Abstract
Obstructive sleep apnea/hypopnea syndrome (OSAHS) is an important and more common public health problem with increasing incidence. Polysomonography (PSG) is the gold standard test in OSAHS diagnosis. Apnea-hypopnea index (AHI) is the main parameter of PSG, which is correlated with OSAHS severity. The main complaint of OSAHS patients is daytime sleepiness and the Epworth Sleepiness Scale (ESS) used for evaluation of disease severity. The correlation of AHI with daytime sleepiness and ESS is well known. But there are many patients, which have uncorrelated daytime sleepiness with AHI. This data calls this hypothesis; Are there any other parameters which may affect daytime sleepiness. 648 patients with complaining of snoring and apnea were evaluated by polysomnography and anthropometric measurements. The cut-off value of ESS was accepted 10 as an indicator of severe daytime sleepiness. Patients were divided to groups with the aim of homogenization, according to AHI values. The patients with similar AHI values were analyzed according to their ESS scores. BMI and neck circumference were elevated in daytime sleepiness patients. The nocturnal hypoxemia markers; apnea number/index, maximum duration of apnea, at least SO2 concentration, duration of SO2 less than 90 % were much effected in the group of daytime sleepiness. Beside the fact that our research, AHI is not enough for predicting the daytime sleepiness; anthropometric measurements and the nocturnal hypoxemia markers should be evaluated.
Keywords: Obstructive sleep apnea syndrome, Daytime sleepiness, Epworth Sleepiness Scale, Nocturnal hypoxemia
Introduction
Obstructive sleep apnea/hypopnea syndrome (OSAHS) is characterized with intermittent, complete or partial obstruction of airway during sleep with the incidence of 2 % in the middle aged women and 4 % in middle aged men [1]. Patients’ complaints are mainly daytime sleepiness, snoring and apnea. The gold standard test is polysomnography (PSG) for detecting OSAHS [2]. The main parameter of PSG is apnea/hypopnea index (AHI) with the correlation of disease severity [3]. Many patients’ disease severity can be predictable according to AHI values. A high AHI value may be thought a severe daytime sleeping complaint vice versa a low AHI value may be thought a mild or not daytime sleeping complaint. However, there are many patients who have uncorrelated AHI values and daytime sleepiness. This situation is the limitation of the gold standard test. There are many patients who have severe daytime sleepiness with low AHI values; vice versa patients have high AHI values with no complaint of daytime sleepiness. The current data are not enough to explain its pathophysiology. Clinicians need additional data for evaluating these patients.
We aimed to investigate PSG parameters in the patients who have uncorrelated daytime sleepiness and AHI values.
Materials and Methods
Study Design
We analyzed 648 patients according to PSG parameters, daytime sleepiness, age, gender, BMI, neck and abdominal circumference retrospectively. The ethic committee approval was taken from The Ethic Commission of Diskapi Yildirim Beyazit Training and Research Hospital prior to study. Epworth Sleepiness Scale (ESS) was applied for evaluating the daytime sleepiness. The patients were accepted as a mild daytime sleepiness that has 10 or lower than 10 points in ESS. The patients were accepted as a severe daytime sleepiness that has more than 10 points in ESS. A standard form was used in routine ENT examination. The patients’ mallampati scores, palatine tonsil sizes, posterior tonsillar plica and uvula sizes were assessed. A routine arterial blood gas analysis and spirometry tests were performed for evaluating the other co morbid diseases. Spirometry test abnormalities and affected daytime arterial blood gas analysis like hypoxia, hypercarbia were accepted as exclusion criteria. The patients were not accepted to study that have chronic obstructive pulmonary disease and obesity hypoventilation syndrome. The routine PSG test evaluated which describes sleep time, sleep efficacy, sleep periods, apnea/hypopnea number, apnea/hypopnea index, obstructive-central-mix sleep apnea number, hypopnea number, mean apnea time, the longest apnea time, mean hypopnea time, the longest hypopnea time, mean SO2 concentration, the lowest SO2 concentration, the time of SO2 concentration under than 90 %, leg movements, PLM index, arousal number, arousal index parameters.
Patients ESS and AHI values were analyzed. Patients were classified according to AHI and ESS values. Patients in the same groups according to AHI values were accepted as homogenised about disease severity. Each group divided into two sub-groups according to ESS. Subgroups were assessed about PSG parameters and anthropometric measurements. The crosshatch analyses were used for elucidating the parameters which make the differences in daytime sleepiness.
