Abstract
Toxins A and B are the main virulence factors of Clostridium difficile and are the targets for molecular diagnostic tests. Here, we describe a new toxin A-negative, toxin B-positive, binary toxin CDT (Clostridium difficile transferase)-negative (A− B+ CDT−) toxinotype (XXXII) characterized by a variant type of pathogenicity locus (PaLoc) without tcdA and with atypical organization of the PaLoc integration site.
TEXT
Clostridium difficile is the main cause of community and nosocomial diarrhea associated with antibiotic treatment and has major health care and economic impacts (1–3).
Three toxins, toxin A (TcdA, enterotoxin), toxin B (TcdB, cytotoxin), and binary toxin CDT (Clostridium difficile transferase), are produced by C. difficile. CDT is present in only a subset of strains, and its role in pathogenesis is increasingly recognized but still unclear (4). Toxins A and B are the main virulence factors causing damage to the intestinal epithelium and producing diarrhea and inflammation. They are also a main target for enzyme-based or molecular diagnostic tests (5, 6). Genes encoding TcdA and TcdB are located on the chromosome and together with three additional genes (tcdR, tcdE, and tcdC) form a 19.6-kb pathogenicity locus (PaLoc). The genes for CDT toxin are located elsewhere on the chromosome (CdtLoc).
C. difficile strains can be differentiated based on different patterns of toxin production. Strains which do not produce any of the toxins are nontoxinogenic and do not cause disease. The majority of toxigenic strains are TcdA and TcdB positive (A+ B+), but some strains produce only TcdB (A− B+). Toxigenic strains can be further differentiated into toxinotypes based on changes (deletions, insertions, single nucleotide polymorphisms [SNPs]) in the PaLoc. By 2008, 24 different toxinotypes had been published (7), and currently, 31 toxinotypes, designated by roman numbers from I to XXXI, are differentiated (see http://www.mf.uni-mb.si/tox/). Here, we describe a case history and the isolation and characterization of a new A− B+ variant of C. difficile.
Case Report.
A 68-year-old Spanish male followed as an outpatient at the Gregorio Marańon University Hospital of Madrid because of ischemic cardiomyopathy, angina, and chronic pancreatitis was admitted to the emergency department in October 2011 with a 21-day history of diarrhea. The patient did not have fever, and his general condition was good, but he reported a recent weight loss of 5 kg. Stool microbiological studies were requested at the emergency department for enteropathogens, antigens of rotavirus and adenovirus, intestinal parasites, and C. difficile culture and toxin detection. A direct cytotoxicity assay was performed by centrifuging stool specimen dilutions (1/40) made with phosphate-buffered saline and filtering 500 μl of supernatant onto monolayers of human MRC-5 fibroblasts. A test result was considered negative only after 48 h of incubation at 37°C. The specificity of the cytopathic effect was confirmed using a neutralizing high-titer C. difficile antitoxin (TechLab) following the manufacturer's instructions.
The rapid detection of glutamate dehydrogenase (GDH) and toxins A and B (C. diff Quik Chek Complete; Techlab, Blacksburg, VA) was also performed on stool specimens showing positive results for GDH but negative results for toxins A and B. Following international recommendations (5, 6), the GeneXpert C. difficile assay (Cepheid, Sunnyvale, CA, USA) was then performed, and the results for toxigenic C. difficile were negative.
As his condition was considered nonurgent, the patient was discharged with a diagnosis of subacute diarrhea, and it was arranged for him to be treated by the digestive medicine department as an outpatient.
On the next day, the direct stool cell culture cytotoxicity neutralization assay was positive, and after 48 h of incubation, C. difficile was isolated from the patient's feces in CLO agar (bioMérieux, Marcy l'Etoile, France). The isolate then tested positive for GDH and toxins A and B; however, the GeneXpert C. difficile assay was negative once again. A report was then issued with the statements “isolation of toxigenic C. difficile” and “direct cytotoxicity positive.” Parasitological examinations and rotavirus and adenovirus antigen detection tests were negative.
The patient was treated with 500 mg of metronidazole every 8 h for 12 days, but his diarrhea persisted, although it was less intense, probably related to chronic pancreatitis. Abdominal ultrasound and a colonoscopy performed a few days later showed only diverticulosis. In the following microbiological examinations, no C. difficile or other pathogens were isolated from the patient's stool.
