Abstract
Background
Patients with cirrhotic ascites (PCA) are susceptible to spontaneous bacterial peritonitis (SBP) which has increased morbidity and mortality. Since some host defense aspects of peritoneal macrophages (PMф) from PCA are altered this study examined factors related to receptor-mediated phagocytosis.
Methods
Twelve PCA were studied. PMɸ were isolated from ascitic fluid (AF) samples removed from these patients. Uptake of mannose receptor (MR)-specific ligand, fluorescein isothiocyanate-mannosylated-bovine serum albumin (FITC-man-BSA), by patients' PMɸ and controls, a human monocytic cell line, was measured pre- and post-IL-4 treatment. Phagocytosis of FITC-labeled yeast particles by patients' PMɸ was measured pre- and post-IL-4 treatment. Fluorescence values were obtained using a spectrofuorometer. MRC1 gene was analyzed in blood samples from PCA and controls, healthy donors, using standard polymerase chain reaction (PCR) technique.
Results
Past SBP episode(s) were reported in 58.3% of patients. Mean AF volume analyzed per patient was 1.3L. PMɸ ratio in cell yield was 53.73% (SD 18.1). Mean uptake absorbance of patients' PMф was 0.0841 (SD 0.077) compared to 0.338 (SD 0.34) of controls, P = 0.023. Following IL-4 treatment absorbance increased to 0.297 (SD 0.28) in patients' PMф (P = 0.018 on paired sample t-test), and to 0.532 (SD 0.398 in controls (P = 0.053 on independent sample t-test). Mean phagocytosis absorbance of patients' PMф was 0.1250 (SD 0.032) before IL-4 treatment compared to 0.2300 (SD 0.104) after (P = 0.026). PCR analysis for MRC1 gene was negative in all PCA samples compared to positive results in all controls.
Conclusion
Since decreased phagocytosis and MR uptake were enhanced post-IL-4 treatment MR downregulation pre-treatment is plausible. Negative PCR results for MRC1 might suggest an anomaly, but this awaits further ellucidation. These altered host defense findings are relevant to infection pathophysiology, and their relevance to SBP susceptibility in PCA is worth verifying.
Keywords: cirrhosis, macrophage host defense, mannose receptor, MRC1 gene, spontaneous bacterial peritonitis
Abbreviations: AF, ascitic fluid; FBS, foetal bovine serum; FITC, fluorescein isothiocyanate; IL-4, interleukin-4; man-BSA, mannosylated bovine serum albumin; MR, mannose receptor; MRC1, gene encoding human MR; PCA, patients with cirrhotic ascites; PCR, polymerase chain reaction; PMф, peritoneal macrophages; RPMI and DMEM, cell culture media
Patients with liver cirrhosis and ascites (PCA) have a low threshold to develop bacterial infection, which is associated with increased morbidity and mortality.1 Spontaneous bacterial peritonitis (SBP), for instance, is considered the prototype infection in cirrhosis and occurs in 30–50%.2,3 With regarding infection pathophysiology host defense of circulating phagocytes from patients with cirrhosis was shown to be impaired.4,5 However, little is known about host defense of tissue-type phagocytes in PCA. The tissue-type peritoneal macrophages (PMф) play a pivotal role in pathophysiological processes in the peritoneal milieu,6,7 and a function anomaly in these cells could be relevant to SBP pathophysiology. Mannose receptor (MR) is a novel example of a host defense receptor that has recently been focused. It is a C-type lectin endocytic receptor with extra-cellular domains that co-ordinate sugar binding of ligands.8,9 Ligand binding and internalization are referred to as receptor-mediated endocytosis.10,11 Mannose-containing glyco-conjugates constitute part of the structure of microorganisms responsible for infection.11 MR expression is upregulated by interleukin-4 (IL-4) and dexamethasone,12,13 and is downregulated by in vivo activation state in which MR gets internalized.14 MRC1, the gene encoding the human MR, is located on chromosome 10p12 and consists of 30 exons.15 More recently MRC1 polymorphism has been associated with susceptibility to certain infections like tuberculosis and leprosy.16,17 We have previously demonstrated that PMф from PCA produced vigorous respiratory burst, and we speculated that an in vivo activation state might be present.5 Since uptake measurement of man-BSA, the MR-specific ligand, may indirectly represent MR expression we performed uptake measurement and also analyzed MRC1 gene in PCA.
