Abstract
Hepatic sinusoidal obstruction syndrome (SOS) is an obliterative venulitis of the terminal hepatic venules, which in its more severe forms imparts a high risk of mortality. SOS, also known as veno-occlusive disease (VOD), occurs as a result of cytoreductive therapy prior to hematopoietic stem cell transplantation (HSCT), following oxaliplatin-containing adjuvant or neoadjuvant chemotherapy for colorectal carcinoma metastatic to the liver and treated by partial hepatectomy, in patients taking pyrrolizidine alkaloid-containing herbal remedies, and in other particular settings such as the autosomal recessive condition of veno-occlusive disease with immunodeficiency (VODI). A central pathogenic event is toxic destruction of hepatic sinusoidal endothelial cells (SEC), with sloughing and downstream occlusion of terminal hepatic venules. Contributing factors are SEC glutathione depletion, nitric oxide depletion, increased intrahepatic expression of matrix metalloproteinases and vascular endothelial growth factor (VEGF), and activation of clotting factors. The clinical presentation of SOS includes jaundice, development of right upper-quadrant pain and tender hepatomegaly, ascites, and unexplained weight gain. Owing to the potentially critical condition of these patients, transjugular biopsy may be the preferred route for liver biopsy to exclude other potential causes of liver dysfunction and to establish a diagnosis of SOS. Treatment includes rigorous fluid management so as to avoid excessive fluid overload while avoiding too rapid diuresis or pericentesis, potential use of pharmaceutics such as defibrotide, coagulolytic agents, or methylprednisolone, and liver transplantation. Proposed strategies for prevention and prophylaxis include reduced-intensity conditioning radiation for HSCT, treatment with ursodeoxycholic acid, and inclusion of bevacizumab with oxaliplatin-based chemotherapeutic regimes. While significant progress has been made in understanding the pathogenesis of SOS and in mitigating against its adverse outcomes, this condition remains a serious complication of a selective group of medical treatments.
Keywords: Liver, Hematopoietic stem cell transplantation, Oxaliplatin, Colorectal cancer, Herbal remedies
Abbreviations: AML, acute myeloid leukemia; APRI, aspartate aminotransferase to platelet ratio; AST, aspartate aminotransferase; Bmab, bevacizumab; DF, defibrotide; FOLFOX, chemotherapy regimen containing Folinic acid, 5-Fluorouracil, and Oxaliplatin; GO, gemtuzumab ozogamicin; GSTM1, glutathione S-transferase M1; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation; l-NAME, N(G)-nitro-l-arginine methyl ester; MOF, multi-organ failure; PML, promyelocytic leukemia protein; RIC-HSCT, reduced-intensity conditioning hematopoietic stem cell transplantation; RILD, radiation-induced liver disease; RT, radiation therapy; SEC, sinusoidal endothelial cells; s-ICAM-1, soluble intercellular adhesion molecular-1; SOS, sinusoidal obstruction syndrome; TBI, total body irradiation; TIPS, transjugular intrahepatic porto-systemic shunt; t-PA, tissue plasminogen activator; UPLC-MS, ultra-performance liquid chromatography-mass spectrometry; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; VOD, veno-occlusive disease; VODI, veno-occlusive disease with immunodeficiency; V-PYRRO/NO, O(2)-vinyl 1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate; v-WF, von Willebrand factor
Obliterative endophlebitis in the terminal hepatic veins of the human liver lobule was first reported by a pathologist from Prague in 1905, with the only etiologic suggestion being syphilis.1 In 1954, terminal vein lesions were described in Jamaican drinkers of bush tea, characterized by obliteration of hepatic vein radicals by varying amounts of subendothelial swelling and fine reticulated tissue.2 At later stages, a fibrous pericentral scar developed. In the early 1960's, studies of the effects of ionizing radiation on mammalian tissues documented that the hepatic vasculature could be damaged by this mechanism,3 in the absence of antecedent vascular thrombosis.4,5 The most striking example of an obliterative venous lesion induced by irradiation was documented in humans with lung tumors receiving radiation treatment; both the lung vasculature and that of the dome of the liver that was included in the radiation field developed vascular obliteration, but not the remainder of the unexposed liver.6 Shortly thereafter, induction of obliterative venopathy following heavy irradiation directly of the human liver for metastatic disease was reported in 12 patients receiving upper abdominal irradiation by the Stanford Linear Accelerator.7 Thus, by the mid-1960s, the concept of hepatic veno-occlusive disease was well-established, induced by either chemical or radiation toxicity, and as a lesion separate from Budd-Chiari syndrome and Banti syndrome.8,9
In the late 1970's, similar histologic lesions were reported from outbreaks in India and Israel, attributed to contamination of wheat and traditional herbal remedies with plant toxins.10,11 The histological lesions resembled previously described hepatic veno-occlusive lesions described in rats poisoned with Senecio plant extracts12 or Crotolaria.13 This form of liver toxicity was ultimately attributed to hepatic exposure to plant pyrrolizidine alkaloids,14 establishing these plant toxins as the cause of veno-occlusive disease in users of herbal teas.
Bone marrow transplantation for humans with leukemias became a therapeutic option during the 1960s. Initial challenges to this new therapy were preservation of harvested marrow, and achieving successful marrow engraftment.15 Reports of hepatic veno-occlusive disease in patients undergoing bone marrow transplantation emerged in the 1970's,16 followed by numerous reports which established the following apparent risk factors: bone marrow transplantation for malignancy, involving intense chemotherapeutic and radiation conditioning regimens; patient age over 15 years; and in particular, abnormal pretransplant serum levels of liver enzymes.17–19 The presence of metastatic liver disease in patients undergoing bone marrow transplantation for solid tumors and lymphomas also predisposed to veno-occlusive disease.20,21 In these initial years after recognition of veno-occlusive disease as a complication of induction regimes prior to bone marrow transplantation, the incidence of veno-occlusive disease varied from 21% to 25% in allogeneic graft recipients,19,21,22 to 5% in recipients of autologous marrow.20,23,24 In the four decades since routine use of bone marrow transplantation for solid malignancies, lymphomas and leukemias, induction regimes and therapies have helped improve, but not eliminate, the incidence of this condition in the transplant population. Its incidence now is primarily in the setting of hematopoietic stem cell transplantation, but SOS may occur in other settings as well (Table 1).
Table 1.
Causes of Sinusoidal Obstruction Syndrome (SOS).
| Hematopoietic stem cell transplantation (HSCT) Adjuvant or neoadjuvant chemotherapy with hepatectomy for metastatic liver disease Radiation-induced liver disease Chemotherapy for acute myeloid leukemia (AML) Liver transplantation Use of herbal remedies Veno-occlusive disease with immunodeficiency (VODI) |
Pathogenesis
Experimental Investigation in Animals
Initial experimental efforts to induce veno-occlusive disease in animals focused on irradiation. Although the non-human primate liver is relatively resistant to radiation-induced veno-occlusive disease,25 veno-occlusive lesions could be induced in primates26 and in non-primates27 by exposure to chronic irradiation regimes. However, the underlying pathogenesis of this condition was not elucidated with these early experimental models. Careful ultrastructural examination of human tissues suggested that the initial morphologic change in hepatic veno-occlusive disease was obstruction at the level of hepatic sinusoids, followed by obliteration of the terminal hepatic veins.28 This observation was confirmed with the report in 1999 of a more economical and reproducible rodent model of veno-occlusive disease.29 In rats gavaged with the pyrrolizidine alkaloid monocrotaline and killed between days 1–10 after exposure, the earliest documented change was damage to the hepatic sinusoids; fibrosis and obliteration of the terminal hepatic veins occurred as a subsequent lesion. This articulation that toxic injury to the hepatic sinusoids was the fundamental lesion of hepatic veno-occlusive disease led to its being renamed sinusoidal obstruction syndrome (SOS).30
The rat model of SOS may be summarized as follows.29,31 Following a single gavage with monocrotaline, within 24 h–48 h there is ultrastructural evidence of damage to hepatic sinusoidal endothelial cells (SEC), but little clinical or histological evidence of hepatic toxicity. By days 3–5 (early SOS), manifestations of sinusoidal obstruction are severe, with severe centrilobular necrosis and hemorrhage, damage to endothelial cells of the terminal hepatic veins, subendothelial hemorrhage, and ultrastructural evidence of extensive destruction of the sinusoidal wall. The clinical manifestations are hepatomegaly, ascites, and hyperbilirubinemia. By days 6–7 (late SOS), the characteristic subendothelial and adventitial fibrosis of terminal hepatic veins becomes evident. There is continued sinusoidal and subendothelial hemorrhage, but gradual resolution of the ultrastructural evidence of damage to the sinusoidal endothelial cells. By days 8–10, SOS has resolved completely in some animals, or persisted as a severe pattern of hepatic damage in others.
Detailed study of the first hours after monocrotaline exposure31 reveal that SECs become swollen, with increased adhesion of leukocytes to the endothelium. Red blood cells dissect beneath the endothelial cells and into the space of Disse, separating the endothelial cells from the underlying hepatocytes and permitting free access of blood to the parenchymal space. In contrast, blood flow in the restricted sinusoidal channel becomes sluggish. The sinusoid is eventually obstructed by an embolism of aggregated sinusoidal lining cells, red blood cells, and adherent monocytes. Kupffer cells are lost along the sinusoidal lining, and are replaced with an influx of circulating phagocytic monocytes which accumulate in the injured centrilobular area. The specific toxicity of monocrotaline is that of its reactive metabolite, monocrotaline pyrrole, which binds covalently to the actin microfilaments of endothelial cells.10 The F-actin depolymerizes leading to disassembly of the actin cytoskeleton and rounding up of the endothelial cells. Increased expression and release of matrix metalloproteinase-9 into the extracellular space leads to breakdown of the extracellular matrix in the space of Disse, permitting further dehiscence of the endothelial cells.31
These experiments gave rise to the premise that SECs are more susceptible to toxic injury than hepatocytes. Key pathogenic factors include SEC glutathione depletion, nitric oxide depletion, increased expression of matrix metalloproteinases and vascular endothelial growth factor (VEGF), and activation of clotting factors.32 In the first instance, drugs leading to SOS are metabolized exclusively by the hepatocellular cytochrome P450 systems, for which glutathione is an antioxidant recovery mechanism. Hepatic exposure to these drugs leads to depletion of glutathione. Severe depletion of glutathione levels in SECs renders them susceptible to cell death; prophylactic infusion of glutathione or N-acetyl cysteine prevents the development of SOS in the monocrotaline-treated rat model31; post-hoc administration of glutathione reduces the degree of sinusoidal injury, but does not prevent it. Additional evidence in favor of the central role of glutathione depletion includes the predisposition to SOS in humans with a glutathione S-transferase M1 (GSTM1) null genotype who are undergoing bone marrow transplantation33 or receiving oxaliplatin for metastatic colon cancer.34 In the second instance, nitric oxide levels in the hepatic vein of rats decrease during induction of SOS,35 and induction of SOS can either be exacerbated by administration of N(G)-nitro-l-arginine methyl ester (l-NAME), an inhibitor of nitric oxide synthase, or be mitigated by infusion of V-YRRO/NO, a liver-selective nitric oxide donor prodrug,36 Beyond providing evidence for nitric oxide depletion in the pathogenesis of SOS,35 a role for vasoconstriction also is implicated, since adequate nitric oxide levels inhibit vasoconstriction of hepatic stellate cells, which invest the hepatic sinusoidal from within the space of Disse.37
Matrix metalloproteinase-9 expression increases early in the rat monocrotaline-induced SOS model; later there is a lower magnitude increase in matrix metalloproteinase-2. SECs are the major source of both basal and monocrotaline-induced enzyme expression and release. Administration of matrix metalloproteinase inhibitors prevents the development of experimental SOS.38 This implicates degradation of the extracellular matrix within the space of Disse as contributing to loss of endothelial cell adhesion.
