Abstract
Myringotomy and ventilation tube placement is an office-based procedure and represents the treatment of choice for middle ear effusions and for transtympanic drug administration. The aim of our study was to study the efficiency of low-pressure spray cryotherapy on protraction of patency time of myringotomies in rats. Bilateral myringotomies were performed with cold instruments. Afterward, one ear in each rat was randomly assigned for liquid nitrogen treatment of the perforated eardrum. Myringotomy patency was recorded daily and histological analysis of the eardrums was performed. In the myringotomy group patency time was 4.75 ± 2.93 days, while in the cryotherapy group was 11.01 ± 4.87 days. The difference is statistically significant: Student t test, p < 0.00001, 95 % CI (−8.82; −3.67). Moreover, according to Kaplan–Meier method, myringotomy survival is significantly longer in the cryotherapy group. The healing processes of the cryotreated eardrums last longer and the patency of myringotomy was longer as compared to untreated myringotomies.
Keywords: Myringotomy, Cryotherapy, Patency time, Survival, Histological analysis
Introduction
Ventilation tube (VT) placement is recognized as the treatment of choice for middle ear effusions and represents one of the most commonly performed otolaryngologic surgical procedure [1]. Moreover, transtympanic drug administration requires patent myringotomies throughout the therapy schedule. Ventilation tubes carry the risk for long-term complications including tympanosclerosis, persistent tympanic membrane perforations, granulation tissue formation and associated hearing loss [1]. On the other hand the most frequent sequelae associated with VTs is recurrent otorrhea, with an estimated incidence up to 83 % [2]. These are the reasons why, alternative therapies aimed at providing long-term patent myringotomies were recently suggested. Among methods employed to maintain a patent myringotomy were the use of CO2 laser and mitomycin C. Mitomycin C is an antineoplastic aminoglycoside and it was demonstrated to be safe and effective at prolonging the duration of myringotomy patency in the guinea pig [3]. These treatments hinder the physiologic processes which lead to spontaneous closure of the perforation. The aim of our study was to study the efficiency of low-pressure spray cryotherapy on protraction of patency time of myringotomies in rats. Based on our previously study on experimental sinusitis [4], we assumed that the use of cryotherapy may significantly delay spontaneous closure of the myringotomy.
Materials and Methods
The study was approved by the Ethics Committee of the Iuliu Hatieganu University and Pharmacy from Cluj-Napoca, Romania. The experimental protocol was in compliance with the institutional and European guidelines for laboratory animal experiments. Forty male Wistar adult male rats each weighing 200–220 g were used and dispersed in two equal groups, one for observational study and the second for histological study. The rats were kept in standard conditions of temperature, humidity, day/night cycle and they had unrestricted access to food and water throughout the experiment. Body weight and clinical signs were recorded regularly.
All procedures were performed with the animals under general anesthesia with Xylazine/Ketamine cocktail using a dosage of 8 mg Xylazine and 80 mg Ketamine per kg of body weight. Initially, all animals in the two study groups underwent bilateral microscopic examination to exclude external and middle ear pathology. Examinations and surgical procedure were carried out by a single surgeon. Subsequently, bilateral myringotomies located posterior to the malleus were performed with cold instruments in each animal under microscopic control. Afterward, one ear in each rat was randomly assigned for liquid nitrogen treatment of the perforated eardrum. The liquid nitrogen treatment of the eardrums was achieved by means of a Cryosurgical Unit operated in the spray mode and currently used for cryotherapy in humans. Before administration of the liquid nitrogen in the selected outer ear canal, small cotton pledged was placed in the mouse’s middle ear to prevent the dispersion of the liquid nitrogen in the middle ear cavity with subsequent freezing effect on the middle ear mucosa and inner ear. After the administration of liquid nitrogen, bilateral otomicroscopy and video recordings were repeated.
Follow-up of the animals included bilateral daily otomicroscopy with video recordings of the myringotomy sites for a period of 4 weeks. In case of otorrhea, ear suction of the outer ear canal was applied followed by topical antibiotic administration. Myringotomy patency was recorded daily for each animal within the study. The statistical analysis was performed by means of SPSS 20.0 (SPSS, Inc., Chicago, IL). Data were expressed as mean ± standard deviation (SD). P values < 0.05 were considered significant. Student t test was used for normally distributed data. The Kaplan–Meier survival curves were used to evaluate myringotomy patency between cryotreated and control ears. The log-rank test was used to compare patency time between the two groups.