Methods
Patients' Samples Collection
Ascitic fluid (AF) was collected from 15 adult patients with established diagnosis of cirrhosis with ascites. Patients were randomly selected and the number was determined arbitrarily. Patients were excluded from the study if they had any of following criteria:
1. malignancy, 2. evidence of current SBP or other infection, 3. an altered level of consciousness, 4. hematemesis within the previous four-weeks to recruitment, 5. a major cardiopulmonary disease, end stage kidney disease or terminal illness, 6. intake of immunomodulator drugs or antibiotics within the previous six-week. These agents could affect some of the functional assays. For the majority of PCA in this study locality secondary prophylaxis with antibiotics is not readily available, and this exclusion criterion was not an obstacle.
An informed consent was obtained in all patients. Patients were then re-assessed where full history was obtained and complete physical examination was performed. History of past episode(s) of SBP was specifically recorded. Patients were originally booked either as day cases or as short-stay admissions for routine therapeutic paracentesis. This procedure was judged indicated by the treating physician. The in-charge physicians have kindly given permission for the use of the aspirated fluid for the study purpose.
Paracentesis procedure was done according to published guidelines.18 The volume of fluid sample that was sent to the lab was no more than 2.0 L per patient and it was analyzed within an hour. The exact fluid volume for each patient received in the lab was recorded on arrival. Also, fluid appearance, neutrophil cell count and protein level in each sample were obtained and recorded at the outset. If AF appearance was very cloudy or heavily blood-stained or if the neutrophil count was >200 cells/mI the specimen was considered unsuitable for further analysis. The in-charge physician was kept informed of all this information.
Approval for this study was obtained from the Hospital's Research Ethics Committee, according to the Declaration of Helsinki.19
Patients Peritoneal Macrophage Preparation
The cell separation process was done as previously.5 Cell viability tests were determined by trypan blue dye exclusion. The ratio of PMф in cell mixture was determined manually by counting during the microscopic examination of cytocentrifuge preparations. These were stained using May Grunwald Giemsa and acid esterase.20,21 In experiments where adherent cells were used the cell adherence was allowed to take place first by incubating the plates for 2 h in complete fresh culture media containing 10% RPMI/FBS, 100 u/mI penicillin G sodium and 100 μg/mI streptomycin sulfate (Sigma Chemicals, Poole, UK) and at 37 °C and 5% CO2 air. The intended assay was then carried out immediately. In experiments where PMɸ were used in suspension the adherent cell layer prepared as above was detached by a quick incubation of plates in trypsin solution for 45 s.22 The cell harvest was re-counted, re-tested for viability as above and then the intended assaying was performed directly.
Control Cells Preparation
The Mono Mac 6 (MM6) human monocytic cell line was originally obtained as a commercial product from German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany. This cell line is a human acute monocytic leukemia cell line which was confirmed suitable for in vitro experiments and the cells were prepared by sub-culturing as previously.23
Phagocytosis
Yeast Labeling
Candida albicans particles were prepared and F1TC-labeled (Sigma Chemicals) as previously.24 Labeled particles were then re-suspended at 5 × 107 cells/ml in 20% DMEM/FBS (Sigma Chemicals) and stored light-protected at 4 °C. The resulted stock was used within ten weeks of preparation.
Phagocytosis Measurement
Phagocytosis measurements were done according to previous method24 with some modification.5 Labeled yeast particles prepared as above were added to wells at a macrophage-to-particles ratio of 1:40 and phagocytosis proceeded for 30 min. Measurements were performed before and after treatment of cells with IL-4 (Sigma Chemicals). Treatment was given for 36 h at a concentration of 5 ng/ml. Normally cells treated this way were assayed in adherent form. At the end of phagocytosis process plates were thoroughly washed out, cells were lysed in 0.1 N NaOH and fluorescence readings from internalized particles were obtained using a spectrofluorometer (Chem 7, Erba Diagnostics, Manheim GmbH, Germany) at an excitation wavelength of 482 nm and emission wavelength of 520 nm. All samples were analyzed in duplicates.
Fluorescence values were expressed as percentages of arbitrary absorbance units and calculations were made using the following formula:
where %A is the percentage of absorbance units, F1 is the absorbance of the wells containing both cells and labeled particles, F2 is the absorbance of the wells containing cells only (auto-fluorescence) and F3 is the absorbance of the wells containing labeled particles only (total fluorescence).