Using the rat model of monocrotaline-induced SOS, DeLeve and associates also have demonstrated that constituitive bone marrow stem cell repair of sinusoidal endothelial cells (SEC) is critical to maintenance of the sinusoidal architecture.39 Bone marrow suppression by irradiation, by impairing this SEC repair mechanism, may contribute to the pathogenesis of SOS.
The role of VEGF as an angiogenic factor is less clearcut. Iguchi et al observed increased serum levels of VEGF during development of SOS in human patients,40 leaving open the question of whether VEGF-induced acceleration of vasopermeability, neovascularization, and/or expression of coagulopathic tissue factors on circulating mononuclear cells may play a role in SOS pathogenesis.32 Nakamura et al41 hypothesized that antiangiogenic agents may protect against SOS, and chose to use sorafenib to test this hypothesis. Sorafenib is a multiple receptor tyrosine kinase inhibitor that inhibits multiple tyrosine kinases including the VEGF receptor-2 (VEGFR-2) and -3 (VEGFR-3). Its primary use is as standard treatment for hepatocellular carcinoma and renal cell carcinoma, but it may also have an antifibrotic effect in the liver and prevent the development of portal hypertension.42,43 Nakamura treated rats with sorafenib before induction of SOS by monocrotaline prior to partial hepatectomy, and demonstrated significant suppression of the morphological features of SOS, with significant improvement in post-hepatectomy survival.41 While loss of endothelial cells was not completely blocked, it did suppress degradation of extracellular matrix in the space of Disse and uplift of the endothelial cells. Hence, although the link to VEGF is not proven owing to the broad inhibitory action of sorafenib on tyrosine kinases, remodeling of the extracellular matrix in the space of Disse is demonstrated to be a contributing factor to the dehiscence of sinusoidal endothelial cells in the development of SOS.
Recent technological advances in radiation therapy have sparked renewed interest in understanding radiation-induced liver disease (RILD)44 Although whole-liver irradiation has generally been restricted to 30–35 Gy in standard daily fractions of 1.8–2.0 Gy owing to risk of lethal RILD above these levels, intensity-modulated radiation therapy, 3-dimensional conformal radiation treatment planning, and organ and tumor motion tracking enable treatment of liver cancer with fewer but larger dose fractions.45 This innovation is called “hypofractionated stereotactic body RT”. Returning to the original Cynomolgus monkey model of RILD,26 Yannam et al44 recently conducted an escalated dose study to examine the effect of newer hypofractionated regimes on the liver. The authors demonstrated a higher tolerance for hypofractionated radiation, but that the characteristic histological lesions of SOS still developed at radiation doses above 40 Gy. However, a key additional finding was that metabolic stress on the liver, such as glucose loading or administration of total parenteral nutrition, produced substantial additional hepatic injury and generated hepatic failure. This points towards radiation-induced hepatocellular injury as a key component of RILD. Although this injury may be secondary to inadequate blood supply to perivenous hepatocytes per se, a broader level of injury to the parenchyma may also be operative owing to upstream impairment of sinusoidal blood flow.
In separate work, a new rodent model for oxaliplatin-induced SOS has been developed,46 owing to the importance of this form of chemotherapy for advanced colorectal cancer (vide infra). The model consisted of intra-peritoneal administration of FOLFOX (folinic acid, 5-fluorouracil, and oxaliplatin) to C57Bl/6 mice, based on the premise that FOLFOX induces endothelial damage and leads to a pro-thrombotic state within the liver. This new model may permit experimental examination of the role of oxidative stress in the pathogenesis of oxaloplatin-induced SOS, and potential amelioration or prevention of SOS by prophylactic administration of antioxidants. This model may also be important in exploring the role of the pro-thrombotic state in SOS,47 unlike the monocrotaline rodent model of SOS, in which there is no evidence of clotting abnormalities.29
Efforts to establish a rodent model of SOS arising from hematopoietic stem cell transplantation also have more recently met with success, using an allogeneic transplantation model involving male C57BL/6 male mice as donors and female BALB/c mice preconditioned with whole body irradiation as recipients.48 This gives further opportunity for productive future studies into the pathogenesis of SOS.
Clinical Pathogenesis of Sinusoidal Obstruction Syndrome
Experimental models of SOS in animals permitted examination of fundamental causes of sinusoidal destruction and vascular compromise. However, clinical investigation of human patients affected by SOS are still required, particularly since the induction pathways vary. Through the 1980s and 1990s, SOS was a complication of induction regimes for a broad set of patients undergoing allogeneic bone marrow transplantation. The fundamental premise was that, while SOS developed in the days and weeks following the actual bone marrow transplantation, it was the marrow ablation regimes prior to transplantation that were the actual cause of the hepatotoxicity. Accordingly, induction regimes were modified so as to reduce the incidence of SOS.
In the past decade, SOS has become primarily an issue with patients undergoing bone marrow stem cell transplantation for widely metastatic solid tumors, or intense neoadjuvant chemotherapy for cancer metastatic to the liver. The reported incidence of SOS in these two patient populations remains high, prompting continuing investigation into pathogenesis. An example is performance of gene expression profiling so as to examine potential mechanisms for oxaliplatin-induced SOS in humans (vide infra).49
A recent study reports on 98 patients undergoing preoperative chemotherapy for colorectal cancer metastatic to the liver followed by hepatic resection.50 Of these patients, 80 received preoperative chemotherapy. SOS occurred in 39 of these patients (39.8%), with development of SOS in patients receiving oxaliplatin-based neoadjuvant chemotherapy significantly higher than those receiving non-oxaliplatin-based chemotherapy. Histological examination of resected non-tumoral liver tissue showed increased CD34 immunohistochemical reactivity in sinusoids, the intensity of which correlated both with preoperative functional studies of indocyanine green retention, and severity of histological SOS. These authors conclude that sinusoidal capillarization, as evidenced by CD34 expression, may be a component of the hepatic functional deterioration in the setting of SOS.
Pathology
SOS may exhibit acute, subacute, and chronic features, depending upon when liver material is obtained. Unfortunately, as this disease is focal in nature, diagnostic features may not be evident on liver biopsy. In acute disease, there is striking centrilobular congestion, with centrilobular hepatocellular necrosis and accumulation of hemosiderin-laden macrophages (Figure 1). The terminal hepatic venules exhibit intimal edema, without obvious fibrin deposition or thrombosis. As discussed above, SOS arises from toxic injury to the sinusoidal endothelium.51 The cells round up and slough off the sinusoidal wall, embolizing downstream and obstructing sinusoidal blood flow. This is accompanied by dissection of erythrocytes into the space of Disse and downstream accumulation of cellular debris in the terminal hepatic vein. Proliferation of perisinusoidal stellate cells and subendothelial fibroblasts in the terminal hepatic vein follows, with deposition of extracellular matrix. Liver biopsies are almost never obtained during the acute stage.
Figure 1.
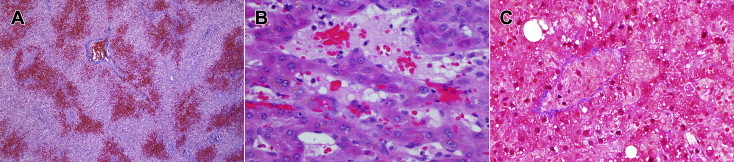
Early histology changes of sinusoidal obstruction syndrome. (A) Post-mortem liver: low power image showing massive pericentral congestion and hemorrhagic necrosis. Masson Trichrome, 4X. (B) Liver biopsy: high power image of pericentral region, showing subtotal occlusion of terminal hepatic venule, with entrapped erythrocytes. Hematoxylin & eosin, 400X. (C) Liver biopsy: high power image showing dilatation of sinusoids and necrosis of hepatocytes. Terminal hepatic vein is occluded, but collagen deposition has not yet occurred. Masson trichrome, 400X.
Over days to weeks (subacute), collagen deposition occurs in and around the affected terminal hepatic venule (Figure 2). This leads to progressive obliteration of the venule, which is easily identified with special stains for either collagen or reticulin.52 Obvious centrilobular congestion without readily identifiable terminal hepatic veins on hematoxylin and eosin stain should prompt performance of connective tissue stains to exclude SOS. With persistence of the SOS lesion into weeks to months (chronic), dense perivenular fibrosis radiating out into the parenchyma develops. The scar tissue contains hemosiderin-laden macrophages, and terminal hepatic vein lumina cannot be identified. Notably, congestion is minimal at this stage. There may be severe destruction of lobular parenchyma, and rarely evolution to cirrhosis.
Figure 2.
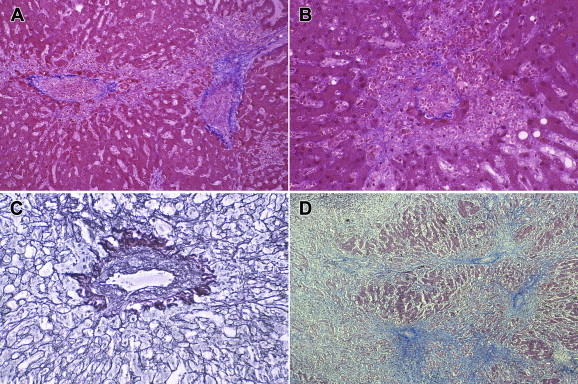
Sinusoidal obstruction syndrome of longer duration, post-mortem livers. (A) Medium power image showing deposition of loose fibrous tissue in pericentral region, with early formation of fibrous bridges to adjacent centrilobular areas. Masson trichrome, 40X. (B) Medium power image of terminal hepatic vein, showing complete loss of pericentral hepatocytes with sharp demarcation zone from viable hepatocytes in the middle of the lobule. The lumen of the terminal hepatic vein is completely occluded, and there is extravasation of erythrocytes in the pericentral space. Masson trichrome, 100X. (C) Higher power image of terminal hepatic vein, showing intraluminal deposition of extracellular matrix, with residual lumen. Reticulin stain, 200X. (D) Low power image of post-mortem liver with SOS of longer duration, with extensive destruction of the parenchyma and central-to-central fibrous bridging. Masson trichrome, 4X.