Patients
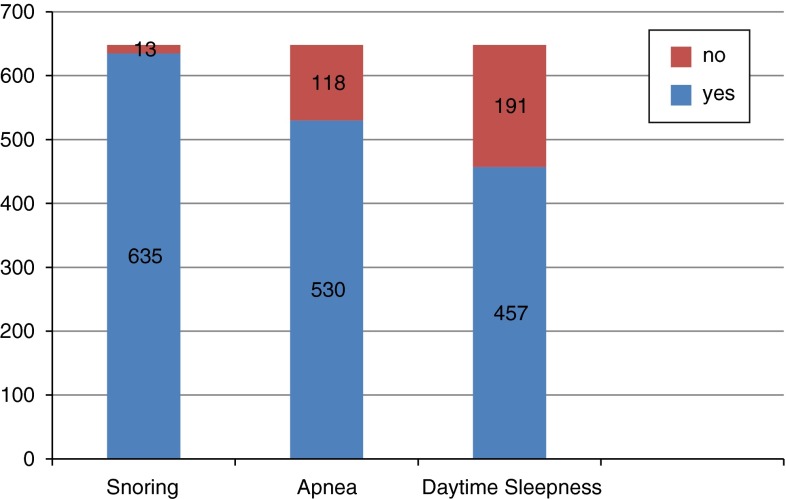
There are 648 patients, 467 male and 181 female with the 47, and 74 ± 11.32 median age (Fig. 1). Snoring was observed in 635 patients, apnea in 530 patients, and daytime sleepiness in 457 patients (Fig. 2). The mean complaining time was 89.42 ± 85.64 months.
Fig. 1.
Gender disturbance
Fig. 2.
Complaining disturbance
The mean height, weight and BMI were 167 ± 9.22, 85 ± 14, 30.34 ± 5.63 respectively. The mean neck circumference was 40.76 ± 4.09. The mean abdominal circumference was 97.30 ± 20.34 (Table 1).
Table 1.
Anthropometric measurement
| Mean | Std. deviation | Minimum | Maximum | |
|---|---|---|---|---|
| Height | 167 | 9.22 | 115 | 192 |
| Weight | 85 | 14 | 42 | 140 |
| BMI | 30.34 | 5.63 | 18 | 62 |
| Neck circumference | 40.76 | 4.09 | 28 | 54 |
| Abdominal circumference | 97.30 | 20.34 | 54 | 151 |
The mean ESS score was 10.90 ± 6 (0–24). Daytime sleepiness was accepted as a mild in the ESS < 10 patients. Daytime sleepiness was accepted as a severe in the patients who have ESS ≥ 10. Patients were divided into two groups according to AHI; 30 was accepted as a cut-off value. Disease severity was accepted severe in the group of AHI ≥ 30; AHI < 30 as a mild or moderate. Then crosshatch analyses were performed in the four subgroups (Table 2). Group I includes the patients who have AHI < 30 and ESS ≤ 10; group II, AHI < 30 and ESS > 10. Group III includes the patients who have AHI ≥ 30 and ESS ≤ 10; group II, AHI ≥ 30 and ESS > 10. There are 253 patients in the group I, 252 patients in the group II, 49 patients in the group II and 94 patients in the group IV.
Table 2.
Patient disturbance according to AHI and ESS
| AHI < 30 | AHI ≥ 30 | |
|---|---|---|
| ESS ≤ 10 | Group I (253) | Group III (49) |
| ESS > 10 | Group II (252) | Group IV (94) |
Statistical Analyses
SPSS-15 was used for statistical analyses. One-way ANOVA test was used for comparing the groups.
Results
Group II was statistically significantly different from group I according to neck circumference; S2 sleep period and arousal index (Table 3). The mean neck circumference was 39.89 ± 4.01 in the group I and 40.06 ± 3.36 in the group II (p < 0.05, one-way ANOVA). The mean S2 sleep period was 73.90 ± 64.22 in the group I, 78.79 ± 74.17 in the group II (p < 0.05, one-way ANOVA). The mean arousal index was 18.45 ± 2.16 in the group I, 26.20 ± 60.13 in the group II (p < 0.05, one-way ANOVA).