Receptor Specific Uptake Measurement
Uptake measurement of FITC-labeled man-BSA by MR in PMф from PCA and controls was performed as previously.25 Yeast mannan 0.1 M was used as a blank ligand and was added to wells at a concentration of 12 μg/ml and FITC-labeled man-BSA (Sigma Chemicals) as specific stable ligand and was added to wells at a concentration of 12 μg/ml. Where an effect of IL-4 treatment was examined, PMф were treated first with this agent at a concentration of 5 ng/ml for 36 h. Fluorescence in cell lysate was obtained using a spectrophotometer at 482- and 520-nm wavelengths.
Specific uptake (Fsp) was calculated as follows:
where F1 is fluorescence of wells containing cells and FITC-labeled man-BSA, F2 is fluorescence of wells containing cells only (autofluoresence), F3 is fluorescence of wells containing cells, yeast mannan and FITC-labeled man-BSA and F4 is fluorescence of wells containing mannan only.
MRC1 analysis
DNA Extraction
Genomic DNA was purified from EDTA-blood samples taken from twelve PCA and eight healthy volunteers using QIAamp Blood Midi Kit (spin protocol) (Qiagen GmbH, Hilden, Germany) and as recommended by the manufacturers.26 Samples were processed within an hour of their withdrawal. Volume eluted from the column was <200 μl which was kept at—20 °C till PCR analysis which was normally carried out the following day. This way 200 μl of whole blood yields 3–12 μg of DNA, and the DNA concentration was 34.0 ng/μl.
Polymerase Chain Reaction (PCR)
MRC1 gene was analyzed by standard PCR technique as previously16 with some modification. Briefly, genomic DNA was purified as described above. PCR Master Mix used was GoTaq® Green Master mix product (Promega Corporation, Madison, USA) and PCR primers were used. The PCR primers included forward: TTGAGGCTGCAATGAGACAT and reverse: AGTGTAAGGTAGACTGCTCT. Final PCR reaction volume (20 μl) was composed of 10 μl GoTaq® Green Master Mix, 0.5 μl forward primer, 0.5 μl reverse primer, 5 μl DNA template and 4 μl distilled water. PCR was performed as follows: denaturing at 95 °C for 5 min, 35 cycles at 95 °C for 50 s, 63 °C for 50 s, and 72 °C for 30 s, and a final extension at 72 °C for 10 min. PCR products were subjected to agarose gel electrophoresis and visualized by ethidium bromide staining.
Statistical Analysis
Statistical package used to analyzed data was SPSS version 17. Paired and independent sample t-tests were used depending on the experiment conducted. Paired sample t-test was used to analyze data in the same group pre- and post intervention e g phagocytosis data and part of uptake data. Independent t-test was used to analyze data in patients' group as compared to that in controls, namely the rest of uptake data. The significant level for P value was taken as <0.05.
Results
Patients Detail
The number of patients included in this study was 12 PCA. Three other patients were excluded because they did not meet the inclusion criteria. Nine (75%) were males and 3 females (25%) (Table 1). Male to female ratio was 3:1. Mean age was 52.86 (SD 11.1) years. There were 10 patients (83.3%) in Child-Pugh class C and 2 patients (16.7%) in Child-Pugh class B (Table 1). The cause of cirrhosis was HBV-related in 8 patients (66.6%) in whom HBsAg test was positive (Table 1). The co-existence of HBV-related cirrhosis and schistosoma peri-portal fibrosis (PPF) was present in 2 out of those 8 patients (Table 1). PPF was mainly an ultrasound finding made on top of cirrhosis. The other causes of cirrhosis were found as follows: HCV-related in 1 patient (8.3%) in whom HCV test was positive, alcohol-related in 2 patients (16.6%), and autoimmune hepatitis (AIH)-related in 1 patient (4.7%) in whom anti-nuclear antibody test was positive (Table 1). Ultrasound diagnosis of cirrhosis was made in all patients but the liver biopsy diagnosis was obtainable in only 2 patients (16.6%) (Table 1). In terms of occurrence of previous episode(s) of SBP 7 patients (58.3%) reported to have had at least one past episode, no previous episodes in 2 patients (16.6%) and in the remaining 3 patients (25%) the information on whether SBP has actually occurred was not definite (Table 1). None of those patients who have had past episode(s) of SBP were kept on prophylaxis antibiotic treatment.
Table 1.
