Abstract
Urinary tract infections (UTIs) are the second most common bacterial infection. Urine culture is the gold standard for diagnosis, but new techniques, such as flow cytometry analysis (FCA), have been introduced. The aim of the present study was to evaluate FCA characteristics regarding bacteriuria, leukocyturia, and erythrocyturia in relation to cultured uropathogens in specimens from patients with a suspected UTI. We also wanted to evaluate whether the FCA characteristics can identify uropathogens prior to culture. From a prospective study, 1,587 consecutive urine specimens underwent FCA prior to culture during January and February 2012. Outpatients and inpatients (79.6% and 19.4%, respectively) were included, of whom women represented 67.5%. In total, 620 specimens yielded growth, of which Escherichia coli represented 65%, Enterococcus spp. 8%, Klebsiella spp. 7%, and Staphylococcus spp. 5%. For the uropathogens, the outcome of FCA was compared against the results for specimens with E. coli and those with a negative culture. E. coli had high bacterial (median, 17,914/μl), leukocyte (median, 348/μl), and erythrocyte (median, 23/μl) counts. With the exception of Klebsiella spp., the majority of the uropathogens had considerable or significantly lower bacterial counts than that of E. coli. High leukocyte counts were found in specimens with Staphylococcus aureus, Proteus mirabilis, Pseudomonas aeruginosa, and group C streptococci. Elevated erythrocyte counts were found for P. vulgaris, P. aeruginosa, and group C streptococci, as well as for Staphylococcus saprophyticus. In essence, FCA adds new information about the bacterial, leukocyte, and erythrocyte counts in urine specimens for different uropathogens. Based on FCA characteristics, uropathogens can be classified and identified prior to culture. E. coli and Klebsiella spp. have similar FCA characteristics.
INTRODUCTION
Urinary tract infections (UTIs) are caused by pathogenic microorganisms in the urinary tract, causing an inflammatory response, as well as the presence of red and white blood cells in the urine. The inflammatory response and clinical manifestations depend on the etiologic organism, severity of infection, and immune status of the patient (1).
UTIs are the second most common bacterial infection and are associated with high morbidity and costs. The annual incidence is estimated to be >175 million UTI episodes worldwide. In the United States, UTIs annually account for >7 million physician visits, >1 million emergency department visits, and >100,000 hospitalizations (2, 3).
In outpatients, UTIs are the most frequent kind of bacterial infection, and antibiotics given for their treatment represent approximately 15% of all antibiotics prescribed to humans in the United States, with an estimated annual cost of >$1 billion (3). In addition, the indirect annual cost is estimated to be approximately $1.6 billion (4).
The bacteria in urine can be classified as primary, secondary, tertiary, or doubtful uropathogens, according to their pathogenic capacity, frequency of appearance, and association with contamination of urine specimens (5). E. coli is the most prevalent uropathogen and is responsible for approximately 80% of lower UTIs, while Staphylococcus saprophyticus, Klebsiella spp., Proteus spp., and other uropathogens are less prevalent (6–11), according to European Centre for Disease Prevention and Control (ECDC) surveillance data from 2012 (12). E. coli more commonly causes community-acquired UTIs than nosocomial UTIs (13, 14).
Urine culture is the gold standard for the diagnosis of UTI, but culture is laborious and moderate costly, with a turnaround time of 24 to 48 h. Recently, fully automated and cost-effective diagnostic instruments, such as flow cytometry analysis (FCA), have been introduced to improve the efficiency of handling urine specimens. FCA makes it possible to rule out urine specimens that contain significant numbers of bacteria from those that do not.
The first-generation automated FCA instrument, Sysmex UF50 (Medical Electronics, Kobe, Japan) showed variable results, but the next-generation Sysmex UF-500i and UF-1000i instruments have improved sensitivity and specificity, as well as a separate measurement channel for detecting bacteria (15–19). In urine specimens, FCA identifies and enumerates bacteria, leukocytes, erythrocytes, and other cells. Culture-negative specimens are identified and ruled out before culture is performed, which reduces the turnaround time, workload, and cost (15–17). However, the instrument has not been evaluated for its ability to predict uropathogens prior to urine culture.
The aim of the present study was to examine if the FCA characteristics, as bacterial, leukocyte, and erythrocyte counts can be associated with different uropathogens in urine specimens among patients with a suspected UTI. Moreover, we aimed to evaluate whether the causative uropathogen could be predicted in urine specimens prior to urine culture based on its FCA characteristics.
MATERIALS AND METHODS
Collection of urine specimens.
During January and February 2012, 1,587 urine specimens from inpatients and outpatients were collected in nonpreservative tubes, stored, and transported at <6°C to the Department of Clinical Microbiology Laboratory at the University Hospital of Umeå, Sweden, for analysis. The specimens were cultured within 3 h after arrival at the laboratory, and all specimens were from the county of Västerbotten, Sweden.
Urinalysis.
Prior to culture, the urine specimens were analyzed with flow cytometry (Sysmex UF-1000i; TOA Medical Electronics, Kobe, Japan). The specimens that arrived after 4 p.m. were analyzed and stored at 6°C until culture was performed the next morning. The instrument was supported by Sysmex UF-1000i software version 00-15.
