Abstract
Early and appropriate blood culture sampling is recommended as a standard of care for patients with suspected bloodstream infections (BSI) but is rarely taken into account when quality indicators for BSI are evaluated. To date, sampling of about 100 to 200 blood culture sets per 1,000 patient-days is recommended as the target range for blood culture rates. However, the empirical basis of this recommendation is not clear. The aim of the current study was to analyze the association between blood culture rates and observed BSI rates and to derive a reference threshold for blood culture rates in intensive care units (ICUs). This study is based on data from 223 ICUs taking part in the German hospital infection surveillance system. We applied locally weighted regression and segmented Poisson regression to assess the association between blood culture rates and BSI rates. Below 80 to 90 blood culture sets per 1,000 patient-days, observed BSI rates increased with increasing blood culture rates, while there was no further increase above this threshold. Segmented Poisson regression located the threshold at 87 (95% confidence interval, 54 to 120) blood culture sets per 1,000 patient-days. Only one-third of the investigated ICUs displayed blood culture rates above this threshold. We provided empirical justification for a blood culture target threshold in ICUs. In the majority of the studied ICUs, blood culture sampling rates were below this threshold. This suggests that a substantial fraction of BSI cases might remain undetected; reporting observed BSI rates as a quality indicator without sufficiently high blood culture rates might be misleading.
INTRODUCTION
With an incidence of 100/100,000 patient-days at risk and with a case fatality rate of 20 to 50%, bloodstream infections (BSI) are a major cause of morbidity and mortality worldwide (1, 2). Identification of the causal pathogen allows a targeted and shortened antibiotic treatment and is thereby associated with reduced case fatality rates (3, 4) and a lower risk for development of antibiotic resistance (5). Thus, blood culture sampling is the most important diagnostic test for bloodstream infections, as it is the only tool that gives reliable information about the causative pathogen and its resistance profile (6). Knowledge about blood culture sampling rates, however, is also important for the interpretation of quality indicators for nosocomial infections, as those quality indicators (e.g., primary BSI rates) give valid estimates only if a sufficient number of blood cultures is taken. Nevertheless, this is only rarely taken into account when primary BSI rates are reported and interpreted.
Recommendations for target blood culture rates have been published in microbiological guidelines. In the United States, the Cumitech guideline recommends 103 to 188 blood culture sets per 1,000 patient-days, referring to a web-based U.S. forum in 1999 (7). However, the relevant discussion in this forum is no longer available and could not be reconstructed by the facilitators of the forum. The German Mikrobiologisch-Infektiologische Qualitätsstandards (MIQ) guideline gives a reference range of 100 to 200 blood culture sets per 1,000 patient-days, referring directly to the U.S. guideline (8). The guidelines do not provide empirical justification for the recommended values; these are apparently based on expert consensus rather than formal evidence. From a technical perspective, it seems a fair assumption that there is a range in which blood culture rates are too low for detecting most cases.
A potential way of deriving a target range for blood culture rates is to define a threshold above which blood culture sampling rates are sufficiently high to detect most BSI cases while a further increase of blood culture rates does not result in an increase of BSI rates. This threshold can then be used as a process indicator for quality management purposes. Therefore, our aim was to assess the form of the association between blood culture rates and BSI rates in order to derive a reference range for blood culture rates in intensive care units (ICUs).
MATERIALS AND METHODS
We used data from 223 German ICUs participating in the German hospital infection surveillance system (KISS). KISS is a voluntary network of German ICUs that collects a variety of data related to health care-associated infections (9). Within KISS, institution-specific characteristics as well as BSI rates are available for each calendar year. BSI is defined according to the criteria for laboratory-confirmed BSI proposed by the Centers for Disease Control and Prevention (Atlanta, GA, USA). To fulfil these criteria, (i) the patient (older than 12 months of age) must have a recognized pathogen cultured from one or more blood cultures, and the organism cultured from blood must not be related to an infection at another site, or (ii) the patient must have at least one of fever (>38°C), chills, or hypotension, and positive laboratory results which are not related to an infection at another site, and a common skin contaminant (i.e., diphtheroids [Corynebacterium spp.], Bacillus spp. [not Bacillus anthracis], Propionibacterium spp., coagulase-negative staphylococci [including Staphylococcus epidermidis], viridans group streptococci, Aerococcus spp., and Micrococcus spp.) must be cultured from 2 or more blood cultures drawn on separate occasions (10).
