Abstract
The current guidelines recommend culture and antibiotic susceptibility testing of Helicobacter pylori following two failed eradication attempts. Where testing is unavailable, epidemiological data for secondary H. pylori resistance are essential to allow for the rational use of antibiotics. The aim of this study was to describe the temporal changes in antibiotic resistance among adults previously treated for H. pylori infections and to identify predictors of resistance. Between 2007 and 2014, consecutive patients undergoing gastroscopy with H. pylori culture and susceptibility testing at our institution following at least two treatment failures were retrospectively identified. Antibiotic susceptibilities were recorded and linked to the demographic data. A total of 1,042 patients were identified, including 739 (70.9%) males, aged 39.3 ± 18.9 years. Resistance to clarithromycin, metronidazole, and levofloxacin was found in 57.2%, 64.4%, and 5.1% of isolates, respectively. Dual resistance to clarithromycin and metronidazole was seen in 39.9%. Over the study period, clarithromycin resistance increased annually in a linear manner (odds ratio [OR], 1.09; 95% confidence interval [CI], 1.03 to 1.14; P < 0.01), levofloxacin resistance decreased annually (OR, 0.78; 95% CI, 0.61 to 0.92; P < 0.01), and metronidazole resistance was nonlinear. Age was an independent predictor of resistance to all antibiotics. Time elapsed predicted resistance for clarithromycin and levofloxacin and dual resistance for clarithromycin-metronidazole. Secondary resistance of H. pylori to clarithromycin and metronidazole remains high. The low secondary resistance to levofloxacin makes it an attractive treatment option in our region for patients following two failed eradication attempts.
INTRODUCTION
Despite 30 years of experience in treating Helicobacter pylori infection, the ideal treatment regimen has not yet been defined. Antibiotic resistance remains the most important impediment to the successful eradication of H. pylori. The current guidelines recommend H. pylori culture and susceptibility testing following two unsuccessful courses of treatment (1). Nevertheless, in regions where antibiotic susceptibility testing is unavailable, epidemiological data for secondary H. pylori resistance is essential to allow for the rational use of antibiotics following several failures in treatment (2). In this manner, the treatment success of a particular antibiotic regimen can be reliably predicted based on epidemiological data, even in the absence of individualized H. pylori culture and susceptibility testing. Due to the dynamic nature of H. pylori resistance, up-to-date data are imperative, and, if possible, temporal trends should be identified. A recent study from China found that between 2000 and 2009 secondary resistance to clarithromycin (CLR) increased from 29% to 100%, and resistance to metronidazole (MET) increased from 71% to 89%. Secondary resistance to levofloxacin (LEV) increased from 38% in 2006 to 82% in 2009. Resistance to ampicillin (AMP) and tetracycline (TET) was consistently negligible (3). A similar trend was observed in a European cohort between 2004 and 2007 where, altogether, secondary CLR resistance was 68%, MET resistance was 75%, and quinolone resistance was 20% (4). The temporal trends in secondary antibiotic resistance have not been previously studied in the Eastern Mediterranean region. The aim of this study was to describe the temporal trends in the secondary antibiotic resistance of H. pylori and to identify the predictors of microbial resistance. This will allow for the rational use of antibiotics in patients who do not achieve successful eradication with the standard treatment regimens.
MATERIALS AND METHODS
Data source.
Consecutive patients who underwent gastroscopy with culture and susceptibility testing for H. pylori between 1 January 2007 and 31 May 2014 were retrospectively identified. Data were retrieved from the Ofeq Database belonging to Clalit Health Services. In addition to data on the susceptibility of H. pylori to AMP, CLR, MET, LEV, and TET, demographic data were available, including age, sex, and country of birth. All of the gastroscopies were performed at Rabin Medical Center. Sterile samples from patients who were H. pylori positive as diagnosed by a [13C]urea breath test, rapid urease test, or histology were excluded. If more than one culture was obtained in a given patient on separate occasions, only the first culture was included in the analysis.
Patients.
Patients were referred from a dedicated H. pylori clinic at Rabin Medical Center. This clinic is the only dedicated H. pylori clinic is Israel and receives referrals from all regions of the country and from every health insurer. Patients in whom at least 2 courses of clarithromycin- and nitroimidazole-based therapies had failed were referred for gastroscopy with culture and susceptibility testing. Generally, the first-line treatment consisted of CLR-based triple therapy (CLR-AMP or CLR-MET with a proton pump inhibitor), sequential therapy, or concomitant therapy for 10 to 14 days, whereas second-line treatment consisted of bismuth-based quadruple therapy for 10 to 14 days. Due to the tight regulation of and lack of insurance coverage for levofloxacin, a negligible number of patients had received this antibiotic prior to referral. Direct referrals to gastroscopy and culture were not allowed.
