide incidence of CML is generally 1.0–1.5/100,000 population/year [Goldman, 2008], although the incidence is estimated to be slightly higher in the US [Surveillance Epidemiology and End Results (SEER) Program, 2014]. Figures for Europe are comparable, with data from the HAEMACARE project, which encompassed 44 European registries, revealing a crude incidence rate (between 2000 and 2002) in Europe of 1.1–1.2/100,000 population/year [Sant et al. 2010].
The clinical development of small-molecule tyrosine kinase inhibitors (TKIs) in the past decade has transformed the nature of chronic phase CML (CP-CML) from a terminal disease to a chronic illness. Physicians and their patients now have improved treatment options that allow long-term disease control [Efficace et al. 2012]. Survival has increased dramatically for patients with CP-CML using BCR-ABL-targeted TKIs, such that life expectancy is expected to approximate that of patients without CP-CML [Björkholm et al. 2011].
Such progress in the field brings a wealth of new data for physicians to consider when determining the most appropriate therapy for individual patients. It also brings fresh challenges, and the chronic nature of the disease has prompted a shift in the overarching objectives of patient management. While clinical response rates and overall survival (OS) remain important clinical endpoints, other more patient-centered outcomes, such as health-related quality of life (QoL), adverse event (AE) profiles and treatment adherence, are also becoming increasingly important. These patient-centered outcomes capture the impact of the disease and its management from the patient’s perspective, and therefore provide crucial additional information to guide treatment decisions. Available data on safety, toxicity, adherence and QoL are largely derived from data from randomized controlled trials (RCTs), which have strict inclusion criteria with respect to patient performance status and comorbidities and rigid study protocols with respect to treatment adherence and patient engagement in the study. Current data on the impact of CML and the long-term use of TKIs on patient-centered outcomes are therefore notably lacking, and questions remain over the best setting to collect these data [Efficace et al. 2012].
This review focuses on the relative merits of research derived from RCTs and observational studies in the CML setting, and how the insights gleaned from this research can provide invaluable guidance that shapes future management practices and optimizes outcomes for patients with CML. It describes what observational research has revealed about CML management outside the setting of clinical trials, highlights some of the unanswered clinical questions in CML, and addresses ongoing observational studies that have been designed to answer these key questions.
Do we ask too much of randomized controlled trials?
It is widely accepted that RCTs are the ‘gold standard’ for evaluating the efficacy and safety of new therapies [Silverman, 2009]. The design of an RCT includes precise and pre-specified clinical endpoints that are used to assess a treatment’s benefits and risks. The design also aims to maximize the homogeneity of enrolled patients through inclusion and exclusion criteria, and the study takes place under controlled conditions, which aim to minimize variance in the clinical context in which the study drug is delivered and to maximize adherence. Finally, patients are randomized to receive either the investigational therapy or the control, and are sometimes blinded to the treatment assigned, so that neither their expectations of treatment effects, nor those of the study investigators or other site staff, bias the study findings, either directly or indirectly, and the patient characteristics are balanced across different treatment groups [Jadad, 1998; Silverman, 2009].
Given this, datasets from double-blind RCTs that have been executed correctly are likely to be internally valid, suggesting that we should have confidence in the benefits of a therapy that has been tested in this way [Rawlins, 2008]. If these results are replicated in another RCT, our confidence grows further [Rawlins, 2008]. The stringent design of RCTs allows researchers to accurately detect even small differences between treatment arms. As such, RCTs merit their position as the cornerstone of clinical development, the definitive means of establishing the safety and efficacy of a novel treatment, and a requirement for regulatory submissions. However, are we asking too much of them?
The various limitations of RCTs have been described extensively elsewhere in the literature [Stables, 2002; Rawlins, 2008; Silverman, 2009], though a brief overview for context is warranted. RCTs are designed to test a ‘null hypothesis’, namely, that there is no difference between the treatments being tested. The results are therefore only helpful if an appropriate and up-to-date null hypothesis has been tested; this may not always be the case, however, for example when an investigational drug is compared with therapy that is no longer the standard of care [Rawlins, 2008]. However, perhaps the most self-evident limitations of RCTs arise from their stringent design: because of the need for homogenous study populations and controlled conditions, their findings cannot be extended to many of the patients that a physician encounters in clinical practice. For example, study populations defined by inclusion and exclusion criteria in RCTs may only represent a proportion of the patients who suffer from the disease or condition in question [Dowd et al. 2000; Heiat et al. 2002; Khan et al. 2005]. Patients who are older, have a poor performance status, have comorbidities or who have received certain treatments previously may be widely represented in clinical practice, but are often excluded from RCTs. This may be an especially pertinent consideration in both the setting of oncology and in the context of treating chronic conditions. For instance, CML is becoming increasingly prevalent in older patients [Redaelli et al. 2004] and therefore these patients may often have comorbidities that can influence treatment choice, management practices and clinical outcomes. Future treatment challenges may well arise from the use of drugs that were tested in a relatively young, homogenous patient population, but are intended for the long-term care of older patients with comorbidities.
Furthermore, patterns of care and treatment adherence may also differ widely between RCTs and clinical practice. Extensive follow-up and patient education, which are both required through informed consent and increasingly offered to patients given an emphasis on structured encounters in RCTs, may lead to an overestimation of treatment adherence compared with physicians’ day-to-day experience. Evidence suggests that these factors make a difference to treatment outcomes. In a multiphase, prospective study including 200 community-based patients aged 65 years or older who were taking at least four chronic medications, continued use of custom-packed medication and patient education significantly increased medication adherence compared with standard care. Medication adherence was associated with improved clinical outcomes [Lee et al. 2006]. Despite this finding, it is rare for adherence in patients with chronic conditions to be assessed and for interventions to be implemented in clinical practice [Ho et al. 2009]. Furthermore, if a physician is unaware that a patient is failing to adhere to a regimen of oral medication, they may attribute disease progression to lack of efficacy and alter the treatment regimen accordingly [Ruddy et al. 2009]. In CML, the detrimental effect of reduced treatment adherence (even modest reduction) on clinical outcomes has been well documented [Ganesan et al. 2011; Ibrahim et al. 2011]. In the future, understanding the factors that contribute to adherence and implementing strategies to ensure long-term adherence will be essential in order to achieve the beneficial outcomes reported in RCTs.