New BSI cases arising at the ICU are reported in KISS on a daily basis. In 2006, KISS collected blood culture sampling rates from all participating institutions within a prospective add-on study. Information on blood culture sampling is not available for any other year in KISS. BSI rates (both including and excluding coagulase-negative staphylococci [CNS]) from 2006 and 2007 were extracted from KISS for the institutions participating in this add-on study and were linked to the 2006 blood culture sampling rates. We assumed that blood culture practice did not change considerably from 2006 to 2007 and used data on BSI rates from both years in order to improve precision in BSI rates, as the number of BSI cases was low in many of the participating institutions. As KISS collects data on primary BSI cases only, no information on secondary BSI was available. Thus, numerators of BSI rates in this study consist only of primary BSI cases, resulting in rates lower than those reported in the literature when primary and secondary BSI cases were included. The data used for this study were derived from the data collection used by Gastmeier et al. (11) but were supplemented for the current analysis with information for BSI rates in 2007 as well as with additional covariates.
BSI rates were defined as the number of BSI cases (including those with CNS) divided by patient-days at risk. Rates of nosocomial infections recorded in KISS have been shown to give a reliable estimate of true nosocomial infection rates in the participating ICUs (12). Blood culture rates were defined as the number of blood culture sets taken in the ICU divided by the number of patient-days at risk.
In the first step, in order to identify the form of association between blood culture rates and BSI rates, locally weighted regression (a nonparametric regression method that is purely based on the data and does not make assumptions about the underlying distribution) was performed using the R package “gam” (13). In the second step, a segmented Poisson regression model with two linear segments (representing two different regression equations applied to distinct ranges of blood culture rates) was used to identify the threshold for blood culture rates above which no further increase in BSI rates could be observed. This was done using the R package “segmented” (14). All analyses were performed in a univariable way. A sensitivity analysis was conducted excluding BSI cases with CNS from the numerator when BSI rates were calculated. In another sensitivity analysis, we replaced the outcome variable BSI rate with the central-line-associated bloodstream infection (CLABSI) rate (calculated as the number of catheter-related BSI cases divided by the number of central-line-days at risk) in order to (i) investigate if our findings remain stable when we split BSI cases into catheter-related and catheter-unrelated ones and (ii) account for differences in a priori risks for BSI between the participating ICUs due to differences in central-line rates. Moreover, we analyzed data for 2006 and 2007 separately in order to check if combining BSI rates from both years might have affected the results of this study.
In the last step, ICUs below the identified threshold for blood culture rates were compared to those above the threshold with respect to institutional characteristics. Chi-square tests were used for categorical variables and Wilcoxon rank sum tests for continuous variables.
KISS is based on anonymous surveillance data which are reported from German hospitals to the National Reference Centre of the Surveillance of Nosocomial Infections as part of an official mandatory reporting process (German Protection against Infection Act, Infektionsschutzgesetz §23). These data are available for secondary analyses. Ethics approval and informed consent were thus not necessary for this study.
RESULTS
In total, data from 223 German ICUs (representative of the German health care system [12]) were analyzed for this study (Table 1). Most of the ICUs were interdisciplinary (50.2%) and were located in medium-size hospitals (median, 500 beds; interquartile range [IQR], 300 to 1,000 beds) with the status of an academic teaching hospital (45.7%) or nonacademic hospital (37.7%). Only a minority of ICUs worked with a microbiology lab within the same institution, while 73.2% of the ICUs sent their blood cultures to private-sector remote labs. The median central-line rate (defined as central-line-days divided by total patient-days) in the participating ICUs was 67.3% (IQR, 49.2 to 82.1%), while the median ventilation rate was 34.2% (IQR, 22.7 to 49.9%). The participating ICUs reported a median blood culture rate of 60/1,000 patient-days (IQR, 33 to 108) and median BSI rates of 0.46/1,000 patient-days (IQR, 0 to 1.19; including those with CNS) and 0.38/1,000 patient-days (IQR, 0 to 0.85; excluding those with CNS).
TABLE 1.