In summary, the flow cytometry instrument aspirates urine, which is split into two volumes in the instrument prior to fluorescent dye staining. In the first volume, the sediment stain, polymethine, stains nucleic acid in the cells, and in the second volume, only nucleic acids in bacteria are stained. After staining, the specimen is delivered to the flow cell for particle analysis by the use of a red semiconductor laser. The particles are characterized according to impedance, scattering, and fluorescence light from forward- and side-scatter lights. The forward scatter provides information on particle size, and the side scatter provides information on the internal complexity and the surface. In addition, fluorescence intensity provides information on the nucleic acid content of each particle.
The particle concentrations of the counted bacteria, leukocytes, and erythrocytes in urine specimens were denoted U bacteria, U leukocytes, and U erythrocytes, respectively.
Culture and bacterial identification.
A quantitative urine culture was performed by inoculation with a 10-μl calibrated plastic loop (Sarstedt, Nümbrecht, Germany) on cystine-lactose electrolyte-deficient (CLED) agar (Acumedia Manufacturers, Inc., Baltimore, MD, USA). The agar plates were incubated in air at 35°C for 18 to 20 h before counting the CFU (CFU/liter).
Species identification was performed as previously described (6). Briefly, Gram-negative and Gram-positive uropathogens were identified by Brilliance UTI agar (Oxoid Ltd., Basingstoke, United Kingdom). Gram-negative bacteria were identified further with biochemical tests, the ornitine, inositol, and triple sugar iron test, as described previously (20). Acinetobacter spp. were also identified by species-specific growth on CHROMagar Acinetobacter (CHROMagar, Paris, France).
Gram-positive bacteria were identified by a catalase reaction. Staphylococci were identified with species-specific growth on CHROMagar. In addition, Staphylococcus aureus was identified by its DNase activity. S. saprophyticus was identified by novobiocin susceptibility (coagulase-negative staphylococci other than S. saprophyticus were denoted CoNS). Enterococci and streptococci were identified by esculin, agglutination to type-specific serum (Streptex; Murex Biotech Ltd., Dartford, England), and species-specific growth on Brilliance UTI agar. In addition, the detection of Streptococcus agalactiae (group B streptococcus [GBS]) was supported by species-specific growth on CHROMagar (StrepB; Bio-Rad, Marnes-la-Coquette, France).
Inclusion criteria.
Urine specimens yielding growth of ≥108 CFU/liter of an identified pathogen were included, irrespective of the presence of UTI symptoms in the patient from whom they were isolated. Urine specimens yielding growth of 106 to 107 CFU/liter of an identified pathogen were included if UTI symptoms were reported in the patient from whom they were isolated and/or laboratory tests indicated the presence of a UTI (nitrite test positive and/or moderate to high leukocyturia). Among these, specimens with one pathogen were included, as well as those with mixed flora containing a single dominating pathogen (bacterial count ≥10 times higher than any other species that was identified)(6). The specimens with <106 CFU/liter were classified as negative cultures. The specimens from patients with a urinary catheter, pregnant women, as well as specimens that lacked clinical information of UTI and that had mixed flora without a dominating uropathogen were excluded.
Statistical analysis.
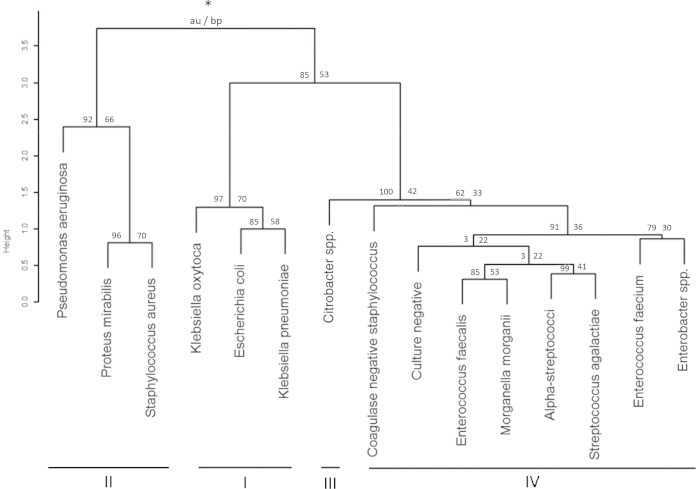
All presented values are median values, unless otherwise stated. The differences between proportions were analyzed using the proportion z-test. Wilcoxon's rank sum test was used to compare the differences between groups, and Spearman's rank correlation was used to study the dependencies between variables. The two-sided alternative hypothesis was used for all tests, and P values of <0.05 were considered to be significant. Importantly, we did not correct the P values for multiple testing. We performed 220 tests, looking at differences between pathogenes, negative controls, and the dominating species E. coli, and 83 of those tests resulted in a P value of <0.05. The expected number of false positives is at most 11 (under the extreme assumption that the null hypothesis is true for all tests), and a conservative estimate of the false-discovery rate is 13.3%. If we assume that the null hypothesis is true for half of the considered tests, an upper limit of the false-discovery rate is 6.6%. In addition, 108 direct comparisons were made between the pathogenes, and for those tests, we presented the observed P values as well as the locally adjusted P values obtained using Bonferroni's correction. For some bacteria/groups of bacteria, the numbers of isolates were rather few, and the corresponding tests had relatively weak power. As a consequence, the observed P values were judged by taking the sample size and the observed effect size into account. The uropathogens were clustered using hierarchical clustering (using average linkage) based on the Manhattan distance between the standardized U-bacterial, U-leukocyte, and U-erythrocyte counts. The R package Pvclust (21) was used to identify robust clusters in the observed dendrogram (see Fig. 3). All statistical analyses were conducted using R version 2.9.1 (R Development Core Team, 2009).