Another limitation of RCTs is that, although they are designed to evaluate clinical benefit with sufficient statistical power, this often does not extend to the analysis of AEs associated with the drug [Rawlins, 2008]. Furthermore, the duration of RCTs is often short, relative to the duration of chronic disease, in order to capture a defined endpoint, making it difficult to reliably capture AEs that have a long latency or are relatively uncommon [Rawlins, 2008]. Although findings stemming from AE data derived from RCTs are valuable, they may not provide the complete picture of what can be expected in clinical practice that is so essential to making the right clinical choices for the individual patient. CML poses a unique challenge as it has introduced a paradigm of chronic indefinite maintenance chemotherapy administration and challenges the confines of a RCT to capture the more complex balance of benefit and risk inherent in this previously unexplored model. Indeed, the focus of RCTs on therapeutic efficacy and shorter-term toxicity direct away from the long-term impact of a disease and/or its management relative to patient-centered outcomes, such as QoL, treatment satisfaction, preference and adherence. Given the increasing importance of these outcomes, both for regulatory authorities and healthcare professionals, the inability of RCTs to fully capture the patient perspective, particularly over the longer term, is a growing concern and an important limitation.
Observational studies: complementary to randomized controlled trials?
Having examined the strengths and limitations of RCTs, it is clear that the evidence base they provide, particularly in the context of chronic disease, may not be as complete as we would like. In order to fill these gaps, what alternative strategies can we consider in conjunction with RCTs?
Data from observational studies can be an important source of evidence about patients’ true experience of long-term treatment, in contrast to the information recorded in the controlled setting of an RCT [Ligthelm et al. 2007]. Observational studies differ considerably from RCTs. In contrast to RCTs, observational studies can evaluate a larger and more heterogeneous group of patients, which may therefore be more representative of patient populations encountered in clinical practice [Silverman, 2009]. Their typically longer study duration and use of large-scale databases can capture newly emergent or rare AEs, and assess the true incidence and impact of AEs in a real-world setting [Silverman, 2009]. Observational studies can also provide data on QoL [Arne et al. 2009], with broad deployment of validated instruments, and on the cost effectiveness of therapies – information that is increasingly required by regulatory authorities and payers. The use of healthcare claims databases to assess treatment cost and outcomes has proved valuable in this regard, and several strategies are now available to optimize datasets obtained from such studies [Motheral and Fairman, 1997]. Furthermore, patient registries allow the natural history and response to treatment of rare diseases to be studied in greater depth than would otherwise be achievable [Clarke et al. 2011]. Overall, the value of observational studies lies in their focus on everyday clinical practice, reflecting the differences between consecutive patients that make treatment decisions so interesting and, in some cases, so challenging.
Nevertheless, the methodologies used in observational studies are associated with disadvantages; examples have been extensively reviewed elsewhere [Garbe and Suissa, 2004; Gallicchio et al. 2008]. Notably, their non-randomized design limits their internal validity and increases the chance of selection bias and confounding [Rochon et al. 2005], as defined in the CONSORT statement [Altman et al. 2001]. The design of an observational study must minimize these problems – for example, by adjusting for confounding variables such as key baseline population, prognostic and predictive characteristics. Similarly, physicians need to assess potential sources of selection bias and confounding when using data from these studies, and place the data into clinical context in order to avoid drawing invalid conclusions [Rochon et al. 2005]. Other limitations are that incomplete datasets are more commonplace than in the RCT setting, which can pose problems in data analysis and interpretation, and observational studies can reveal longitudinal trends, but often cannot detect small differences between different patient subpopulations, given their less stringent design. If these limitations are borne in mind when interpreting the findings of observational studies, physicians can obtain important insights into the impact of long-term treatment in clinical practice, as well as the patient experience of their disease and its management. How, then, can consideration of data from observational studies add to what we have currently learnt from RCTs in the context of CML and its management?
Exploring the role of observational studies in CML
The approval of imatinib for the treatment of patients with newly-diagnosed CML changed the disease trajectory, making long-term treatment strategies possible for these patients. Now, with the availability of the next-generation TKIs, such as dasatinib and nilotinib, patients have more treatment options that allow them to manage their disease effectively over the long term. Efficacy data from the pivotal phase III RCTs of these three drugs in the first-line treatment of CML have been published elsewhere [O’Brien et al. 2003; Kantarjian et al. 2010; Saglio et al. 2010] and will not be considered in detail here.
RCTs may have established new standards for the management of CML, but they still have relatively short follow-up times compared with the overall duration of the disease. In addition, the current choice between three available TKIs (imatinib, dasatinib and nilotinib) means that additional guidance is needed about appropriate treatment choice in individual patients, and long-term outcomes – such as persistent AEs, QoL, adherence, treatment switching, potential treatment cessation, cost and mutation status – are key factors determining this choice. Consequently, a shift in focus from data provided by RCTs to more longitudinal, patient-centric outcomes, such as those addressed in observational studies, may be helpful.