Characteristics of the ICUs enrolled in this study, divided according to blood culture rate
| Characteristic | No. of ICUs |
P valuea | ||
|---|---|---|---|---|
| All (n = 223) | With blood culture rates below threshold (n = 145) | With blood culture rates above threshold (n = 78) | ||
| Type of ICU | ||||
| Interdisciplinary | 112 (50.2%) | 82 (56.6%) | 30 (38.5%) | 0.048 |
| Internal medicine | 47 (21.1%) | 25 (17.3%) | 22 (28.2%) | |
| Surgery | 41 (18.4%) | 26 (17.9%) | 15 (19.2%) | |
| Othersb | 23 (10.3%) | 12 (8.3%) | 11 (14.1%) | |
| Hospital size (beds)c | 500 (300–1,000) | 500 (300–800) | 800 (400–1,400) | <0.001 |
| Hospital type | ||||
| University | 37 (16.6%) | 13 (9.0%) | 24 (30.8%) | <0.001 |
| Academic teaching | 102 (45.7%) | 69 (47.6%) | 33 (42.3%) | |
| Nonacademic | 84 (37.7%) | 63 (43.5%) | 21 (26.9%) | |
| Own lab | 82 (36.8%) | 40 (27.6%) | 42 (53.8%) | <0.001 |
| Short-stay patientsd | ||||
| Less than 1/3 | 71 (31.8%) | 35 (24.1%) | 36 (46.2%) | 0.003 |
| 1/3 to 2/3 | 131 (58.8%) | 94 (64.8%) | 37 (47.4%) | |
| More than 2/3 | 21 (9.4%) | 16 (11.0%) | 5 (6.4%) | |
| Central line ratec | 67.3 (49.2–82.1%) | 62.5 (47.4–77.4%) | 74.5 (56.2–85.2%) | 0.006 |
| Ventilation ratec | 34.2 (22.7–49.9%) | 29.7 (21.5–44.5%) | 43.6 (32.9–56.1%) | <0.001 |
| Blood culture ratee | 60 (33–108) | 39 (25–59) | 131 (103–194) | <0.001 |
| Bloodstream infection ratee | 0.46 (0–1.19) | 0.42 (0–0.81) | 0.97 (0.34–1.72) | <0.001 |
| Bloodstream infection rate (CNS excluded)e | 0.38 (0–0.85) | 0.27 (0–0.68) | 0.65 (0.27–1.02) | <0.001 |
P values for the comparison between ICUs below and above the threshold using chi-square tests for categorical variables and Wilcoxon rank-sum tests for continuous variables.
Includes pediatrics (n = 3), neurology (n = 2), cardiology (n = 2), neurosurgery (n = 1), trauma unit (n = 1), burn unit (n = 1), heart surgery (n = 1).
Displayed are medians and interquartile ranges (in brackets).
Short stay patients are defined as having stayed less than 48 h at the ICU before discharge from ICU.
Per 1,000 patient-days at risk.
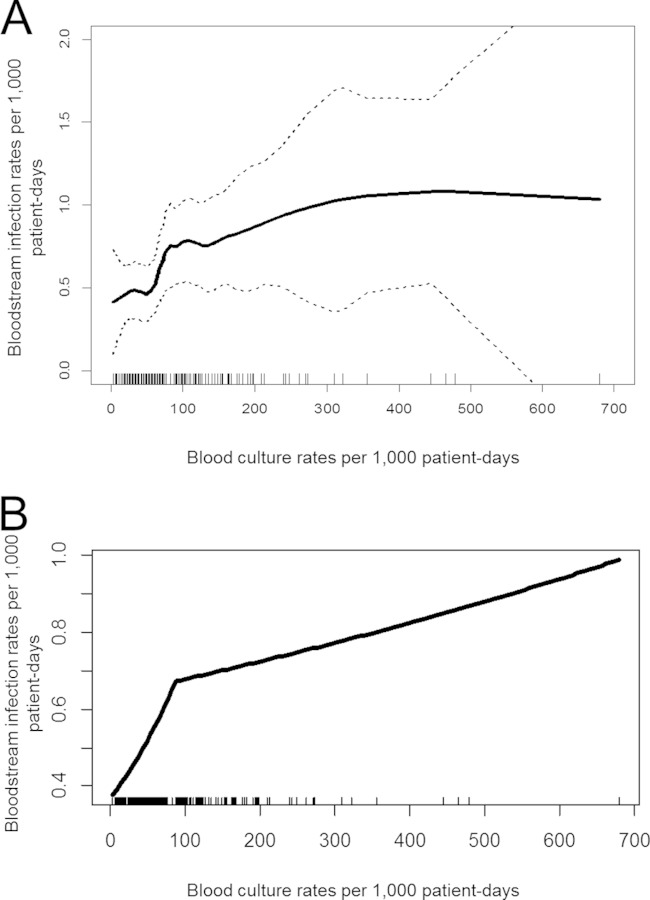
Locally weighted regression showed an increase in observed BSI rates with increasing blood culture rates until a plateau was reached (Fig. 1A). Segmented regression with two linear segments localized the breaking point at 87 (95% CI, 55 to 120) blood culture sets per 1,000 patient-days. The first segment showed an increase in observed BSI rates with increasing blood culture rates. In the second segment, the slope was substantially smaller and not significantly different from zero (Fig. 1B). A sensitivity analysis excluding CNS BSI cases produced virtually unchanged results. In the sensitivity analysis based on observed CLABSI rates instead of observed BSI rates, the threshold was estimated as slightly lower, at 83 (95% CI, 53 to 114) blood culture sets per 1,000 patient-days. Analyzing data for 2006 and 2007 separately gave threshold estimates similar to those from the main analyses (with, however, wider confidence intervals).
