Abstract
An unusual increase in the number of Campylobacter concisus isolates found in stool cultures provoked an outbreak investigation at Bern University Hospital. No epidemiological links were found between the cases, and the Campylobacter isolates were clonally unrelated. A change in culture conditions to a hydrogen-rich atmosphere enhancing growth of C. concisus was deemed responsible for this pseudo-outbreak.
TEXT
Campylobacter concisus is a fastidious Campylobacter species whose pathogenic role in human disease has not been established. Isolation of C. concisus from samples has been reported in association with periodontal disease, Barrett's esophagus (1, 2), enteritis, and inflammatory bowel disease (IBD) (3), and the pathogen has been proposed to be linked to certain hepatobiliary and kidney conditions in children (4). High prevalences of C. concisus in stool samples were encountered not only in children and adults suffering from diarrhea (detection rate, 0.7% to 49%) but also in healthy controls (detection rate, 0% to 52%) (1, 3). Immunodeficiency (5) and age extremes (6, 7) appear to be determinants of a higher prevalence in stool. Moreover, C. concisus might be detected by PCR almost universally in human saliva samples (3). Thus, it is unresolved whether C. concisus is merely a commensal bacterium of the human digestive tract or a true pathogen. In light of its genetic variability, both of these may be true (1, 2). In late 2013, a substantial increase in the number of stool cultures testing positive for C. concisus was observed at the Bern University Hospital. In order to rule out an outbreak, an epidemiological investigation was conducted.
Bern University Hospital is a 950-bed tertiary care teaching hospital in Switzerland. In the microbiology laboratory, approximately 2,000 stool samples are cultured for enteropathogenic bacteria each year. For Campylobacter cultures, clinical stool specimens were inoculated onto Preston agar plates and incubated in a microaerobic atmosphere at 35°C or 42°C for 48 h. Microaerobic conditions were obtained with gas generator packs (CampyGen, Oxoid, United Kingdom), producing a final atmosphere of 5% O2, 10% CO2, and 85% N2, or with evacuation and gas replacement of anaerobic jars (TriLab, Jenny Science, Switzerland) containing approximately 5% O2, 8% CO2, 15% H2, and 72% N2 (replacing 76% of the air with an anaerobic gas mixture containing 70% N2, 20% H2, and 10% CO2). Isolates were identified by matrix-assisted laser desorption–ionization time of flight mass spectrometry (MALDI-TOF MS) (Bruker Biotyper; Bruker Daltonics, Bremen, Germany) and sequence analysis using the MicroSeq 500 16S rRNA gene PCR and sequencing kits (Applied Biosystems, Foster City, CA). Genetic relatedness of the isolates was analyzed by repetitive extragenic palindromic PCR (rep-PCR) (8). A case was defined as any patient with C. concisus isolated from a stool sample between 2003 and 2013. Retrospective and prospective case finding was performed, including patients who met the case definition during 2013. Incidence data were taken from electronic data on all samples processed at the microbiology laboratory. The laboratory incidence was defined as the number of C. concisus identifications divided by the total number of stool cultures processed in the given time period. Epidemiological and clinical data were taken from the hospital's electronic patient chart (CGM Phoenix; Parametrix Solutions, Lachen, Switzerland), primarily focusing on acquisition mode (nosocomial versus community acquisition). Nosocomial acquisition was defined as a diagnosis >48 h after hospital admission. Patients diagnosed as outpatients with hospitalization within the previous month were considered to have nosocomial C. concisus (3, 9). This outbreak investigation was part of the infection prevention mandate and therefore was not subject to review by the ethics committee.
(This work was partially presented as a poster at the 24th European Congress of Clinical Microbiology and Infectious Diseases, Barcelona, Spain, 10 to 13 May 2014.)
In the decade prior to the increase, C. concisus was rarely detected in routine stool cultures (average, 1.1 isolates annually). In 2013, C. concisus was isolated from stool specimens from 21 individual patients and from an intestinal biopsy specimen from another patient. In all instances, C. concisus was the sole organism with pathogenic potential detected. The incidence increased from an average of 0.03% (January 2012 to May 2013) to 1.92% (June to December 2013) (P < 0.001, chi-square test; Fig. 1).
FIG 1.
(A) Annual number of clinical samples and patients positive for C. concisus from 2003 to 2013. (B) Absolute numbers (squares) and incidence (solid line) of C. concisus isolates from January 2012 to December 2013. The arrow indicates the introduction of the new culture method.
The mean age of the 22 patients included in the analysis was 46.7 years (standard deviation, ±25.9 years; range, 3 months to 85 years). Eleven of 22 patients were female. Eight of 22 patients were outpatients. C. concisus was detected >48 h after the first admission in 8 of 14 inpatients and more than 48 h into the admission in 3 of 14 patients, during which the diagnosis was made. Two patients (3 and 5) were hospitalized on the same ward during the same time period prior to C. concisus detection, with patient 3 being on contact precautions due to diarrhea of unknown etiology. Prior to detection of C. concisus, 3 of 22 patients had a colonoscopy at our hospital and 1 of 22 had one at an external hospital (with intervals of 1, 4, 122, and 140 days prior to diagnosis). Two patients had colonoscopy on the same ward but months apart from each other. In one additional patient, C. concisus was cultured from biopsy material. Putative risk factors for colonization/infection were found in 13 of 22 patients (immunodeficiency, 6 patients [3 with IBD]; extremes of age, 6 patients; extremes of age and immunodeficiency, 1 patient). Seven of 22 cases suffered from either IBD (n = 4) or chronic kidney disease (n = 3), among which 4 of 7 cases were also immunodeficient. Figure 2 summarizes epidemiological data and the results of rep-PCR-based genotyping.