FIG 3.
Dendrogram (hierarchical cluster analysis) presenting the relationship between bacteria and bacterial clusters based on bacteria, leukocyte, and erythrocyte counts/μl (median values) for bacteria of ≥5 in number. *, Data are presented as the au value (approximated unbiased P values in percentage) (left) and bp value (bootstrap probability values in percentage) (right). The uropathogenes were grouped in clusters I to IV. High au/bp values (%) indicate robust clusters.
RESULTS
A total of 1,587 consecutive urine specimens from patients with a suspected UTI were eligible for urine culture and flow cytometer analysis. Of these, 772 were excluded due to being from urine catheters (n = 125), having mixed flora without a dominating pathogen (n = 608), being isolated from a pregnant woman (n = 128), and being without clinical information (n = 5). The remaining 823 specimens were included, of which 620 were culture positive and 203 were culture negative. The majority (67.5%, P < 0.001) of the specimens were collected from women. The mean ages for men and women were 61.9 years (standard deviation, 20.8 years) and 59.9 years (standard deviation, 25.5 years), respectively. Outpatients represented 79.6% and inpatients 19.4% of the subjects. Of the included specimens, 54.4% yielded growth of >108 CFU/liter, 17.3% had 107 to 108 CFU/liter, 4.4% had 106 to 107 CFU/liter, and 24.7% were culture negative.
The dominating species was E. coli at 64.5%, followed by Enterococcus spp. at 8.2%, Klebsiella spp. at 7.2%, and Staphylococcus spp. at 5.0% (Table 1).
TABLE 1.
Bacterial, leukocyte, and erythrocyte counts in urine specimens from patients with suspected urinary tract infection estimated by flow cytometer analysis
| Groupa | Bacteria | No. | % positive | Cell count (median value/μl) inb: |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Bacterial cells |
WBC |
RBC |
|||||||
| EC | Escherichia coli | 400 | 64.5 | 17,914 | 348 | 23 | |||
| KC | Klebsiella pneumoniae | 33 | 5.3 | 20,866 | +,+* | 153 | −*,+* | 16 | −,+ |
| Klebsiella oxytoca | 10 | 1.6 | 27,239 | +,+ | 351 | +,+* | 17 | −,+ | |
| Other Klebsiella | 2 | 0.3 | 29,817 | +,+* | 70 | −,+ | 55 | +,+ | |
| All Klebsiella spp.c | 45 | 7.2 | 24,240 | +,+* | 160 | −*,+* | 18 | −,+* | |
| Enterobacter spp. | 5 | 1.5 | 4,345 | −,+* | 166 | −*,+* | 30 | +,+ | |
| Citrobacter spp. | 9 | 0.2 | 8,405 | −,+* | 144 | −,+* | 9 | −*,+ | |
| Citrobacter freundii | 1 | 0.2 | 15,220 | −,+ | 226 | −,+ | 7 | −,− | |
| Acinetobacter spp. | 1 | 0.2 | 163 | −,+ | 118 | −,+ | 24 | +,+ | |
| Pantoea spp. | 1 | 0.2 | 7,143 | −,+* | 56 | −,+ | 15 | −,+ | |
| Aggregated | 62 | 10.0 | 15,346 | −,+* | 157 | −*,+* | 17 | −,+* | |
| PR | Proteus vulgaris | 3 | 0.5 | 13,107 | −,+* | 79 | −,+ | 197 | +,+ |
| Proteus mirabilis | 12 | 1.9 | 1,955 | −*,+* | 902 | +,+* | 52 | +,+* | |
| Morganella morganii | 5 | 0.8 | 249 | −*,+ | 207 | −,+* | 19 | −,+ | |
| Providencia rettgeri | 1 | 0.2 | 30,689 | +,+ | 9 | −,+ | 8 | −,− | |
| Aggregated | 21 | 3.4 | 2,128 | −*,+* | 207 | −,+* | 38 | +,+* | |
| PS | Pseudomonas aeruginosa | 11 | 1.8 | 6,888 | −*,+* | 1,278 | +,+* | 73 | +*,+* |
| Other Pseudomonas | 1 | 0.2 | 3,467 | −,+ | 294 | −,+ | 12 | −,+ | |
| Aggregated | 12 | 1.9 | 5,177 | −*,+* | 1,175 | +,+* | 62 | +*,+* | |
| ST | Staphylococcus saprophyticus | 4 | 0.6 | 3,732 | −,+* | 200 | −,+ | 154 | +,+ |
| Staphylococcus aureus | 10 | 1.6 | 474 | −*,+* | 936 | +,+* | 32 | +,+* | |
| CoNSd | 17 | 2.7 | 4,383 | −*,+* | 462 | +,+* | 19 | −,+* | |
| Aggregated | 31 | 5.0 | 3,392 | −*,+* | 466 | +,+* | 24 | +,+* | |
| EN | Enterococcus faecalis | 42 | 6.8 | 2,623 | −*,+* | 200 | −*,+* | 19 | −*,+* |
| Enterococcus faecium | 9 | 1.5 | 1,116 | −*,+* | 34 | −,+* | 35 | +,+* | |
| Aggregated | 51 | 8.2 | 2,103 | −*,+* | 120 | −*,+* | 20 | −,+* | |
