Abstract
A 23-year-old male died of severe pneumonia and respiratory failure in a tertiary hospital in Beijing, and 4 out of 55 close contacts developed fever. Molecular analysis confirmed human adenovirus type 7 (HAdV7) as the causative agent. We highlight the importance of early diagnosis and treatment and proper transmission control of HAdV7.
CASE REPORT
Human adenovirus type 7 (HAdV7) infection is associated with acute respiratory disease syndrome, pharyngoconjunctival fever, pneumonia, and central nervous system disease (1–4). According to the last global survey, approximately one-fifth of all HAdV infections reported to the World Health Organization (WHO) were attributed to HAdV7 (5). HAdV7 outbreaks generally occur in settings with close living conditions, such as military barracks, hospital wards, and chronic care facilities (1, 6–8). In hospitals, HAdV has emerged as a nosocomial pathogen, and nosocomial outbreaks caused by HAdV7 with fatal cases have been reported worldwide (8–10). Although some prevention measurements have been carried out to control nosocomial transmission, questions regarding the efficacy of these programs persist, and the contribution of HAdV7 (and adenoviruses in general) to nosocomial infection is likely to be underestimated. Here, we report a case of HAdV7 infection associated with severe pneumonia and fatal acute lower respiratory disease and nosocomial transmission.
The index case, a 23-year-old male in Wuhan City, Hubei Province, China, presented with a fever of approximately 39°C on 18 January 2014, but discontinuous treatment did not alleviate his symptoms. On 26 January, he was admitted to the emergency department of a tertiary hospital in Beijing. A routine blood test showed high neutrophil levels (71.4%) and elevated plasma C reactive protein (19 mg/ml), and chest radiographs revealed an increase of right lung markings. However, the use of cefoxitin sodium by injection did not prevent his condition from worsening, with symptoms including severe fever, cough, and phlegm. He was diagnosed with pneumonia and transferred to the respiratory department on 28 January. Six days later, he was transferred to an intensive care unit (ICU) because of lung consolidation, and he passed away on 5 February 2014.
During his hospitalization, 4 of 55 close contacts developed fevers higher than 38.0°C, including a family member, a bedside clinician, and two patients in the same ward (Table 1 and Fig. 1). The initial secondary case (patient 1) was his cousin, a 19-year-old girl who visited the index case on 26 January for 30 min in the emergency department and on 2 February for 5 min in the respiration department. The distance between them was more than 2 meters, but neither wore a mask. She experienced a fever of 38.5°C on 3 February. Patient 2, a 29-year-old male with acute pancreatitis and a fatty liver, was admitted to the same ward of the emergency department on 27 January. He developed a fever on 3 February, and a chest X-ray showed pulmonary shadows. Patient 3, a bedside clinician of the index case during treatment from 28 January to 3 February, developed a fever and sore throat on 6 February. During this period, both the clinician and the index case did not always wear masks during treatment and the clinician did not have close contact with other patients with fever. Patient 4, a 12-year-old boy with a respiratory infection, spent 3 h in the same room with the index case on 27 January and went home the same day. On 7 February, he developed a fever, 11 days after first contact with the index case.
TABLE 1.
Patient characteristics and laboratory results
| Patient | Age (yr) | Underlying condition | Clinical symptoms | Laboratory results | X-ray results | Outcome |
|---|---|---|---|---|---|---|
| Index case | 23 | None | Fever, cough, sore throat | Neutrophilia, 71.94%; C-reactive protein, 19 mg/ml | Increase of right lung markings; later, lung consolidation developed | Death |
| 1 | 19 | None | Fever, sore throat | Mononucleosis, 11.14%; lymphocytosis, 54.24% | Normal | Recovered after 6 days of hospitalization and was discharged 7 days later |
| 2 | 29 | Acute pancreatitis, fatty liver | Fever | Normal leukocyte count; mononucleosis, 11.74% | Pulmonary shadows | Recovered after 9 days of hospitalization and was discharged 7 days later |
| 3 | 29 | None | Fever, sore throat | Normal leukocyte count; mononucleosis, 10.74% | Normal | Recovered after 6 days of hospitalization and was discharged 7 days later |
| 4 | 12 | Respiratory infections | Fever | Mononucleosis, 10.85%; lymphocytosis, 52.68% | Normal | Recovered after 7 days of hospitalization and was discharged 7 days later |
FIG 1.
Timeline of critical events for the index patient and nosocomial infection. (Top) Key events during the index patient's illness are shown. (Bottom) Each bar indicates the period for an infected patient from first contact with the index case to symptom onset.