Patients' Clinical Information and Laboratory Results.
| No. of patients | % | |
|---|---|---|
| Sex: | ||
| Male | 9 | 75% |
| Female | 3 | 25% |
| M:F ratio 3:1 | ||
| Mean age: 52.8 yrs (SD 11.1) | ||
| Child-Pugh’s class: | ||
| C | 10/12 | 83.3% |
| B | 2/12 | 16.7% |
| No. of patients with past SBP episode(s) reported | 7/12 | 58.3% |
| Means of diagnosis | ||
|
12/12 | 100% |
|
2/12 | 16.6% |
| Etiology of Cirrhosis: | ||
| HBsAg positive | 8 | 66.6% |
| HCV positive | 1 | 8.3% |
| Alcoholic | 2 | 16.6% |
| Auto-immune | 1 | 8.3% |
| Total | 12 | 100% |
Ascitic Fluid Analysis
The mean volume of sample of ascitic fluid analyzed per patient was 1.3 (SD 0.357) L (Table 2). The mean total number of cells isolated was 23.87 × 106 cells (SD 17.2) per sample, and 53.73% (SD 18.1) of these were PMф as determined by counting under light microscopy (Figure 1a) (Table 2). After overnight incubation the plates were examined in an inverted microscope and striking morphological changes were observed (Figure 1b). These morphological changes were suggestive of cell activation. The mean cell viability was 93% (SD 2.8). Fluid appearance was described as clear in 8 (66.6%) patients, as hazy in 3 (25%) patients and as cloudy or slightly blood-stained in one patient (Table 2). Fluid protein level was 2.25 mg/dl (SD 0.57) (Table 2), which was in keeping with a clear fluid appearance in the majority of samples.
Table 2.
Results of Ascitic Fluid (AF) Analysis.
| Parameter | Result |
|---|---|
| Fluid volume | 1.3L (SD 0.357) |
| Mean fluid protein level | 2.25 mg/dl (SD 0.57) |
| Mean total cell no. isolated | 23.87 x106 cells (SD 17.2) |
| Mean percentage of PMф in total cells no. isolated | 53.73% (SD 18.1). |
| Cell viability | 93% (SD 2.8) |
| Fluid appearance was: | |
| clear in | 8/12 patients (66.6%) |
| hazy in | 3/12 patients (25%) |
| cloudy/slightly blood-stained in | 1/12 patients (8.3%) |
Figure 1.

Photographs of stained sections of patients' PMф at high magnifications (A & B) and phase contrast views of tissue culture plates (C & D). Slide A & B were stained using May Grunwald Giemsa stain. Observe the triking morphological changes following overnight incubation (D). No stimulus was added to these cells.
Phagocytosis Results
Phagocytosis measurement was made in six samples (A, B, C, D, E and F) of patients' PMф before and after treatment with IL-4 (Figure 2). The absorbance increased in patients' PMф samples as follows: from 0.175 to 0.365 in sample A, from 0.08 to 0.17 in B, from 0.10 to 0.15 in C, from 0.14 to 0.24 in D, from 0.10 to 0.15 in E and from 0.125 to 0.18 in sample F (Figure 2). These values gave a mean absorbance of 0.2300 (SD 0.104) in IL-4-treated PMɸ as compared to 0.1250 (SD 0.032) in untreated PMɸ, P = 0.026 on paired sample t-test (Figure 2). The vertical axis shows absorbance values and the letters on the horizontal axis represent the samples tested (Figure 2). The black diamond symbols represent absorbance value before IL-4 treatment and the light square symbols represent absorbance values after IL-4 treatment (Figure 2).
Figure 2.
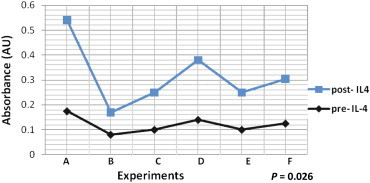
Effect of IL-4 on phagocytosis in patients' macrophages. The bottom curve (dark diamond symbols) represents phagocytosis measurements before IL-4 treatment and the top curve (square symbols) represents measurements after IL-4 treatment. Letters in horizontal axis denote samples of patients' PMɸ. Values in vertical axis represent absorbance.
Receptor Uptake Results
The mean absorbance of specific uptake of PMф from 6 PCA was 0.0841 (SD 0.077) (dark bars) as compared to 0.338 (SD 0.34) of controls (light bars), P = 0.023 on independent sample t-test (Figure 3). Following treatment with IL-4 the mean uptake absorbance increased from 0.0841 to 0.297 (SD 0.28) (dark bars) and from 0.338 to 0.532 (SD 0.398) (light bars) in patients' PMф and in controls respectively, P = 0.053 on independent sample t-test and P = 0.018 on paired sample t-test (Figure 3).