| SR | Alpha−streptococci | 11 | 1.8 | 2,877 | −*,+* | 64 | −,+* | 19 | −,+* |
| Group C streptococci | 4 | 0.6 | 1,286 | −,+* | 1,399 | +,+* | 59 | +,+ | |
| Group A streptococci | 1 | 0.2 | 111 | −,+ | 782 | +,+ | 6 | −,− | |
| Group G streptococci | 3 | 0.5 | 467 | −,+* | 96 | −,+* | 28 | +,+ | |
| Gemella haemolysans | 1 | 0.2 | 4,503 | −,+ | 973 | +,+ | 16 | −,+ | |
| Aggregated | 20 | 3.2 | 1,583 | −*,+* | 269 | −,+* | 22 | −,+* | |
| GB | Streptococcus agalactiae | 20 | 3.2 | 272 | −*,+* | 36 | −*,+* | 19 | −,+* |
| OT | Candida glabrata | 1 | 0.2 | 518 | −,+ | 94 | −,+ | 4 | −,− |
| Diphtheroid rod | 1 | 0.2 | 18,266 | +,+ | 65 | −,+ | 40 | +,+ | |
| Haemophilus influenzae | 1 | 0.2 | 2,263 | −,+ | 434 | +,+ | 108 | +,+ | |
| Aggregated | 3 | 0.5 | 2,263 | −,+* | 94 | −,+* | 40 | +,+ | |
| NE | Culture negative/no growth | 195 | NA | 33 | 7 | 9 | |||
Denotes all uropathogens or group of uropathogens presented in the “Bacteria” column.
WBC, white blood cells/leukocytes; RBC, red blood cells/erythrocytes. Each pair of symbols indicates that the observed value is higher (+) or lower (−) than the value for E. coli (first symbol) and negative cultures (second symbol). *, P < 0.05.
Denotes all Klebsiella species; this group is denoted K.
Coagulase-negative staphylococci.
Among the culture-positive specimens, 91% had 108 CFU/liter, 7% had 107 CFU/liter, and 2% had 106 CFU/liter of growth, of which mixed-flora populations were found in 17%, 53%, and 0% of these specimens, respectively. Among patients with 108 and <108 CFU/liter of growth, E. coli represented 65.6 and 63.4% of the uropathogens, respectively. The distributions of uropathogens other than E. coli were also similar in the two groups.
Overall, specimens with Gram-negative bacteria were associated with significantly (P < 0.001) higher U-bacterial counts (median, 16,777/μl) than those with Gram-positive bacteria (2,271/μl).
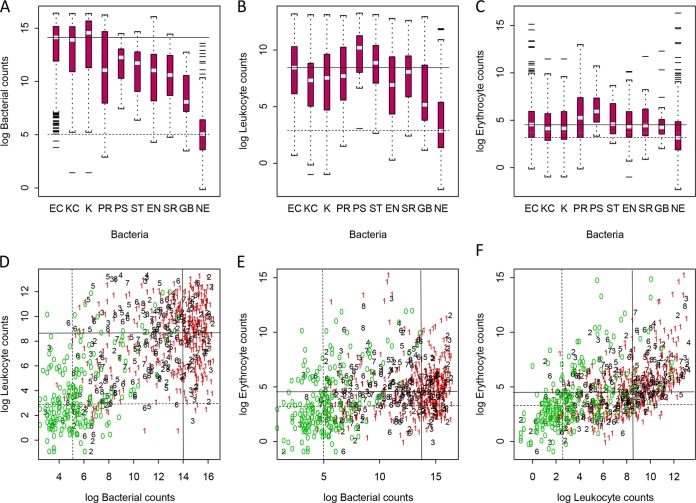
We found significant correlations between the concentration of uropathogens (bacteria/μl) and U-leukocyte counts (Spearman's rho = 0.26, P < 0.001), the concentration of U-bacterial and U-erythrocyte counts (Spearman's rho = 0.15, P < 0.001), and the concentration of U-leukocyte counts and U-erythrocyte counts (Spearman's rho = 0.51, P < 0.001) (Fig. 1D, E, and F). The outcomes of the bacterial, leukocyte, and erythrocyte counts (particles/μl) for groups or individual uropathogens were compared against the FCA characteristics for E. coli as well as specimens with a negative culture result (Table 1 and Fig. 1, 2, and 3).
FIG 1.
Bacterial, leukocyte, and erythrocyte counts per μl (logarithmic scale with base 2) in urine for uropathogens, groups of uropathogens, and specimens with negative culture estimated by flow cytometry analysis. (A) Bacterial counts for bacterial spp./bacterial groups. (B) Leukocyte counts for bacterial spp./bacterial groups. (C) Erythrocyte counts for bacterial spp./bacterial groups. (D) Leukocyte counts versus bacterial counts. (E) Erythrocyte counts versus bacterial counts. (F) Erythrocyte counts versus leukocyte counts. EC (lane 1), E. coli; KC (lane 2), Klebsiella spp., Enterobacter spp., Citrobacter spp., Acinetobacter spp., Pantoea spp.; K (lane 3), all Klebsiella spp.; PR (lane 4), Proteus spp., Morganella spp., and Providencia rettgeri group; PS (lane 5), Pseudomonas spp.; ST (lane 6), Staphylococcus spp.; EN (lane 7), Enterococcus spp.; SR (lane 8), streptococci groups A, C, and G, alpha-hemolytic streptococci, and Gemella haemolysans group; GB (lane 9), S. agalactiae (GBS); NE (lane 10), specimens with negative culture. (D to F) Red, E. coli; green, specimens with negative culture. (A to F) The median values for E. coli and the negative culture are shown with solid and dashed lines, respectively.