Rats from the histological study group were sacrificed 1, 2, 3 and 4 weeks after the surgical procedure. The tissue samples from the skull including the outer ear canal, the eardrum and the middle ear were harvested and immediately fixed for light microscopy in 10 % neutral-buffered formalin. After 48 h the samples were submerged to decalcification in an acidic mixture composed by 8 % formic acid and 8 % clorhidric acid in a 1/1 report. When decalcification was completed the tissues were processed by routine paraffin-embedding techniques. The tissue sections were cut from each paraffin block at 4 µm thickness with a rotary microtome and stored overnight in a thermostatically controlled oven. Finally, sections were stained with hematoxylin and eosin (H&E) and examined under an Olympus BX41 microscope. The bright field microscopic images were taken with Olympus UC30 camera and processed using Olympus Stream Basic image analysis software.
Results
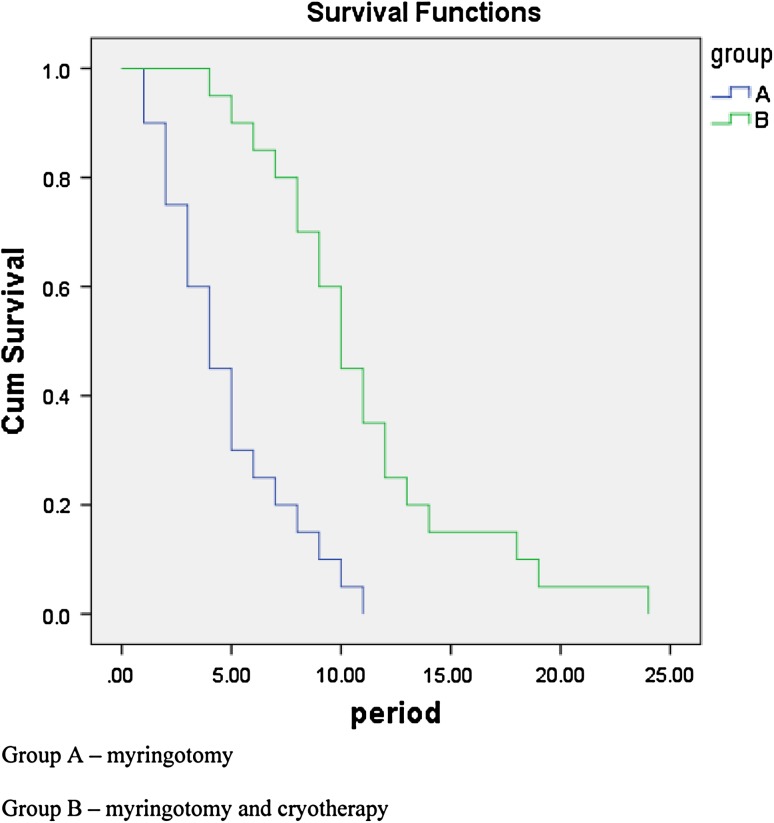
There were 20 tympanic membranes (Group A) subjected to simple myringotomy and 20 eardrums underwent myringotomy and cryotherapy (Group B). In Group A the mean patency time of the myringotomy was 4.75 ± 2.93 days, while in Group B was 11.01 ± 4.87 days. The difference is statistically significant: Student t test, p < 0.00001, 95 % CI (−8.82; −3.67). On the other hand, according to the Kaplan–Meier method, myringotomy survival is significantly longer in the cryotherapy group (see Fig. 1): log-rank test = 19.88, p < 0.00001.
Fig. 1.
