Abstract
Data on the performance of rapid molecular point-of-care use platforms for diagnosis of influenza are lacking. We validated nasopharyngeal (NP) flocked specimens in universal transport medium (UTM) and evaluated the clinical sensitivity and specificity of the Alere i influenza A&B test compared to those of the Xpert flu A/B assay. The Alere i influenza A&B test had an overall sensitivity and specificity of 93.8% and 62.5% for influenza A, respectively, and of 91.8% and 53.6% for influenza B, respectively. The poor specificity was due to influenza virus samples determined positive for both type A and B.
TEXT
Rapid and accurate diagnoses of influenza prompt necessary infection control, public health notification, tracking, and accurate administration of antiviral therapy. During the pandemic H1N1 outbreak of 2009, the performance of rapid antigen detection tests for influenza was shown to be inferior to the performance of molecular methods, with sensitivity ranging from 10% to 70% (1–3). Rapid molecular testing was not available in many hospitals, clinics, and physician offices due to either cost of equipment or cost of reagents, use of complex molecular diagnostics requiring skilled technologists to perform testing, and/or slow turnaround time to results (2, 4, 5). Recently, the Alere i influenza A&B assay (Alere, Scarborough, ME) became a FDA-cleared molecular test for detection of influenza viruses A and B.
The Alere i influenza A&B system (Alere i system) is a rapid, semiautomated in vitro diagnostic test for the detection and differentiation of influenza A virus and influenza B virus with objective results available in less than 15 min. The Alere i system incorporates isothermal nucleic acid amplification technology using primers and fluorescent probes specific for amplification of RNA targets for influenza A and B virus in samples from patients presenting with influenza-like illness (ILI). The test is performed with an Alere i instrument and three test components: sample receiver, containing elution buffer, test base with two sealed reaction tubes containing a lyophilized pellet containing reagents for amplification of target RNA, and a transfer cartridge for transferring the eluted sample to test base. The Alere i influenza A&B test is intended for direct nasal swab specimen testing for influenza A and B viral infections in conjunction with clinical and epidemiological risk factors. Similar to the earlier IQuum Liat influenza A/B assay (Roche Diagnostics, Indianapolis, IN), the Alere i system was developed to address the unmet clinical need for rapid point-of-care testing of influenza at clinical sites with low test volume and limited easy-to-use molecular technology. Performance characteristics of such devices are lacking, as well as comparisons to traditional PCR methods.
The Alere i influenza A&B assay is equipped with sterile-foam-tipped applicator swabs (Puritan Medical Products LLC, Guilford, ME) for fresh specimen collection; however, rayon or flocked nasal swabs have been validated for use by Alere. Specimen transportation and storage have also been validated for media such as saline, Vircell, and universal transport medium (UTM) in leak-proof containers with a suggested 0.5- to 3.0-ml dilution range to maximize sensitivity. The objective of this study was to evaluate the clinical sensitivity and specificity of the Alere i influenza A&B test versus PCR results from residual frozen nasopharyngeal (NP) swab specimens eluted in UTM (NP UTM) from Rhode Island Hospital (RIH) patients. Evaluation of NP UTM specimens is appropriate, as it is a common respiratory sample collection method for many laboratories, since it allows for subsequent testing without the need to collect additional samples from the patient (6, 7).