FIG 1.
Association between blood culture rates and bloodstream infection rates in the sample of participating ICUs. (A) Locally weighted regression for the association of blood culture rates and bloodstream infection rates. Dotted lines represent 95% confidence intervals. Each ICU is represented by a small vertical line at the x axis. This nonparametric regression method is based purely on the data and does not make assumptions about the underlying distribution. It was used for understanding the type of association between blood culture rates and bloodstream infection rates. (B) Segmented regression representing the association between blood culture rates and bloodstream infection rates in the samples from participating ICUs (slope 1, 0.69; 95% CI, 0.31 to 1.06; slope 2, 0.06; 95% CI, −0.01 to 0.14). This model with two linear segments (representing two different regression equations applied for distinct ranges of blood culture rates) was used to identify the threshold for blood culture rates above which no further increase in rates of detection of bloodstream infections could be observed.
Nearly two thirds (65%, n = 145) of the ICUs in this study showed blood culture rates below the proposed threshold. These ICUs were more likely to represent interdisciplinary ICUs in smaller, nonacademic hospitals with a remote microbiology lab and a high proportion of short-stay patients (Table 1). Ventilation rates and central-line rates were lower in ICUs below the threshold.
DISCUSSION
Using data from a large surveillance study in German ICUs, we demonstrated that below a specific threshold of blood culture rates, observed BSI rates increase with higher blood culture rates. Above this threshold further increase of blood culture rates does not increase the number of observed BSI cases. These results suggest that the identified threshold represents the lower boundary for a recommended target range for blood culture rates that can be used for quality management purposes. Since there is no substantial gain in detection of BSI cases when blood culture rates are increased further, the rationale of cost reduction would suggest the upper boundary of a target range to be only slightly above this threshold.
The identified threshold of 87 blood culture sets per 1,000 patient-days at risk was quite close to the lower boundaries of previous recommendations of 100 to 200 and 103 to 188 blood culture sets per 1,000 patient-days at risk.
Our study has implications for both the assessment of quality of patient care in ICUs and the interpretation of established quality indicators in the context of health care-associated infections. With respect to the former, blood culture sampling rates below the proposed threshold are likely to be associated with a lower quality of individual patient care and might represent a problem either in the indication or in the correct performance of blood culture sampling. With respect to the latter, the clear implication of this study is that quality indicators based on observed BSI rates are meaningful only when blood culture sampling rates are sufficiently high (i.e., above the proposed threshold); thus, achieving target blood culture rates is a prerequisite for quality control. In Germany, a median of 60 blood culture sets per 1,000 patient-days has been reported for ICUs (11). This indicates that traditional quality indicators in these institutions (such as BSI or CLABSI rates) are likely to be biased and in fact might not be suitable as quality indicators. There is a danger that mandatory and public reporting of these established quality indicators for health care-associated infections will serve as an incentive for reduced blood culture sampling rates and will finally result in an underreporting of BSI rates (“no screening, no health care-associated infections, no punishment”) (15, 16). This problem has recently been recognized and has led to a debate about the potential of surveillance bias associated with mandatory reporting of health care-associated infections (17–19). Thus, the results of this study underline the potential problems and limitations associated with mandatory and public reporting of established quality indicators for health care-associated infections (20). Sufficiently high blood culture sampling rates as well as preanalytic quality control (sample withdrawal techniques and transport times) should be established as a precondition for quality assurance in the field of nosocomial bloodstream infections. We therefore propose that the interpretation of established result quality indicators (such as primary BSI rates or CLABSI rates) take into account the process quality indicator blood culture sampling rate in order to allow appropriate conclusions to be drawn.