The patient had only occasional contact with health care settings, and his medical history did not reflect antibiotic consumption. Although toxigenic C. difficile was the only detected pathogen, the diarrhea had not resolved after treatment, and the clinical significance of C. difficile in this particular case is unknown.
Strain characterization.
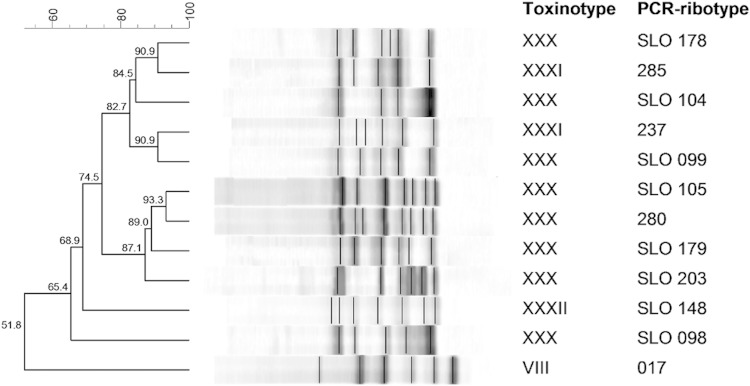
Because of the discrepancies in microbiological results, the C. difficile isolate was further characterized. Several approaches were used to amplify parts of the toxin genes. By means of a multiplex PCR used to detect tcdA, tcdB, cdtA, and cdtB (8, 9), positive results were obtained for the tcdB gene only. PCR amplification of tcdC was negative (10). These results were further confirmed by PCRs covering the entire PaLoc as part of a toxinotyping scheme (11). All three PCRs covering the tcdA gene were negative, and only the B1 and B2 fragments covering the tcdB gene were positive. Whole-genome sequencing (genomic sequencing and analysis were performed as described previously [12]) has finally clarified the structure of the PaLoc region in this strain, and it represents a new toxinotype (XXXII) (Fig. 1A). In this toxinotype, the genes tcdR and tcdE are conserved. A complete tcdB gene is also present, but several SNPs were found in this region aligning to primers used for toxinotyping, explaining the negative PCR result for the B3 fragment and possibly also the negative GeneXpert toxin B-based molecular test. However, toxin B is produced and was detected with an enzyme immunoassay (EIA) toxin test and a cell culture cytotoxicity assay. The other two genes within the PaLoc, tcdA and tcdC, are absent and were not detected even in truncated form in the PaLoc or anywhere else in the genome. Hence, toxinotype XXXII is one of the A− B+ toxinotypes. Up until recently, all known A− B+ toxinotypes had at least a part of the tcdA gene present and the entire or deleted version of tcdC (7). An Australian group described the first A− B+ variants (toxinotypes XXX and XXXI) lacking the complete tcdA gene and two accessory genes (tcdE and tcdC); however, each of these were binary toxin positive (A− B+ CDT+) (13, 14). In addition to lacking tcdA and tcdC, toxinotype XXXII also has no binary toxin CDT genes (A− B+ CDT−). The strain was PCR ribotyped using agarose-based and capillary-based approaches. The PCR ribotype profile was not previously recognized in the Leeds collection (typed in October 2013) or in the WEBRIBO collection and was designated by the internal designation SLO 148 (Fig. 2). In silico multilocus sequence typing (MLST) demonstrated that the strain belongs to sequence type 200.