Figure 3.

Mannose receptor (MR) specific uptake measurement of PMɸ from patients and controls. The panel on the left represents uptake measurements in patients' PMɸ (dark bar) and controls (light bar) before IL-4 treatment P = 0.023 on independent sample t-test. The panel on the right represents uptake measurements after IL-4 treatment in both groups P = 0.053 on independent sample t-test and P = 0.018 on paired sample t-test. The figures on the vertical axis represent absorbance.
Specific uptake absorbance values were calculated using the formula in Material and Methods.
Experimental conditions were identical and experiments were carried out in duplicates.
Polymerase Chain Reaction Results for MRC1
There were no bands in lanes 1, 2, 3 and 5 (Figure 4a). These were negative results in four out of twelve PCA's samples tested. The only positive result (visible band) was in lane 4 which represents a sample from a healthy donor (Figure 4a). Likewise no bands were visualized in the remaining 8 patients' samples (film not shown). Positive results (visible bands) in lanes 1–7 were present in the remaining seven control samples from healthy donors. 550 bp on 1.5% agarose gel M: 50 bp DNA size marker (Figure 4b).
Figure 4.

a. PCR Results of MRC1 gene in patients with cirrhosis. In lanes 1, 2, 3 and 5 no bands for MRC1 gene were visualized (negative PCR results) in four out of twelve samples from PCA. In lane 4 a clear band is visualized (positive PCR result) for MRC1 gene in a healthy donor's sample, serving as a normal reference. In the remaining eight patients' samples the results were also negative (film not shown). 550 bp on 1.5% agarose gel M: 50 bp DNA size marker. b. PCR Results of MRC1 gene in normal subjects. In lanes 1–7 clear bands were visualized for MRC1 gene (positive PCR results). No patients' samples were included here. M: 50 bp DNA size marker. 550 bp on 1.5% agarose gel.
Discussion
In this study we examined the host defense function of PMф from PCA. Since these cells are tissue-resident phagocytes they are expected to play a pivotal role in the pathophysiology of SBP, namely host defense aspect of pathophysiology. The function of circulating phagocytes has already been extensively studied in cirrhosis patients,27–30 but only little information is available on the function of PMф from these patients. Ninety percent of our patients were in Child-Pugh class C indicating an advanced stage of cirrhosis. Because of the study's nature and size we were uncertain if a convincing correlation of data would be achieved betdifferent aetiological subgroups or other clinical parameters of cirrhosis. At this stage of cirrhosis ascites is present in about 50–70% of cases and SBP and its complications are known to be prevalent.31–33 This high risk group of cirrhotic patients was targeted because of the dismal outcome of infection in them. Depending on what type of new information about infection pathophysiology is gained such knowledge might help in reducing susceptibility to infection.
In a previous study we demonstrated for the first time that quantitative phagocytosis measurement in PMф from PCA was lower than that in controls (P = 0.0001).5 In the same study PMф showed vigorous respiratory burst (RB) and we hypothesized that this heightened RB could be associated with decreased phagocytosis.5 MR internalizes rapidly in the presence of reactive oxygen intermediates released in RB and MR also internalizes in the presence of other inflammatory mediators like trypsin and antigenic products.11,14,34,35 In such an environment clearance of products from inflammatory milieu by host defense receptors is reduced.14
This study showed a significantly lower MR specific uptake in patients' PMф than that in controls (P = 0.023). One plausible explanation to this finding is receptor downregulation in an activated peritoneal environment in cirrhosis. In a few studies IL-4 has upregulated MR expression in healthy macrophages.12,35–37 In this study we examined the effect of this anti-inflammatory cytokine and we found uptake measurements in both groups were higher in IL-4-treated cells than that in untreated cells. However, this increase in uptake did not reach statistical significance on independent sample t-test which compared data in the two groups (P = 0.053). However, the increase in uptake was significant on two-tailed sample t-test which compared patients-only data pre- and post-IL-4 treatment (P = 0.018).
We then measured phagocytosis before and after treatment of cells with IL-4. in the patients' group only. Phagocytosis increased significantly following IL-4 treatment as compared to pre-treatment levels, P = 0.026 paired sample t-test (Figure 3).
