Abstract
Albumin is a negatively charged, relatively small protein synthesized by liver cells. Is the most abundant protein in extracellular fluid and accounts for about 70% of the plasma colloid osmotic pressure. Therefore it plays a crucial role in regulating fluid distribution in the body. In addition, albumin possesses functional domains with important non-oncotic properties, such as potent anti-oxydant and scavenging activities, binding of highly toxic reactive metal species and a great amount of endogenous and exogenous substances.
We have recently learned that albumin in cirrhosis undergoes a number of post-transcriptional changes that greatly impair its non-oncotic properties. The overall assessment of these changes clearly shows that the relative abundance of the native form of albumin is significantly reduced in hospitalized patients with cirrhosis and that these abnormalities worsen in parallel with the increasing severity of the disease. Thus, it is time to abandon the concept of serum albumin concentration and refer to the effective albumin concentration, that is the native intact albumin.
Given the pathophysiological context in which we use human albumin in patients with cirrhosis, who are characterized by peripheral vasodilation and a low-grade but sustained inflammatory state, the use of albumin in patients with cirrhosis should aim at enhancing effective hypovolemia and exploiting its antioxidant and scavenging activities.
The indications for the use of albumin in cirrhosis that clearly emerge from evidence-based medicine are represented by conditions characterized by an acute aggravation of effective hypovolemia and inflammation, such as such post-paracentesis circulatory dysfunction, spontaneous bacterial peritonitis, and hepatorenal syndrome. Other indications to the use of albumin that still require further studies are represented by bacterial infections other than spontaneous bacterial peritonitis, hepatic encephalopathy and long-term treatment of ascites, which has been debated for the last half-century.
Keywords: albumin, cirrhosis of the liver, ascites, bacterial infections, non-oncotic properties of albumin
Abbreviations: ACB, albumin cobalt binding; ACLF, acute-on-chronic liver failure; EASL, European Association for the Study of the Liver; EPR, electron paramagnetic resonance; HE, hepatic encephalopathy; HPLC, high performance liquid chromatography; HRS, hepatorenal syndrome; IMA, ischemia-modified albumin; MALDI-TOF, Matrix-Assisted Laser Desorption/Ionization with Time of flight technique; MARS, Molecular Adsorbent Recirculating Systems; MELD, model for end stage liver disease; NO, nitric oxide; PPCD, post-paracentesis circulatory dysfunction; RAAS, renin-angiotensin-aldosteron axis; ROS, reactive oxygen species; SBP, spontaneous bacterial peritonitis; SNS, sympathetic nervous system
Structure and functions
Albumin is the most abundant protein in plasma, constituting approximately 50% of the total protein content (3.5–5 g/dl). It is a globular protein, with a primary sequence made up of 585 amino acid residues and weighing 66.5 kDa. In its native form, albumin is moderately elongated, flexible and with a stable structure. Its high solubility and low viscosity are well suited to its role as a vector, scavenger and plasma expander. It consists of a single chain of amino acids that develops through nine loops, which are organized into three domains. In the molecular structure of albumin there are 35 cysteine residues, 34 of which are involved in internal disulfide bonds stabilizing the spatial conformation of the molecule, while the cysteine at position 34 (Cys-34) remains free.1
In the human body, albumin assumes the tertiary structure of an ellipsoid, formed by 67% of α-helices, where we can recognize three homologous domains (I-II-III), each containing two sub-domains (A and B). The different domains are capable of folding into hydrophobic pockets, which can open and close, and accommodate large insoluble anions as fatty acids. On the molecule surface there also are cationic groups capable of forming ionic bonds with many ligands.2,3
Liver cells are the only site where albumin is synthesized. They produce about 10–15 g per day, but, if needed, albumin production can increase upto 3–4 fold. The osmolarity and, subsequently, the oncotic pressure of the interstitial fluid in the hepatic parenchyma play a fundamental role in the regulation of albumin synthesis. In health, its production is quite constant, so that only a small intracellular deposit of the protein is required.