Table 3.
Statistical compeering of group I and group II
| AHI < 30; ESS ≤ 10 | AHI < 30; ESS > 10 | P value | |
|---|---|---|---|
| (Group I) | (Group II) | ||
| BMI | 39.95 ± 9.9 | 30.15 ± 6.73 | 0.09 |
| Age | 47.96 ± 10.98 | 46.52 ± 11.76 | 0.19 |
| Neck circumference | 39.89 ± 4.01 | 40.06 ± 3.36 | 0.00 |
| S1 | 9.12 ± 11.8 | 11.65 ± 28.18 | 0.16 |
| S2 | 73.90 ± 64.22 | 78.79 ± 74.17 | 0.01 |
| Obst apnea | 11.93 ± 18.68 | 13.25 ± 26.39 | 0.18 |
| Hypopnea | 36.86 ± 32.19 | 41.84 ± 36.92 | 0.06 |
| Mean apnea duration | 11.98 ± 8.15 | 11.90 ± 6.44 | 0.06 |
| The longest apnea duration | 19.48 ± 17.07 | 18.91 ± 14.71 | 0.05 |
| Mean hypopnea duration | 14.74 ± 5.62 | 15.17 ± 5.10 | 0.66 |
| The longest hypopnea duration | 30.43 ± 19.73 | 33.72 ± 20.68 | 0.21 |
| OD | 15.27 ± 21.03 | 15.77 ± 23.94 | 0.72 |
| Mean SO2 | 92.98 ± 3.37 | 92.50 ± 4.17 | 0.09 |
| The lowest SO2 saturation | 81.04 ± 12.75 | 81.56 ± 10.38 | 0.42 |
| Mean desaturation | 5.53 ± 5.39 | 5.19 ± 1.15 | 0.13 |
| The time of SO2 ≤ 90 | 11.88 ± 26.94 | 15.98 ± 53.16 | 0.07 |
| PLM | 2.65 ± 7.93 | 2.79 ± 7.60 | 0.95 |
| Arousal | 119.07 ± 148.60 | 127.24 ± 149.155 | 0.46 |
| Arousal index | 18.45 ± 2.16 | 26.20 ± 60.13 | 0.01 |
Bold values signify P < 0.05 value accepted as a meaningful difference (Confident interval: 95 %)
Group IV was statistically significantly different from group III according BMI, S2 sleep period, obstructive apnea number, the lowest SO2 saturation, the time of SO2 ≤ 90 (Table 4). The mean BMI was 31.24 ± 4.47 in the group III, 33.69 ± 9.37 in the group IV (p < 0.05, one-way ANOVA). The mean S2 sleep period was 72.26 ± 67.04 in the group III, 84.98 ± 89.14 in the group IV (p < 0.05, one-way ANOVA). The mean obstructive apnea number was 123.83 ± 141.73 in the group III, 199.98 ± 197.87 in the group IV (p < 0.05, one-way ANOVA). The mean value of the lowest SO2 saturation was 74.46 ± 11.35 in the group III, 69.76 ± 11.91 in the group IV (p < 0.05, one-way ANOVA). The mean value of the time of SO2 ≤ 90 was 29.01 ± 33.05 min in the group III, 45.18 ± 37.88 min in the group IV (p < 0.05, one-way ANOVA).
Table 4.