When liver biopsy is performed, either in the acute stage or during a more chronic stage, the histologic findings of SOS may be strikingly evident (Figure 3). That being said, the pathologist must be alert to SOS being the cause of these findings, since failure to recognize the centrilobular venopathy may lead to misinterpretation of the histologic findings.
Figure 3.
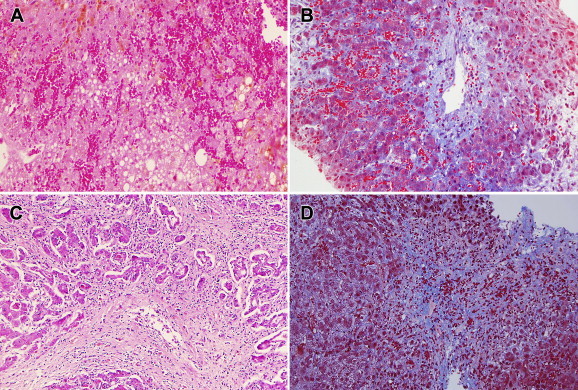
Liver biopsies. (A) Biopsy from patient with acute SOS, demonstrating extensive dilatation and congestion of sinusoids, and lack of evident terminal hepatic veins. Hematoxylin and eosin, 100X. (B) Same liver biopsy, showing terminal hepatic vein with narrowed lumen, intraluminal deposition of extracellular matrix with entrapped erythrocytes. Masson trichrome, 100X. (C) Biopsy from patient with chronic consumption of herbal teas, showing extensive destruction of pericentral parenchyma with deposition of fibrous tissue, inflammation, and partial obliteration of the terminal hepatic vein lumen. Hematoxylin and eosin, 100X. (D) Liver biopsy from a different patient, also consuming herbal teas, showing inapparent terminal hepatic vein, extensive extravasation of erythrocytes into pericentral parenchyma, and diffuse pericentral fibrosis. Masson trichrome, 100X.
Clinical considerations
Hematopoietic Stem Cell Transplantation
SOS developing after allogeneic hematopoietic stem cell transplantation (HSCT) typically occurs within the first month after HSCT, although later onset has been described.53 Conditioning regimens associated with onset of SOS typically consist of cyclophosphamide combined either with total body irradiation or busulfan.54
Liver disease occurs in up to 80% of patients following hematopoietic stem cell transplantation (HSCT) for malignancies, ranging from mild and reversible elevation of serum transaminases, to fatal hepatic failure.52 The incidence of SOS is much lower following HSCT for idiopathic aplastic anemia, reported as 7%, but still carries a high fatality rate (21%) if it occurs.55 SOS is part of a broader pattern of toxic liver damage occurring following HSCT,56 in which generalized impairment of liver function in the immediate post-transplantation period, may be further complicated by SOS in the weeks following transplantation, or nodular regenerative hyperplasia months later.28 Although initial reports of SOS incidence were in the 60% range,17 a more recent meta-analysis of 135 studies performed between 1979 and 2007 place the overall mean incidence of SOS following HSCT at 13.7% (95% confidence interval = 13.3%–14.1%)57 In one report, the cumulative incidence was significantly higher 20 years ago (1985–1996) than in more recent years (1997–2008), attributed to reduced-intensity conditioning regimes for HSCT, and the reduction in use of unrelated donors for HSCT.58
Onset of SOS is characterized by painful hepatomegaly, jaundice, weight gain, and ascites. Severe SOS is typically associated with multiorgan failure (MOF) and high mortality rates (>80%)57–59 The sudden weight gain and the development of hepatomegaly and tenderness typically occur on day 0 or 1 following HSCT, or 8–10 days after initiation of cytoreductive therapy. From the standpoint of clinical parameters, when serum bilirubin values exceed 15 mg/dl and weight gains approach 10–15 kg, mortality exceeds 90%.60 While persistent severe liver dysfunction is a harbinger of a fatal outcome, liver disease per se is not usually the direct cause of death; most patients succumb to septicemia, pneumonia, and bleeding and/or multiorgan failure.57 Pretransplant hepatitis (elevation of serum transaminases) is a risk factor for liver toxicity after transplantation.
Criteria for a clinical diagnosis of SOS are the occurrence of at least two of the three following symptoms during the first month after bone marrow transplantation: jaundice, development of tender hepatomegaly and right-upper quadrant pain, ascites, and/or unexplained weight gain.61 Serum transaminase levels may also rise substantially, but do not differentiate between potential causes of liver injury. Recommended clinical indices for assessing the severity of SOS are given in Table 2 (62). Given the risks of complications, liver biopsy is usually reserved for patients in whom the diagnosis of SOS is unclear and there is a need to exclude other diagnoses.61 Owing to the potentially severe condition of patients requiring liver biopsy, the transjugular approach has been recommended to reduce the risks associated with the procedure. With the passage of time after transplantation, liver biopsy may be increasingly necessary to sort out the diagnostic possibilities in these patients. In these later time frames, coagulation status may be more intact, permitting a percutaneous approach.
Table 2.
Clinical Grading of Sinusoidal Obstruction Syndrome.a
| Mild | Moderate | Severe | |
|---|---|---|---|
| Bilirubin, mg/dL | <5 | 5.1–8.0 | >8.0 |
| Liver enzymes (AST, ALT) | <3X normal | 3–8X normal | >8X normal |
| Weight above baseline | <2% | 2–5% | >5% |
| Serum creatinine | Normal | <2X normal | >2X normal |
| Clinical rate of change | Slow | Moderate | Rapid |
AST, aspartate transferase. ALT, alanine transferase.
Per reference. 62
Differential Diagnosis
Diagnoses to be excluded, if possible, include: graft-versus-host disease (GVHD); other causes of venous outflow obstruction such as Budd-Chiari syndrome and congestive heart failure; drug reactions, including the toxic effects of hyperalimentation; and infections such as viral hepatitis, fungi, and sepsis. The pathology of Budd-Chiari syndrome features centrilobular congestion and potentially parenchymal destruction, but without the occlusion of terminal hepatic veins seen in SOS. Drug reactions exhibit parenchymal damage with hepatocellular apoptosis, cholestasis, and parenchymal inflammation. While severe SOS may cause similar histology in its more severe stages, identification of the terminal hepatic vein lesions is readily made in such cases. Importantly, the clinical features of post-transplantation “drug toxicity” and the specific condition of SOS are similar, and there is no specific management of either condition except supportive. Hence, there is less need to make a specific diagnosis of SOS by liver biopsy in these unstable patients, as there is need to exclude other potential causes of hepatic dysfunction. Lastly, patients who undergo stem cell transplantation for nonmalignant conditions are less likely to develop liver toxicity, as the doses of cytoreductive therapy are generally lower than those given for malignancy.19
Owing to the concomitant risk of post-transplant GVHD, sirolimus has been used to reduce that risk. However, there is an increased risk of SOS after sirolimus-based GVHD prophylaxis63 Sirolimus may act as an endothelial toxin, and has been associated with another endothelial injury syndrome, thrombotic microangiopathy, after transplantation.64 Cutler et al have proposed that biomarkers of endothelial injury can be used to predict the development of SOS following HSCT.65 These include pre- and post-transplant measurement of circulating von Willebrand Factor (vWF), thrombomodulin, E-selectin, and soluble intercellular adhesion molecule-1 (sICAM-1). Increased serum vascular endothelial growth factor (VEGF) after HSCT also has been correlated with development of SOS.40
Cytoreductive Conditioning Prior to Hepatectomy for Metastatic Disease
The liver is the most common site of metastatic tumor spread, and SOS may occur in patients with metastatic solid tumors to the liver who are given alkylating agents such as oxaliplatin prior to surgical resection of the metastatic tumors.66 In particular, nearly half of patients with colorectal cancer develop liver metastases during the course of their disease.67 For patients with limited metastatic burden of colorectal cancer in the liver, surgical resection of the hepatic tumor deposits without chemo-inductive therapy offers the best opportunity for cure,68 with a five-year survival rate of 40%–58%.69,70 However, 60%–70% of patients undergoing initial curative resection suffer recurrent disease, of whom 10%–15% may be eligible for repeated resections.71 The remainder are relegated to chemotherapy. For patients with initially unresectable colorectal metastatic disease, chemotherapeutic regimes can rescue a reported 12.5% by promoting tumor down-sizing and enabling successful surgical resection of residual disease.72 Widely utilized regimens for this latter purpose consist of a thymidylate synthase inhibitor such as 5-fluorouracil, folinic acid, or capecitabine, in combination with either oxaliplatin or irinotecan. More recently, antibody therapy in the form of bevacizumab or cetuximab has been included in these regimes.73 Thus, there is a potentially large population of patients with advanced colorectal carcinoma who will be subjected to either adjuvant or neoadjuvant chemotherapy. In the pediatric population, chemotherapy accompanying hepatectomy for metastatic solid cancer such as Wilms tumor may also cause hepatic SOS.74,75
Oxaliplatin is a key agent in such regimes, and may cause sinusoidal injury, onset of SOS, and increased morbidity before or after hepatectomy.76–78 Histologic evidence of high-grade lesions of SOS has been reported to be present in 59% of patients receiving preoperative oxaliplatin treatment before hepatectomy, based on examination of the surrounding resected liver.79 The presence of such sinusoidal injury had a detrimental effect both on the intra- and post-operative course of patients undergoing major liver resection. In the first instance, both intraoperative bleeding and transfusion rate were increased in patients with high grade SOS lesions, when compared to those with low grade SOS lesions. In the second instance, post-operative complications were significantly more frequent and severe, including development of a large amount of post-operative ascites and liver insufficiency. Ironically, oxaliplatin has also been demonstrated to induce focal hepatic SOS that can mimic metastatic liver disease. Specifically, a 40 year old woman is described as receiving adjuvant oxaliplatin chemotherapy for advanced rectal cancer without clinically evident hepatic metastases. She then developed three hypovascular tumors in the liver which, on resection, were shown to be focal peliotic space-occupying lesions resulting from SOS.80
The occurrence of post-hepatectomy SOS is also associated with early tumor recurrence and decreased long-term survival.81 Parenthetically, conditioning pre-hepatectomy regimens for metastatic colorectal carcinoma involving irinotecan are not subject to concerns about SOS, but there is risk of chemotherapy-associated steatohepatitis.82
Preoperative laboratory indices designed to predict liver fibrosis in patients with hepatitis C virus-related liver disease involve such calculations as aspartate aminotransferase (AST) to platelet ratio index (APRI) and fibrosis scoring systems.83,84 Recent application of these clinical parameters to patients undergoing oxiplatin-based induction chemotherapy for metastatic colorectal carcinoma suggest that the APRI score, along with a low preoperative platelet count, may be reliable predictors of SOS severity following hepatectomy.79 In the post-hepatectomy time-frame, measurement of circulating Hyaluronic Acid as a measure of endothelial function also has been advocated as a clinical marker of risk for SOS.85 More recently, monitoring of post-hepatectomy splenic volume by imaging methods has been found to be a useful predictor of risk for the development of SOS.69 Lastly, polymorphisms in the ATP7B gene, which encodes for a transmembrane drug transporter, have been identified which may explain varying susceptibility to SOS among patients following oxaliplatin-based chemotherapy.86
Radiation-Induced Liver Disease
Radiation therapy (RT) without chemotherapy or hepatectomy remains as a central approach for treatment of cancer metastatic to the liver, and host conditioning for bone marrow transplantation. Considered separately from the combined chemoradiation regimes for stem cell transplantation, the development of radiation-induced liver disease (RILD) limits the amount of irradiation that can be given, and hence, the potential efficacy of treatment. Patients with RILD typically present with fatigue, weight gain, hepatomegaly, ascites, and an isolated elevation in alkaline phosphatase in months following hepatic RT.87 Liver biopsy reveals the characteristic features of SOS affecting terminal and sublobular hepatic veins.17
RILD is a severe complication of RT. In patients who do not succumb to this condition, liver healing occurs over several months as the vascular congestion resolves.7 Hepatic architecture remains distorted, however, with persistent fibrosis of terminal hepatic veins and the residua of parenchymal destruction throughout the centrilobular region.87
Chemotherapy for Acute Myeloid Leukemia
A singular example of SOS occurring in the setting of intensive cytotoxic chemotherapy is the use of Mylotarg® (gemtuzumab ozogamicin, GO) for acute myeloid leukemia (AML)88 GO is composed of a humanized conjugated antibody carrier linked to a potent antitumor antibiotic (calicheamicin). It targets the CD33 antigen expressed on the surface of AML cells, which is relatively absent from non-hematopoietic tissues and primitive hematopoietic stem cells. The incidence of SOS in this population of GO-treated patients is reported as 9.1%.89 Although the 6-month mortality rate in this population of SOS-affected patients was high (68%), three quarters of the deaths were due to progression of the AML. Overall risk of death from SOS and multiorgan failure was 2.7%.