Approximately 30%–40% of the albumin pool is retained in the blood stream, while the remaining is distributed in the interstitium, where its concentration is low (1.4 g/dl), as well as in muscles and skin. The exchange of albumin between the plasma and the interstitium is a highly dynamic process: the protein leaves the vascular compartment at a rate of 5% per hour, returning to it via the lymphatic system in an amount equaling the output. The circulatory half-life of albumin, which is approximately 16–18 h, is therefore much lower than its total half-life, which varies from 12.7 to 18.2 days in a young healthy adult.
Albumin synthesis is stimulated by hormonal factors such as insulin, cortisol and growth hormone while, conversely, mediators of inflammation, such as cytokines IL-6 and TNF-α, act as inhibitors. Albumin is mainly degraded by the muscles, liver and kidneys, although its catabolism is a widespread process involving many tissues, which is influenced by the plasma concentrations of atrial natriuretic peptide.4,5
Functions
Albumin exerts important physiological roles. A most prominent feature is that albumin is the main modulator of fluid distribution in the various compartments of the body. In fact, about 70–75% of the oncotic pressure of the plasma is determined by albumin due to its direct osmotic property. Moreover, binding of cations, such as sodium, to the negative charges of the protein leads water to move from interstitium to the intravascular compartment (Gibbs–Donnan effect).6
Albumin binds and carries a great variety of hydrophobic molecules such as metals, fatty acids, metabolites and drugs, with consequent implications on solubilization, transport and metabolism of many endogenous and exogenous substances (Figure 1). Indeed, through the binding with albumin, many potentially toxic ligands are neutralized and definitively catabolized with the degradation of the protein.
Figure 1.
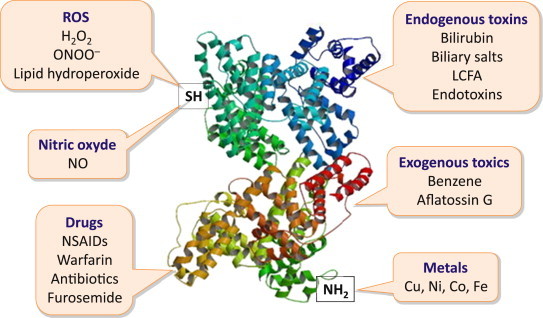
Albumin possesses functional domains with important properties, such as the free cysteine residue (SH) in position 34, which exerts potent anti-oxydant and scavenging activities, the aminoterminal (NH2) that binds to and removes highly toxic reactive metal species, and other domains that bind a variety of endogenous and exogenous substances including endotoxins and various drugs (ROS: reactive oxygen species; LCFA: long-chain fatty acids; NSAIDs: non-steroideal anti-inflammatory drugs).
Furthermore, albumin is the major source of extracellular reduced sulfhydryl groups (–SH), which act as potent scavengers of reactive oxygen species (ROS) derived from oxidative stress, thus constituting the main circulating antioxidant system in the body. In healthy adults, approximately 70–80% of albumin contains a free sulfhydryl group in Cys-34 position, the so called human mercaptalbumin. About 25%, instead, presents the Cys-34 involved in a disulfide bond with plasma sulfhydryl compounds, such as cysteine, homocysteine or glutathione. This form of albumin is called non-mercaptalbumin 1. Finally, only a small percentage of albumin circulates in the form of non-mercaptalbumin 2, where the residue Cys-34 is completely and irreversibly oxidized to sulfonic or sulphinic acid (albumin-SOH). In chronic diseases, such as diabetes, renal failure and ischemic heart disease, the percentage of totally oxidized albumin rises dramatically, causing a drastic decrease in the amount of albumin available for the detoxification of free radicals. Albumin also binds various metals, including copper, cobalt, nickel, zinc and iron, to its N-terminal portion through a high affinity binding, and this contributes to the antioxidant activity of the protein.7,8
Albumin exerts anti-inflammatory properties, being involved in the regulation of signaling systems between inflammatory cells, especially neutrophils and endothelial cells, through the reversible inhibition of pro-inflammatory cytokines (TNF-α) and complement factors (C5a).