FIG 2.
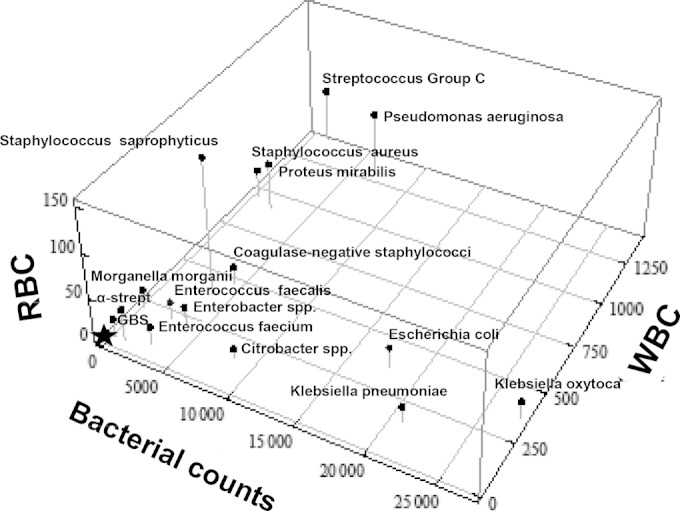
Three-dimensional plot of the median bacterial, leukocyte, and erythrocyte counts per μl in urine estimated from flow cytometry analysis for different uropathogens isolated from patients with suspected urinary tract infection. Star, culture-negative specimens. y axis: RBC, red blood cell counts/μl (erythrocytes). x axis: bacterial counts/μl. z axis: WBC, white blood cell counts/μl (leukocytes). α-strept, alpha-hemolytic streptococci.
The specimens with E. coli had high U-bacterial (17,914/μl), U-leukocyte (348/μl), and U-erythrocyte counts (23/μl) compared with those of the culture-negative specimens (33, 7, and 9/μl, respectively) (Table 1).
Among the Gram negatives, urine specimens with Klebsiella spp. were associated with higher U-bacterial counts (24,240/μl, P = 0.46) but lower U-leukocyte (160/μl, P < 0.05) and U-erythrocyte (18/μl, P = 0.67) counts than those with E. coli. Among Klebsiella spp., K. oxytoca was associated with higher U-leukocyte counts than was K. pneumoniae (351 versus 153/μl, respectively), but the two species had similar U-erythrocyte counts (16 and 17/μl, respectively) (Table 1).
In comparison, the specimens with Citrobacter spp. (9 isolates) had significantly lower U-erythrocyte counts (9/μl, P < 0.05) and lower U-bacterial counts (8,405/μl, P = 0.27) and U-leukocyte counts (144/μl, P = 0.09) than those with E. coli. The 12 isolates of P. mirabilis had significantly lower U-bacterial counts (1,955/μl, P < 0.05) and higher U-leukocyte (902/μl, P = 0.48) and U-erythrocyte counts (52/μl, P = 0.15) than the isolates of E. coli. In contrast, Proteus vulgaris had high U-bacterial counts (13,107/μl), low U-leukocyte counts (79/μl), and high U-erythrocyte counts (197/μl). Of the 12 Pseudomonas isolates, 11 represented P. aeruginosa, which had significantly lower U-bacterial counts (6,888/μl, P < 0.05), higher U-leukocyte counts (1,278/μl, P = 0.08), and significantly higher U-erythrocyte counts (73/μl, P < 0.05) than did the E. coli isolates (Table 1).
Among the Gram-positive bacteria, staphylococci were the second most common group after enterococci (31 isolates versus 51). Staphylococci had significantly lower U-bacterial counts (3,392/μl, P < 0.05) and higher U-leukocyte counts (466/μl, P < 0.05), as well as higher and U-erythrocyte counts (24/μl, P = 0.53) than did E. coli.
Enterococci (Enterococcus faecalis and E. faecium, with 42 and 9 isolates, respectively) were associated with significantly lower bacterial counts (2,103/μl, P < 0.05) than those of E. coli, and E. faecalis had higher U-leukocyte counts than did E. faecium (200 versus 34/μl, respectively; P = 0.45). Alpha-hemolytic streptococci and GBS were both associated with low leukocyte counts (64 and 36/μl, respectively) and low erythrocyte counts (19 and 19/μl, respectively). However, GBS had low bacterial counts (272/μl).
The relationships between the most common uropathogens are summarized in a 3-dimensional plot of the outcome of FCA (U-leukocyte, U-erythrocyte, and U-bacterial counts) (Fig. 2), and the relationships between the uropathogens are presented in the dendrogram (Fig. 3). According to these findings, uropathogens can be classified into four groups (I to IV, Fig. 3). The first group consists of E. coli, K. pneumoniae, and K. oxytoca, the second of P. aeruginosa, P. mirabilis, and S. aureus, and the third of Citrobacter species. Finally, the fourth group consists of alpha-streptococci, CoNS, E. faecalis, E. faecium, Morganella morganii, Enterobacter spp., and GBS that cluster together with the culture-negative specimens.