In the field of CML, observational studies have already provided some important insights. Perhaps most importantly, recent epidemiologic analyses suggest there is a significant age difference between patients with CML treated within or outside of clinical trials. In one such study in Southwest Germany, patients with CML participating in a clinical study were on average 10.7 years younger than those who did not participate (median age 54.1 and 64.8 years, respectively), and patients younger than 65 years were 3.8 times more likely to be enrolled in a clinical trial than patients aged 65 years and over [Rohrbacher et al. 2009]. Numerous studies have shown differences in clinical outcomes between older patients compared with those typically observed in patients enrolled into clinical trials. A study of 117 consecutive patients treated with imatinib in a single institution revealed, for example, that grade 3–4 hematologic toxicity occurred more frequently in older patients (aged ≥ 65 years) compared with younger patients (aged < 65 years; 25% versus 9.1%; p = 0.02). Similar findings were reported for grade 3–4 nonhematologic toxicity (27.5% versus 10.1%; p = 0.017). Despite deriving similar efficacy from imatinib, this increased toxicity leads to higher rates of treatment discontinuation and dose reduction in older patients, thereby questioning appropriate dosing in this subset of patients [Latagliata et al. 2010].
The Unmet Needs in CML study is an observational, retrospective study for which enrollment took place in September 2006–March 2007. It was designed to assess the proportion of patients with chronic CML who received imatinib and the proportion of those patients experiencing imatinib resistance or intolerance, across eight European countries. Results from the subset of 654 French patients have been published; 95.9% of patients received imatinib at some point following diagnosis, with 14.8% experiencing imatinib resistance and 31.4% being classed as imatinib intolerant at any time during the follow-up period. The rate of imatinib intolerance reported was higher than that reported in clinical trials, possibly owing to its broader definition as ‘experiencing an AE that led to change or discontinuation of imatinib’ [Michallet et al. 2010]. For comparison, in a UK observational study of 204 consecutive patients with CML who received imatinib between June 2000 and August 2006, 25% had discontinued treatment at 5 years because of an unsatisfactory response or AEs [De Lavallade et al. 2008]. These rates of discontinuation and intolerance contrast with the reported incidence of discontinuation from imatinib in the IRIS trial; 68 patients (12.3%) receiving imatinib (400 mg daily) discontinued treatment and, of these, 18% discontinued owing to AEs and 26% owing to disease progression [O’Brien et al. 2003]. An additional finding of the French subset analysis was that the frequency of response monitoring did not meet European recommendations, with 45.5% of patients never having had a mutational analysis. This has highlighted an area for improvement in patient care: to identify those likely to benefit from treatment switching owing to imatinib resistance or intolerance [Michallet et al. 2010].
Insights into the long-term clinical experience with imatinib have also stemmed from the long-term follow up of patients enrolled in the pivotal phase 3 study, IRIS [O’Brien et al. 2003]. Recent data from 8-year follow up reveals 304 (55%) patients remained on imatinib therapy and 249 (45%) had discontinued owing to unsatisfactory therapeutic outcome (16%), AEs (6%), stem cell transplant (3%), death (3%) and other (17%; including withdrawal of consent). There were no unexpected safety issues. The relationship between early cytogenetic response (CyR) and long-term outcomes was also explored; minor CyR at 3, 6 and 12 months, and complete CyR (CCyR) at 18 months, were associated with stable CCyR throughout the remaining follow-up period [Deininger et al. 2009].
The Imatinib Long-Term (Side) Effects (ILTE) study was another important observational study that provided a deeper understanding of toxicity reported in imatinib-treated CP-CML. The study was an independent, multicenter, observational study that investigated the long-term effects of imatinib in a patient population with CML in stable cytogenetic remission (excluding the initial treatment period in which the risk is highest). A total of 832 patients were enrolled, from 1999 to 2004, in 27 centers located in 12 different countries on five continents. The median duration of imatinib therapy in the patient cohort was 5.8 years. The results showed that the incidence of nonserious side effects was 53%; the most frequent side effects were muscle cramps, asthenia, edema, skin fragility, diarrhea, and tendon or ligament lesions. Furthermore, the long-term toxicity burden was found to be modest, with 9% of patients discontinuing imatinib because of AEs, relapse or insufficient response, or persistent polymerase chain reaction (PCR) negativity. The study also revealed that most patients with stable CCyR after approximately 2 years of treatment with imatinib maintained long-term CCyR with continued treatment. CML-related deaths were uncommon and survival rates comparable with the general population [Gambacorti-Passerini et al. 2011]. The long-term nature of the ILTE study is a particularly important aspect of the trial and, indeed, of many observational studies. It is this longevity that distinguishes the findings of this study from those of previous RCTs that have investigated safety and efficacy of imatinib in these patients.
Observational studies can also provide insights into other potential risks or late effects of TKI therapy. In a study of 1445 patients with CML(n = 1342) or myeloproliferative neoplasms (n = 103) treated with TKIs at the MD Anderson Cancer Center between November 1998 and April 2010, the number of secondary malignancies that developed following TKI therapy was compared with the expected number of malignancies from the SEER database. A total of 66 patients (4.6%) developed 80 secondary malignancies, at a median time from initiation of TKI therapy of 39 months (range 2–98 months). The risk of developing a secondary malignancy was lower than expected (observed to expected ratio 0.6; 95% confidence interval 0.44–0.81), dispelling concerns over the potential link between exposure to TKI therapy and the development of secondary malignancies [Verma et al. 2011].