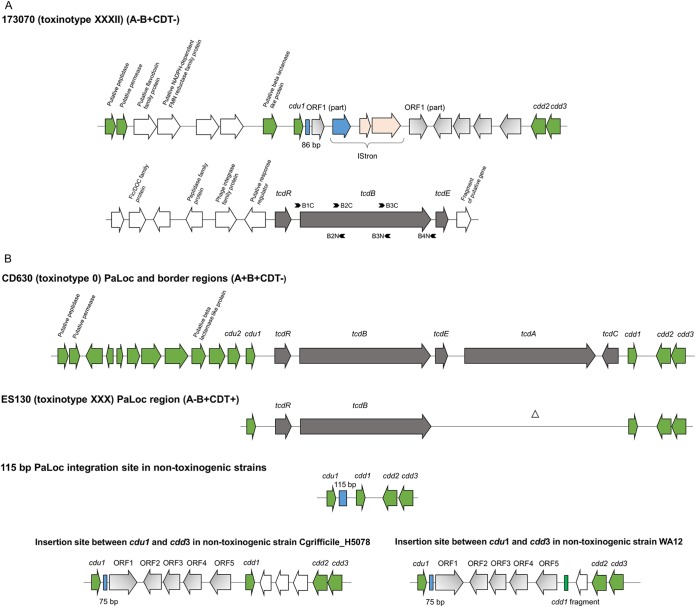
FIG 1.
Schematic representation of organization of PaLoc and flanking regions. (A) PaLoc and flanking genes in toxinotype XXXII (strain 173070). Primer pairs B1C-B2N, B2C-B3N, and B3C-B4N were used to amplify the B1, B2, and B3 fragments of the tcdB gene, respectively. The putative functions of the predicted genes were identified by a BLAST search in NCBI. (B) From top to bottom, PaLoc and flanking region of toxinotype 0 (strain CD630); reference strain of toxinotype XXX (strain ES130) (12), first variant toxinotype with PaLoc characterized by absence of the tcdA gene; PaLoc insertion site in nontoxigenic strains; the 7.2-kb region (shaded in gray) inserted in the PaLoc insertion site in nontoxinogenic isolates. Bottom left, Cgrifficile_H5078 (GenBank accession number HG002397.1) (12); bottom right, WA12 (GenBank accession number HG002390.1) (15).
FIG 2.
PCR ribotyping (agarose-based) profiles of representative A− B+ strains. PCR ribotypes from our library belonging to new toxinotype XXXII, similar toxinotypes XXX and XXXI, and the most prevalent toxinotype, toxinotype VIII, are shown.
Analysis of regions upstream and downstream of the PaLoc indicated that the PaLoc in toxinotype XXXII may be inserted at a genomic location that is different from that in other strains studied to date (Fig. 1A). Furthermore, five genes that had already been identified inserted between cdu1 and cdd1 in strains WA12 (15) and Cgrifficile_H5078 (12) have been found inserted upstream of the cdd2 gene (Fig. 1B). In toxinotype XXXII, ORF1 is fragmented and was found on two different contigs. The subsequent linking of the contigs by PCR and sequencing demonstrated insertion of the mobile element (IStron) within the ORF1 (Fig. 1A). In addition, a shorter (86-bp) section of the 115-bp stretch that is normally present in nontoxinogenic strains replacing the PaLoc region was found adjacent to cdu1. To date, these two regions have been described in nontoxinogenic strains only (12, 15, 16).
Here, we report a new type of A− B+ C. difficile strain (toxinotype XXXII) with a variant form of PaLoc and an atypical organization of the PaLoc integration site. The strain is characterized by the absence of the tcdA and tcdC genes, while tcdR, tcdB, and tcdE are present and TcdB is produced and detected by diagnostic toxin-specific tests. Conserved toxin genes are located within a yet-undescribed region of the C. difficile genome. The known boundaries of PaLoc are conserved (cdu1/cdd2), and genes between the boundaries were already described in at least two other (nontoxinogenic) C. difficile strains. The strain was isolated from a diarrheic patient, and despite the unknown clinical significance, it is important to keep in mind that new variants of C. difficile strains might be present in patients, and because of the changes in PaLoc, they might not be detected by molecular C. difficile tests.
ACKNOWLEDGMENTS
We thank Kate Dingle and Derrick Crook for the genomic sequencing.