The present study has some limitations. Blood culture rates have been recorded in an aggregated way, so linkage of blood cultures to BSI cases is not possible. Thus, we do not have information on the number of blood cultures collected for an individual patient. If there was heterogeneity in the adherence to existing blood culture sampling guidelines (7, 8), with some ICUs taking just one blood culture set instead of the recommended 2 to 4 sets for patients suspected of having BSI, this might have resulted in an underestimation of the proposed reference threshold. Moreover, we cannot exclude the possibility that a proportion of reported blood cultures was taken due to conditions other than BSI; however, this mainly affects blood cultures taken for endocarditis, which represents a small patient group compared to sepsis. Secondary BSI cases are not included in KISS, but collected blood culture rates include cultures taken for secondary BSI cases as well. This might have resulted in biased estimates for the threshold, as the proportion of secondary BSI cases might vary considerably among ICUs. We included both catheter-related and catheter-unrelated infections in this study as long as they fulfilled CDC criteria. As the two have very different risk factors, this might have resulted in estimates not applicable to either entity alone. However, in the sensitivity analyses, we confirmed that the main results of this study remain unchanged when we restrict the study to catheter-related infections (and CLABSI rates) only. As the present study was based on data collected within a German voluntary network for health care-associated infections in ICUs, the resulting reference threshold might not be generalizable to other countries and health care systems. Moreover, many ICUs in this study used remote microbiological labs, which is specific to the German health care system and might therefore make these results less generalizable to other countries. These ICUs were more likely to have blood culture sampling rates below the proposed threshold, which might be attributable to longer transport times resulting in low blood culture positivity rates and therefore a reduced a priori motivation of the respective physicians to take blood cultures (21).
Due to the limited sample size, there is remaining uncertainty regarding the form of association between observed BSI rates and blood culture rates. However, the form of association remained stable when the data set was split into the individual years of 2006 and 2007. For reasons of limited sample size, the proposed threshold could not be identified with high precision. In addition, it is likely that ICUs differ in the way in which patients are selected for blood cultures. Therefore, the percentage of BSI patients identified might vary between settings, although blood culture rates are the same. When the patient mix suggests higher BSI rates, it is likely that the target range for blood culture rates should be higher. Unfortunately, given the limited number of data, we were not able to use stratification in order to estimate institution-specific target ranges. By confirming our study results using CLABSI rates instead of BSI rates as the outcome of interest, we took differing central-line rates into account for the threshold estimation. Nevertheless, future prospective studies are necessary in order to provide reference thresholds stratified by a priori institutional risk factors for BSI, such as central-line rates or level of care.
Conclusion.
We provided an empirical justification for the recommended target blood culture rates in ICUs of around 100 blood cultures per 1,000 patient-days. Future studies are necessary to derive target blood culture rates that account for different institutional characteristics.
ACKNOWLEDGMENTS
We thank all hospitals, ICUs, doctors, and nurses participating in KISS for data collection and processing. The data used in this study were kindly provided by the German National Reference Center for the Surveillance of Healthcare-Associated Infections via KISS. KISS is funded by the German Ministry of Health.
This study was primarily funded by institutional funds of the Helmholtz Centre for Infection Research. As the present study is associated with AlertsNet, additional funding was provided through this project. AlertsNet is funded by the German Ministry of Health (grant IIA5-2512FSB114) and by the Thuringian Ministry for Education, Science and Culture.