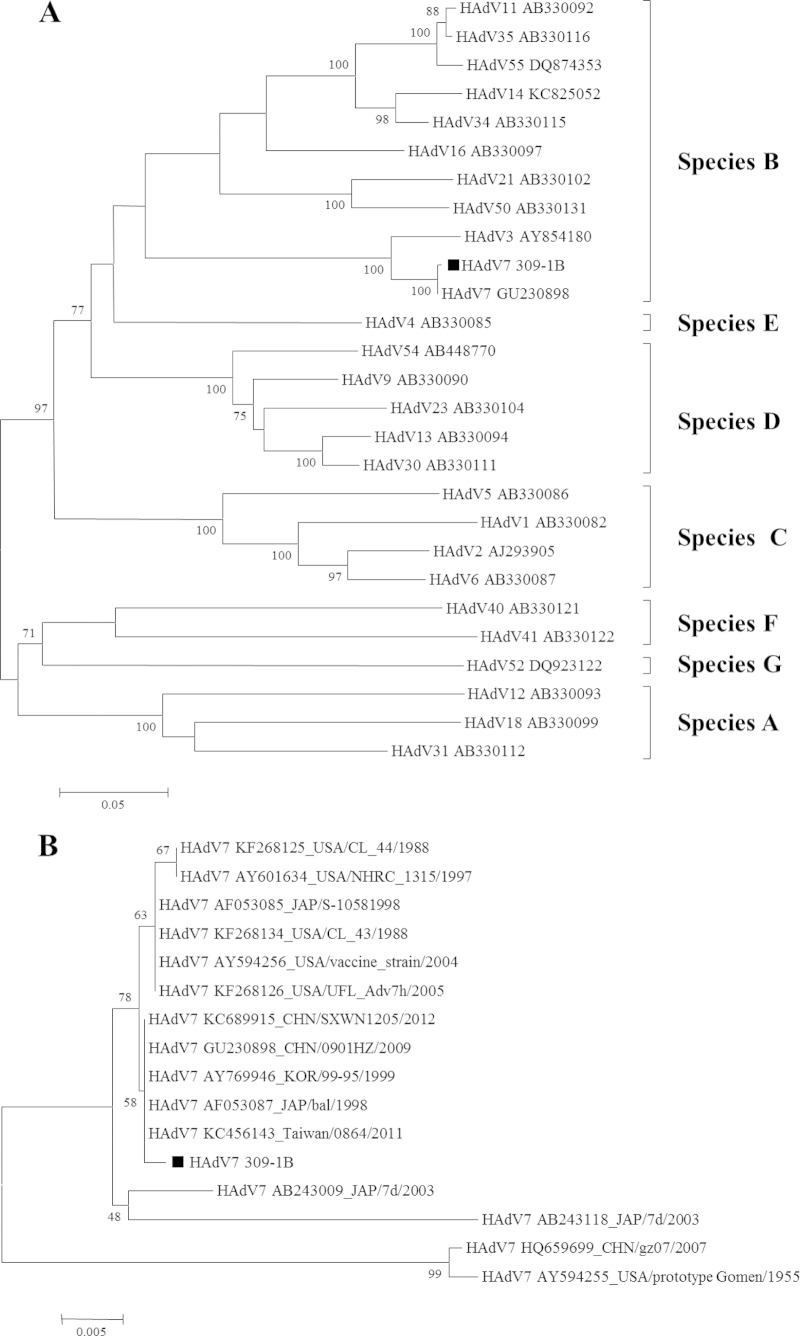
Nasopharyngeal specimens were collected from the index case on 3 February and from four close contacts on 7 and 8 February. DNA and RNA were extracted from the swab specimens using the QIAamp MinElute virus spin kit (Qiagen). Commercially available multiplex PCR assays (Seeplex RV15 ACE and PneumoBacter ACE; Seegene, South Korea) were used to test for respiratory pathogens; the results showed that the index case and the four close contacts were not infected by bacteria but were positive for HAdV. Then, a portion of the hexon gene was amplified and sequenced as described by Madisch et al. (11). Nucleotide BLAST analysis of the sequences revealed that they were all HAdV7. Comparison of the nucleic acid sequences of samples from these patients indicated that the isolates were 100% identical, strongly suggesting that all isolates were derived from a common ancestor (HAdV_7_309-1B from the index case). To assess the genetic relationships of different virus serotypes and determine their origin, phylogenetic analysis of the partial sequences was performed (Fig. 2A and B). Strain HAdV_7_309-1B displayed one substitution at position 1228 of the hexon gene, which was a silent mutation. The strains identified in this report are closely related to the predominant HAdV7 isolates reported in China in recent years (12).