S.J. and M.R. are supported by Slovenian Research Agency grant P3-0387. S.J. was supported by a FEMS research grant.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2012. Vital signs: preventing Clostridium difficile infections. MMWR Morb Mortal Wkly Rep 61:157–162. [PubMed] [Google Scholar]
- 2.Eyre DW, Cule ML, Wilson DJ, Griffiths D, Vaughan A, O'Connor L, Ip CL, Golubchik T, Batty EM, Finney JM, Wyllie DH, Didelot X, Piazza P, Bowden R, Dingle KE, Harding RM, Crook DW, Wilcox MH, Peto TEA, Walker AS. 2013. Diverse sources of C. difficile infection identified on whole-genome sequencing. N Engl J Med 369:1195–1205. doi: 10.1056/NEJMoa1216064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team . 2014. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerding DN, Johnson S, Rupnik M, Aktories K. 2014. Clostridium difficile binary toxin CDT: mechanism, epidemiology, and potential clinical importance. Gut Microbes 5:15–27. doi: 10.4161/gmic.26854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll KC. 2011. Tests for the diagnosis of Clostridium difficile infection: the next generation. Anaerobe 17:170–174. doi: 10.1016/j.anaerobe.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Crobach MJ, Dekkers OM, Wilcox MH, Kuijper EJ. 2009. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): data review and recommendations for diagnosing Clostridium difficile-infection (CDI). Clin Microbiol Infect 15:1053–1066. doi: 10.1111/j.1469-0691.2009.03098.x. [DOI] [PubMed] [Google Scholar]
- 7.Rupnik M. 2008. Heterogeneity of large clostridial toxins: importance of Clostridium difficile toxinotypes. FEMS Microbiol Rev 32:541–555. doi: 10.1111/j.1574-6976.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- 8.Lemee L, Dhalluin A, Testelin S, Mattrat M-A, Maillard K, Lemeland J-F, Pons J-L. 2004. Multiplex PCR targeting tpi (triose phosphate isomerase), tcdA (toxin A), and tcdB (toxin B) genes for toxigenic culture of Clostridium difficile. J Clin Microbiol 42:5710–5714. doi: 10.1128/JCM.42.12.5710-5714.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Persson S, Torpdahl M, Olsen KEP. 2008. New multiplex PCR method for the detection of Clostridium difficile toxin A (tcdA) and toxin B (tcdB) and the binary toxin (cdtA/cdtB) genes applied to a Danish strain collection. Clin Microbiol Infect 14:1057–1064. doi: 10.1111/j.1469-0691.2008.02092.x. [DOI] [PubMed] [Google Scholar]
- 10.Spigaglia P, Mastrantonio P. 2002. Molecular analysis of the pathogenicity locus and polymorphism in the putative negative regulator of toxin production (TcdC) among Clostridium difficile clinical isolates. J Clin Microbiol 40:3470–3475. doi: 10.1128/JCM.40.9.3470-3475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rupnik M. 2010. Clostridium difficile toxinotyping. Methods Mol Biol 646:67–76. doi: 10.1007/978-1-60327-365-7_5. [DOI] [PubMed] [Google Scholar]
- 12.Dingle KE, Elliott B, Robinson E, Griffiths D, Eyre DW, Stoesser N, Vaughan A, Golubchik T, Fawley WN, Wilcox MH, Peto TE, Walker AS, Riley TV, Crook DW, Didelot X. 2014. Evolutionary history of the Clostridium difficile pathogenicity locus. Genome Biol Evol 6:36–52. doi: 10.1093/gbe/evt204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott B, Squire MM, Thean S, Chang BJ, Brazier JS, Rupnik M, Riley TV. 2011. New types of toxin A-negative, toxin B-positive strains among clinical isolates of Clostridium difficile in Australia. J Med Microbiol 60:1108–1111. doi: 10.1099/jmm.0.031062-0. [DOI] [PubMed] [Google Scholar]
- 14.Squire MM, Carter GP, Mackin KE, Chakravorty A, Noren T, Elliott B, Lyras D, Riley TV. 2013. Novel molecular type of Clostridium difficile in neonatal pigs, Western Australia. Emerg Infect Dis 19:790–792. doi: 10.3201/eid1905.121062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott B, Reed R, Chang BJ, Riley TV. 2009. Bacteremia with a large clostridial toxin-negative, binary toxin-positive strain of Clostridium difficile. Anaerobe 15:249–251. doi: 10.1016/j.anaerobe.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Braun V, Hundsberger T, Leukel P, Sauerborn M, von Eichel-Streiber C. 1996. Definition of the single integration site of the pathogenicity locus in Clostridium difficile. Gene 181:29–38. doi: 10.1016/S0378-1119(96)00398-8. [DOI] [PubMed] [Google Scholar]