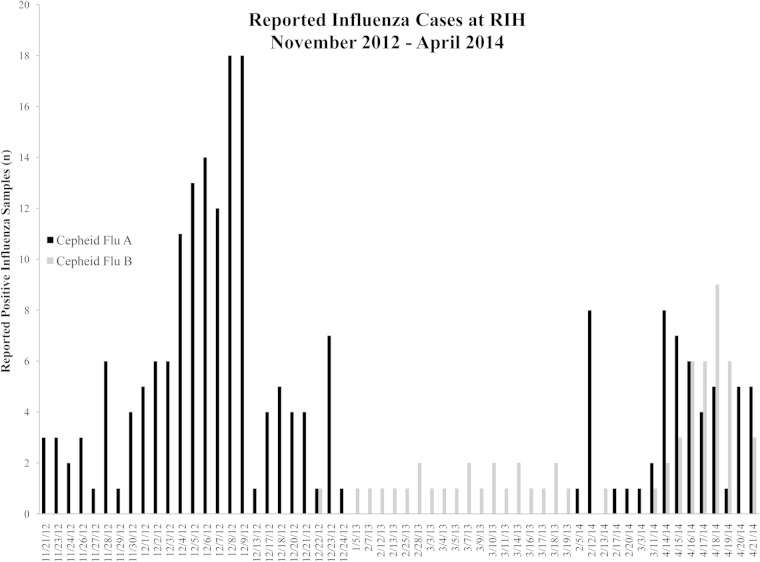
A total of 291 previously tested respiratory specimens from two influenza seasons, November 2012 to March 2013 and February 2014 to April 2014, were selected to be evaluated on the Alere i system. Figure 1 shows the calendar time line and distribution of influenza A and B viral isolates tested. The demographic characteristics of the patients were as follows: 43% male and 57% female, with 21% pediatric patients (range, 3 weeks to 17 years) and 79% adult patients (18 to 96 years). Per the standard of care at RIH, a rapid influenza A/B test request is performed using NP flocked swab in 1 ml of UTM (BD universal viral transport combo kit; BD, Sparks, MD) from patients presenting with influenza-like illness (ILI) and subsequently tested using the Xpert flu A/B assay (Cepheid, Sunnydale, CA). Labeled remnant UTM respiratory samples stored at −70°C were thawed at room temperature, and 200-μl portions were tested with the Alere i system. Specimens with discrepant results were stored at 4°C and tested within a week using the xTAG respiratory viral panel (RVP) (Luminex, Austin, TX). Cohen's kappa coefficient was computed to assess the agreement in results obtained with our laboratories' current clinical standard, Xpert flu A/B assay, and the Alere i system. True-positive influenza specimens were the specimens with two positive test results. Specimens positive for both influenza A and B virus, or incorrectly typed by the Alere i system that were historically influenza A virus positive using the Xpert flu A/B assay and confirmed influenza A positive by xTAG, were considered Alere i influenza B false positive and vice versa. STATA/SE 12.1 (College Station, TX) was used to calculate sensitivity and specificity, with 95% confidence intervals (95% CI), and Cohen's kappa coefficient. The study was approved by the Lifespan/Rhode Island Hospital Institutional Review Board (IRB).
FIG 1.
Distribution of positive influenza cases at RIH for two influenza seasons, November 2012 to March 2013 and February 2014 to April 2014, tested with the Xpert flu A/B assay.
The Alere i system reported 180 positive results for influenza A virus, 45 positive results for influenza B virus, and 15 negative results, after analysis of the samples with discrepant results (Table 1). Thirteen samples were eliminated, four samples gave invalid Alere i results (invalid rate of 1.4% [4/283]) due to internal control failure, and nine samples gave indeterminate results in more than one molecular assay (limited volume when retested on xTAG RVP), leaving 278 samples for final analysis. We observed a kappa coefficient of 0.36, denoting significant agreement (86.33% agreement) between diagnostic methods (P < 0.0001). The results for the 38 discrepant samples are displayed in Table 2. After resolution by xTAG RVP, 22 of 38 (58%) discrepant samples yielded positive results for influenza virus but yielded an incorrect type on the Alere i system. Of the incorrectly typed samples, 17 of 22 (77%) were positive for both influenza A and B virus. The Alere i influenza A&B test had an overall sensitivity and specificity of 93.8% and 62.5% for influenza A virus and 91.8% and 53.6% for influenza B virus, respectively, after discrepant resolution with PCR.
TABLE 1.
Performance of the Alere i influenza A&B nucleic acid amplification test compared to that of the Xpert flu A/B assaya
| Influenza type | No. of samples giving the following results by Alere vs Xpertb: |
% Sensitivity (95% CI) | % Specificity (95% CI) | |||
|---|---|---|---|---|---|---|
| +/+ | +/− | −/− | −/+ | |||
| Flu A | 180 | 9 | 15 | 12 | 93.8 (89.3–96.7) | 62.5 (40.6–81.2) |
| Flu B | 45 | 13 | 15 | 4 | 91.8 (80.4–97.7) | 53.6 (33.9–72.5) |
Influenza virus is abbreviated in the table as follows: Flu A or B for influenza virus A or B.