Culture and susceptibility.
The biopsy specimens were inoculated directly onto Columbia blood agar (Difco, Detroit, MI) supplemented with yeast extract (5 g/liter), laked/lysed horse blood (7%), vancomycin (3 mg/liter), colistin sulfate (7.5 mg/liter), nystatin (12,500 IU/liter), and cotrimoxazole (5 mg/liter). The cultures were incubated for at least 72 h at 37°C under microaerobic conditions. H. pylori isolates were identified by colony morphology, characteristic spiral morphology on Gram staining, and positive findings on catalase, urease, and oxidase tests. The culture success rate of our reference laboratory is >95%.
The MICs for CLR, MET, AMP, TET, and LEV were determined by Etest (Biodisk, Solna, Sweden). Suspensions of a 3 McFarland standard were prepared in Columbia broth (Difco) from 72-h blood agar subcultures and spread (100 AL) on 90-mm-diameter petri plates containing Mueller-Hinton agar (BBL; Becton Dickinson and Company, Cockeysville, MD) supplemented with yeast extract (5 g/liter), horse serum (12%), and NAD (25 mg/liter). Etest strips were placed on the plates as soon as the inocula were absorbed into the agar (5, 6). The plates were incubated at 37°C under microaerobic conditions. The MIC values were determined after a 72-h incubation. Resistance was defined in accordance with the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines (7): AMP, MIC of >1.2 mg/liter; TET, MIC of >1 mg/liter; CLR, MIC of > 0.5 mg/liter; MET, MIC of ≥8 mg/liter (8); and LEV, MIC of >1 mg/liter (9). The H. pylori ATCC 43526 strain was used for quality control of the selective medium and the ATCC 43504 strain was used for the quality control of the susceptibility assay (10). Susceptibility testing for amoxicillin and tetracycline was not performed subsequent to 1 March 2009 due to the overwhelming susceptibility of H. pylori to these agents.
Statistical analysis.
All analyses were performed using SPSS version 21.0 statistical analysis software (IBM Inc., Chicago, IL, USA). The distributions of continuous variables were assessed for normality using the Kolmogorov-Smirnov test (cutoff at P < 0.01) and are described as means ± standard deviations (SD). The continuous variables were compared by antibiotic susceptibility using the t test for independent samples or the Mann-Whitney U test as appropriate. Nominal variables are described as frequency counts and are presented as number (%). The nominal variables were compared by antibiotic susceptibility using the chi-square test. The antibiotic resistance was modeled using regression analysis to develop odds ratios (OR) with 95% confidence intervals (CI), and a Bonferroni correction was performed. A multivariate prediction model included the following variables: year, origin, age, and sex. All tests were two-sided, and the results were considered significant at a P value of <0.05.
RESULTS
Patients.
A total of 1,042 cultures from 1,042 patients, including 739 (70.9%) males, were tested. The mean age was 39.3 years (SD, 18.9 years). Of the subjects, 740 (71.0%) were born in Israel, 174 (16.7%) were born in Eastern Europe and the former Soviet Union (FSU), and 110 (10.6%) were born in other Middle/Near Eastern and North African countries. The patient characteristics are displayed in Table 1.
TABLE 1.
Patient characteristics
| Patient characteristic | Value |
|---|---|
| Total no. | 1,042 (100) |
| Male (no. [%]) | 739 (70.9) |
| Age, yr (mean [SD]) | 39.3 (18.9) |
| Origin (no. [%]) | |
| Israel | 740 (71.0) |
| Western Europe/USA | 8 (0.8) |
| Eastern Europe/FSUa | 174 (16.7) |
| Near/Middle East | 65 (6.2) |
| Africa | 45 (4.3) |
| South America | 6 (0.5) |
| Asia | 4 (0.4) |
FSU, former Soviet Union.
Culture and susceptibility.
A total of 596 (57.2%) isolates of H. pylori were resistant to CLR, 671 (64.4%) were resistant to MET, and 53 (5.1%) were resistant to LEV (Table 2). Dual CLR-MET resistance was seen in 416 (39.9%) isolates, and simultaneous resistance for CLR-MET-LEV was seen in 29 (2.8%) cases. All 191 cultures tested for AMP and TET showed antibiotic susceptibility. Therefore, testing for AMP and TET was discontinued after March 2009.