With regard to adherence to TKI therapy in CML, observational study results have differed widely. A single-center study in Sweden, which included 38 patients with CML treated with imatinib, revealed a self-reported Morisky (mean) score of 12.3 on a scale of 1–13, with higher scores indicative of better adherence. Predictors of high adherence included patients feeling well informed about their treatment and taking an active part in treatment decisions, and follow-up with a single hematologist [Jonsson et al. 2012]. These findings are in line with the experiences at a US center, where an outpatient coordinated team care approach for patients with CML – involving effective patient education about their disease and monitoring requirements, as well as clear communication pathways between all members of the multidisciplinary team and the patient – was found to boost patient engagement and adherence, both to clinic visits and TKI therapy [Holloway et al. 2012]. In contrast, other studies have reported poor adherence in patients with CML receiving long-term treatment with imatinib. One study of 87 patients found that 26.4% of patients had an adherence rate of 90% or lower [Marin et al. 2010], while another study of 202 patients in Belgium considered one-third of patients to be nonadherent [Noens et al. 2009]; reduced adherence to this extent is likely to impact long-term outcomes [Ganesan et al. 2011; Ibrahim et al. 2011].
Observational studies and cancer registries in patients with CML have also revealed disparities in management practices and clinical outcomes between clinical trials and clinical practice. A 2011 study, for example, evaluated 5-year survival rates in patients with CML treated in clinical trials (early trials: 1980–1987; late trials: 2004–2005) compared with patients with CML in the general population using SEER data (1973–2006). The median age of patients enrolled in the 29 clinical studies included in the analysis (trial size of 40–1109 patients) was between 37 and 60 years, whereas patients in the SEER database had a median age of 62 years. The survival rate in patients enrolled in clinical trials was higher than that of all patients with CML: in the early trials, the 5-year survival rate was 30–40% compared with 22% in the general population, whereas in the later trials, the 5-year survival rate was 96% compared with 44% in the general population. These differences persisted even after adjusting for differences in the median age of patients in the two populations. The authors speculate that this difference is likely the result of improved access to treatment and the inclusion of younger, healthier patients in clinical studies, and differences in socioeconomic factors, such as therapy adherence, medical literacy and access to medical care, between the two settings [Pulte et al. 2011]. However, a prospective population-based cohort conducted by the UK’s Haematological Malignancy Research Network revealed that survival rates in patients with CML in clinical practice was comparable with those reported in clinical trials. In a sample of 242 patients with CML, treated mainly with imatinib, the 5-year survival rate was 89.1% and 90.1% for men and women, respectively. This suggests that disparities seen in the US may reflect financial barriers to accessing TKI therapy. The study also revealed that, despite having access to TKI therapy, clinical outcomes were poorer in patients from lower socioeconomic groups in the UK; this is thought to be the result of differences in therapy adherence in these groups [Smith et al. 2014].
An analysis using data from the Glivec International Patient Assistance Program has also revealed that there are significant differences in age of diagnosis and OS between different geographic regions. An analysis of 33,985 patients with CML low- and middle-income countries showed that CML was diagnosed at a much earlier age in these countries compared with the US (37.8 versus 64.0 years); this suggests the presence of one or more biologic, environmental or socioeconomic factors that influence the pattern of CML within these countries. The same study also revealed regional differences in OS, with Southern and Eastern Europeans having better outcomes than patients from Latin America and Africa. These differences are thought to be, at least partially, related to differences in therapy adherence between these geographic regions, resulting from differences in infrastructure, patient insight and understanding (apathy) and political climate. Time from diagnosis to treatment was also found to be an independent and modifiable risk factor for OS [Mendizabal et al. 2013].
Unanswered clinical questions in CML
Although observational studies have already contributed to our knowledge base about optimal CML management, many unanswered questions remain.
First, there is a clear need for more data on patient-centric outcomes, such as treatment adherence, treatment satisfaction, symptom burden and QoL. These outcomes capture the long-term burden of disease and treatment from a patient perspective. Unanswered questions include the following: What is the true symptom burden from the patient’s perspective? What are the factors that lead to treatment discontinuation and treatment switching? What are the intolerances associated with first-line TKI treatment and how do they influence treatment satisfaction, QoL and overall outcomes? These questions are highly relevant, given the prolonged nature of treatment and the importance of treatment satisfaction and QoL in determining adherence, and thus whether a patient derives clinical benefit from administered treatments. The long-term nature of observational research makes it ideal for capturing the patient perspective of long-term treatment of a chronic disease, and it can provide invaluable clues about how management should be optimized. Prolonged follow-up could also increase our understanding of chronic low-grade AEs that may be linked to prolonged use of TKIs or to the disease itself, and of their effect on patients’ QoL and the subsequent impact on patient satisfaction and adherence. A report on the levels of symptom burden (the combined impact of CML itself and of treatment on daily functioning) experienced by patients receiving TKI treatment has highlighted the importance of this factor in clinical decision making [Williams et al. 2010]. Identifying patients at particular risk of experiencing AEs or poor QoL, as well as knowing how to manage these factors, will be one of the key features of future patient care in CML.
Second, there is a need for further information on management practices outside the clinical trial setting and how well these mirror the National Comprehensive Cancer Network (NCCN) and European LeukemiaNet (ELN) guidelines for CML management. Questions that remain unanswered include the following: How frequently are patients monitored in clinical practice? What TKIs are used in the first-line setting and which factors determine treatment choice? What are the patterns of treatment switching and how do these patterns impact on outcomes? Observing clinical practice over several years can reveal whether physicians are following guidelines for monitoring and the impact these practice patterns have on patient outcomes. Evidence that can identify gaps between routine clinical practice and recommended best practice is key, as it highlights the need for physician education and provides definitive guidance for these physicians on how they can further optimize CML management.