Statistical compeering of group III and group IV
| AHI ≥ 30; ESS ≤ 10 | AHI ≥ 30; ESS > 10 | P value | |
|---|---|---|---|
| (Group III) | (Group IV) | ||
| BMI | 31.24 ± 4.47 | 33.69 ± 9.37 | 0.04 |
| Age | 48.30 ± 49.36 | 49.36 ± 10.81 | 0.14 |
| Neck circumference | 43.17 ± 4.25 | 43.43 ± 3.84 | 0.45 |
| S1 | 10.33 ± 12.48 | 14.46 ± 38.07 | 0.28 |
| S2 | 72.26 ± 67.04 | 84.98 ± 89.14 | 0.00 |
| Obst apne | 123.83 ± 141.73 | 199.98 ± 197.87 | 0.00 |
| Hypopnea | 103.22 ± 61.98 | 104.08 ± 79.04 | 0.10 |
| Mean apnea duration | 18.45 ± 5.47 | 19.11 ± 5.62 | 0.49 |
| The longest apnea duration | 45.41 ± 52.00 | 24.11 ± 29.29 | 0.50 |
| Mean hypopnea duration | 17.45 ± 3.82 | 17.66 ± 6.28 | 0.18 |
| The longest hypopnea duration | 49.12 ± 26.85 | 50.14 ± 27.70 | 0.33 |
| OD | 60.74 ± 97.26 | 86.18 ± 93.73 | 0.23 |
| Mean SO2 | 91.02 ± 4.49 | 93.61 ± 59.85 | 0.23 |
| The lowest SO2 saturation | 74.46 ± 11.35 | 69.76 ± 11.91 | 0.01 |
| Mean desaturation | 8.54 ± 9.51 | 9.76 ± 8.25 | 0.61 |
| The time of SO2 ≤ 90 | 29.01 ± 33.05 | 45.18 ± 37.88 | 0.02 |
| PLM | 2.19 ± 4.61 | 1.99 ± 3.90 | 0.57 |
| Arousal | 86.10 ± 145.14 | 105.01 ± 157.97 | 0.28 |
| Arousal index | 12.51 ± 21.42 | 16.94 ± 32.60 | 0.23 |
Bold values signify P < 0.05 value accepted as a meaningful difference (Confident interval: 95 %)
Discussion
PSG is the gold standard test for evaluating OSA, but it is not enough to explain some patients’ DS. Some patients have severe OSA according to AHI values despite none DS; some patients have mild OSA according to AHI values despite severe DS. This situation is the limitation of the gold standard test and AHI values which is the main parameter of PSG. The ethiopathogenesis of OSA is not completely clear yet. Is not it the main determining parameter for predicting DS, apnea + hypopnea index? Do they have the same severity of disease; a patient has 40 apnea indexes with no hypopnea and a patient has 40 hypopnea index with no apnea? We investigated to explain these questions in this research.
We tried to make homogenization in the groups with classification the all patients according to AHI values. Then the homogeny groups were divided into two groups according to their ESS scores. ESS was described in 1991 as a simple questionnaire form by Johns [3]. Many clinicians use it for evaluating the DS. It depends on patients’ cultures, daily habits and socioeconomic situations [4]. Karakoç et al. [4] were reported as an association of ESS and AHI value in 264 moderate/severe OSA patients. A score of more than 10 is accepted as a severe DS in many investigations.
The parameter were analyzed which may influence the DS. The results were associated with that BMI, neck circumference, obstructive apnea number, the longest apnea duration, the lowest SO2 concentration, the time of SO2 lower than 90 %, mean apnea duration, OD may influence the DS in the 95 % confident interval. Many parameters are related with nocturnal hypoxemia. This result indicates the main parameter of the DS is nocturnal hypoxemia.
Obesity is a well-known risk factor for OSA. Airway obstruction occurs due to increased adipose tissue on the neck. Increased BMI may affect the severity of OSA. Katz et al. [5] reported the association of BMI and neck circumference with AHI. This situation points out the possible role of obesity hypoventilation syndrome (OHS). OHS is characterized with; morbid obesity, daytime sleepiness, morning headaches, depressed mood, memory and learning problems, hypoxemia and hypercarbia at blood gas analysis [6]. We excluded the patients who have hypoxia or hypercarbia at daytime arterial blood gas analyses for avoiding the OHS. We detected that, patients have severe DS who have increased BMI in the same groups. It indicates the obesity as an independent risk of DS.
The main complaint of OSA patients is DS. Its pathophysiology is not clear yet [7]. We can observe variated degree of DS at the patients who have same AHI values [8]. If we accept the AHI as a main determining factor of disease severity, we assume the same influence of apnea and hypopnea on the disease. We detect that apneas influence the DS more than hypopnea in our investigation.
Sleep stages disturbance may be changed by severity of OSAHS. Severe patients couldn’t pass the deep sleep (S3, S4, and REM) with increased duration of S1 and S2 stages. As a result of this pathologic disturbance, patients have decreased sleep quality with increased DS. Increased slow wave sleep duration was detected in the severe patients in our investigation.