TABLE 2.
Prevalence of secondary antibiotic resistance in Helicobacter pylori isolates according to year
| Antibiotic(s)a | Resistance in isolates (no. [%]) according to yr: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | |
| Total | 1,042 (100) | 74 (7.1) | 95 (9.1) | 103 (9.9) | 114 (10.9) | 140 (13.4) | 184 (17.7) | 233 (22.3) | 99 (9.5) |
| CLR | 596 (57.2) | 479 (46.0) | 560 (53.7) | 572 (54.9) | 518 (49.7) | 498 (47.8) | 723 (69.4) | 656 (63.0) | 590 (56.6) |
| MET | 671 (64.4) | 676 (64.9) | 659 (63.2) | 597 (57.3) | 563 (54.0) | 683 (65.5) | 686 (65.8) | 766 (73.5) | 617 (59.2) |
| LEV | 53 (5.1) | 127 (12.2) | 88 (8.4) | 93 (8.9) | 19 (1.8) | 16 (1.5) | 57 (5.5) | 46 (4.4) | 21 (2.0) |
| CLR-MET | 416 (39.9) | 325 (31.2) | 373 (35.8) | 384 (36.9) | 320 (30.7) | 357 (34.3) | 516 (49.5) | 510 (48.9) | 347 (33.3) |
| CLR-MET-LEV | 29 (2.8) | 56 (5.4) | 33 (3.2) | 60 (5.8) | 9 (0.9) | 7 (0.7) | 46 (4.4) | 18 (1.7) | 21 (2.0) |
CLR, clarithromycin; MET, metronidazole; LEV, levofloxacin.
Temporal trends in antibiotic susceptibility.
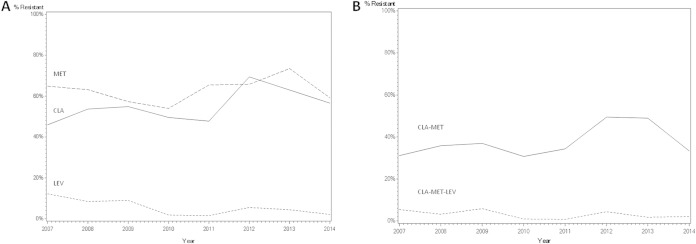
With linear regression modeling, resistance to CLR increased 8.9% each year relative to that in the preceding year (OR, 1.09; 95% CI, 1.03 to 1.14; P < 0.01) (Table 3; Fig. 1). The likelihood of LEV resistance decreased by 22.3% each year (OR, 0.78; 95% CI, 0.61 to 0.92; P < 0.01). Dual CLR-MET resistance increased by 8.4% each year (OR, 1.08; 95% CI, 1.03 to 1.14; P < 0.01) (Fig. 1). Resistance to MET and simultaneous CLR-MET-LEV resistance did not follow a linear pattern.
TABLE 3.
Linear effect of year (time elapsed) on the prevalence of secondary antibiotic resistance, univariate analysis
| Antibiotic(s)a | Odds ratio | 95% confidence limits |
P | |
|---|---|---|---|---|
| Lower | Upper | |||
| CLR | 1.1 | 1.0 | 1.2 | <0.01 |
| MET | 1.1 | 1.0 | 1.1 | 0.07 |
| LEV | 0.8 | 0.6 | 0.9 | <0.01 |
| CLR-MET | 1.1 | 1.0 | 1.1 | <0.01 |
| CLR-MET-LEV | 0.9 | 0.6 | 1.0 | 0.12 |
CLR, clarithromycin; MET, metronidazole; LEV, levofloxacin.
FIG 1.
Secondary resistance of Helicobacter pylori to clarithromycin, metronidazole, and levofloxacin, according to year. (A) Resistance to clarithromycin, metronidazole and levofloxacin. (B) Dual clarithromycin-metronidazole resistance and simultaneous clarithromycin-metronidazole-levofloxacin resistance. Abbreviations: CLA, clarithromycin; MET, metronidazole; LEV, levofloxacin.
Factors associated with antibiotic resistance.
Increasing age was significantly associated with CLR, MET, LEV, dual CLR-MET, and simultaneous CLR-MET-LEV resistance (Table 4). Gender was not associated with resistance to any antibiotic. The frequencies of LEV, dual CLR-MET, and simultaneous CLR-MET-LEV resistance were all significantly higher in subjects born in Eastern Europe/FSU and the Middle/Near East and less common in Israeli-born subjects.