Ongoing observational studies in CML
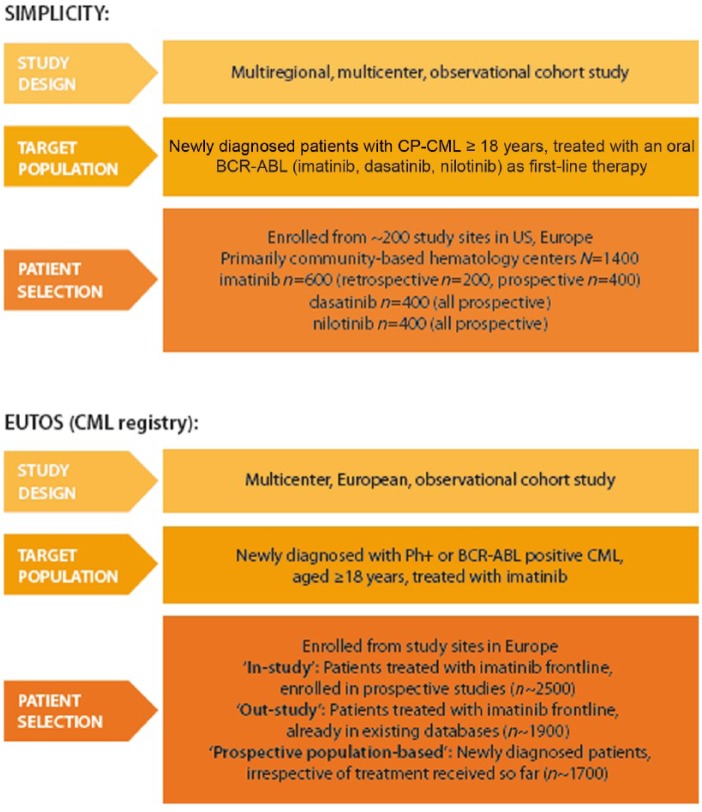
Two large observational studies are ongoing in chronic CML (Figure 1): The European Treatment and Outcome Study (EUTOS) [EUTOS, 2012] and the ‘Studying Interventions for Managing Patients with CML in Chronic Phase: The 5-Year Prospective Cohort Study’ (SIMPLICITY) [ClinicalTrials.gov identifier: NCT01244750].
Figure 1.
Key features of the European Treatment and Outcome Study (EUTOS) and the ‘Studying Interventions for Managing Patients with CML in Chronic Phase: The 5-Year Prospective Cohort Study’ (SIMPLICITY) studies. CP-CML, chronic phase chronic myeloid leukemia; Ph+, Philadelphia chromosome-positive.
EUTOS for CML is a program of activities initiated in 2007 by the ELN and Novartis and focuses solely on patients receiving imatinib treatment. It aims to improve understanding of CML, promote best practice and enhance treatment outcomes through a number of key projects, including a CML registry that is collecting baseline, treatment and outcome data across 11 European countries. These patient data will be derived from three sources: patients from national study groups who are enrolled in prospective studies (n ~ 2500); patients already registered in existing databases (n ~ 1900); and newly-diagnosed patients not previously in registries or clinical studies (n ~ 1700). Objectives of the registry include providing a clear epidemiologic picture of CML, patient treatment and outcomes; understanding how patients are treated outside of the trial setting; assessing implementation of the ELN management guidelines; evaluating quality-controlled outcomes; and developing a comprehensive prognostic model to optimize treatment.
Much has been published on the last of these objectives: the evaluation of known CML-related prognostic factors. Analysis of data from the overall EUTOS initiative has given rise to the EUTOS score for CML. This is a new prognostic score that can be used to predict the probability of achieving a CCyR within 18 months. It has been shown to have superior prognostic power to the Sokal and Euro scores, and was derived from a database of around 2000 prospectively diagnosed, treated and monitored patients with CP-CML. The EUTOS score can identify patients with a significantly higher risk of progression and impaired survival, thereby allowing physicians to tailor their treatment approach to optimize outcomes [Hasford et al. 2011]. Discussion is continuing regarding the value and difference between the EUTOS score and currently validated scores (Sokal, Hasford) as well as its ability to predict patient outcomes and identify those at high risk of disease progression [Marin et al. 2011].
The SIMPLICITY study was designed to enroll 1400 newly-diagnosed patients with CML treated with imatinib, dasatinib or nilotinib as first-line therapy in the US and a number of key European countries. It aims to improve our understanding of the pattern of use of these TKIs in first-line CML, outside of the setting of clinical trials. SIMPLICITY includes three ‘prospective’ cohorts of patients who initiated treatment with first-line imatinib, dasatinib or nilotinib during or after 2010 (the study opened after first-line approval of all three TKIs) and therefore began treatment at the time of their enrollment in SIMPLICITY. The study also includes a historical ‘retrospective’ cohort, including patients who started first-line treatment with imatinib any time after 2008, and therefore may have begun their treatment before enrollment in SIMPLICITY. All patients will be followed for up to 5 years from the index date (initiation of first-line treatment). The primary objective of SIMPLICITY is to understand TKI use and management patterns in clinical practice. Secondary objectives include evaluating the impact of treatment on patient-reported outcomes, such as symptom burden, treatment satisfaction, health-related QoL and treatment adherence, as well as the assessment of patterns of disease monitoring and resource utilization associated with CML management in clinical practice. In the absence of head-to-head data for imatinib, dasatinib and nilotinib, SIMPLICITY is a means of obtaining meaningful comparative data on clinical outcomes with the different TKIs and of collecting data on patient-reported outcomes to ensure that the patients and their needs will continue to be the primary driving force behind future management strategies in this setting.