The increase in specific uptake and in phagocytosis following IL-4 supported our claim that IL-4 had an enhancing effect on a previously downregulated MR, but more specific MR expression studies in PMɸ from PCA are required to substantiate this claim. However, an increase of upto four fold in phagocytosis has been reported before in IL-4-treated macrophages from healthy volunteers and also in macrophages from patients with chronic obstructive airway disease.38 IL-4-enhancing effect on MR expression both in health and in certain disease states is possibly through its anti-inflammatory properties. IL-4 serum levels are expected to show wide variations, particularly in cirrhosis and these may cause limitation in interpretation. However, since our data were essentially obtained from in vitro experiments it is perhaps too early to discuss in depth facts about clinical or in vivo applications. Dexamthasone, is another agent that has been shown to increase MR activity by increasing receptor mRNA levels,13 but again this was only at an in vitro level. Phagocytosis of alveolar macrophages increased with in vitro treatment of azithromycin more than the increase in untreated cells. Of interest, this increase in phagocytosis measurement was associated with MR upregulation.38 However, when comparing the enhancing effect of IL-4 on phagocytosis shown in this study to that of azithromycin reported in another study38 the IL-4 effect is less than that of azithromycin. The increase is <2 fold vs. 2–4 fold respectively. It is prudent to add that macrophage heterogeneity could account, at least in part, for such a difference in effect because the two macrophage populations were from different habitat.
Novel receptor characterization and expression studies have applied radioisotope techniques.11,12,34–37 Unfortunately we were unable to perform these techniques. However, in order to back-up the initial results we obtained from fluorescence technique we opted for PCR analysis. Needleless to say PCR has been used in host defense research and it provided valuable information in various disease settings including cirrhosis.16,17,39,40 For instance, in a recent study the investigators demonstrated molecular evidence of bacterial antigenic material in cirrhotic ascites in the absence of a microbiological evidence for SBP.40 MR studies, in particular, identified an association between polymorphic allele of MRC1 and susceptibility to certain infections including tuberculosis and leprosy.16,17 In those studies standard PCR technique was used. This study has tested the gene encoding MR and in all patients' samples we were unable to visualize a band representative of MRC1on agarose gel electrophoresis (Figure 4a). In contrast, clear bands for the gene were visualized in all control samples (Figure 4b). The negative PCR finding in the patients' group is interesting.
First, it is in line with the finding of decreased fluorescence uptake measurement reported above. Second, it might suggest the presence of an anomaly such as polymorphism, but this needs further elucidation. Given that the results in control samples were consistently positive it is fair to say that the standard PCR technique is adequate for gene detection and that patients' results are true negatives. In a previous study the initial PCR results were not shown separately as we did in this study, instead the investigators proceeded to sequence analysis using a sophisticated sequencer.16 According to this study's protocol the sequence analysis was meant to be dependent on obtaining positive PCR results beforehand. Since patients' results were negative we were therefore unable to undertake sequence analysis. We presume that a larger study size is required if a suitable material was required for sequencing, and this is our next task in a future study.
Conclusion
Decreased phagocytosis could be attributed to by the finding in this study of reduced MR uptake. The increase in phagocytosis following IL-4 treatment suggests that MR was possibly downregulated prior this treatment, and hence uptake was reduced. The negative PCR result for MRC1 might also suggest a possible mutation but this awaits further verification. Since the factors examined in this study relate mainly to pathophysiology the findings have provided useful information that could help in a better understanding of the complex issue of infection pathophysiology. Whether this could help in reducing the increased susceptibility to infection in PCA remains to be seen.
Funding
The research was partly funded by a grant from Research Directorate at Ministry of Higher Education (grant number 462/2010).
Conflicts of interest
All authors have none to declare.