TABLE 4.
Demographic factors associated with secondary antibiotic resistance, univariate analysis
| Demographic factor | Result for antibiotic(s)a: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CLR |
MET |
LEV |
CLR-MET |
CLR-MET-LEV |
||||||
| S | R | S | R | S | R | S | R | S | R | |
| Male (no. [%]) | 305 (41.3) | 434 (58.7) | 258 (34.9) | 481 (65.1) | 698 (94.4) | 41 (5.6) | 433 (58.6) | 306 (41.4) | 714 (96.6) | 25 (3.4) |
| Female (no. [%]) | 141 (46.5) | 162 (53.5) | 113 (37.3) | 190 (62.7) | 291 (96.1) | 12 (3.9) | 193 (63.7) | 110 (36.3) | 299 (98.7) | 4 (1.32) |
| Age, yr (mean [SD]) | 37.2 (18.9) | 40.6 (18.8)b | 35.8 (19.6) | 41.0 (18.3)c | 38.5 (18.8) | 50.6 (16.8)c | 36.7 (19.2) | 42.7 (18.0)c | 38.7 (18.8) | 54.3 (16.0)c |
| Origin (no. [%])d | ||||||||||
| Israel | 319 (43.1) | 421 (56.9) | 280 (37.8) | 460 (62.2) | 715 (96.1) | 25 (3.4))c | 463 (62.6) | 277 (37.4) c | 726 (98.1) | 14 (1.9)b |
| Other | 7 (38.9) | 11 (61.1) | 5 (27.6) | 13 (72.2) | 18 (100) | 0 (0))c | 8 (44.4) | 10 (55.6) c | 18 (100) | 0 (0)b |
| Eastern Europe/FSUe | 78 (44.8) | 96 (55.2) | 48 (27.8) | 126 (72.4) | 158 (91.3) | 16 (8.7))c | 95 (54.6) | 79 (45.4) c | 165 (94.8) | 9 (5.2)b |
| Near/Middle East | 20 (30.8) | 45 (69.2) | 20 (30.8) | 45 (69.2) | 57 (87.3) | 8 (12.7))c | 28 (43.1) | 37 (56.9) c | 60 (92.3) | 5 (7.7)b |
| Africa | 22 (48.8) | 23 (51.2) | 18 (40.0) | 27 (60.0) | 41 (91.1) | 4 (8.9))c | 32 (71.1) | 13 (28.9) c | 44 (97.8) | 1 (2.2) |
CLR, clarithromycin; MET, metronidazole; LEV, levofloxacin; S, susceptible; R, resistant.
P < 0.05.
P < 0.01.
P value refers to differences across all geographical origins for a particular antibiotic drug or combination.
FSU, former Soviet Union.
Multivariate regression analysis.
The period of observation (time elapsed) was independently linked to CLR, LEV, and dual CLR-MET resistance (Table 5). Increasing age was found to independently predict CLR, MET, LEV, dual CLR-MET, and simultaneous CLR-MET-LEV resistance. Gender and country of birth did not predict antibiotic resistance in our model.
TABLE 5.
Predictors of secondary antibiotic resistance, multivariate analysis
| Antibiotica and predictor | P |
|---|---|
| CLR | |
| Yr | <0.01 |
| Gender | 0.16 |
| Origin | 0.32 |
| Age | 0.03 |
| MET | |
| Yr | 0.14 |
| Gender | 0.65 |
| Origin | 0.38 |
| Age | <0.01 |
| LEV | |
| Yr | <0.01 |
| Gender | 0.25 |
| Origin | 0.64 |
| Age | <0.01 |
| CLR-MET | |
| Yr | <0.01 |
| Gender | 0.20 |
| Origin | 0.07 |
| Age | <0.01 |
| CLR-MET-LEV | |
| Yr | 0.08 |
| Gender | 0.10 |
| Origin | 0.78 |
| Age | <0.01 |
CLR, clarithromycin; MET, metronidazole; LEV, levofloxacin.
DISCUSSION
Antibiotic resistance is the main factor accounting for unsuccessful eradication of H. pylori (1). Our 8-year historical perspective provides insight into the relationship between secondary antibiotic resistance and covariates, including time elapsed, age, gender, and geographical origin. Among adults previously treated for H. pylori, across the study period we found a linear increase in CLR resistance, a linear decrease in LEV resistance, and an erratic pattern for MET resistance. Dual CLR-MET resistance remained steady with the exception of a spike in 2012-2013, parallel to peak CLR and MET resistance rates. Simultaneous resistance to CLR-MET-LEV followed a downward trend; however, this was not significant.