Liver Transplantation
SOS as a cause of liver graft dysfunction after liver transplantation is rare, but has a poor prognosis.90 This is to be distinguished from post-transplant perivenulitis, as part of graft rejection.91 If present, SOS is more likely to occur in living-related transplant grafts92 or in patients receiving azothiaprine as part of their immunosuppressive regime.93 Better optimization of immunosuppression is suggested as an approach to reduce the incidence of this rare event.93
Exposure to Herbal Toxins
The use of hepatotoxic herbal remedies by humans has recently been extensively reviewed.94 An estimated 500 herbal products are distributed worldwide, and are represented as harmless healthy products without side effects95,96 Over 60% of patients in the U.S. being seen by a physician report use of herbal remedies.97 Because herbal products are considered dietary supplements in many countries including the U.S., proof of efficacy and safety does not have to be provided by the manufacturer. However, hepatotoxicity has been widely reported, up-to-and-including development of SOS. It has been estimated that more than 6000 plant species contain pyrrolizidine alkalioids with different chemical structures.98
One of the well-known herbs containing pyrrolizidine alkaloids is Symphytum officinale, commonly known as comfrey.99 This herb has been used as an external remedy for bone fractures, joint inflammation, and wound healing, and internally for gastroduodenal ulcers and gastritis.100 Comfrey-induced injury involves fibrous obliteration and destruction of hepatic veins, leading to cirrhosis.101,102 However, hepatotoxicity, including SOS, acute or chronic hepatitis, cholestasis, hepatic necrosis or fibrosis, cirrhosis, and outright liver failure, can occur with a much broader array of herbal products,103 owing to variability in what plant components have been used, storage conditions for the plant products, mislabeling or misidentification of the plant, and outright contaminations,104,105 Even Echinacea, widely touted as being completely free of toxicities, is known to contain pyrrolizidine alkaloids and may induce hepatotoxicity.106
Clinical presentation of hepatotoxicity arising in the setting of herbal product use is non-specific, involving fatigue, loss of appetite, and potentially jaundice. Hepatomegaly, ascites, and hyperbilirubinemia may be evident. Liver enzymes may be elevated in either a hepatitic, cholestatic, or combined pattern. Similar to onset of SOS in the post stem cell transplantation setting (vide infra), there are no symptoms or laboratory findings that specifically implicate SOS.
The presence of this form of liver injury requires liver biopsy, and attentiveness to this possibility in examining the biopsy tissue. The presence of perivenular necrosis, endophlebitis and fibrotic alterations of terminal and sub-lobular hepatic veins are consistent with SOS.107 More severe chronic exposure to the hepatotoxins in comfrey can manifest clinically in hepatomegaly and ascites, with SOS again confirmed by liver biopsy,108,109 even in the very young child given comfrey tea.110 However, histological examination of liver tissue may be difficult to perform owing to ascites and coagulopathy. In such instances, diagnosis of SOS may have to rely on clinical features and a retrospective history of drug or herb exposure. Recently, development of an ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) assay for identification of pyrrole-protein adducts in peripheral blood may prove to be a useful supplement to conventional diagnostic methods.111
With either mild or moderate cases of SOS, removal of the offending agent may offer good prospects for clinical recovery. In the most severe cases of herbal toxin-induced SOS in adults, hepatic failure may ensue. Notably, a fatal case of SOS has been reported in the preterm infant born of a mother consuming herbal teas.102
Because botanicals are self-prescribed, self-administered, and widely available, they are difficult to control and difficult to document.94 Unspecified mixed formulations are common. The point should be made that severe hepatotoxicity, including SOS, may be quite rare, given the massive use of herbal products and the relative infrequency of severe clinical presentations.107 Nevertheless, healthcare providers are well-advised to obtain accurate information about their patients' use of herbal remedies, and to ensure that they are well educated about their potential hepatotoxicities.
Veno-occlusive Disease with Immunodeficiency
In 1976, Mellis and Bale described five infants from three families who died between the ages of 2 and 7 months with veno-occlusive disease of the liver.112 All infants had some evidence of immune deficiency, including hypogammaglobulinemia, multiple infections including infection with Pneumocystic jerovici, enteroviral infections, and/or mucocutaneous candidiasis, and lymphoid tissues devoid of germinal centers and mature plasma cells. An additional 19 cases of what is now termed autosomal recessive hepatic veno-occlusive disease with immunodeficiency syndrome (VODI) have been identified over the ensuing years. VODI is associated with an 85% mortality if unrecognized and untreated with intravenous immunoglobulin and P. jerovici prophylaxis. The lack of hepatic veno-occlusive disease in other forms of inherited immunodeficiency suggest that this is a primary feature of VODI.
In 2006, Roscioli et al113 described mutations in the promyelocytic leukemia protein (PML) nuclear body protein Sp110 in six children from five families of Lebanese ethnicity, who met the clinical criteria for VODI. The immunodeficiency consisted of a combined T and B cell immunodeficiency. The B cell immunodeficiency was characterized by evolving severe hypogammaglobulinemia, absent memory (CD19+ CD27+ IgD−) B cells and tonsillar lymph nodes, and circulating CD19+ B cell numbers and percentages within the normal range. The T cell immunodeficiency was characterized by reduced numbers of memory (CD4+ CD45RO+ CD27−) T cells, and low or reduced intracellular T cell cytokine expression after antigen stimulation. SP110 is an immunoregulatory gene expressed in T and B lymphocytes within lymph nodes, spleen and liver. The affected infants in four of the families had a homozygous single-base deletion, 642delC (P214PfsX15) in exon 5. There were no living affected individuals in the fifth family, but the consanguineous parents and unaffected children of these families exhibited a heterozygous single-base deletion, 40delC (Q14SfsX25) in exon 2 of SP110. The central role of the SP110 mutation in VODI, along with identification of novel mutations and key clinical and immunological features, has been confirmed in an extensive additional study.114 This and a limited number of other studies have shown that early disease recognition and management (e.g., immunoglobulin replacement therapy and trimethoprim-sulfamethoxazole prophylaxis) can improve the dismal mortality rates.115 HSCT, when carried out before disease onset, can be curative. In one publication, antenatal diagnosis was possible for a family with a known risk of this disease.116
Interestingly, SP110 is a single-copy gene in humans. However, in all inbred strains of laboratory mice, it is part of a rearranged and amplified genomic region containing approximately 60–2000 copies of Sp110 and 20 adjacent genes.117 It therefore has not been possible to produce a genetic knockout model of Sp110 for study of the tissue pathobiology of this syndrome. Thus, despite the potential unique opportunity to gain insights from SP110 mutation into the immunopathogenesis of hepatic veno-occlusive disease (SOS), the evolution of the hepatic vascular lesions in VODI has not been elucidated.
Therapy and prevention
Supportive Measures
Current management of SOS consists primarily of supportive care, with a focus toward fluid management, adequate oxygenation and transfusional support to minimize ischemic liver injury, and avoidance of hepato/nephrotoxins.118,119 Specifically, patients with mild SOS, as defined by clinical indicators (Table 2) can be observed.62 Patients with evidence of moderate SOS should be treated expectantly with mild diuretics. This helps mitigate against fluid overload, while preserving renal blood flow and so as to avoid prerenal azotemia and potential hepatorenal syndrome. If ascites occurs, paracentesis may be required. However, the amount of ascites removed should be modest, in the range of 1 liter per day, so as to not harm renal blood flow.62 Patients with severe SOS require treatment.
Defibrotide
The investigational drug Defibrotide (DF) has shown the most promising results in clinical trials to date. The use of Defibrotide (DF) for the treatment of SOS/VOD is supported by a large number of clinical trials showing that DF improves both complete response and survival. Richardson et al119 first reported on 19 patients with SOS and multiorgan failure who had received Defibrotide for the management of severe SOS in the United States. The efficacy of Defibrotide (DF) for the treatment showed complete resolution of SOS in 8 patients (42%), 6 of who survived for longer than 100 days with no significant bleeding observed.120 A number of trials have subsequently confirmed the efficacy of DF in this setting, including a similar European multicenter compassionate-use study, which treated 40 patients and demonstrated a 55% complete response (CR) rate, with 43% patients alive after 100 days.121 Importantly, the US Food and Drug Administration permitted access to DF in the United States through an investigational new drug compassionate-use treatment protocol in December 2007. An interim analysis of 269 patients enrolled between December 2007 and March 2011 at 67 US centers revealed that 32% of patients achieved a CR at day-100 post-HSCT, with an overall day-100 survival of 50%.122 To date, DF has been used in more than 240 transplantation centers across 33 countries as a result of a large compassionate-use program and the ongoing named patient programs.