DISCUSSION
In this study, we evaluated the presence of leukocyturia and erythrocyturia for different uropathogens present in the urine samples from patients with suspected UTI, as measured by FCA analysis. In addition, the FCA results for different uropathogens were compared with those of E. coli, the most prevalent uropathogen, and to the culture-negative specimens.
The distribution of uropathogens in the present study was consistent with previous reports (6, 22). S. saprophyticus represented only <1% of the uropathogens, but a similar prevalence has been reported by others (23). The low prevalence of S. saprophyticus in the present study is explained by the high mean age (59.9 years) of the included women and that the study was performed during the winter season. In addition, elderly persons are more likely to have asymptomatic bacteriuria, an altered sense of UTI symptoms, and probably also a weakened immune response (24), factors that differ from those of younger adults and may influence the conclusions of the present study for an overall population of patients with UTIs. Gram-positive bacteria were often associated with lower bacterial counts than those of Gram-negative bacteria, presumably because Gram-positive bacteria often form aggregates (staphylococci) or chains (streptococci) (25). The bacterial counts of aggregated bacteria may be underestimated by the instrument and incorrectly reported with lower counts than those of Gram-negative bacteria, which are present in the urine as single planktonic bacteria. In other studies, the urinalysis instrument has been shown to correlate well with manual microscopy (26).
Bacterial, leukocyte, and erythrocyte counts differ among Gram-negative uropathogens. Citrobacter spp. were associated with a near lack of erythrocyturia (<10 erythrocytes/μl), indicating that they are less invasive, virulent, and immunostimulating than other uropathogens. In contrast, high erythrocyte counts were observed for P. vulgaris (197/μl) and S. saprophyticus (154/μl). Whether the high erythrocyte count is an effect of invasiveness or an inflammatory response to a uropathogen or both is unclear. However, both of these species are urease producers, a property that has been reported as an important bacterial virulence factor essential for colonization and associated with gastric peptic ulcers in humans (27). For Pseudomonas spp. and P. mirabilis, we found high erythrocyte and leukocyte counts despite low bacterial counts. Virulence factors as exotoxins and at least four different proteases have been reported in Pseudomonas spp. that may cause bleeding and tissue necrosis (28, 29).
However, specimens with high bacterial counts were associated with high leukocyte and erythrocyte counts (Fig. 1D and E), indicating that the bacterial load is of importance for the inflammatory response of leukocytes and erythrocytes in urine during a UTI.
Finally, the U-leukocyte and U-erythrocyte counts for the most common uropathogen species were plotted and presented in a 3-dimensional figure (Fig. 2). As can be seen, we found E. coli and K. pneumoniae/K. oxytoca to have similar characteristics. However, E. coli is by far a more common cause of UTI and has virulence factors of importance that are not measured by FCA (30). Despite this, the FCA characteristics of the uropathogens differ from the present Swedish classification in primary, secondary, and doubtful uropathogens (5). In particular, urine specimens with S. saprophyticus show lower U-leukocyte counts than those with P. aeruginosa, S. aureus, and P. mirabilis, although S. saprophyticus is classified as a primary UTI pathogen.
The fourth group of uropathogens (execept CoNS) represented by alpha-streptococci, GBS, E. faecalis, E. faecium, Enterobacter spp., and M. morganii is associated with low leukocyte and erythrocyte counts that were similar to what was seen in the culture-negative specimens.
Urinalysis with automated FCA has four diagnostic potentials. First, it has the ability to separate Gram-negative from Gram-positive bacteria based on bacterial counts (31, 32). Second, it can predict bacterial species (e.g., Citrobacter spp.) or groups of bacteria (e.g., E. coli/Klebsiella spp.). Such information can assist a clinician in deciding which antimicrobial (or not) to use for treatment while awaiting the outcome of urine culture. Third, the quality of a urine specimen can be examined by the numbers of epithelial cells present in the specimen. High epithelial cell counts indicate suboptimal sampling, which may affect the interpretation of the culture results. Fourth, the outcome of FCA can guide laboratory staff in improving diagnosis in culture-negative specimens with high U-leukocyte counts. In the present study, 24.7% of the specimens were culture negative despite clinical signs of UTI, which is in accordance with the findings in other studies (6, 33, 34). Culture-negative specimens may be due to suboptimal culture conditions, in particular those for anaerobic bacteria. In the present study, 203 specimens were culture negative, of which 30 and 22 specimens had >100 or 200 U leukocytes, respectively. In these specimens, an expanded urine culture protocol beyond the standard protocols should have been applied for the detection of fastidious bacteria.
The Sysmex UF-1000i instrument also measures results beyond bacterial, leukocyte, and erythrocyte counts. Taking other FCA results (e.g., squamous cells, conductivity, pH, etc.) into consideration might improve the characterization and diagnosis of uropathogens. The inclusion of improved staining techniques (fluorophores) that identify the four most prevalent uropathogens would identify nearly 90% of the causative uropathogens. Further studies are warranted to improve the diagnosis of uropathogens in urine specimens using FCA and to confirm the results from the present study, especially as some of the bacteria were present in a low number.