+/+, true positive; +/−, false positive; −/−, true negative; −/+, false negative.
TABLE 2.
Breakdown and final resolution of 38 discrepant results between Alere i influenza A&B test and Xpert flu A/B assaya
| Test resultb |
Resolution | Final Alere resultb,c | ||
|---|---|---|---|---|
| Alere (38) | Xpert | xTAG | ||
| Flu A (3) | Flu B (3) | Flu B (3) | Positive Flu B | FP Flu A (3) |
| Flu B (2) | Flu A (2) | Flu A (2) | Positive Flu A | FP Flu B (2) |
| Flu A&B (17) | Flu A (11) | Flu A (11) | Positive Flu A | FP Flu B (11) |
| Flu B (6) | Flu B (6) | Positive Flu B | FP Flu A (6) | |
| Negative (16) | Flu A (12) | Flu A (12) | Positive Flu A | FN Flu A (12) |
| Flu B (4) | Flu B (4) | Positive Flu B | FN Flu B (4) | |
The xTAG RVP was used to resolve discrepant Alere and Xpert results.
The test result is the type (influenza A or B) of influenza virus found if the test result was positive or negative on any of the 3 molecular assays. The number of samples with the indicated test result is shown in parentheses.
FP, false-positive; FN, false-negative.
The limitations of this study include the use of frozen specimens, the selection of positive specimens for the majority of testing, which hampered our ability to infer the true positive predictive value (PPV) and negative predictive value (NPV) of this test, and the inability to compare directly with another point-of-care molecular platform, like the IQuum Liat influenza A/B test (Roche Diagnostics, Indianapolis, IN). Because previously reported issues with waived influenza tests have been poor sensitivity, and data reported by other investigators with the Alere i molecular test showed excellent specificity, we focused our testing on the ability to detect positive cases. Previously, our team assessed the performance of the IQuum Liat influenza A/B assay to the xTAG RVP and Xpert flu A/B assay, and our results indicated a 91.5% agreement with 54/59 NP samples being concordant with the molecular platforms mentioned above (K. C. Chapin and R. Dickenson, presented at the 30th Annual Clinical Virology Symposium, Daytona Beach, FL, April 2014) (8). Strengths of the study include the use of specimens from two consecutive seasons and influenza type distribution, inclusion of pediatric and adult patient samples, as well as the first report of the Alere i system compared to the Clinical Laboratory Improvement Amendments (CLIA) moderate-complexity Xpert flu A/B assay. In comparing both molecular diagnostics, the Alere i system requires less technician time, minimal capital equipment outlay, and allows greater flexibility for personnel performing the assay in a point-of-care environment.
This retrospective study evaluating the performance of the Alere i influenza A&B test on previously tested influenza virus NP samples in UTM showed decreased specificity for influenza A virus and influenza B virus compared to the Xpert flu assay. Although proper specimen handling, control testing, and decontamination were followed in our lab, we had a high number of dual positive influenza virus results from the Alere i system, which were not previously reported in other studies using this novel system. The package insert provided prior to FDA approval did not call for repeat testing of such samples, and this has subsequently been added. In 2014, Nie et al. (9) and Bell and Selvarangan (10) reported high sensitivity and specificity ranges of 87.2% to 93.3% and 93.3% to 100% for influenza A virus, and 97.4% to 100% and 100% for influenza B virus, respectively. Nie et al. evaluated the Alere i system with the FilmArray respiratory panel (RP) (BioFire Diagnostics, Salt Lake City, UT) using frozen NP swabs in viral transport media, using the Prodesse ProFlu+ assay (Gen-Probe, San Diego, CA) for discrepant analysis (9). Bell and Selvarangan reported similar findings comparing the results of Alere i system to those of viral culture, with discrepant analysis by the Prodesse ProFlu+ assay in their pediatric study (10). Validating performance parameters with UTM means continued use of a common collection system currently in place in our laboratory and the availability of additional specimens without having to collect additional samples for subsequent testing, which may be necessary to clarify respiratory diagnoses, such as coinfections, or address specific infection control requirements (1, 2, 11). Overall, the Alere i influenza A&B assay provided rapid results in less than 15 min with 2 min of hands-on time and a high sensitivity for detection of influenza virus, making it a viable point-of-care molecular diagnostic. The Alere i system is currently awaiting consideration for CLIA waiver (http://www.alere.com/content/dam/alere/docs/australia/ID/Alere_i_US_release_6_16_2014.pdf).