FIG 2.
Phylogenetic analysis of a portion of the hexon gene for strain HAdV7 309-1B described in this study and other reference strains of adenovirus. The phylogenetic tree was generated using the neighbor-joining method. Bootstrap values are shown at branching points. Scale bars indicate nucleotide substitutions per site. (A) Strains HAdV7 309-1B compared to other subgenotype of HAdV reference strains; (B) HAdV7 309-1B compared to other HAdV7 reference strains.
As shown by the case described above, early identification and treatment of adenovirus infection is vital. Initially, the index case only presented with mild acute respiratory symptoms. However, without prompt treatment, the viral load may increase by replication in human lungs, leading to severe pneumonia (13, 14). There are no approved antiviral agents with proven efficacy for the treatment of severe pneumonia caused by HAdV (15). Thus, the patient's condition deteriorated quickly even after later antiviral and hormone therapy. In contrast, the other four patients recovered well after timely antiviral treatment with ribavirin. The combined use of Chinese patent medicines (Lianhua Qingwen capsule and Radix Isatidis) that have antiviral curative effects may also have played a role.
Nosocomial control of HAdV7 transmission is another challenge faced by health care centers. HAdV7 transmission generally occurs through respiratory droplets and close contact, which can lead to rapid and wide-spread infection. Large community and regional outbreaks of acute respiratory disease with an increased incidence of severe and fatal outcomes caused by HAdV7 have been described in Europe and Asia (7, 12, 16, 17). Thus, control of HAdV7 spread is crucial. However, it is difficult to differentiate pneumonia caused by HAdVs from other types of viral pneumonia, and culturing and typing of HAdVs are not routinely performed in hospitals (18). Therefore, the inevitable delay of several days in the recognition of HAdV infections probably promoted the transmission. One study of adenovirus infection reported the incubation period to be 5 to 6 days (19), and another study of secondary infections in families found the mean incubation period to be 10 days (20). In this study, therefore, it was concluded that patient 4, who developed fever 11 days after first contact with the index case, was also infected by him. When the pathogen was identified, an infection control response was initiated immediately, on 8 February. Efforts to prevent the spread of HAdV7 included the use of disposable gowns and gloves, strict hand hygiene, and disinfection of common areas, such as floors and toilets. Furthermore, the four patients with HAdV7 infection were transferred to single rooms. The symptomatic patients had recovered well after 5 to 8 days of hospitalization and were discharged after 7 days of isolation.
In summary, we suggest that more importance should be given to the early diagnosis and treatment and proper transmission control of HAdV7, which causes acute respiratory illness. Therefore, hospitals should improve their diagnostic capabilities for rapid detection of HAdV infection, as well as their infrastructure to institute proper case management and transmission control.
Nucleotide sequence accession numbers.
The hexon gene sequences of the isolates were deposited in the GenBank database (accession numbers KJ783432 to KJ783436).
ACKNOWLEDGMENTS
We thank all the participants at the hospital for help in recruiting patients and all the patients for agreeing to be included in this study.
This work was supported by Mega-projects of Science and Technology Research grants (grant no. 2012ZX10004215, 2013ZX10004607, 2013ZX10004218, and 2012ZX10004801) and by the National Nature Science Foundation of China (grant no. 81171554 and 81202252).
The authors declare that they have no competing interests.