An evaluation of preliminary patient-reported outcomes has been reported from a relatively small sample size of 74 patients enrolled in SIMPLICITY (79.7% received imatinib, 10.8% received nilotinib and 9.5% received dasatinib). Overall, newly-diagnosed patients with CML appear to be satisfied with treatment and report good health, although approximately 30% reported low adherence to their CML treatment [Cortes et al. 2011]. These aspects will be examined in greater detail once larger datasets are available. More recently, results from the prospective cohorts of SIMPLICITY have been presented. Demographics were consistent across all three cohorts; however, overall, the SIMPLICITY population was noted to be older with potentially more comorbidities than patients enrolled in the pivotal Phase 3 clinical trials with restrictive inclusion criteria [O’Brien et al. 2003; Kantarjian et al. 2010; Saglio et al. 2010]. Choice of the pivotal Phase 3 therapy appears to be driven by perceived effectiveness, cost and familiarity rather than baseline comorbidity [Cortes et al. 2013], despite a wide acceptance that comorbidities should drive treatment choice, particularly in older patients with CML [Cervantes and Mauro, 2011]. Furthermore, Hasford/Sokal scores were not recorded in the majority of patients prior to starting first-line TKI therapy; regional analysis reveals, however, that 68.4% of European patients had available Hasford or Sokal scores compared with 14.3% of US patients (p < 0.001) [Cortes et al. 2013].
About a quarter of all patients with CP-CML followed for at least 12 months discontinued or switched first-line TKI therapy during the first year. Primary reasons for discontinuation were intolerance, as defined and reported by the treating physician, or resistance (imatinib only). Types of intolerance varied by TKI: gastrointestinal disorders were most common and equally distributed across all TKI cohorts (18% all cohorts); skin disorders were the most common type of intolerance leading to discontinuation of imatinib and nilotinib (22% and 20%, respectively; compared with 2% with dasatinib); and blood and lymphatic system disorders were the most common type of intolerance leading to discontinuation of dasatinib (28% compared with 5% with imatinib and 12% with nilotinib) [Hehlmann et al. 2014].
In patients with CP-CML with at least 12 months of follow-up, the frequency of monitoring for CyR and molecular response is noticeably lower than recommended in the NCCN and ELN guidelines [Goldberg et al. 2014a]. By 12 months the following predictors of monitoring emerged: patients younger than 65 years at initiation of first-line TKI, those who had switched from first-line TKI, and those seen in academic centers were more likely to be monitored by 12 months [Goldberg et al. 2014b].
Conclusion: time to adopt a multifaceted approach?
RCTs and observational studies provide valuable insights into the management of chronic diseases. It is entirely appropriate that, although we should strive to optimize the design of RCTs and overcome some of their limitations, even in their current form, they should maintain their prominent and unique role in clinical research. In addition, observational trials can extend the knowledge base gained from RCTs beyond the clinical trial setting and capture the patient’s experience of their disease and its treatment. In doing so, it is hoped that future observational research will answer some of the key unanswered questions surrounding the management of CML in clinical practice. By capturing the impact of long-term treatment on patient-reported outcomes, as well as mapping management practices outside of the clinical trial setting, observational studies promise to reveal insights that will help to tailor patient and physician education, thereby enhancing future CML management practices. Physicians bear the responsibility to ensure that choice of treatment not only maximizes efficacy outcomes, but also does not compromise QoL or have a detrimental effect on potential future therapies. It is hoped that results from ongoing clinical and observational studies in CML will provide the CML community with a comprehensive evidence base that enables us to meet this responsibility.
Acknowledgments
We thank AXON Communications who provided medical writing services on behalf of Bristol-Myers Squibb.
Footnotes
Conflict of interest statement: MM has acted as a consultant for BMS, Novartis Oncology, Ariad and Pfizer. HJK has acted as a consultant for BMS and has received honoraria from BMS, Pfizer, Ariad and Teva. Both MM and HJK act as consultants on the SIMPLICITY study. TZ and CD are both employees of BMS.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Michael J. Mauro, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, Box 489, New York, NY 10065, USA
Catherine Davis, Bristol-Myers Squibb, Oncology, Global Health Outcomes, Princeton, NJ, USA.
Teresa Zyczynski, Bristol-Myers Squibb, Oncology, Global Health Outcomes, Princeton, NJ, USA.
H. Jean Khoury, Emory University School of Medicine, Atlanta, GA, USA.
References
- Altman D., Schulz K., Moher D., Egger M., Davidoff F., Elbourne D., et al. (2001) The revised consort statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 134: 663–694. [DOI] [PubMed] [Google Scholar]
- Arne M., Janson C., Janson S., Boman G., Lindqvist U., Berne C., et al. (2009) Physical activity and quality of life in subjects with chronic disease: chronic obstructive pulmonary disease compared with rheumatoid arthritis and diabetes mellitus. Scand J Prim Health Care 27: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkholm M., Ohm L., Eloranta S., Derolf A., Hultcrantz M., Sjöberg J., et al. (2011) Success story of targeted therapy in chronic myeloid leukemia: a population-based study of patients diagnosed in Sweden from 1973 to 2008. J Clin Oncol 29: 2514–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes F., Mauro M. (2011) Practical management of patients with chronic myeloid leukemia. Cancer 117: 4343–4354. [DOI] [PubMed] [Google Scholar]
- Clarke J., Giugliani R., Sunder-Plassmann G., Elliott P., Pintos-Morell G., Hernberg-Stahl E., et al. (2011) Impact of measures to enhance the value of observational surveys in rare diseases: the Fabry Outcome Survey (FOS). Value Health 14: 862–866. [DOI] [PubMed] [Google Scholar]
- Cortes J., Hehlmann R., Gambacorti-Passerini C., Goldberg S., Khoury H., Mauro M., et al. (2013) Baseline characteristics of patients with chronic myeloid leukemia in a prospective observational study (SIMPLICITY). 55th Annual Meeting of the American Society of Hematology, December 6–9, 2013, New Orleans, LA Blood 122: 4026. [Google Scholar]
- Cortes J., Mauro M., Goldberg S., Paquette R., Khoury H., Hirji I., et al. (2011) Quality of life during early tyrosine kinase inhibitor treatment as self-reported by chronic myeloid leukemia patients participating in a prospective observational study (Simplicity). 53rd Annual Meeting of the American Society of Hematology, December 10–13, 2011, San Diego, CA Blood 118: 4435. [Google Scholar]
- Deininger M., O’Brien S., Guilhot F., Goldman J., Hochhaus A., Hughes T., et al. (2009) International randomized study of interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. 51st Annual Meeting of the American Society of Hematology, December 5–8, 2009, New Orleans, LA Blood 114: 462. [Google Scholar]
- De Lavallade H., Apperley J., Khorashad J., Milojkovic D., Reid A., Bua M., et al. (2008) Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol 26: 3358–3363. [DOI] [PubMed] [Google Scholar]
- Dowd R., Recker R., Heaney R. (2000) Study subjects and ordinary patients. Osteoporos Int 11: 533–536. [DOI] [PubMed] [Google Scholar]
- Efficace F., Cocks K., Breccia M., Sprangers M., Meyers C., Vignetti M., et al. (2012) Time for a new era in the evaluation of targeted therapies for patients with chronic myeloid leukemia: inclusion of quality of life and other patient-reported outcomes. Crit Rev Oncol Hematol 81: 123–135. [DOI] [PubMed] [Google Scholar]
- European Treatment and Outcome Study for CML (EUTOS). Available at: http://www.eutos.org (accessed 10 November 2014).