There also are hemostatic properties, which are likely due to the interaction between the sulfhydryl groups of albumin and the nitric oxide (NO), with subsequent formation of the complex albumin-NO, also known as nitroso-albumin (with stable groups S-nitroso-thiol). This compound, whose role in health and disease is still debated, seems to play an important role in platelet aggregation and vasodilatation.9 Finally, albumin is involved in the regulation of acid-base equilibrium. The 16 imidazole residues of histidine within its molecular structure provide the protein a role of extracellular buffer system. In fact, albumin acts as a weak acid and, at the same time, it can help in buffering non-volatile acids.10
Alterations of albumin in cirrhosis
Recent studies have clearly shown that, in patients with cirrhosis, albumin undergoes post-transcriptional abnormalities that can be assessed by different techniques. Oettl et al used high performance liquid chromatography (HPLC) to separate fractions of albumin according to the redox state of cysteine-34. Carbonyl groups were measured as a marker of oxidative modification in plasma proteins and albumin. The oxidative forms of albumin non-mercaptalbumin 1 (oxidation state reversible in vitro) and non-mercaptalbumin 2 (oxidation state irreversible in vitro) were increased in parallel with the severity of liver failure.11 Jalan et al assessed the affinity of the fatty acid binding sites by using spin label (16 doxyl-stearate) titration and electron paramagnetic resonance (EPR) spectroscopy: this albumin function was reduced in patients with cirrhosis and worsened along with the disease severity. They also used the albumin cobalt binding (ACB) assay to measure the ischemia-modified albumin (IMA), a marker of oxidative damage to the molecule N-terminal. IMA was significantly increased in patients with acute or chronic liver failure (ACLF) and related to a poorer survival.12 Finally, our group assessed the post-transcriptional alterations of albumin by applying the Matrix-Assisted Laser Desorption/Ionization with Time of flight technique (MALDI-TOF) combined with HPLC, which has the advantage of simultaneously assessing the different structural changes of albumin.13 This study showed that extensive post-transcriptional changes of albumin, involving several molecular sites and increasing in parallel with disease severity, occur in patients with cirrhosis. Namely, cysteinylation was the more frequent alteration, which occurred alone or in combination with other molecular changes, including truncation of N-terminal portion and glycosylation. These altered isoforms were independently associated with specific complications of cirrhosis, such as ascites and renal failure. Moreover, the reduced relative abundance of native albumin isoform independently predicted 1-year patient survival with greater prognostic accuracy than total serum albumin concentration. In another study, we found that elevated IMA was an independent predictor of bacterial infection.14
Therefore, serum albumin in patients with cirrhosis is not only reduced, but also dysfunctional. This likely has important and clinically relevant consequences, such alterations of the redox balance, hemostatic disorders, modifications in transport, metabolism and excretion of several substances, acid-base imbalance and, mainly, reduced detoxification capacity and antioxidant activity. Thus, it may be time to abandon the concept of reduced serum albumin concentration and focus, instead, on the effective albumin concentration.7
Use of albumin in patients with cirrhosis: Pathophysiological basis
Reduced serum albumin concentration is a typical feature of cirrhosis and represents an important and adverse prognostic factor. It results from both a decreased hepatocyte production and various events closely related to the course of the disease. For example, renal sodium and water retention leads to plasma volume expansion and dilution of extracellular fluid protein content, thus contributing to lower serum albumin concentration. Another factor, at least in the most advanced stage of cirrhosis, is represented by an increase in the trans-capillary escape rate of the molecule that leads the protein to be lost towards the extravascular space.