Based on an overall experience in more than 1800 patients, DF continues to demonstrate remarkable safety and tolerability despite a very ill patient population, with manageable toxicities and low rates of attributable hemorrhage.59 In a study from Spain of 845 patients over 24 years receiving allogeneic HSCT, a total of 117 patients developed SOS.57 Overall mortality from SOS decreased, from a reported 22% in 1997 to 14% in 2008, despite no change in clinical severity of the SOS.57 For those patients with severe SOS and multiorgan failure, only 2 of 8 (25%) of patients receiving defibrotide died, versus 14 of 18 (78%) for those severely ill patients receiving other treatments.
Tissue-Plasminogen Activator (t-PA)
Anticoagulation and thrombolytic therapies using tissue-plasminogen activator (t-PA) have been used, but are reported to be ineffective and are associated with significant bleeding complications.59,123 The use of tissue plasminogen activator with or without heparin has been evaluated in a number of studies and small case series. However, results have generally been disappointing; although about one-third of patients show improvement in their SOS with thrombolytic therapy, life-threatening hemorrhages are common, and no survival advantage is apparent.118
For pediatric patients developing SOS after chemotherapy and hepatectomy, careful supportive measures and anti-fibrinolytic drugs are the standard treatment,74,75 In one report, two patients with life-threatening SOS who failed to respond to the standard therapeutic approach were successfully treated with non-activated protein C supplementation.124
Methylprednisolone
High dose methylprednisolone has also been used in the treatment of SOS. Some studies reported that response was defined as a reduction in the total serum bilirubin by 50% or more within 10 d of initiation of corticosteroids. Sixty-three percent of patients responded and 28/48 (58%) were alive at 100 days from transplant.61 A recent pediatric retrospective study described by Myers et al, 2013, 9 patients who received methylprednisolone.125 Eight of these patients had multi-organ failure secondary to SOS. Response was defined as a 50% reduction in bilirubin level by 10 days after the commencement of therapy. Six patients responded to treatment. Four patients also received treatment with defibrotide. Overall survival was 78%.125 Methylprednisolone may be considered for the use in the treatment of SOS with the appropriate caveats of caution regarding infection.
TIPS
Transjugular intrahepatic portosystemic shunt (TIPS) insertion has been used to decompress the portal circulation, and relieve ascites in some patients with hepatic SOS, but in some others this procedure has been shown to worsen the process and did not improve the outcome.126–128
Liver Transplantation
Liver transplantation has been reported as a treatment for SOS.129–131 However, it should be considered only in patients with severe liver failure who are expected to have a good outcome in the absence of liver disease, and those who have undergone bone marrow transplantation for benign disease. Liver transplantation is usually contra-indicated when malignancy is present because of the high rates of recurrence.
Prevention
Holding to the adage that “an ounce of prevention is worth a pound of cure”, prevention remains the primary tool in the clinician's arsenal for managing SOS in the HSCT population.62 The first step is identification of patients at higher risk for developing SOS, namely those with pre-existing liver injury or global systemic inflammation.132 These include patients with pre-existing viral hepatitis, alcoholic hepatitis, or steatohepatitis, or other causes of elevated liver enzymes, heavy exposure to hepatotoxic agents, a history of infection and long antibiotic exposure, or patients with reduced diffusing capacity of the lung.62 Risk factors arising directly from HSCT include those receiving high doses of total body irradiation (TBI), cyclophosphamide exposure and dosage, oral busulfan use, and exposure to other hepatotoxic medications.133–137 The reported incidence of SOS following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade.138 This has been attributed to reduced-intensity conditioning HSCT (RIC-HSCT) for patients myeloablative-HSCT from unrelated donors.
Patients identified as being at higher risk for SOS should be considered for prophylaxis. Ursodeoxycholic acid (ursodiol) beginning two weeks before transplantation is commonly used. That being said, the evidence as to whether early prophylactic use of ursodeoxycholic acid (ursodiol) reduces the incidence of SOS in the HSCT population is equivocal.139–142 Ursodeoxycholic acid is a naturally occurring hydrophilic bile acid that functions to reduce the fraction of potentially hepatotoxic hydrophobic bile acids in the hepatobiliary system. It can also have modulating effects on cytokine expression and reduce inflammation.143 Institution of low-dose heparin prophylaxis is a more aggressive approach, as it requires an infusion regime, and the evidence for efficacy of this prophylaxis also is equivocal.144–146 Glutamine supplementation, use of peripheral blood progenitor cells as opposed to those derived from the bone marrow, and T-cell depletion as prophylaxis against graft-versus-host disease have been reported as having reduced incidence of SOS.147,148 In the pediatric population undergoing HSCT, a prophylactic regime of enoxaparin or ursodeoxycholic acid and vitamin E has been of potential value.149
Lastly, aggressive fluid management and control of fluid balance during the course of HSCT are essential. Although not proven to prevent SOS, successful fluid management can reduce hepatic congestion and potentially eliminate a source of hepatic insult. Inputs, outputs, and weight are strictly recorded daily, with aggressive diuresis to maintain euvolemia.
In the population of patients with metastatic colorectal carcinoma, the addition of bevacizumab to oxaliplatin-based chemotherapy can attenuate the incidence and severity of SOS.40,150,151 Bevacizumab (Bmab) is a monoclonal humanized antibody directed against VEGF, and is an approved drug for first-line treatment of metastatic colorectal cancer, but only as part of combination chemotherapy.152 It is gratifying that this drug may also help prevent one of the life-limiting complications of one of the companion chemotherapeutic agents, oxaliplatin.
Conflicts of interest
All authors have none to declare.
References
- 1.Hess J. Fatal obliterative endophlebitis of the hepatic vein. Am J Med Sci. 1905;130:986–1001. [Google Scholar]
- 2.Bras G., Jelliffe D.B., Stuart K.I. Veno-occlusive disease of liver with nonportal type of cirrhosis, occurring in Jamaica. Arch Pathol Lab Med. 1954;57:285–300. [PubMed] [Google Scholar]
- 3.Hahn P.F., Jackson M.A., Goldi H. Liver cirrhosis with ascites, induced in dogs by chronic massive hepatic irradiation with radioactive colloidal gold. Science. 1951;114:303–305. doi: 10.1126/science.114.2960.303. [DOI] [PubMed] [Google Scholar]
- 4.Stirling G.A., Bras G., Urquhart A.E. The early lesions in veno-occlusive disease of the liver. Arch Dis Child. 1962;37:535–538. doi: 10.1136/adc.37.195.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bras G., McLean E. Toxic factors in veno-occlusive disease. In: Sterling J.A., editor. Fetal and Infant Liver Function and Structure. Vol. 111. 1963. pp. 392–398. (Ann N Y Acad Sci). [DOI] [PubMed] [Google Scholar]
- 6.Ogata K., Hizawa K., Yoshida M. Hepatic injury following irradiation – a morphologic study. Tokushima J Exp Med. 1963;9:240–251. [PubMed] [Google Scholar]
- 7.Reed G.B., Cox A.J., Jr. The human liver after radiation injury: a form of veno-occlusive disease. Am J Pathol. 1966;48:597–611. [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson J.B. Chiari's disease and the Budd-Chiari syndrome. J Path Bact. 1960;79:381–401. doi: 10.1002/path.1700790220. [DOI] [PubMed] [Google Scholar]
- 9.Shillam D.S. Congestive splenomegaly (Banti's syndrome) due to portal stenosis. Calif Med. 1947;67:379–381. [PMC free article] [PubMed] [Google Scholar]
- 10.Tandon B.N., Tandon H.D., Tandon R.K., Narmdranathan M., Joshi Y.K. An epidemic of hepatic veno-occlusive disease in central India. Lancet. 1976;ii:271. doi: 10.1016/s0140-6736(76)90727-3. [DOI] [PubMed] [Google Scholar]
- 11.Ghanem J., Hershko C. Veno-occlusive disease and primary hepatic vein thrombosis in Israeli Arabs. Isr J Med Sci. 1981;17:339–347. [PubMed] [Google Scholar]
- 12.Selzer G., Parker R.G.F., Sapeika N. An experimental study of Senecio poisoning in rats. Br J Exp Path. 1951;32:14–20. [PMC free article] [PubMed] [Google Scholar]
- 13.McLean E., Bras G., György P. Veno-occlusive lesions in livers of rats fed Crotolaria fulva. Br J Exp Path. 1964;45:242–247. [PMC free article] [PubMed] [Google Scholar]
- 14.McLean E.K. The toxic actions of pyrrolizidine (Senecio) alkaloids. Pharmacol Rev. 1970;22:429–483. [PubMed] [Google Scholar]
- 15.Thomas E.D., Epstein R.B. Bone marrow transplantation in acute leukemia. Cancer Res. 1965;25:1521–1524. [PubMed] [Google Scholar]
- 16.Jacobs P., Miller J.L., Uys C.J., Dietrich B.E. Fatal veno-occlusive disease of the liver after chemotherapy, whole-body irradiation and bone marrow transplantation for refractory anemia. S Afr Med J. 1979;55:5–10. [PubMed] [Google Scholar]
- 17.Shulman H.M., McDonald G.B., Matthews D. An analysis of hepatic veno-occlusive disease and centrilobular hepatic degeneration following bone marrow transplantation. Gastroenterology. 1980;79:1178–1191. [PubMed] [Google Scholar]
- 18.Woods W.G., Dehner L.P., Nesbit M.E. Fatal veno-occlusive disease of the liver following high dose chemotherapy, irradiation and bone marrow transplantation. Am J Med. 1980;68:285–290. doi: 10.1016/0002-9343(80)90368-x. [DOI] [PubMed] [Google Scholar]
- 19.McDonald G.B., Sharma P., Matthews D.E., Shulman H.M., Thomas E.D. Veno-occlusive disease of the liver after bone marrow transplantation: diagnosis, incidence and predisposing factors. Hepatology. 1984;4:116–122. doi: 10.1002/hep.1840040121. [DOI] [PubMed] [Google Scholar]