- Gallicchio L., Boyd K., Matanoski G., Tao X., Chen L., Lam T., et al. (2008) Carotenoids and the risk of developing lung cancer: a systematic review. Am J Clin Nutr 88: 372–383. [DOI] [PubMed] [Google Scholar]
- Gambacorti-Passerini C., Antolini L., Mahon F., Guilhot F., Deininger M., Fava C., et al. (2011) Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst 103: 553–561. [DOI] [PubMed] [Google Scholar]
- Ganesan P., Sagar T., Dubashi B., Rajendranath R., Kannan K., Cyriac S., et al. (2011) Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol 86: 471–474. [DOI] [PubMed] [Google Scholar]
- Garbe E., Suissa S. (2004) Hormone replacement therapy and acute coronary outcomes: methodological issues between randomized and observational studies. Hum Reprod 19: 8–13. [DOI] [PubMed] [Google Scholar]
- Goldberg S., Cortes J., Gambacorti-Passerini C., Hehlmann R., Khoury H., Mauro M., et al. (2014a) Cytogenetic and molecular testing in patients with chronic myeloid leukemia (CML) in a prospective observational study (SIMPLICITY). J Clin Oncol 32(Suppl.): abstract 7050. [Google Scholar]
- Goldberg S., Cortes J., Gambacorti-Passerini C., Khoury H., Mauro M., Michallet M., et al. (2014b) Predictors of performing response monitoring in patients with chronic-phase chronic myeloid leukemia (CP-CML) in a prospective observational study (SIMPLICITY). J Clin Oncol 32(Suppl. 30): abstract 116. [Google Scholar]
- Goldman J. (2008) Chronic myeloid leukaemia. Leukaemia 37: 195–197. [Google Scholar]
- Hasford J., Baccarani M., Hoffmann V., Guilhot J., Saussele S., Rosti G., et al. (2011) Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood 118: 686–692. [DOI] [PubMed] [Google Scholar]
- Hehlmann R., Cortes J., Gambacorti-Passerini C., Goldberg S., Khoury H., Mauro M., et al. (2014) Tyrosine kinase inhibitor (TKI) switching: experience from simplicity, a prospective observational study of chronic-phase chronic myeloid (CP-CML) patients in clinical practice. 19th Annual Meeting of the European Haematology Association, June 12–14, 2013, Milan, Italy: abstract P883. Available at: https://b-com.mci-group.com/EventProgramme/EHA19.aspx (accessed 10 November 2014). [Google Scholar]
- Heiat A., Gross C., Krumholz H. (2002) Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med 162: 1682–1688. [DOI] [PubMed] [Google Scholar]
- Ho P., Bryson C., Rumsfeld J. (2009) Medication adherence: its importance in cardiovascular outcomes. Circulation 119: 3028–3035. [DOI] [PubMed] [Google Scholar]
- Holloway S., Lord K., Bethelmie-Bryan B., Shepard M., Neely J., Mclemore M., et al. (2012) Managing chronic myeloid leukemia: a coordinated team care perspective. Clin Lymphoma Myeloma Leuk 12: 88–93. [DOI] [PubMed] [Google Scholar]
- Ibrahim A., Eliasson L., Apperley J., Milojkovic D., Bua M., Szydlo R., et al. (2011) Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood 117: 3733–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadad A. (1998) Randomised Controlled Trials: A User’s Guide. London: BMJ Books. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson S., Olsson B., Soderberg J., Wadenvik H. (2012) Good adherence to imatinib therapy among patients with chronic myeloid leukemia-a single-center observational study. Ann Hematol 91: 679–685. [DOI] [PubMed] [Google Scholar]
- Kantarjian H., Shah N., Hochhaus A., Cortes J., Shah S., Ayala M., et al. (2010) Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 362: 2260–2270. [DOI] [PubMed] [Google Scholar]
- Khan A., Preskorn S., Baker B. (2005) Effect of study criteria on recruitment and generalizability of the results. J Clin Psychopharmacol 25: 271–275. [DOI] [PubMed] [Google Scholar]
- Latagliata R., Breccia M., Carmosino I., Cannella L., De Cuia R., Diverio D., et al. (2010) ‘Real-life’ results of front-line treatment with imatinib in older patients (≥65 years) with newly diagnosed chronic myelogenous leukemia. Leuk Res 34: 1472–1475. [DOI] [PubMed] [Google Scholar]
- Lee J., Grace K., Taylor A. (2006) Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA 296: 2563–2571. [DOI] [PubMed] [Google Scholar]
- Ligthelm R., Borzi V., Gumprecht J., Kawamori R., Wenying Y., Valensi P. (2007) Importance of observational studies in clinical practice. Clin Ther 29 Spec No: 1284–1292. [PubMed] [Google Scholar]
- Marin D., Bazeos A., Mahon F., Eliasson L., Milojkovic D., Bua M., et al. (2010) Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 28: 2381–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin D., Ibrahim A., Goldman J. (2011) European Treatment and Outcome Study (EUTOS) score for chronic myeloid leukemia still requires more confirmation. J Clin Oncol 29: 3944–3945. [DOI] [PubMed] [Google Scholar]