References
- 1.Sheer T.A., Runyon B.A. Spontaneous bacterial peritonitis. Dig Dis. 2005;23:39–46. doi: 10.1159/000084724. [DOI] [PubMed] [Google Scholar]
- 2.Conn H.O. Spontaneous peritonitis and bacteraemia in Laennec's cirrhosis caused by enteric organisms: a relatively common but rarely recognized syndrome. Ann Int Med. 1964;60:568–580. doi: 10.7326/0003-4819-60-4-568. [DOI] [PubMed] [Google Scholar]
- 3.Conn H.O. Spontaneous Bacterial Peritonitis: The Disease, Pathogenesis and Treatment. Marcel Dekker; New York: 2005. The prevalence of spontaneous bacterial peritonitis; pp. 62–70. Print ed, Taylor and Francis. [Google Scholar]
- 4.Hassner A., Kietter V., Schlag D., Yedvab M., Aronson M., Shibolet S. Impaired monocyte function in liver cirrhosis. Br Med J. 1981;282(12):62–1263. doi: 10.1136/bmj.282.6272.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed A.M.M., Bomford A., Nouri-Aria K.T., Davies T., Smith R., Williams R. Peritoneal macrophages from patients with cirrhotic ascites show impaired phagocytosis and vigorous respiratory burst. Results Immunol. 2011;1:53–59. doi: 10.1016/j.rinim.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanner A.R., Arthur M.J.P., Wright R. Macrophage activation, chronic inflammation and gastrointestinal disease. Gut. 1985;25:760–783. doi: 10.1136/gut.25.7.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strossel T.P. The mechanism of phagocytosis. J Reticuloendothel Soc. 1976;19:237–245. [PubMed] [Google Scholar]
- 8.Harding C., Levy M.A., Stahl P. Morphological analysis of ligand uptake and processing: the role of multivesicular endosomes and CURL in receptor ligand processing. Eur J Cell Biol. 1985;36(2):230–238. [PubMed] [Google Scholar]
- 9.Stahl P., Schlesinger P.H., Sigardson E., Rodman J.S., Lee Y.C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980;19(1):207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- 10.Kang B.K., Schlesinger L.S. Characterization of mannose receptor-dependent phagocytosis mediated by mycobacterium tuberculosis lipoarabinomannan. Infect Immun. 1998;66(6):2769–2777. doi: 10.1128/iai.66.6.2769-2777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linehan S.A., Martinez-Pomares L., da Silva R.P., Gordon S. Endogenous ligands of carbohydrate recognition domains of the mannose receptor in murine macrophages, endothelial cells and secretory cells; potential relevance to inflammation and immunity. Eur J Immunol. 2001;31(6):1857–1866. doi: 10.1002/1521-4141(200106)31:6<1857::aid-immu1857>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 12.Stein M., Keshav S., Harris N., Gordon S. Interleukin 4 potently enhances alternative murine macrophage mannose receptor activity: a marker of immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowan H.B., Vick S., Conary J.T., Shepherd V.L. Dexamethazone upregulates MMR activity by increasing mRNA levels. Arch Biophys. 1992 July;296(1):314–320. doi: 10.1016/0003-9861(92)90578-k. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd V.L., Hoidal J.R. Clearance of neutrophil-derived myeloperoxidase by the macrophage mannose receptor. Am J Respir Cell Mol Biol. 1990;2(4):335–340. doi: 10.1165/ajrcmb/2.4.335. [DOI] [PubMed] [Google Scholar]
- 15.East L., Isacke C.M. The mannose receptor family. Biochim Biophys Acta. 2002;1572:364–386. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X., Jiang F., Wei L. Polymorphic allele of human MRC1 confer protection against tuberculosis in a Chinese population. Int J Biol Sci. 2012;8(3):375–382. doi: 10.7150/ijbs.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alter A., de Leseleuc L., Van Thuc N. Genetic and functional analysis of common MRC1 exon 7 polymorphisms in leprosy susceptibility. Hum Genet. 2010;127:337–348. doi: 10.1007/s00439-009-0775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Runyon B.A. Management of adult patients with ascites due to cirrhosis. Hepatology. 2009;49(6):2087–2107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 19.Human Experimentation. Code of ethics of the World Medical association, declaration of helsinki. Br Med J. 1964;ii:177. doi: 10.1136/bmj.2.5402.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eischen A., DucIos B., Schmitt-Goguel M. Human resident peritoneal macrophages: phenotype and biology. Br J Haematol. 1994;88:712–722. doi: 10.1111/j.1365-2141.1994.tb05109.x. [DOI] [PubMed] [Google Scholar]
- 21.van Furth R., Raeburn I.A., Van Zwet T.L. Characteristics of human mononuclear phagocytes. Blood. 1979;54:485–498. [PubMed] [Google Scholar]
- 22.Mackaness G.B. The imuunological basis of acquired cellular resistance. J Exp Med. 1964;120:105–112. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright E.L., Quenelle D.C., Suling W.J., Barrow W.W. Use of Mono Mac 6 human monocytic cell line and J774 murine macrophage cell line in parallel antimicrobial drug studies. Antimicrob Agents Chemother. 1996;40(9):2206–2208. doi: 10.1128/aac.40.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ragsdale R.L., Grasso R.I. An improved spectrofluorometry assay for quantitating yeast phagocytosis in cultures of murine peritoneal macrophages. J Immunol Methods. 1989;123:259–267. doi: 10.1016/0022-1759(89)90230-5. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto Y., Klein T.W., Friedman H. Involvement of mannose receptor in cytokine interleukin-1beta (IL-1beta), IL-6, and granulocyte-macrophage colony-stimulating factor responses, but not in chemokine macrophage inflammatory protein 1beta (MIP-1beta), MIP-2, and KC responses, caused by attachment of Candida albicans to macrophages. Infect Immun. 1997 March;65(3):1077–1082. doi: 10.1128/iai.65.3.1077-1082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartmann C, Lennartz K, Ibrahim H et al. Stable 16-Year Storage of DNA Purified with the QIAamp® DNA Blood Mini Kit. Qiagen GmbH, Qiagen Straße 1, Hilden, Germany. www.qiagen.com.