ACKNOWLEDGMENTS
We thank the Rhode Island Hospital Microbiology Laboratory for providing initial PCR data and helping conduct discrepant analysis.
This study was funded in part by Alere Scarborough Inc.
We declare that we have no conflicts of interest.
REFERENCES
- 1.Ginocchio CC, Lotlikar M, Falk L, Arora S, Kowerska M, Bornfreund M, Manji R. 2009. Clinical performance of the 3M Rapid Detection Flu A+B test compared to R-Mix culture, DFA and BinaxNOW Influenza A&B test. J Clin Virol 45:146–149. doi: 10.1016/j.jcv.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Ginocchio CC, Zhang F, Manji R, Arora S, Bornfreund M, Falk L, Lotlikar M, Kowerska M, Becker G, Korologos D. 2009. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol 45:191–195. doi: 10.1016/j.jcv.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2012. Evaluation of 11 commercially available rapid influenza diagnostic tests – United States, 2011-2012. MMWR Morb Mortal Wkly Rep 61:873–876. [PubMed] [Google Scholar]
- 4.Tayo A, Ellis J, Linden Phillips L, Simpson S, Ward DJ. 2012. Emerging point of care tests for influenza: innovation or status quo. Influenza Other Respir Viruses 6:291–298. doi: 10.1111/j.1750-2659.2011.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peaper DR, Landry ML. 2014. Rapid diagnosis of influenza: state of the art. Clin Lab Med 34:365–385. doi: 10.1016/j.cll.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scansen KA, Bonsu BK, Stoner E, Mack K, Salamon D, Leber A, Marcon MJ. 2010. Comparison of polyurethane foam to nylon flocked swabs for collection of secretions from the anterior nares in performance of a rapid influenza virus antigen test in a pediatric emergency department. J Clin Microbiol 48:852–856. doi: 10.1128/JCM.01897-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larios OE, Coleman BL, Drews SJ, Mazzulli T, Borgundvaag B, Green K, McGeer AJ. 2011. Self-collected mid-turbinate swabs for the detection of respiratory viruses in adults with acute respiratory illnesses. PLoS One 6:e21335. doi: 10.1371/journal.pone.0021335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapin K, Dickenson R. 2014. Comparison of IQuum Liat influenza A/B assay to Cepheid flu A/B and Luminez RVP. Abstr 30th Annu Clin Virol Symp, abstr S50. [Google Scholar]
- 9.Nie S, Roth RB, Stiles J, Mikhlina A, Lu X, Tang Y-W, Babady NE. 2014. Evaluation of Alere i influenza A&B for rapid detection of influenza viruses A and B. J Clin Microbiol 52:3339–3344. doi: 10.1128/JCM.01132-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell JJ, Selvarangan R. 2014. Evaluation of the Alere i influenza A&B nucleic acid amplification test by use of respiratory specimens collected in viral transport medium. J Clin Microbiol 52:3992–3995. doi: 10.1128/JCM.01639-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell J, Bonner A, Cohen DM, Birkhahn R, Yogev R, Triner W, Cohen J, Palavecino E, Selvarangan R. 2014. Multicenter clinical evaluation of the novel Alere i influenza A&B isothermal nucleic acid amplification test. J Clin Virol 61:81–86. doi: 10.1016/j.jcv.2014.06.001. [DOI] [PubMed] [Google Scholar]