All funders had no role in design, in the collection, analysis, and interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
REFERENCES
- 1.Engel C, Brunkhorst FM, Bone HG, Brunkhorst R, Gerlach H, Grond S, Gruendling M, Huhle G, Jaschinski U, John S, Mayer K, Oppert M, Olthoff D, Quintel M, Ragaller M, Rossaint R, Stuber F, Weiler N, Welte T, Bogatsch H, Hartog C, Loeffler M, Reinhart K. 2007. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med 33:606–618. doi: 10.1007/s00134-006-0517-7. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS. 2012. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther 10:701–706. doi: 10.1586/eri.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacArthur RD, Miller M, Albertson T, Panacek E, Johnson D, Teoh L, Barchuk W. 2004. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS trial. Clin Infect Dis 38:284–288. doi: 10.1086/379825. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer LG, Parmigiani G, Reller LB. 1997. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis 24:584–602. doi: 10.1093/clind/24.4.584. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup . 2013. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 6.Hall KK, Lyman JA. 2006. Updated review of blood culture contamination. Clin Microbiol Rev 19:788–802. doi: 10.1128/CMR.00062-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baron EJ, Weinstein MP, Dunne WM Jr, Yagupsky P, Welch DF, Wilson DM. 2005. Cumitech 1c, Blood cultures IV. ASM Press, Washington, DC. [Google Scholar]
- 8.Seifert H, Abele-Horn M, Fätkenheuer G, Glück T, Jansen B, Kern WV, Mack D, Plum G, Reinert RR, Roos R, Salzberger B, Shah PM, Ullmann U, Weiß M, Welte T, Wisplinghoff H. 2007. Blutkulturdiagnostik: Sepsis, Endokarditis, Katheterinfektionen. In Podbielski A, Herrmann M, Kniehl E, Mauch H, Russmann H (ed), Mikrobiologisch-infektiologische Qualitätsstandards (MiQ). Elsevier, München, Germany. [Google Scholar]
- 9.Geffers C, Koch J, Sohr D, Nassauer A, Daschner F, Ruden H, Gastmeier P. 2000. Establishment of a national database for ICU-associated infections; first results from the “Krankenhaus-Infections-Surveillance-System” (KISS). Anaesthesist 49:732–737. (In German.) doi: 10.1007/s001010070068. [DOI] [PubMed] [Google Scholar]
- 10.Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Gastmeier P, Schwab F, Behnke M, Geffers C. 2011. Less blood culture samples: less infections? Anaesthesist 60:902–907. (In German.) doi: 10.1007/s00101-011-1889-9. [DOI] [PubMed] [Google Scholar]
- 12.Zuschneid I, Rucker G, Schoop R, Beyersmann J, Schumacher M, Geffers C, Ruden H, Gastmeier P. 2010. Representativeness of the surveillance data in the intensive care unit component of the German nosocomial infections surveillance system. Infect Control Hosp Epidemiol 31:934–938. doi: 10.1086/655462. [DOI] [PubMed] [Google Scholar]
- 13.Hastie T, Tibshirani R. 1990. Generalized additive models. Chapman and Hall, London, United Kingdom. [Google Scholar]
- 14.Muggeo VM. 2003. Estimating regression models with unknown break-points. Stat Med 22:3055–3071. doi: 10.1002/sim.1545. [DOI] [PubMed] [Google Scholar]
- 15.Haut ER, Pronovost PJ. 2011. Surveillance bias in outcomes reporting. JAMA 305:2462–2463. doi: 10.1001/jama.2011.822. [DOI] [PubMed] [Google Scholar]
- 16.Pierce CA, Haut ER, Kardooni S, Chang DC, Efron DT, Haider A, Pronovost PJ, Cornwell EE III. 2008. Surveillance bias and deep vein thrombosis in the national trauma data bank: the more we look, the more we find. J Trauma 64:932–937. doi: 10.1097/TA.0b013e318166b808. [DOI] [PubMed] [Google Scholar]
- 17.Fung CH, Lim YW, Mattke S, Damberg C, Shekelle PG. 2008. Systematic review: the evidence that publishing patient care performance data improves quality of care. Ann Intern Med 148:111–123. doi: 10.7326/0003-4819-148-2-200801150-00006. [DOI] [PubMed] [Google Scholar]
- 18.Lin MY, Hota B, Khan YM, Woeltje KF, Borlawsky TB, Doherty JA, Stevenson KB, Weinstein RA, Trick WE, CDC Prevention Epicenter Program . 2010. Quality of traditional surveillance for public reporting of nosocomial bloodstream infection rates. JAMA 304:2035–2041. doi: 10.1001/jama.2010.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pronovost PJ, Colantuoni E. 2009. Measuring preventable harm: helping science keep pace with policy. JAMA 301:1273–1275. doi: 10.1001/jama.2009.388. [DOI] [PubMed] [Google Scholar]
- 20.Haustein T, Gastmeier P, Holmes A, Lucet JC, Shannon RP, Pittet D, Harbarth S. 2011. Use of benchmarking and public reporting for infection control in four high-income countries. Lancet Infect Dis 11:471–481. doi: 10.1016/S1473-3099(10)70315-7. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz RP, Keller PM, Baier M, Hagel S, Pletz MW, Brunkhorst FM. 2013. Quality of blood culture testing—a survey in intensive care units and microbiological laboratories across four European countries. Crit Care 17:R248. doi: 10.1186/cc13074. [DOI] [PMC free article] [PubMed] [Google Scholar]