Interestingly, between 2012 and 2014, resistance to CLR tapered from 69.4% to 56.6% (Fig. 1). Time will tell whether or not this decline will continue. Our findings are consistent with the pattern observed in the Netherlands between 1997 and 2002, where the rate of CLR resistance decreased (11). However, these results are difficult to interpret due to a small sample size and a low overall rate of secondary CLR resistance (5.3%). In other regions, resistance did not change significantly. For example, in France, CLR resistance was 18% in 1994 and 20% in 2005 (12). Nevertheless, the same study group subsequently found that between 2004 and 2007 secondary CLR resistance in France increased from 29% to 77% (4). Indeed, in most of the regions in which CLR susceptibility has been studied, resistance has risen over time. In Bulgaria, CLR resistance rose from 10% in 1996-1999 to 19% in 2007-2009 (13), and in Belgium from 6% in 1990 to 56% in 2009 (14). In China, secondary resistance rose from 29% in 2000 to 100% in 2009 (3). We found that the period of observation was an independent predictor of CLR resistance. This is probably related to a surge in the use of macrolides for respiratory tract infections worldwide and also in Israel (15–17). In this setting, it is reassuring that in Israel CLR resistance has tapered since 2012.
The seroprevalence of H. pylori in Israel is approximately 45% (18). Temporal antibiotic susceptibility data for our geographical region have not previously been published. However, data from a small Israeli cohort (n = 28) in 2000-2001 showed that secondary resistance to CLR and MET was 46% and 61%, respectively (19). Zevit et al. found that secondary resistance to CLR and MET was 42% and 52%, respectively, in an Israeli pediatric cohort during 2005-2007 (n = 19) (20). Yahav et al. found that secondary resistance to CLR, MET, and LEV was 66%, 57%, and 19%, respectively, in an Israeli cohort during 2003-2004 (n = 70) (21). The dual CLR-MET resistance was 33%, which is comparable to our observations for 2007-2014; however, simultaneous CLR-MET-LEV resistance was 13%, which is considerably higher. This might be reflective of the tightened regulation surrounding fluoroquinolone prescriptions over the past decade in Israel and the lack of reimbursement (22, 23).
We found that age was an independent predictor of secondary resistance to CLR, MET, LEV, and combinations thereof, presumably related to a longer duration of infection and increased prior antibiotic exposure. We excluded subjects younger than 18 years in our analysis since they are not a target for treatment (1). Nevertheless, in studies which stratify for age, resistance appears to be lowest in children (14). This is especially true for LEV, presumably because fluoroquinolones are rarely used in this age group. On the other hand, some data suggest that CLR resistance is higher in children than in adults, due to widespread macrolide use for respiratory tract infections (5, 13, 24–27).
Gender did not predict antibiotic resistance in our study. Other studies have reported variable associations with gender. A female predilection for resistance was seen in the Belgian cohort (14) and hypothesized to be related to macrolide and nitroimidazole prescription for gynecological infections. A male predilection has been described in other geographical regions (28–30).
Geographical origin was associated with various patterns of antibiotic resistance; however, these did not remain significant when analyzed in a multivariate regression model (Table 4). The antibiotic resistance was highest among subjects born in Eastern Europe/FSU and the Near/Middle Eastern countries. This is presumably confounded by the older age of the immigrant population in Israel.
Low rates of secondary fluoroquinolone resistance in our region make levofloxacin an attractive option for second- or third-line treatment, in combination with AMP and a proton pump inhibitor. In penicillin-allergic individuals, LEV may be combined with CLR. Secondary analyses showed that the rate of dual CLR-LEV resistance in our cohort was only 3.5% (5.4% in 2007 and 2.0% in 2014), making this treatment combination feasible.
The strength of our study lies in the large sample size, the reliability of our data, and the homogeneity of our cohort. Limitations include a possible referral bias and the single center design which may overrepresent an urban, high socioeconomic demographic. However, our center is the only site in Israel to perform H. pylori culture and as such receives referrals from the entire country. We did not include clinical and endoscopic data such as underlying gastric disease or details of prior treatment attempts. Retrospective, manual data retrieval was forgone in favor of a very large sample and data accuracy. We did not include data on primary antibiotic resistance, which is necessary for the rational choice of first-line treatment.