- 27.Rimola A., Soto R., Bory F., Arroyo B., Piera C., Rodes J. Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology. 1984;4(1):53–58. doi: 10.1002/hep.1840040109. [DOI] [PubMed] [Google Scholar]
- 28.Yousif-Kadaru A.G.M., Rajkovic I.A., Wyke R.J., Williams R. Defects in serum attractant activity in different types of chronic liver disease. Gut. 1984;25:79–84. doi: 10.1136/gut.25.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajkovic I.A., Williams R. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity of alcoholic cirrhosis and hepatitis. Hepatology. 1986;6:252–262. doi: 10.1002/hep.1840060217. [DOI] [PubMed] [Google Scholar]
- 30.Lahnborg G., Friman L., Berghem L. Reticuloendothelial function in patients with alcoholic liver cirrhosis. Scand J Gastroenterol. 1981;16:481–489. doi: 10.3109/00365528109182002. [DOI] [PubMed] [Google Scholar]
- 31.Wyke R.J. Bacterial infections complicating liver disease. Bailliere's Clin Gastroenterol. 1989;3(1):187–210. doi: 10.1016/0950-3528(89)90052-3. [DOI] [PubMed] [Google Scholar]
- 32.Caly W.R., Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993;18(3):353–358. doi: 10.1016/s0168-8278(05)80280-6. [DOI] [PubMed] [Google Scholar]
- 33.Parsi M.A., Atreja A., Zein N.N. Spontaneous bacterial peritonitis: recent data on incidence and treatment. Cleve Clin J Med. 2004;71(7):569–576. doi: 10.3949/ccjm.71.7.569. [DOI] [PubMed] [Google Scholar]
- 34.Shepherd V.L., Abdolrasulnia R., Stephenson I.D., Crenshaw C. Modulation of mannose receptor activity by proteolysis. Biochem J. 1990;270:771–776. doi: 10.1042/bj2700771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abramson S.L., Gallin J.I. IL-4 inhibits superoxide production by human mononuclear phagocytes. J Immunol. 1990;144:625–630. J Leukoc Biol 2006; 80(3): 563–571. [PubMed] [Google Scholar]
- 36.Crawford R.M., Finbloom D.S., Ohara J., Paul W.E., Meltzer M.S. B cell stimulatory factor-1 (interleukin 4) activates macrophages for increased tumoricidal activity and expression of Ia antigens. J Immunol. 1987;139:135. [PubMed] [Google Scholar]
- 37.Hodge S., Hodge G., Scicchitano R., Reynolds P.N., Holmes M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in the ability to phagocytose apoptotic airway epithelial cells. Immunol Cell Biol. 2003;81:289–296. doi: 10.1046/j.1440-1711.2003.t01-1-01170.x. [DOI] [PubMed] [Google Scholar]
- 38.Hodges S., Hodges G., Jersmann H. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in COPD. Am J Respir Crit Care Med. 2008;178:139–148. doi: 10.1164/rccm.200711-1666OC. [DOI] [PubMed] [Google Scholar]
- 39.Frances R., Munoz C., Zapater P. Bacterial DNA activates cell mediated immune response and nitric oxide overproduction in peritoneal macrophages from patients with cirrhosis and ascites. Gut. 2004;53:860–864. doi: 10.1136/gut.2003.027425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Such I., Frances R., Munoz C. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36:135–141. doi: 10.1053/jhep.2002.33715. [DOI] [PubMed] [Google Scholar]