- 20.Ayash L.J., Hunt M., Antman K. Hepatic veno-occlusive disease in autologous bone marrow transplantation of solid tumors and lymphomas. J Clin Oncol. 1990;8:1699–1706. doi: 10.1200/JCO.1990.8.10.1699. [DOI] [PubMed] [Google Scholar]
- 21.Shibayama Y., Hashimoto K., Nakata K. Focal veno-occlusive lesions following metastasis of cancer in the liver with special reference to obstruction of lymphatics in hepatic veins. Virchows Arch [A] 1991;418:169–174. doi: 10.1007/BF01600293. [DOI] [PubMed] [Google Scholar]
- 22.Jones R.J., Lee K.S., Beschorner W.E. Veno-occlussive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–783. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Dulley F.L., Kanfer E.J., Appelbaum R.F. Veno-occlusive disease of the liver after chemoradiotherapy and autologous bone marrow transplantation. Transplantation. 1987;43:870–873. [PubMed] [Google Scholar]
- 24.Brugieres L., Hartman O., Benhamou E. Veno-occlusive disease of the liver following high-dose chemotherapy and autologous bone marrow transplantation in children with solid tumors: incidence, clinical course and outcome. Bone Marrow Transplant. 1988;3:53–58. [PubMed] [Google Scholar]
- 25.Stephens L.C., Peters L.J., Ang K.K. Tolerance of rhesus monkey liver to ionizing radiation. Radiat Oncol Invest. 1994;1:279–284. [Google Scholar]
- 26.Allen J.R., Carstens L.A., Katagiri G.J. Hepatic veins of monkeys with veno-occlusive disease. Sequential ultrastructural changes. Arch Pathol. 1969;87:279–289. [PubMed] [Google Scholar]
- 27.Shulman H.M., Luk K., Deeg H.J., Shuman W.B., Storb R. Induction of hepatic veno-occlusive disease in dogs. Am J Pathol. 1987;126:114–125. [PMC free article] [PubMed] [Google Scholar]
- 28.Shulman H.M., Fisher L.B., Schoch H.G., Henne K.W., McDonald G.B. Veno-occlusive disease of the liver after marrow transplantation: histological correlates of clinical signs and symptoms. Hepatology. 1994;19:1171–1181. [PubMed] [Google Scholar]
- 29.DeLeve L.D., McCuskey R.S., Wang X. Characterization of a reproducible rat model of hepatic veno-occlusive disease. Hepatology. 1999;29:1779–1791. doi: 10.1002/hep.510290615. [DOI] [PubMed] [Google Scholar]
- 30.DeLeve L.D., Shulman H.M., McDonald G.B. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (venoocclusive disease) Semin Liver Dis. 2002;22:623–638. doi: 10.1055/s-2002-23204. [DOI] [PubMed] [Google Scholar]
- 31.DeLeve L.D., Ito Y., Bethea N.W., McCusky M.K., Wang X., McCuskey R.S. Embolization by sinusoidal lining cells obstructs the microcirculation in rat sinusoidal obstruction syndrome. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1045–G1052. doi: 10.1152/ajpgi.00526.2002. [DOI] [PubMed] [Google Scholar]
- 32.Helmy A. Review article: updates in the pathogenesis and therapy of hepatic sinusoidal obstruction syndrome. Aliment Pharmacol Ther. 2006;23:11–25. doi: 10.1111/j.1365-2036.2006.02742.x. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava A., Poonkuzhali B., Shaji R.V. Glutathione S-transferase M1 polymorphism: a risk factor for hepatic venoocclusive disease in bone marrow transplantation. Blood. 2004;104:1574–1577. doi: 10.1182/blood-2003-11-3778. [DOI] [PubMed] [Google Scholar]
- 34.Vreuls C.P.H., Olde Damink S.W.M., Koek G.H. Glutathione S-transferase M1-null genotype as risk factor for SOS in oxaliplatin-treated patients with metastatic colorectal cancer. Br J Cancer. 2013;108:676–680. doi: 10.1038/bjc.2012.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeLeve L.D., Wang X. Decrease in nitric oxide production contributes to hepatic venoocclusive disease. Hepatology. 1999;30:218A. [Google Scholar]
- 36.DeLeve L.D., Wang X., Kanel G.C. Decreased hepatic nitric oxide production contributes to the development of rat sinusoidal obstruction syndrome. Hepatology. 2003;38:900–908. doi: 10.1053/jhep.2003.50383. [DOI] [PubMed] [Google Scholar]
- 37.Ueno T., Bioulac-Sage P., Balabaud C., Rosenbaum J. Innervation of the sinusoidal wall: regulation of the sinusoidal diameter. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:868–873. doi: 10.1002/ar.a.20092. [DOI] [PubMed] [Google Scholar]
- 38.DeLeve D.L., Wang X., Tsai J., Kanel G., Strasberg S., Tokes Z.A. Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology. 2003;125:882–890. doi: 10.1016/s0016-5085(03)01056-4. [DOI] [PubMed] [Google Scholar]
- 39.Harb R., Xie G., Lutzko C. Bone marrow progenitor cells repair hepatic sinusoidal endothelial cells after liver injury. Gastroenterology. 2009;137:704–712. doi: 10.1053/j.gastro.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iguchi A., Kobayashi R., Yoshida M. Vascular endothelial growth factor (VEGF) is one of the cytokines causative and predictive of hepatic veno-occlusive disease (VOD) in stem cell transplantation. Bone Marrow Transplant. 2001;27:1173–1180. doi: 10.1038/sj.bmt.1703061. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura K., Hatano E., Narita M. Sorafenib attenuates monocrotaline-induced sinusoidal obstruction syndrome in rats through suppression of JNK and MMP-9. J Hepatol. 2012;57:1037–1043. doi: 10.1016/j.jhep.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Mejias M., Garcia-Pras E., Tiani C., Miquel R., Bosch J., Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2008;49:1245–1256. doi: 10.1002/hep.22758. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y., Gao J., Zhang D., Ma J., Jiang H. New insights into the antifibrotic effects of sorafenib on hepatic stellate cells and liver fibrosis. J Hepatol. 2010;53:132–144. doi: 10.1016/j.jhep.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 44.Yannam G.R., Han B., Setoyama K. A nonhuman primate model of human radiation-induced venoocclusive liver disease and hepatocyte injury. Int J Radiat Oncol Biol Phys. 2013 doi: 10.1016/j.ijrobp.2013.10.037. Accessed 1.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rusthoven K.E., Kavanagh B.D., Cardenes H. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 46.Robinson S.M., Mann J., Vasilaki A. Pathogenesis of FOLFOX induced sinusoidal obstruction syndrome in a murine chemotherapy model. J Hepatol. 2013;59:318–326. doi: 10.1016/j.jhep.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson S.M., Mann D.A., Manas D.M., Oakley F., Mann J., White S.A. The potential contribution of tumour-related factors to the development of FOLFOX-induced sinusoidal obstruction syndrome. Br J Cancer. 2013;109:2396–2403. doi: 10.1038/bjc.2013.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng L., An L., Fang T. A murine model of hepatic veno-occlusive disease induced by allogeneic hematopoietic stem cell transplantation. Cell Biochem Biophys. 2013;67:939–948. doi: 10.1007/s12013-013-9587-7. [DOI] [PubMed] [Google Scholar]
- 49.Rubbia-Brandt L., Tauzin S., Brezault C. Gene expression profiling provides insights into pathways of oxaliplatin-related sinusoidal obstruction syndrome in humans. Mol Cancer Ther. 2011;10:687–696. doi: 10.1158/1535-7163.MCT-10-1072. [DOI] [PubMed] [Google Scholar]
- 50.Narita M., Oussoultzoglou E., Chenard M.-P. Liver injury due to chemotherapy-induced sinusoidal obstruction syndrome is associated with sinusoidal capillarization. Ann Surg Oncol. 2012;19:2230–2237. doi: 10.1245/s10434-011-2112-6. [DOI] [PubMed] [Google Scholar]
- 51.Bearman S.I. Avoiding hepatic veno-occlusive disease: what do we know and where are we going? Bone Marrow Transplant. 2001;27:1113–1120. doi: 10.1038/sj.bmt.1703014. [DOI] [PubMed] [Google Scholar]
- 52.McDonald G.B., Shulman H.M., Wolford J.L., Spencer G.D. Liver disease after human marrow transplantation. Semin Liver Dis. 1987;7:210–229. doi: 10.1055/s-2008-1040578. [DOI] [PubMed] [Google Scholar]
- 53.Toh H.C., McAfee S.L., Sackstein R. Late onset veno-occlusive disease following high-dose chemotherapy and stem cell transplantation. Bone Marrow Transplant. 1999;24:891–895. doi: 10.1038/sj.bmt.1701994. [DOI] [PubMed] [Google Scholar]
- 54.Kalayoglu-Besisik S., Yeneral M.N., Caliskan Y., Ozturk S., Besisik F., Sargin D. Time-related changes in the incidence, severity, and clinical outcome of hepatic veno-occlusive disease in hematopoietic stem cell transplantation patients during the past 10 years. Transplant Proc. 2005;37:2285–2289. doi: 10.1016/j.transproceed.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 55.Kim H., Lee K.H., Sohn S.K. Hepatic sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation in adult patients with idiopathic aplastic anemia. Leuk Res. 2013;37:1241–1247. doi: 10.1016/j.leukres.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 56.Crawford J., Ferrell L. The liver in transplantation. In: Rustgi V., Van Thiel D., editors. The Liver in Systemic Diseases. Raven Press; New York: 1993. pp. 337–364. [Google Scholar]
- 57.Coppell J.A., Richardson P.G., Soiffer R. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16:157–168. doi: 10.1016/j.bbmt.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carreras E., Díaz-Beyá M., Rosiñol L. The incidence of venoocclusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transplant. 2011;17:1713–1720. doi: 10.1016/j.bbmt.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Richardson P.G., Ho V.T., Cutler C., Glotzbecker B., Antin J.H., Soiffer R. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: novel insights to pathogenesis, Current status of treatment, and future Directions. Biol Blood Marrow Transplant. 2013;19:S88–S90. doi: 10.1016/j.bbmt.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 60.McDonald G.B., Sharma P., Matthews D.E., Shulman H.M., Thomas E.D. The clinical course of 53 patients with venocclusive disease of the liver after marrow transplantation. Transplantation. 1985;39:603–608. doi: 10.1097/00007890-198506000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Dignan F.L., Wynn R.F., Hadzic N. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol. 2013;163:444–457. doi: 10.1111/bjh.12558. [DOI] [PubMed] [Google Scholar]
- 62.Chao N. How I treat sinusoidal obstruction syndrome. Blood. 2014;123:4023–4026. doi: 10.1182/blood-2014-03-551630. [DOI] [PubMed] [Google Scholar]