Kaplan–Meier curve for myringotomy patency
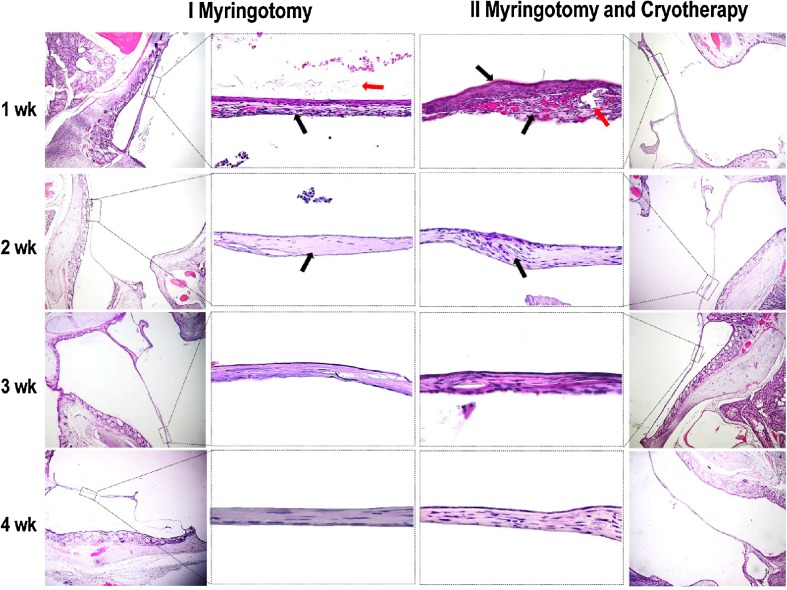
Histological analysis of the two groups is represented in Fig. 2. As one can see, at 1 week following myringotomy and cryotheraphy the proliferation of epithelial cells (indicated by the superior black arrow from the center-right column) covers the tympanic aperture (indicated by the red arrow). In the group undergoing myringotomy and cryotheraphy epithelial cells are disposed on 5–7 layers of cubical or slightly flattened appearance. The inferior black arrow from the same image indicates the abundant angiogenesis and inflammation associated with cryotheraphy.
Fig. 2.
Microscopic images of the tympanic membrane at different time intervals following myringotomy and myringotomy and cryotheraphy. In the left and right columns the general aspect of the ear canal, tympanic membrane and meddle ear can be observed. In the central columns the detailed structure of the eardrum is represented. See text for explanations
At 2 weeks the focal proliferation of the fibrous connective tissue and flattening of the epithelial cells can be observed in both groups. In the group undergoing cryotheraphy one can see few inflammatory cells and incomplete regeneration of the fibrous connective tissue from the eardrum lamina (indicated by the arrow).
At 3 and 4 weeks after myringotomy, one can see that de eardrum defect is completely closed, the perforation being covered by flattened epithelial cells and several layers of mature connective tissue in the lamina propria.
Discussion
The healing process of the eardrum following complete perforation is intricate, displays several stages, including an inflammatory phase and epithelial migration and conjunctive tissue proliferation period [5]. The initial epithelial migration covering the outer surface of the tympanic membrane in the form of epithelial buds is followed by fibrous proliferation and, finally remodeling [6]. Thus, the major initial regenerative occurrence consists of epithelial migration leading to covering of the tympanic defect. Subsequent fibrous-conjunctive proliferation follows the centripetal migration of squamous cells [6, 7].
Cell proliferation starts at the centre of the tympanic membrane and is moving towards the defect [7]. This process is modulated by several local factors like EGF, TGF-α, and PDGF [7, 8]. This healing pattern is contrary to the regenerative processes of the skin where squamous cells migrate at the surface of a fibrous supportive tissue which precedes the development of the epithelial layer [5, 7].
Immediate effects of cryotherapy on the squamous epithelium include both reversible alterations such as vacuolization of the cytoplasm or fragmentation of the mitochondria, and irreversible injuries within the nucleus 11 like alteration of chromatin distribution 12 and coagulation necrosis [9, 10]. Cryotherapy generates intracellular and extra cellular water crystallization, alteration of cellular electrolytes distribution. An alteration of cell membrane function brings about intracellular penetration of Na and Cl, cell dehydration, enzymes inhibition, cell metabolism collapse and consecutive lipoprotein distorsion in cell membranes [10], and secondary tissue hypoxia [11].
Cryotherapy involves not only the epithelium but also the sub epithelial structures like the connective tissue [11] and the vascular network in the lamina propria [12, 13]. An important inflammatory reaction follows these tissue injuries. Its landmarks include acute vascular phenomena and white cells migration, and finally, granulation tissue occurrence as a healing phenomenon [14, 15].
The healing phenomena associated with cryotherapy are a function of the depth of thermal injury. Nevertheless, cryotherapy induces a more intense vascular proliferation and, paradoxically, slows the healing processes when compared to mechanical injuries [13]. Thrombosis and vascular injury, in capillaries, arterioles and veins seem to be the principal mechanism for tissue injury following cryotherapy [14]. Following cryotherapy induced injury, platelet factors like VEGF, PDGF, IL-6, TGF-β or thrombin stimulate thrombosis and angiogenesis being strongly and proportionally linked together [14, 15].