The overestimation of resistance to MET is of concern in this study. Previous reports have shown that incubation for 24 h in anaerobic conditions before transferring to a microaerobic atmosphere yields significantly lower MET resistance than incubation which is exclusively microaerobic (31). The reason is that the nitro group of metronidazole must be reduced for drug activation. Anaerobic conditions are required since oxygen has a higher reduction potential than metronidazole and will be otherwise preferentially reduced (32). Another criticism of our study relates to the clinical relevance of in vitro testing; however, with the exception of MET, the concern that susceptibility results do not predict treatment efficacy is largely unfounded (33–36). Furthermore, the future of susceptibility testing may be noninvasive stool PCR-based testing, which will obviate direct culture (37). A future study might be enhanced by including resistance data for additional antibiotics which are sometimes used to treat refractory H. pylori, such as furazolidone, rifabutin, and nitazoxanide (38–40).
In conclusion, although secondary resistance of H. pylori to CLR and MET remains high, rates do not appear to be increasing at this stage. Low secondary resistance to LEV makes it an attractive treatment option in our region for patients after two failed eradication attempts.
ACKNOWLEDGMENTS
We acknowledge Nili Golan for her assistance in data processing and Haim Shmuely for his assistance in data acquisition.
Author contributions: D.B. prepared the manuscript and designed the study, H.B.-Z. and Z.S. retrieved the data, T.T.P. and Z.K. provided technical assistance, and R.D. and Y.N. oversaw implementation at all stages.
We declare no conflicts of interest.
REFERENCES
- 1.Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon Anthony TR, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ. 2012. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut 61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 2.Graham DY, Lee Y, Wu M. 2014. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol 12:177−186.e3; Discussion e12–e13. doi: 10.1016/j.cgh.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao W, Cheng H, Hu F, Li J, Wang L, Yang G, Xu L, Zheng X. 2010. The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter 15:460–466. doi: 10.1111/j.1523-5378.2010.00788.x. [DOI] [PubMed] [Google Scholar]
- 4.Raymond J, Lamarque D, Kalach N, Chaussade S, Burucoa C. 2010. High level of antimicrobial resistance in French Helicobacter pylori isolates. Helicobacter 15:21–27. doi: 10.1111/j.1523-5378.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- 5.Glupczynski Y, Mégraud F, Lopez-Brea M, Andersen LP. 2001. European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur J Clin Microbiol Infect Dis 20:820–823. doi: 10.1007/s100960100611. [DOI] [PubMed] [Google Scholar]
- 6.Osato MS, Reddy R, Reddy SG, Penland RL, Graham DY. 2001. Comparison of the Etest and the NCCLS-approved agar dilution method to detect metronidazole and clarithromycin resistant Helicobacter pylori. Int J Antimicrob Agents 17:39–44. doi: 10.1016/S0924-8579(00)00320-4. [DOI] [PubMed] [Google Scholar]
- 7.EUCAST. 2011. EUCAST clinical breakpoints for Helicobacter pylori. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_4.0.pdf.
- 8.King A. 2001. Recommendations for susceptibility tests on fastidious organisms and those requiring special handling. J Antimicrob Chemother 48(Suppl 1):77–80. doi: 10.1093/jac/48.suppl_1.77. [DOI] [PubMed] [Google Scholar]
- 9.Tankovic J, Lascols C, Sculo Q, Petit J, Soussy C. 2003. Single and double mutations in gyrA but not in gyrB are associated with low- and high-level fluoroquinolone resistance in Helicobacter pylori. Antimicrob Agents Chemother 47:3942–3944. doi: 10.1128/AAC.47.12.3942-3944.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mégraud F, Lehn N, Lind T, Bayerdörffer E, O'Morain C, Spiller R, Unge P, van Zanten SV, Wrangstadh M, Burman CF. 1999. Antimicrobial susceptibility testing of Helicobacter pylori in a large multicenter trial: the MACH 2 study. Antimicrob Agents Chemother 43:2747–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janssen MJR, Hendrikse L, de Boer SY, Bosboom R, de Boer WA, Laheij RJF, Jansen JBMJ. 2006. Helicobacter pylori antibiotic resistance in a Dutch region: trends over time. Neth J Med 64:191–195. [PubMed] [Google Scholar]