Mediano et al. have been reported the association of nocturnal hypoxemia with DS. They advocated the nocturnal hypoxemia as a main determining factor of DS [9, 10]. Intermittent hypoxemia during sleep may start the oxidative stress and chronic hypoxemia. Recently published investigations report that the neurovascular damage and apoptosis via oxidative stress and chronic hypoksemia [11].This data supports the association of OSA with DM, HT and HL. Roure et al. [7] reported an effective and longer sleep period with better nocturnal okigenisation in mild DS patients than severe DS patients. Another report advocated the association of DS with daytime PaPO2 concentration [12]. Our results indicate the increased nocturnal hypoxemia in patients who have severe DS. A statistical significantly difference was found at the longest apnea duration, the lowest SO2 concentration, duration of SO2 lower than 90 %.
The limitation of our study is that patients were not evaluated about their co-morbid diseases.
AHI value is not enough alone for predicting DS according to our results and literature. When we mainly use the AHI value for classification of OSAHS, we accept the same effectively of apnea and hypopnea to disease severity. Our results indicate that, apnea dependent patients have more DS than hypopnea dependent patients. We found the nocturnal hypoxemia as an important parameter for predicting of DS. These findings may explain the patients’ DS who have extensive nocturnal hypoxemia intervals with mild AHI values.
The association of obesity and OSA is well known. We analyzed the patients who have same AHI values. Our results indicate the extensive DS in patients who have more much BMI and neck circumference.
Conclusion
We report that, AHI is not enough for predicting DS; anthropometric measurements, apnea/hypopnea index and nocturnal hypoxemia should be evaluated.
Conflict of interest
There is no conflict of interest for any authors in this study.
References
- 1.Carlson J, Davies R, Ehlen ZK, et al. Obstructive sleep apnea and blood pressure elevation : what is the relation? Blood Press. 1993;2:166–182. doi: 10.3109/08037059309077548. [DOI] [PubMed] [Google Scholar]
- 2.Dahlqvist J, Dahlqvis A, Marklund M, et al. Physical findings in the upper airways related to obstructive sleep apnea in men and women. Otolaryngology. 2007;127:623–630. doi: 10.1080/00016480600987842. [DOI] [PubMed] [Google Scholar]
- 3.Jons MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 4.Karakoç Ö, Akçam T, Gerek M, et al. Horlama ve obstüriktif uyku apneli hastalarda epworth uykululuk skalasının güvenilirliği. KBB Forum. 2007;6:86–89. [Google Scholar]
- 5.Katz I, Stradling J, Slutsky AS, et al. Do patients with obstructive sleep apnea have thick necks? Am Rev Respir Dis. 1990;141:1228–1231. doi: 10.1164/ajrccm/141.5_Pt_1.1228. [DOI] [PubMed] [Google Scholar]
- 6.Chau EH, Lam D, Wong J, et al. Obesity hypoventilation syndrome: a review of epidemiology, pathophysiology, and perioperative considerations. Anesthesiology. 2012;117:188–205. doi: 10.1097/ALN.0b013e31825add60. [DOI] [PubMed] [Google Scholar]
- 7.Roure N, Gomez S, Mediano O, et al. Daytime sleepiness and polysomnography in obstructive sleep apnea patients. Sleep Med. 2008;9:727–731. doi: 10.1016/j.sleep.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Banno K, Kryger MH. Sleep apnea: clinical investigations in humans. Sleep Med. 2007;8(4):400–426. doi: 10.1016/j.sleep.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Mediano O, Barceló A, de la Peña M, et al. Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur Respir J. 2007;30:110–111. doi: 10.1183/09031936.00009506. [DOI] [PubMed] [Google Scholar]
- 10.Chen R, Xiong KP, Lian YX, et al. Daytime sleepiness and its determining factors in Chinese obstructive sleep apnea patients. Sleep Breath. 2011;15:129–135. doi: 10.1007/s11325-010-0337-4. [DOI] [PubMed] [Google Scholar]
- 11.Zhan G, Fenik P, Pratico D, Veasey SC, et al. Inducible nitric oxide synthase in long-term intermittent hypoxia: hypersomnolence and brain injury. Am J Respir Crit Care Med. 2005;171:1414–1420. doi: 10.1164/rccm.200411-1564OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong ZQ, Tao Y, Zhang ZP, et al. Relationship between excessive daytime sleepiness and oxygen saturation in obstructive sleep apnea-hypopnea syndrome. Zhonghua Yi Xue Za Zhi. 2011;4(91):40–43. [PubMed] [Google Scholar]