The urine specimens analyzed in this study probably do not represent specimens from an average population but rather specimens from a selected population of patients who were examined due to suspected UTI, pyelonephritis, failure of treatment, or control after treatment. On the contrary, the results are representative for urine specimens that were examined in our laboratory.
In essence, except for ruling out culture-negative urine specimens, FCA improves the handling of urine specimens and diagnosis of UTI. The outcome of FCA adds information about the numbers of uropathogens and the leukocyte and erythrocyte counts in urine specimens among patients with suspected UTI. According to these FCA characteristics, uropathogens can be classified into four classes, of which Klebsiella spp. and E. coli had similar characteristics. Specimens containing Citrobacter spp. were associated with the near absence of erythrocyturia. Urine specimens examined by FCA may allow a prediction of the causative uropathogen by its FCA characteristics, which will have clinical implications in guiding clinicians toward appropriate antimicrobial treatment while awaiting culture results.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Tamara Matti, Department of Infectious Diseases, University Hospital of Umeå for support and registration of the data sets and Kjell Leonardsson, Swedish University of Agricultural Science, Umeå for assistance in the presentation of Fig. 2.
The study was financed by the University of Umeå, Umeå, Sweden.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01974-14.
REFERENCES
- 1.Grover S, Srivastava A, Lee R, Tewari AK. 2011. Role of inflammation in bladder function and interstitial cystitis. Ther Adv Urol 3:19–33. doi: 10.1177/1756287211398255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stamm WE, Hooton TM. 1993. Management of urinary tract infections in adults. N Engl J Med 329:1328–1334. doi: 10.1056/NEJM199310283291808. [DOI] [PubMed] [Google Scholar]
- 3.Mazzulli T. 2002. Resistance trends in urinary tract pathogens and impact on management. J Urol 168:1720–1722. doi: 10.1016/S0022-5347(05)64397-2. [DOI] [PubMed] [Google Scholar]
- 4.Foxman B. 2002. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 113(Suppl 1A):5S–13S. doi: 10.1016/S0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 5.Aspevall O, Hallander H. 2000. Reference methodology for laboratory diagnostics at clinical bacteriology laboratories. I. Infectious diagnostics. I 5. Urinary tract infections/bacteriuria (in Swedish). 2 ed Swedish Institute for Infectious Disease Control, Solna, Sweden. [Google Scholar]
- 6.Ferry SA, Holm SE, Stenlund H, Lundholm R, Monsen TJ. 2004. The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomized placebo controlled study. Scand J Infect Dis 36:296–301. doi: 10.1080/00365540410019642. [DOI] [PubMed] [Google Scholar]
- 7.Ejrnaes K. 2011. Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli. Dan Med Bull 58:B4187. [PubMed] [Google Scholar]
- 8.Kahlmeter G. 2000. The ECO.SENS Project: a prospective, multinational, multicentre epidemiological survey of the prevalence and antimicrobial susceptibility of urinary tract pathogens–interim report. J Antimicrob Chemother 46(Suppl 1):S15–S22; discussion 63–65. doi: 10.1093/jac/46.suppl_1.15. [DOI] [PubMed] [Google Scholar]
- 9.Hooton TM, Stamm WE. 1997. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am 11:551–581. doi: 10.1016/S0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- 10.Lewis DA, Gumede LY, van der Hoven LA, de Gita GN, de Kock EJ, de Lange T, Maseko V, Kekana V, Smuts FP, Perovic O. 2013. Antimicrobial susceptibility of organisms causing community-acquired urinary tract infections in Gauteng Province, South Africa. S Afr Med J 103:377–381. doi: 10.7196/samj.6722. [DOI] [PubMed] [Google Scholar]
- 11.Graninger W. 2003. Pivmecillinam–therapy of choice for lower urinary tract infection. Int J Antimicrob Agents 22(Suppl 2):S73–S78. doi: 10.1016/S0924-8579(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 12.ECDC. 2012. Annual epidemiological report: reporting on 2010 surveillance data and 2011 epidemic intelligence data. European Centre for Disease Prevention and Control, Stockholm, Sweden: http://www.ecdc.europa.eu/en/publications/publications/annual-epidemiological-report-2012.pdf. [Google Scholar]
- 13.Horcajada JP, Shaw E, Padilla B, Pintado V, Calbo E, Benito N, Gamallo R, Gozalo M, Rodríguez-Baño J, ITUBRAS group, Grupo de Estudio de Infección Hospitalaria (GEIH), Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC) . 2013. Healthcare-associated, community-acquired and hospital-acquired bacteraemic urinary tract infections in hospitalized patients: a prospective multicentre cohort study in the era of antimicrobial resistance. Clin Microbiol Infect 19:962–968. doi: 10.1111/1469-0691.12089. [DOI] [PubMed] [Google Scholar]