- 12.Kalach N, Serhal L, Asmar E, Campeotto F, Bergeret M, Dehecq E, Spyckerelle C, Charkaluk M, Decoster A, Dupont C, Raymond J. 2007. Helicobacter pylori primary resistant strains over 11 years in French children. Diagn Microbiol Infect Dis 59:217–222. doi: 10.1016/j.diagmicrobio.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Boyanova L, Nikolov R, Gergova G, Evstatiev I, Lazarova E, Kamburov V, Panteleeva E, Spassova Z, Mitov I. 2010. Two-decade trends in primary Helicobacter pylori resistance to antibiotics in Bulgaria. Diagn Microbiol Infect Dis 67:319–326. doi: 10.1016/j.diagmicrobio.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Miendje Deyi VY, Bontems P, Vanderpas J, de Koster E, Ntounda R, Van den Borre C, Cadranel S, Burette A. 2011. Multicenter survey of routine determinations of resistance of Helicobacter pylori to antimicrobials over the last 20 years (1990 to 2009) in Belgium. J Clin Microbiol 49:2200–2209. doi: 10.1128/JCM.02642-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlowsky JA, Lagacé-Wiens Philippe RS, Low DE, Zhanel GG. 2009. Annu macrolide prescription rates and the emergence of macrolide resistance among Streptococcus pneumoniae in Canada from 1995 to 2005. Int J Antimicrob Agents 34:375–379. doi: 10.1016/j.ijantimicag.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Adriaenssens N, Coenen S, Versporten A, Muller A, Minalu G, Faes C, Vankerckhoven V, Aerts M, Hens N, Molenberghs G, Goossens H. 2011. European Surveillance of Antimicrobial Consumption (ESAC): outpatient antibiotic use in Europe (1997-2009). J Antimicrob Chemother 66(Suppl 6):vi3–vi12. doi: 10.1093/jac/dkr453. [DOI] [PubMed] [Google Scholar]
- 17.Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, Andersen LP, Goossens H, Glupczynski Y. 2013. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 62:34–42. doi: 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 18.Muhsen K, Cohen D, Spungin-Bialik A, Shohat T. 2012. Seroprevalence, correlates and trends of Helicobacter pylori infection in the Israeli population. Epidemiol Infect 140:1207–1214. doi: 10.1017/S0950268811002081. [DOI] [PubMed] [Google Scholar]
- 19.Samra Z, Shmuely H, Niv Y, Dinari G, Passaro DJ, Geler A, Gal E, Fishman M, Bachor J, Yahav J. 2002. Resistance of Helicobacter pylori isolated in Israel to metronidazole, clarithromycin, tetracycline, amoxicillin and cefixime. J Antimicrob Chemother 49:1023–1026. doi: 10.1093/jac/dkf041. [DOI] [PubMed] [Google Scholar]
- 20.Zevit N, Levy I, Shmuely H, Samra Z, Yahav J. 2010. Antibiotic resistance of Helicobacter pylori in Israeli children. Scand J Gastroenterol 45:550–555. doi: 10.3109/00365521003663688. [DOI] [PubMed] [Google Scholar]
- 21.Yahav J, Shmuely H, Niv Y, Bechor J, Samra Z. 2006. In vitro activity of levofloxacin against Helicobacter pylori isolates from patients after treatment failure. Diagn Microbiol Infect Dis 55:81–83. doi: 10.1016/j.diagmicrobio.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Nitzan O, Low M, Lavi I, Hammerman A, Klang S, Raz R. 2010. Variability in outpatient antimicrobial consumption in Israel. Infection 38:12–18. doi: 10.1007/s15010-009-9065-8. [DOI] [PubMed] [Google Scholar]
- 23.Low M, Nitzan O, Bitterman H, Cohen C, Hammerman A, Lieberman N, Raz R, Balicer RD. 2013. Trends in outpatient antibiotic use in Israel during the years 2000-2010: setting targets for an intervention. Infection 41:401–407. doi: 10.1007/s15010-012-0332-8. [DOI] [PubMed] [Google Scholar]
- 24.Agudo S, Perez-Perez G, Alarcon T, Lopez-Brea M. 2010. High prevalence of clarithromycin-resistant Helicobacter pylori strains and risk factors associated with resistance in Madrid, Spain. J Clin Microbiol 48:3703–3707. doi: 10.1128/JCM.00144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alarcón T, Vega AE, Domingo D, Martinez MJ, Lopez-Brea M. 2003. Clarithromycin resistance among Helicobacter pylori strains isolated from children: prevalence and study of mechanism of resistance by PCR-restriction fragment length polymorphism analysis. J Clin Microbiol 41:486–499. doi: 10.1128/JCM.41.1.486-488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cabrita J. 2000. Features and trends in Helicobacter pylori antibiotic resistance in Lisbon area, Portugal (1990-1999). J Antimicrob Chemother 46:1029–1031. doi: 10.1093/jac/46.6.1029. [DOI] [PubMed] [Google Scholar]