REFERENCES
- 1.Gerber SI, Erdman DD, Pur SL, Diaz PS, Segreti J, Kajon AE, Belkengren RP, Jones RC. 2001. Outbreak of adenovirus genome type 7d2 infection in a pediatric chronic-care facility and tertiary-care hospital. Clin Infect Dis 32:694–700. doi: 10.1086/319210. [DOI] [PubMed] [Google Scholar]
- 2.Munoz FM, Piedra PA, Demmler GJ. 1998. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin Infect Dis 27:1194–1200. doi: 10.1086/514978. [DOI] [PubMed] [Google Scholar]
- 3.Murtagh P, Cerqueiro C, Halac A, Avila M, Kajon A. 1993. Adenovirus type 7h respiratory infections: a report of 29 cases of acute lower respiratory disease. Acta Paediatr 82:557–561. doi: 10.1111/j.1651-2227.1993.tb12753.x. [DOI] [PubMed] [Google Scholar]
- 4.Sutton RN, Pullen HJ, Blackledge P, Brown EH, Sinclair L, Swift PN. 1976. Adenovirus type 7; 1971-74. Lancet ii:987–991. doi: 10.1016/S0140-6736(76)90832-1. [DOI] [PubMed] [Google Scholar]
- 5.Schmitz H, Wigand R, Heinrich W. 1983. Worldwide epidemiology of human adenovirus infections. Am J Epidemiol 117:455–466. [DOI] [PubMed] [Google Scholar]
- 6.Alpert G, Charney E, Fee M, Plotkin SA. 1986. Outbreak of fatal adenoviral type 7a respiratory disease in a children's long-term care inpatient facility. Am J Infect Control 14:188–190. doi: 10.1016/0196-6553(86)90100-8. [DOI] [PubMed] [Google Scholar]
- 7.Dudding BA, Wagner SC, Zeller JA, Gmelich JT, French GR, Top FH Jr. 1972. Fatal pneumonia associated with adenovirus type 7 in three military trainees. N Engl J Med 286:1289–1292. doi: 10.1056/NEJM197206152862403. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell LS, Taylor B, Reimels W, Barrett FF, Devincenzo JP. 2000. Adenovirus 7a: a community-acquired outbreak in a children's hospital. Pediatr Infect Dis J 19:996–1000. doi: 10.1097/00006454-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Sakata H, Taketazu G, Nagaya K, Shirai M, Sugai R, Ikegami K, Maruyama S. 1998. Outbreak of severe infection due to adenovirus type 7 in a paediatric ward in Japan. J Hosp Infect 39:207–211. doi: 10.1016/S0195-6701(98)90259-6. [DOI] [PubMed] [Google Scholar]
- 10.Straube RC, Thompson MA, Van Dyke RB, Wadell G, Connor JD, Wingard D, Spector SA. 1983. Adenovirus type 7b in a children's hospital. J Infect Dis 147:814–819. doi: 10.1093/infdis/147.5.814. [DOI] [PubMed] [Google Scholar]
- 11.Madisch I, Harste G, Pommer H, Heim A. 2005. Phylogenetic analysis of the main neutralization and hemagglutination determinants of all human adenovirus prototypes as a basis for molecular classification and taxonomy. J Virol 79:15265–15276. doi: 10.1128/JVI.79.24.15265-15276.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang L, An J, Yu P, Xu W. 2013. Complete genome sequence of human adenovirus type 7 associated with fatal infant pneumonia. Genome Announc 1:e00182–12. doi: 10.1128/genomeA.00182-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginsberg HS, Moldawer LL, Sehgal PB, Redington M, Kilian PL, Chanock RM, Prince GA. 1991. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A 88:1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pacini DL, Dubovi EJ, Clyde WA Jr. 1984. A new animal model for human respiratory tract disease due to adenovirus. J Infect Dis 150:92–97. doi: 10.1093/infdis/150.1.92. [DOI] [PubMed] [Google Scholar]
- 15.Gavin PJ, Katz BZ. 2002. Intravenous ribavirin treatment for severe adenovirus disease in immunocompromised children. Pediatrics 110:e9. doi: 10.1542/peds.110.1.e9. [DOI] [PubMed] [Google Scholar]
- 16.Carballal G, Videla C, Misirlian A, Requeijo PV, Aguilar Mdel C. 2002. Adenovirus type 7 associated with severe and fatal acute lower respiratory infections in Argentine children. BMC Pediatr 2:6. doi: 10.1186/1471-2431-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto D, Okamoto M, Lupisan S, Suzuki A, Saito M, Tamaki R, Tandoc A III, Mercado E, Sombrero L, Olveda R, Oshitani H. 2014. Impact of human adenovirus serotype 7 in hospitalized children with severe fatal pneumonia in the Philippines. Jpn J Infect Dis 67:105–110. doi: 10.7883/yoken.67.105. [DOI] [PubMed] [Google Scholar]
- 18.Tsou TP, Tan BF, Chang HY, Chen WC, Huang YP, Lai CY, Chao YN, Wei SH, Hung MN, Hsu LC, Lu CY, Shao PL, Mu JJ, Chang LY, Liu MT, Huang LM, Unknown Pathogen Discovery/Investigation Group . 2012. Community outbreak of adenovirus, Taiwan, 2011. Emerg Infect Dis 18:1825–1832. doi: 10.3201/eid1811.120629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anonymous. 1947. Experimental transmission of minor respiratory illness to human volunteers by filter-passing agents; demonstration of two types of illness characterized by long and short incubation periods and different clinical features. J Clin Invest 26:957–973. doi: 10.1172/JCI101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruuskanen O, Mertsola J, Meurman O. 1988. Adenovirus infection in families. Arch Dis Child 63:1250–1253. doi: 10.1136/adc.63.10.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]