- 14.Bean DC, Krahe D, Wareham DW. 2008. Antimicrobial resistance in community and nosocomial Escherichia coli urinary tract isolates, London 2005–2006. Ann Clin Microbiol Antimicrob 18:7–13. doi: 10.1186/1476-0711-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jolkkonen S, Paattiniemi EL, Kärpänoja P, Sarkkinen H. 2010. Screening of urine samples by flow cytometry reduces the need for culture. J Clin Microbiol 48:3117–3121. doi: 10.1128/JCM.00617-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broeren MA, Bahçeci S, Vader HL, Arents NL. 2011. Screening for urinary tract infection with the Sysmex UF-1000i urine flow cytometer. J Clin Microbiol 49:1025–1029. doi: 10.1128/JCM.01669-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pieretti B, Brunati P, Pini B, Colzani C, Congedo P, Rocchi M, Terramocci R. 2010. Diagnosis of bacteriuria and leukocyturia by automated flow cytometry compared with urine culture. J Clin Microbiol 48:3990–3996. doi: 10.1128/JCM.00975-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marschal M, Wienke M, Hoering S, Autenrieth IB, Frick JS. 2012. Evaluation of 3 different rapid automated systems for diagnosis of urinary tract infections. Diagn Microbiol Infect Dis 72:125–130. doi: 10.1016/j.diagmicrobio.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Zhang Y, Xu D, Shao W, Lu Y. 2010. Evaluation of the Sysmex UF-1000i for the diagnosis of urinary tract infection. Am J Clin Pathol 133:577–582. doi: 10.1309/AJCP1GT2JXOCQBCZ. [DOI] [PubMed] [Google Scholar]
- 20.Burman LG, Ostensson R. 1978. Time- and media-saving testing and identification of microorganisms by multipoint inoculation on undivided agar plates. J Clin Microbiol 8:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki R, Shimodaira H. 2006. Pvclust: an R package for assessing the uncertain in hierarchical clustering. Bioinformatics 22:1540–1542. doi: 10.1093/bioinformatics/btl117. [DOI] [PubMed] [Google Scholar]
- 22.Nys S, van Merode T, Bartelds AI, Stobberingh EE. 2006. Urinary tract infections in general practice patients: diagnostic tests versus bacteriological culture. J Antimicrob Chemother 57:955–958. doi: 10.1093/jac/dkl082. [DOI] [PubMed] [Google Scholar]
- 23.Barrett SP, Savage MA, Rebec MP, Guyot A, Andrews N, Shrimpton SB. 1999. Antibiotic sensitivity of bacteria associated with community-acquired urinary tract infection in Britain. J Antimicrob Chemother 44:359–365. doi: 10.1093/jac/44.3.359. [DOI] [PubMed] [Google Scholar]
- 24.Beveridge LA, Davey PG, Phillips G, McMurdo MET. 2011. Optimal management of urinary tract infections in older people. Clin Interv Aging 6:173–180. doi: 10.2147/CIA.S13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry MLL, Warnock DW (ed). 2011. Manual of clinical microbiology, 10th ed. ASM Press, Washington, DC. [Google Scholar]
- 26.Ottiger C, Huber AR. 2003. Quantitative urine particle analysis: integrative approach for the optimal combination of automation with UF-100 and microscopic review with KOVA cell chamber. Clin Chem 49:617–623. doi: 10.1373/49.4.617. [DOI] [PubMed] [Google Scholar]
- 27.Konieczna I, Zarnowiec P, Kwinkowski M, Kolesinska B, Fraczyk J, Kaminski Z, Kaca W. 2012. Bacterial urease and its role in long-lasting human diseases. Curr Protein Pept Sci 13:789–806. doi: 10.2174/138920312804871094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ben Haj Khalifa A, Moissenet D, Vu Thien H, Khedher M. 2011. Virulence factors in Pseudomonas aeruginosa: mechanisms and modes of regulation. Ann Biol Clin (Paris) 69:393–403. (In French.) doi: 10.1684/abc.2011.0589. [DOI] [PubMed] [Google Scholar]
- 29.Mittal R, Aggarwal S, Sharma S, Chhibber S, Harjai K. 2009. Urinary tract infections caused by Pseudomonas aeruginosa: a minireview. J Infect Public Health 2:101–111. doi: 10.1016/j.jiph.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Ejrnaes K, Stegger M, Reisner A, Ferry S, Monsen T, Holm SE, Lundgren B, Frimodt-Møller N. 2011. Characteristics of Escherichia coli causing persistence or relapse of urinary tract infections: phylogenetic groups, virulence factors and biofilm formation. Virulence 2:528–537. doi: 10.4161/viru.2.6.18189. [DOI] [PubMed] [Google Scholar]
- 31.Dai Q, Jiang Y, Shi H, Zhou W, Zhou S, Yang H. 2014. Evaluation of the automated urine particle analyzer UF-1000i screening for urinary tract infection in nonpregnant women. Clin Lab 60:275–280. [DOI] [PubMed] [Google Scholar]
- 32.Wada A, Kono M, Kawauchi S, Takagi Y, Morikawa T, Funakoshi K. 2012. Rapid discrimination of Gram-positive and Gram-negative bacteria in liquid samples by using NaOH-sodium dodecyl sulfate solution and flow cytometry. PLoS One 7:e47093. doi: 10.1371/journal.pone.0047093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pappas PG. 1991. Laboratory in the diagnosis and management of urinary tract infections. Med Clin North Am 75:313–325. [DOI] [PubMed] [Google Scholar]
- 34.Hilt EE, McKinley K, Pearce MM, Rosenfeld AB, Zilliox MJ, Mueller ER, Brubaker L, Gai X, Wolfe AJ, Schreckenberger PC. 2014. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol 52:871–876. doi: 10.1128/JCM.02876-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.