- 63.Cutler C., Stevenson K., Kim H.T. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood. 2008;112:4425–4431. doi: 10.1182/blood-2008-07-169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cutler C., Li S., Ho V.T. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109:3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cutler C., Kim H.T., Ayanian S. Prediction of veno-occlusive disease using biomarkers of endothelial injury. Biol Blood Marrow Transplant. 2010;16:1180–1185. doi: 10.1016/j.bbmt.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friedman S.L. Focus: a bad transition and a good step forward. Hepatol. 2012;57:935–936. doi: 10.1016/j.jhep.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 67.Leporrier J., Maurel J., Chiche L. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006;93:465–474. doi: 10.1002/bjs.5278. [DOI] [PubMed] [Google Scholar]
- 68.Lochan R., White S.A., Manas D.M. Liver resection for colorectal liver metastasis. Surg Oncol. 2007;16:33–45. doi: 10.1016/j.suronc.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 69.Imai K., Emi Y., Iyama K.I. Splenic volume may be a useful indicator of the protective effect of bevacizumab against oxaliplatin-induced hepatic sinusoidal obstruction syndrome. Eur J Surg Oncol. 2014;40:559–566. doi: 10.1016/j.ejso.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 70.Choti M.A., Sitzmann J.V., Tiburi M.F. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khatri V.P., Petrelli N.J., Belghiti J. extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? J Clin Oncol. 2005;23:8490–8499. doi: 10.1200/JCO.2004.00.6155. [DOI] [PubMed] [Google Scholar]
- 72.Adam R., Delvart V., Pascal G. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–657. doi: 10.1097/01.sla.0000141198.92114.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maughan T.S., Adams R.A., Smith C.G., Meade A.M., Seymour M.T., Wilson R.H. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomized phase 3 MRC COIN trial. Lancet. 2011;377:2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jagt C.T., Zuckermann M., Ten Kate F. Veno-occlusive disease as a complication of preoperative chemotherapy for Wilms tumor: a clinic-pathological analysis. Pediatr Blood Cancer. 2009;53:1211–1215. doi: 10.1002/pbc.22202. [DOI] [PubMed] [Google Scholar]
- 75.Cesaro S., Spiller M., Sartori M.T. Veno-occlusive disease in pediatric patients affected by Wilms tumor. Pediatr Blood Cancer. 2011;57:258–261. doi: 10.1002/pbc.22841. [DOI] [PubMed] [Google Scholar]
- 76.Rubbia-Brandt L., Audard V., Sartoretti P. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 77.Karoui M., Penna C., Amin-Hashem M. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1–7. doi: 10.1097/01.sla.0000193603.26265.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakano H., Oussoultzoglou E., Rosso E. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2007;247:118–124. doi: 10.1097/SLA.0b013e31815774de. [DOI] [PubMed] [Google Scholar]
- 79.Soubrane O., Brouquet A., Zalinski S. Predicting high grade lesions of sinusoidal obstruction syndrome related to oxaliplatin-based chemotherapy for colorectal liver metastases: correlation with post-hepatectomy outcome. Ann Surg. 2010;251:454–460. doi: 10.1097/SLA.0b013e3181c79403. [DOI] [PubMed] [Google Scholar]
- 80.Arakawa Y., Shimada M., Utsunomya T. Oxaliplatin-related sinusoidal obstruction syndrome mimicking metastatic liver tumors. Hepatol Res. 2013;43:685–689. doi: 10.1111/j.1872-034X.2012.01114.x. [DOI] [PubMed] [Google Scholar]
- 81.Tamandl D., Klinger M., Eipeldauer S. Sinusoidal obstruction syndrome impairs long-term outcome of colorectal liver metastases treated with resection after neoadjuvant chemotherapy. Ann Surg Oncol. 2011;18:421–430. doi: 10.1245/s10434-010-1317-4. [DOI] [PubMed] [Google Scholar]
- 82.Zorzi D., Laurent A., Pawlik T.M. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–286. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 83.Wai C.T., Greenson J.K., Fontana R.J. A simple noninvasive index can predict both significant fibrosis and cirrhosis inpatients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 84.Vallet-Pichard A., Mallet V., Nalpas B. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 85.van den Brock M.A.J., Vreuls C.P.H., Winstanley A. Hyaluronic acid as a marker of hepatic sinusoidal obstruction syndrome secondary to oxaliplatin-based chemotherapy in patients with colorectal liver metastases. Ann Surg Oncol. 2013;20:1462–1469. doi: 10.1245/s10434-013-2915-8. [DOI] [PubMed] [Google Scholar]
- 86.Robinson S.M., Mann J., Manas D.M., Mann D.A., White S.A. An experimental study to identify the potential role of pharmacogenomics in determining the occurrence of oxaliplatin-induced liver injury. HPB. 2013;15:581–587. doi: 10.1111/hpb.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lawrence T.S., Robertson J.M., Anscher M.S. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237–1248. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 88.Rajvanish P., Shulman H.M., Sievers E.L. Hepatic sinusoidal obstruction after gemtuzumab ozogamicin (Mylotarg) therapy. Blood. 2002;99:2310–2314. doi: 10.1182/blood.v99.7.2310. [DOI] [PubMed] [Google Scholar]
- 89.Tallman M.S., McDonald G.B., DeLeve L.D., Baer M.R., Cook M.N., Graepel G.J. Incidence of sinusoidal obstruction syndrome following Mylotarg (gembuzumab ozogamicin): a prospective observational study of 482 patients in routine clinical practice. Int J Hematol. 2013;97:456–464. doi: 10.1007/s12185-013-1275-2. [DOI] [PubMed] [Google Scholar]
- 90.Fiel M.I., Schiano T.D., Klion F.M. Recurring fibro-obliterative venopathy in liver allografts. Am J Surg Pathol. 1999;23:734–737. doi: 10.1097/00000478-199906000-00015. [DOI] [PubMed] [Google Scholar]
- 91.Hubscher S.G. Central perivenulitis: a common and potentially important finding in late posttransplant liver biopsies. Liver Transpl. 2008;14:596–600. doi: 10.1002/lt.21451. [DOI] [PubMed] [Google Scholar]
- 92.Izaki T., Inomata Y., Asonuma K. Early graft failure due to a veno-occlusive disease after a pediatric living donor liver transplantation. Pediatr Transplant. 2004;8:301–304. doi: 10.1111/j.1399-3046.2004.00171.x. [DOI] [PubMed] [Google Scholar]
- 93.Sebagh M., Azoulay D., Roche B. Significance of isolated hepatic veno-occlusive disease/sinusoidal obstruction syndrome after liver transplantation. Liver Transpl. 2011;17:798–808. doi: 10.1002/lt.22282. [DOI] [PubMed] [Google Scholar]
- 94.Abdualmjid R.J., Sergi C. Hepatotoxic botanicals – an evidence-based systematic review. J Pharm Pharm Sci. 2013;16:376–404. doi: 10.18433/j36g6x. [DOI] [PubMed] [Google Scholar]
- 95.Shad J.A., Chinn C.G., Brann O.S. Acute hepatitis after ingestion of herbs. South Med J. 1999;92:1095. doi: 10.1097/00007611-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 96.Laliberté L., Villeneuve J.P. Hepatitis after the use of germander, a herbal remedy. CMAJ. 1996;154:1689–1692. [PMC free article] [PubMed] [Google Scholar]
- 97.Harnack L.J., Rydell S.A., Stang J. Prevalence of use of herbal products by adults in the Minneapolis/St Paul, Minn, metropolitan area. Mayo Clin Proc. 2001;76:688–694. doi: 10.4065/76.7.688. [DOI] [PubMed] [Google Scholar]
- 98.Stegelmeier B.L., Edgar J.A., Colegate S.M. Pyrrolizidine alkaloid plants, metabolism and toxicity. J Nat Toxins. 1999;8:95–116. [PubMed] [Google Scholar]
- 99.Stickel F., Seitz H.K. The efficacy and safety of comfrey. Public Health Nutr. 2000;3:501–508. doi: 10.1017/s1368980000000586. [DOI] [PubMed] [Google Scholar]
- 100.Rode D. Comfrey toxicity revisited. Trends Pharmacol Sci. 2002;23:497–499. doi: 10.1016/s0165-6147(02)02106-5. [DOI] [PubMed] [Google Scholar]
- 101.Yeong M.L., Swinburn B., Kennedy M., Nicholson G. Hepatic veno-occlusive disease associated with comfrey ingestion. J Gastroenterol Hepatol. 1990;5:211–214. doi: 10.1111/j.1440-1746.1990.tb01827.x. [DOI] [PubMed] [Google Scholar]
- 102.Consolato S., Bernhard B., Otwin L., Walter J.H. Fatal course of veno-occlusive disease of the liver (endophlebitis hepatica obliterans) in a preterm infant. Pathol Res Pract. 1999;195:847–851. doi: 10.1016/S0344-0338(99)80108-3. [DOI] [PubMed] [Google Scholar]
- 103.Huxtable R.J. The myth of beneficial nature: the risk of herbal preparations. Ann Intern Med. 1992;117:165–166. doi: 10.7326/0003-4819-117-2-165. [DOI] [PubMed] [Google Scholar]
- 104.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005;4:489–499. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- 105.Robinson O., Want E., Coen M. Hirmi Valley liver disease: a disease associated with exposure to pyrrolizidine alkaloids and DDT. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.07.039. Accessed 7.12.13. [DOI] [PubMed] [Google Scholar]
- 106.Abebe W. Herbal medication: potential for adverse interactions with analgesic drugs. J Clin Pharm Ther. 2002;27:391–401. doi: 10.1046/j.1365-2710.2002.00444.x. [DOI] [PubMed] [Google Scholar]
- 107.Schoepfer A.M., Engel A., Fattinger K. Herbal does not mean innocuous: Ten cases of severe hepatotoxicity associated with dietary supplements from Herbalife® products. J Hepatol. 2007;47:521–526. doi: 10.1016/j.jhep.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 108.Bach N., Thung S.N., Schaffner F. Comfrey herb tea-induced hepatic venoocclusive disease. Am J Med. 1989;87:97–99. doi: 10.1016/s0002-9343(89)80492-9. [DOI] [PubMed] [Google Scholar]
- 109.Weston C.F., Cooper B.T., Davies J.D., Levine D.F. Veno-occlusive disease of the liver secondary to ingestion of comfrey. Br Med J (Clin Res Ed) 1987;295:183. doi: 10.1136/bmj.295.6591.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sperl W., Stuppner H., Gassner I., Judmaier W., Dietze O., Vogel W. Reversible hepatic veno-occlusive disease in an infant after consumption of pyrrolizidine-containing herbal tea. Eur J Pediatr. 1995;154:112–116. doi: 10.1007/BF01991912. [DOI] [PubMed] [Google Scholar]
- 111.Gao H., Li N., Wang J.Y., Zhang S.C., Lin G. Definitive diagnosis of hepatic sinusoidal obstruction syndrome induced by pyrrolizidine alkaloids. J Dig Dis. 2012;13:33–39. doi: 10.1111/j.1751-2980.2011.00552.x. [DOI] [PubMed] [Google Scholar]