FIG 2.
Results of genotyping and epidemiologic data of all 22 patients diagnosed with C. concisus in stool samples taken in 2013. One strain was isolated from an intestinal biopsy specimen (patient 17). Patients are numbered in the order of collected culture. A strain (X) isolated in 2010 was included as an unrelated control for typing purposes. NA, not available; m, male; f, female.
After reviewing the cases, a change in microaerobic culture conditions was identified as the most likely explanation for the putative outbreak. Shortly before the C. concisus incidence started to increase, an automated system for the evacuation and gas replacement of anaerobic jars had been introduced. In contrast to the previously used microaerobic gas generator packs, which did not produce hydrogen, the resulting atmosphere of the new system contained approximately 15% hydrogen. Some Campylobacter species, such as C. concisus, appear to require increased hydrogen concentrations for optimal growth (10). When subculturing five frozen C. concisus isolates (not the original stool samples) from the study period under both culture conditions, only weak or no growth was encountered with the previous methodology (Fig. 3).
FIG 3.
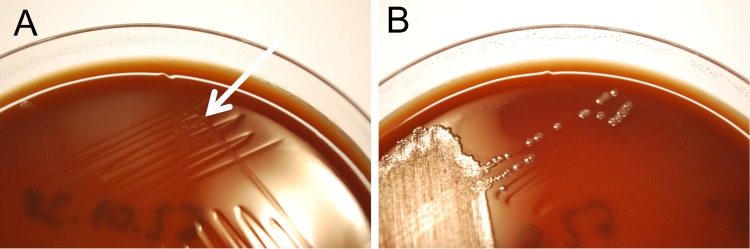
C. concisus isolate subcultured under previous (A, gas generator pack, only a few pinpoint colonies visible [arrow]) and new culture conditions (B, anaerobic jar supplemented with hydrogen) for 3 days at 42°C.
In conclusion, a pseudo-outbreak of C. concisus due to a change in laboratory procedures was identified. A pseudo-outbreak is defined as an episode of increased disease incidence due to enhanced surveillance or other factors but not related to the disease under study (11). Except for one patient, no epidemiological links suggesting nosocomial transmission were found. In addition, genotyping revealed no close relationship between the isolates available for testing. Unfortunately, the isolate of the first—and potential index—case (3) was not available for genotyping. The introduction of a new microaerobic culture system containing a high hydrogen concentration compared to that of conventional microaerobic conditions presumably led to a better recovery of C. concisus from fecal samples. The clinical significance of C. concisus remains unclear to date but may be easier to determine as diagnostic procedures improve and permit the differentiation between pathogenic and nonpathogenic strains.
ACKNOWLEDGMENTS
We thank Regula Tinguely and Andrea Endimiani for rep-PCR analysis.
REFERENCES
- 1.Kaakoush NO, Mitchell HM. 2012. Campylobacter concisus—a new player in intestinal disease. Front Cell Infect Microbiol 2:4. doi: 10.3389/fcimb.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Man SM. 2011. The clinical importance of emerging Campylobacter species. Nat Rev Gastroenterol Hepatol 8:669–685. doi: 10.1038/nrgastro.2011.191. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Lee H, Grimm MC, Riordan SM, Day AS, Lemberg DA. 2014. Campylobacter concisus and inflammatory bowel disease. World J Gastroenterol 20:1259–1267. doi: 10.3748/wjg.v20.i5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lastovica AJ. 2009. Clinical relevance of Campylobacter concisus isolated from pediatric patients. J Clin Microbiol 47:2360. doi: 10.1128/JCM.00568-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aabenhus R, Permin H, On SL, Andersen LP. 2002. Prevalence of Campylobacter concisus in diarrhoea of immunocompromised patients. Scand J Infect Dis 34:248–252. doi: 10.1080/00365540110080566. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen HL, Engberg J, Ejlertsen T, Bucker R, Nielsen H. 2012. Short-term and medium-term clinical outcomes of Campylobacter concisus infection. Clin Microbiol Infect 18:E459–E465. doi: 10.1111/j.1469-0691.2012.03990.x. [DOI] [PubMed] [Google Scholar]
- 7.Engberg J, On SL, Harrington CS, Gerner-Smidt P. 2000. Prevalence of Campylobacter, Arcobacter, Helicobacter, and Sutterella spp. in human fecal samples as estimated by a reevaluation of isolation methods for campylobacters. J Clin Microbiol 38:286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilty M, Betsch BY, Bogli-Stuber K, Heiniger N, Stadler M, Kuffer M, Kronenberg A, Rohrer C, Aebi S, Endimiani A, Droz S, Mühlemann K. 2012. Transmission dynamics of extended-spectrum beta-lactamase-producing Enterobacteriaceae in the tertiary care hospital and the household setting. Clin Infect Dis 55:967–975. doi: 10.1093/cid/cis581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georges-Courbot MC, Beraud-Cassel AM, Gouandjika I, Georges AJ. 1987. Prospective study of enteric Campylobacter infections in children from birth to 6 months in the Central African Republic. J Clin Microbiol 25:836–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzgerald CNI. 2011. Campylobacter and Arcobacter, p 885–899. In Versalovic J, Carroll KC, Jorgensen JH, Funke G, Landry ML, Warnock DW (ed), Manual of clinical microbiology, 10th ed, vol 2 ASM Press, Washington, DC. [Google Scholar]
- 11.Stedman T. 2005. Stedman's medical dictionary for the health professions and nursing, 28th ed, p 1659 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]