- 27.Tolia V, Brown W, El-Baba M, Lin CH. 2000. Helicobacter pylori culture and antimicrobial susceptibility from pediatric patients in Michigan. Pediatr Infect Dis J 19:1167–1171. doi: 10.1097/00006454-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Koletzko S, Richy F, Bontems P, Crone J, Kalach N, Monteiro ML, Gottrand F, Celinska-Cedro D, Roma-Giannikou E, Orderda G, Kolacek S, Urruzuno P, Martínez-Gómez MJ, Casswall T, Ashorn M, Bodanszky H, Mégraud F. 2006. Prospective multicentre study on antibiotic resistance of Helicobacter pylori strains obtained from children living in Europe. Gut 55:1711–1716. doi: 10.1136/gut.2006.091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi I, Murakami K, Kato M, Kato S, Azuma T, Takahashi S, Uemura N, Katsuyama T, Fukuda Y, Haruma K, Nasu M, Fujioka T. 2007. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol 45:4006–4010. doi: 10.1128/JCM.00740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prazeres Magalhães P, De Magalhães Queiroz DM, Campos Barbosa DV, Aguiar Rocha G, Nogueira Mendes E, Santos A, Valle Corrêa PR, Camargos Rocha AM, Martins TL, Affonso de Oliveira C. 2002. Helicobacter pylori primary resistance to metronidazole and clarithromycin in Brazil. Antimicrob Agents Chemother 46:2021–2023. doi: 10.1128/AAC.46.6.2021-2023.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsen AL, Ragnhildstveit E, Moayeri B, Eliassen L, Melby KK. 2013. Resistance rates of metronidazole and other antibacterials in Helicobacter pylori from previously untreated patients in Norway. APMIS 121:353–358. doi: 10.1111/apm.12009. [DOI] [PubMed] [Google Scholar]
- 32.van Zwet AA, Thijs JC, Graaf de B. 1995. Explanations for high rates of eradication with triple therapy using metronidazole in patients harboring metronidazole-resistant Helicobacter pylori strains. Antimicrob Agents Chemother 39:250–252. doi: 10.1128/AAC.39.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Wouden EJ, Thijs JC, van Zwet AA, Sluiter WJ, Kleibeuker JH. 1999. The influence of in vitro nitroimidazole resistance on the efficacy of nitroimidazole-containing anti-Helicobacter pylori regimens: a meta-analysis. Am J Gastroenterol 94:1751–1759. doi: 10.1111/j.1572-0241.1999.01202.x. [DOI] [PubMed] [Google Scholar]
- 34.Graham DY. 1998. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology 115:1272–1277. doi: 10.1016/S0016-5085(98)70100-3. [DOI] [PubMed] [Google Scholar]
- 35.Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. 2013. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion 88:33–45. doi: 10.1159/000350719. [DOI] [PubMed] [Google Scholar]
- 36.Malfertheiner P, Bazzoli F, Delchier J, Celinski K, Giguere M, Riviere M, Megraud F. 2011. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet 377:905–913. doi: 10.1016/S0140-6736(11)60020-2. [DOI] [PubMed] [Google Scholar]
- 37.Xiong LJ, Tong Y, Wang Z, Mao M. 2013. Detection of clarithromycin-resistant Helicobacter pylori by stool PCR in children: a comprehensive review of literature. Helicobacter 18:89–101. doi: 10.1111/hel.12016. [DOI] [PubMed] [Google Scholar]
- 38.Tay CY, Windsor HM, Thirriot F, Lu W, Conway C, Perkins TT, Marshall BJ. 2012. Helicobacter pylori eradication in Western Australia using novel quadruple therapy combinations. Aliment Pharmacol Ther 36:1076–1083. doi: 10.1111/apt.12089. [DOI] [PubMed] [Google Scholar]
- 39.D'Elios MM, Silvestri E, Emmi G, Barnini T, Prisco D. 2012. Helicobacter pylori: usefulness of an empirical fourth-line rifabutin-based regimen. Expert Rev Gastroenterol Hepatol 6:437–439. doi: 10.1586/egh.12.32. [DOI] [PubMed] [Google Scholar]
- 40.Basu PP, Rayapudi K, Pacana T, Shah NJ, Krishnaswamy N, Flynn M. 2011. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am J Gastroenterol 106:1970–1975. doi: 10.1038/ajg.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]