- 112.Mallis C., Bale P.M. Familial hepatic venoocclusive disease with probable immune deficiency. J Pediatr. 1976;88:236–242. doi: 10.1016/s0022-3476(76)80988-2. [DOI] [PubMed] [Google Scholar]
- 113.Roscioli T., Cliffe S.T., Bloch D.B. Mutations in the gene encoding the PML nuclear body protein Sp110 are associated with immunodeficiency and hepatic veno-occlusive disease. Nat Genet. 2006;38:620–622. doi: 10.1038/ng1780. [DOI] [PubMed] [Google Scholar]
- 114.Cliffe S.T., Bloch D.B., Suryani S. Clinical, molecular, and cellular immunologic findings in patients with SP110-associated veno-occlusive disease with immunodeficiency syndrome. J Allergy Clin Immunol. 2012;130:735–742. doi: 10.1016/j.jaci.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 115.Wang T., Ong P., Roscioli T., Cliffe S.T., Church J.A. Hepatic veno-occlusive disease with immunodeficiency (VODI): first reported case in the U.S. and identification of a unique mutation in SP110. Clin Immunol. 2012;145:102–107. doi: 10.1016/j.clim.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 116.Cliffe S.T., Wong M., Taylor P.J. The first prenatal diagnosis for veno-occlusive disease and immunodeficiency syndrome, an autosomal recessive condition associated with mutations in SP110. Prenat Diagn. 2007;27:674–676. doi: 10.1002/pd.1759. [DOI] [PubMed] [Google Scholar]
- 117.Weichenhan D., Kunze B., Winking H. Source and component genes of a 6-200 Mb gene cluster in the house mouse. Mamm Genome. 2001;12:590–594. doi: 10.1007/s00335-001-3015-9. [DOI] [PubMed] [Google Scholar]
- 118.DeLeve L.D., Valla D.C., Garcia-Tsao G. Vascular disorders of the liver. Hepatology. 2009;49:1729–1764. doi: 10.1002/hep.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Richardson P.G., Soiffer R.J., Antin J.H. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose-finding trial. Biol Blood Marrow Transplant. 2010;16:1005–1017. doi: 10.1016/j.bbmt.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Richardson P.G., Elias A.D., Krishnan A. Treatment of severe venoocclusive disease with defibrotide: compassionate use results in response without significant toxicity in a high-risk population. Blood. 1998;92:737–744. [PubMed] [Google Scholar]
- 121.Chopra R., Eaton J.D., Grassi A. Defibrotide for the treatment of hepatic veno-occlusive disease: results of the European compassionate-use study. Br J Haematol. 2000;111:1122–1129. doi: 10.1046/j.1365-2141.2000.02475.x. [DOI] [PubMed] [Google Scholar]
- 122.Richardson P.G., Smith A.R., Grupp S.A. Defibrotide (DF) in the treatment of hepatic veno-occlusive disease (VOD) in stem cell transplant (SCT) and non-SCT patients (Pts): early intervention improves outcome: updated results of a treatment IND expanded accessprotocol. Blood (ASH Annual Meeting Abstract) 2011;118 abstract 487. [Google Scholar]
- 123.Yoon J.-H., Min W.S., Kim H.J. Experiences of t-PA use in moderate-to-severe hepatic veno-occlusive disease after hematopoietic SCT: is it still reasonable to use t-PA? Bone Marrow Transplant. 2013;48:1562–1568. doi: 10.1038/bmt.2013.101. [DOI] [PubMed] [Google Scholar]
- 124.De Leonardia F., Koronica R., Bruno S.D., Santoro N. Non-activated protein C rescue treatment in Wilms tumour associated hepatic sinusoidal obstructive syndrome. Pediatr Blood Cancer. 2014;61:940–941. doi: 10.1002/pbc.24859. [DOI] [PubMed] [Google Scholar]
- 125.Myers K.C., Lawrence J., Marsh R.R., Davies S.M., Jodele S. High-dose methylprednisolone for veno-occlusive disease of the liver in pediatric hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2013;19:492–503. doi: 10.1016/j.bbmt.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Azoulay D., Castaing D., Lemoine A. Transjugular intrahepatic porto- systemic shunt (TIPS) for severe veno- occlusive disease of the liver following bone marrow transplantation. Bone Marrow Transplant. 2000;25:987–992. doi: 10.1038/sj.bmt.1702386. [DOI] [PubMed] [Google Scholar]
- 127.Rajvanshi P., McDonald G.B. Expanding the use of transjugular intrahepatic portosystemic shunts for veno-occlu- sive disease. Liver Transplant. 2001;7:154–159. doi: 10.1053/jlts.2001.0070154. [DOI] [PubMed] [Google Scholar]
- 128.Zenz T., Rossle M., Bertz H. Severe veno-occlusive disease after allogeneic bone marrow or peripheral stem cell transplantation-role of transjugular intrahepatic portosystemic shunt (TIPS) Liver. 2001;21:31–36. doi: 10.1034/j.1600-0676.2001.210105.x. [DOI] [PubMed] [Google Scholar]
- 129.Nimer S.D., Milewicz A.L., Champlin R.E., Busuttil R.W. Successful treatment of hepatic venoocclusive disease in a bone marrow transplant patient with orthotopic liver transplantation. Transplantation. 1990;49:819–821. [PubMed] [Google Scholar]
- 130.Rappaport A.P., Doyle H.R., Starzl T. Orthotopic liver transplantation for lifethreatening veno-occlusive disease of the liver after allogeneic bone marrow transplant. Bone Marrow Transplant. 1991;8:421–424. [PubMed] [Google Scholar]
- 131.Kim I.D., Egawa H., Marui Y. A successful liver transplantation for refractory hepatic veno-occlusive disease originating from cord blood transplantation. Am J Transplant. 2002;2:796–800. doi: 10.1034/j.1600-6143.2002.20815.x. [DOI] [PubMed] [Google Scholar]
- 132.Johnson D.B., Savani B.N. How can we reduce hepatic veno-occlusive disease–related deaths after allogeneic stem cell transplantation? Exp Hematol. 2012;40:513–517. doi: 10.1016/j.exphem.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 133.McDonald G.B., Hinds M.S., Fisher L.D. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–267. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 134.McDonald G.B., Slattery J.T., Bouvier M.E. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043–2048. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- 135.Carreras E., Bertz H., Arcese W. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukemia Working Party. Blood. 1998;92:3599–3604. [PubMed] [Google Scholar]
- 136.Clift R.A., Buckner C.D., Appelbaum F.R. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76:1867–1871. [PubMed] [Google Scholar]
- 137.Lee J.H., Choi S.J., Lee J.H. Decreased incidence of hepatic venoocclusive disease and fewer hemostatic derangements associated with intravenous busulfan vs oral busulfan in adults conditioned with busulfan? Cyclophosphamide for allogeneic bone marrow transplantation. Ann Hematol. 2005;84:321–330. doi: 10.1007/s00277-004-0982-4. [DOI] [PubMed] [Google Scholar]
- 138.Carreras E., Díaz-Beyá M., Rosinol L., Fernández-Avilés F., Rovira M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transplant. 2011;17:1698–1720. doi: 10.1016/j.bbmt.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 139.Tay J., Tinmouth A., Fergusson D., Huebsch L., Allan D.S. Systematic review of controlled clinical trials on the use of ursodeoxycholic acid for the prevention of hepatic veno-occlusive disease in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:206–217. doi: 10.1016/j.bbmt.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 140.Essell J.H., Schroeder M.T., Harman G.S. Ursodiol prophylaxis against hepatic complications of allogeneic bone marrow transplantation. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:975–981. doi: 10.7326/0003-4819-128-12_part_1-199806150-00002. [DOI] [PubMed] [Google Scholar]
- 141.Park S.H., Lee M.H., Lee H. A randomized trial of heparin plus ursodiol vs. heparin alone to prevent hepatic veno-occlusive disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:137–143. doi: 10.1038/sj.bmt.1703342. [DOI] [PubMed] [Google Scholar]
- 142.Ruutu T., Eriksson B., Remes K. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100:1977–1983. doi: 10.1182/blood-2001-12-0159. [DOI] [PubMed] [Google Scholar]
- 143.Yoshikawa M., Tsujii T., Matsumura K. Immunomodulatory effects of ursodeoxycholic acid on immune responses. Hepatology. 1992;16:358–364. doi: 10.1002/hep.1840160213. [DOI] [PubMed] [Google Scholar]
- 144.Attal M., Huguet F., Rubie H. Prevention of hepatic veno-occlusive disease after bone marrow transplantation by continuous infusion of low-dose heparin: a prospective, randomized trial. Blood. 1992;79:2834–2840. [PubMed] [Google Scholar]
- 145.Or R., Nagler A., Shpilberg O. Low molecular-weight heparin for the prevention of veno-occlusive disease of the liver in bone marrow transplantation patients. Transplantation. 1996;61:1067–1071. doi: 10.1097/00007890-199604150-00014. [DOI] [PubMed] [Google Scholar]
- 146.Marsa-Vila L., Gorin N.C., Laporte J.P. Prophylactic heparin does not prevent liver veno-occlusive disease following autologic bone marrow transplantation. Eur J Haematol. 1991;47:346–354. doi: 10.1111/j.1600-0609.1991.tb01859.x. [DOI] [PubMed] [Google Scholar]
- 147.Fisher D.C., Vredenburgh J.J., Petros W.P. Reduced mortality following bone marrow transplantation for breast cancer with the addition of peripheral blood progenitor cells is due to a marked reduction in veno-occlusive disease of the liver. Bone Marrow Transplant. 1998;21:117–122. doi: 10.1038/sj.bmt.1701068. [DOI] [PubMed] [Google Scholar]
- 148.Brown S.A., Goringe A., Fegan C. Parenteral glutamine protects hepatic function during bone marrow transplantation. Bone Marrow Transplant. 1998;22:281–284. doi: 10.1038/sj.bmt.1701321. [DOI] [PubMed] [Google Scholar]
- 149.Gökce M., Kuskonmaz B., Cetin M., Uckan Cetinkaya D., Tuncer M. Coexisting or underlying risk factors of hepatic veno-occlusive disease in pediatric hematopoietic stem cell transplant recipients receiving prophylaxis. Exp Clin Transpl. 2013;11:440–446. doi: 10.6002/ect.2012.0265. [DOI] [PubMed] [Google Scholar]
- 150.Ribero D., Wang H., Donadon M. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer. 2007;110:2761–2767. doi: 10.1002/cncr.23099. [DOI] [PubMed] [Google Scholar]
- 151.Rubbia-Brandt L., Lauwers G.Y., Wang H. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology. 2010;56:430–439. doi: 10.1111/j.1365-2559.2010.03511.x. [DOI] [PubMed] [Google Scholar]
- 152.Hurwitz H., Fehrenbacher L., Novotny W. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]