- Mendizabal A., Garcia-Gonzalez P., Levine P. (2013) Regional variations in age at diagnosis and overall survival among patients with chronic myeloid leukemia from low and middle income countries. Cancer Epidemiol 37: 247–254. [DOI] [PubMed] [Google Scholar]
- Michallet M., Tulliez M., Corm S., Gardembas M., Huguet F., Oukessou A., et al. (2010) Management of chronic myeloid leukaemia in clinical practice in France: results of the French subset of patients from the UNIC study. Curr Med Res Opin 26: 307–317. [DOI] [PubMed] [Google Scholar]
- Motheral B., Fairman K. (1997) The use of claims databases for outcomes research: rationale, challenges, and strategies. Clin Ther 19: 346–366. [DOI] [PubMed] [Google Scholar]
- Noens L., Van Lierde M., De Bock R., Verhoef G., Zachee P., Berneman Z., et al. (2009) prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood 113: 5401–5411. [DOI] [PubMed] [Google Scholar]
- O’Brien S., Guilhot F., Larson R., Gathmann I., Baccarani M., Cervantes F., et al. (2003) Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 348: 994–1004. [DOI] [PubMed] [Google Scholar]
- Pulte D., Gondos A., Redaniel M., Brenner H. (2011) Survival of patients with chronic myelocytic leukemia: comparisons of estimates from clinical trial settings and population-based cancer registries. Oncologist 16: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins M. (2008) De testimonio: on the evidence for decisions about the use of therapeutic interventions. Lancet 372: 2152–2161. [DOI] [PubMed] [Google Scholar]
- Redaelli A., Bell C., Casagrande J., Stephens J., Botteman M., Laskin B., et al. (2004) Clinical and epidemiologic burden of chronic myelogenous leukemia. Expert Rev Anticancer Ther 4: 85–96. [DOI] [PubMed] [Google Scholar]
- Rochon P., Gurwitz J., Sykora K., Mamdani M., Streiner D., Garfinkel S., et al. (2005) Reader’s guide to critical appraisal of cohort studies: 1. Role and design. BMJ 330: 895–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbacher M., Berger U., Hochhaus A., Metzgeroth G., Adam K., Lahaye T., et al. (2009) Clinical trials underestimate the age of chronic myeloid leukemia (CML) patients. Incidence and median age of Ph/BCR-ABL-positive CML and other chronic myeloproliferative disorders in a representative area in Germany. Leukemia 23: 602–604. [DOI] [PubMed] [Google Scholar]
- Ruddy K., Mayer E., Partridge A. (2009) Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin 59: 56–66. [DOI] [PubMed] [Google Scholar]
- Saglio G., Kim D., Issaragrisil S., Le Coutre P., Etienne G., Lobo C., et al. (2010) Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 362: 2251–2259. [DOI] [PubMed] [Google Scholar]
- Sant M., Allemani C., Tereanu C., De Angelis R., Capocaccia R., Visser O., et al. (2010) Incidence of hematologic malignancies in Europe by morphologic subtype: results of the Haemacare project. Blood 116: 3724–3734. [DOI] [PubMed] [Google Scholar]
- Silverman S. (2009) From randomized controlled trials to observational studies. Am J Med 122: 114–120. [DOI] [PubMed] [Google Scholar]
- Smith A., Painter D., Howell D., Evans P., Smith G., Patmore R., et al. (2014) Determinants of survival in patients with chronic myeloid leukaemia treated in the new era of oral therapy: findings from a UK population-based patient cohort. BMJ Open 4: e004266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stables R. (2002) Observational research in the evidence based environment: eclipsed by the randomised controlled trial? Heart 87: 101–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surveillance Epidemiology and End Results (SEER) Program, 2014. Incidence database. Available at: http://seer.cancer.gov/ (accessed 10 November 2014).
- Verma D., Kantarjian H., Strom S., Rios M., Jabbour E., Quintas-Cardama A., et al. (2011) Malignancies occurring during therapy with tyrosine kinase inhibitors (TKIs) for chronic myeloid leukemia (CML) and other hematologic malignancies. Blood 118: 4353–4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L., Garcia-Gonzalez A., Ault P., Williams J., Mobley G., Mendoza T., et al. (2010) Symptom burden in patients with chronic myeloid leukemia (CML) on kinase inhibitor (KI) therapy. American Society of Hematology 2010 Annual Meeting, Orlando FL, Dec 4–7, 2010 Blood 116: 1570–1571.20495074 [Google Scholar]
- Xie Y., Davies S., Xiang Y., Robison L., Ross J. (2003) Trends in leukemia incidence and survival in the United States (1973–1998). Cancer 97: 2229–2235. [DOI] [PubMed] [Google Scholar]