It has been suggested that 2–3 weeks of middle ear aeration might be adequate for the treatment of serous otitis media [16]. We have demonstrated in this experiment that cryotherapy is able to significantly increase the patency time of cold instrument myringotomy. It was demonstrated that the use of CO2 laser is associated with higher patency rates [16]. We can suppose that the use of CO2 laser in combination with cryotherapy may bring about longer patency times of the myringotomies.
Conclusion
Cryotherapy proved to be a safe and effective method in delaying closure of myringotomies in rats. The healing processes of the cryotreated eardrums last longer and the patency of myringotomy was longer as compared to untreated myringotomies.
Conflict of Interest
The authors declare that they have no conflict of interest. No funding was received for this study.
References
- 1.Bluestone CD, Klein JO. Otitis media, atelectasis, and eustachian tube dysfunction. In: Bluestone CD, Stool S, editors. Pediatric otolaryngology. 3. Philadelphia: WB Saunders Co; 1996. pp. 540–548. [Google Scholar]
- 2.Ah-Tye C, Paradise JL, Colborn DK. Otorrhea in young children after tympanostomy-tube placement for persistent middle-ear effusion: prevalence, incidence, and duration. Pediatrics. 2001;107(6):1251–1258. doi: 10.1542/peds.107.6.1251. [DOI] [PubMed] [Google Scholar]
- 3.Jassir D, Buchman CA, Gomez-Marin O. Safety and efficacy of topical mitomycin C in myringotomy patency. Otolaryngol Head Neck Surg. 2001;124:368–373. doi: 10.1067/mhn.2001.114255. [DOI] [PubMed] [Google Scholar]
- 4.Gocea A, Taulescu M, Trombitas V, Albu S. Effects of cryotherapy on the maxillary antrostomy patency in a rabbit model of chronic rhinosinusitis. Biomed Res Int. 2013;2013:101534. doi: 10.1155/2013/101534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somers T, Goovaerts G, Schelfhout L, Peeters S, Govaerts PJ, Offeciers E. Growth factors in tympanic membrane perforations. Otol Neurotol. 1998;19(4):428–434. [PubMed] [Google Scholar]
- 6.Boedts D, Ars B. Histopathological research on eardrum perforations. Arch otorhinolaryngol. 1977;215(1):55–59. doi: 10.1007/BF00463191. [DOI] [PubMed] [Google Scholar]
- 7.Makino K, Amatsu M, Kinishi M, Mohri M. Epithelial migration in the healing process of tympanic membrane perforations. Eur Arch Otorhinolaryngol. 1990;247(6):352–355. doi: 10.1007/BF00179005. [DOI] [PubMed] [Google Scholar]
- 8.Ma Y, Zhao H, Zhou X. Topical treatment with growth factors for tympanic membrane perforations: progress towards clinical application. Acta Otolaryngol. 2002;122(6):586–599. doi: 10.1080/000164802320396259. [DOI] [PubMed] [Google Scholar]
- 9.Larson TR, Rrobertson DW, Corica A, Bostwick DG. In vivo interstitial temperature mapping of the human prostate during cryosurgery with correlation to histopathologic outcomes. Urology. 2000;55(4):547–552. doi: 10.1016/S0090-4295(99)00590-7. [DOI] [PubMed] [Google Scholar]
- 10.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37(3):171–186. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 11.Goertz O, Baerreiter S, Ring A, Jettkant B, Hirsch T, Daigeler A, Langer S. Determination of microcirculatory changes and angiogenesis in a model of frostbite injury in vivo. J Surg Res. 2011;168(1):155–161. doi: 10.1016/j.jss.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Pimente CB, Moraes AMD, Cintra ML. Angiogenic effects of cryosurgery with liquid nitrogen on the normal skin of rats, through morphometric study. Anais brasileiros de dermatologia. 2014;89(3):410–413. doi: 10.1590/abd1806-4841.20142249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kardos TB, Ferguson MM. Comparison of cryosurgery and the carbon dioxide laser in mucosal healing. Int J Oral Maxillofac Surg. 1991;20(2):108–111. doi: 10.1016/S0901-5027(05)80720-9. [DOI] [PubMed] [Google Scholar]
- 14.Carmeliet P. Clotting factors build blood vessels. Science. 2001;293(5535):1602–1604. doi: 10.1126/science.1064981. [DOI] [PubMed] [Google Scholar]
- 15.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 16.Estrem SA, Batra PS. Preventing myringotomy closure with topical mitomycin C in rats. Otolaryngol Head Neck Surg. 1999;120(6):794–798. doi: 10.1016/S0194-5998(99)70316-5. [DOI] [PubMed] [Google Scholar]