The therapeutic use of albumin in liver disease dates back to the 1960s, when hypoalbuminemia was thought to play an important role in the pathophysiology of ascites, primarily due to the alteration of Starling force balance in the intrahepatic microcirculation. Indeed, it was found that serum albumin lower than 3 g/dl was almost constantly associated with the presence of ascites, which did not develop with a concentration greater than 4 g/dl. However, it should be outlined that the net fluid flow from the intravascular compartment to the interstitium is regulated by the trans-capillary colloid osmotic gradient, rather than by its absolute intravascular concentration. Coherent to this concept, the intravascular – interstitial colloid osmotic gradient did not significantly differ between healthy controls and patients with cirrhosis and ascites.15
It is now clear that the cardiovascular abnormalities occurring in advanced cirrhosis represent the main systemic pathogenetic factor favoring ascites formation. In fact, an increased production of vasodilating substances (i.e. NO, carbon monoxide, endocannabinoids) reduce the tone of resistance vessels and hamper their response to vasoconstrictors. As a result, arterial vasodilation ensues, mainly located in the splanchnic circulatory area, that leads to a reduction in effective volemia. This circulatory abnormality evokes compensatory responses, such as the increase in cardiac output, leading to the picture of hyperdynamic circulatory syndrome, and the activation of neuro-humoral systems, such as the renin-angiotensin-aldosteron axis (RAAS), the sympathetic nervous system (SNS) and arginine-vasopressin, leading to the renal retention of sodium and water. In the most advanced stages of cirrhosis, effective hypovolemia is further impaired by cardiac dysfunction, as witnessed by a relative reduction in cardiac output, ultimately leading to renal failure (hepatorenal syndrome).16
From this pathophysiological background (Figure 2), it emerges quite clearly that the preservation of effective blood volume in patients with advanced cirrhosis is crucial, and represents a major aim in the management of these patients. Indeed, several case-control studies and trials have shown that the administration of albumin is effective in preventing post-paracentesis circulatory dysfunction and the renal failure induced by spontaneous bacterial peritonitis, and in treating hepatorenal syndrome in association with vasoconstrictor drugs. Thus, albumin is currently administered to patients with cirrhosis to preserve or improve effective volemia in acute conditions at risk of developing further complications, first of all acute kidney injury. Therefore, serum albumin concentration should not be a guide for its use and the correction of hypoalbuminemia per se should not be a goal to pursue.17
Figure 2.
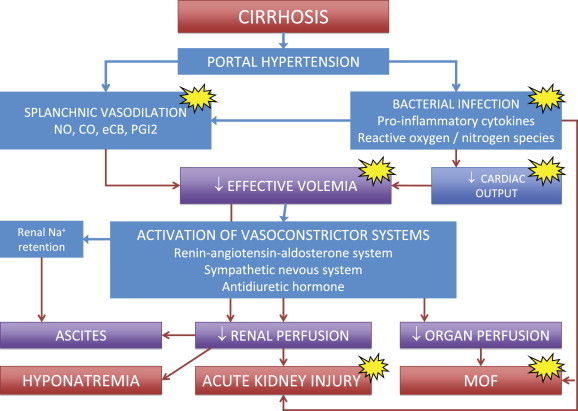
Schematic representation of the main pathophysiological events leading to renal sodium and water retention, ascites, hyponatremia, hepatorenal syndrome and multiorgan failure (MOF) in cirrhosis. Patients with cirrhosis are characterized by peripheral, mainly splanchnic, vasodilation, due to portal hypertension and a low-grade but sustained inflammatory state secondary to the intestinal translocation of bacteria and bacterial products. The ensuing reduction in effective volemia evokes the activation of vasoconstrictor and sodium and water retaining systems aimed at compensation through renal fluid retention and increased cardiac output. However, compensation is only partial and these changes lead to adverse effects, such as ascites formation and reduced renal perfusion that represents the background for dilutional hyponatremia and acute kidney injury. In this setting, the storm of pro-inflammatory cytokines and reactive oxygen and nitrogen species provoked by bacterial infections further enhances vasodilation, depresses cardiac function and endanger microcirculation potentially leading to multiorgan failure. The clinical use of albumin should aim not only at enhancing effective hypovolemia, but also at exploiting its antioxidant, scavenging and immunomodulating activities (yellow spots).
Use of albumin in patients with cirrhosis: Current indications (Figure 4)
Figure 4.
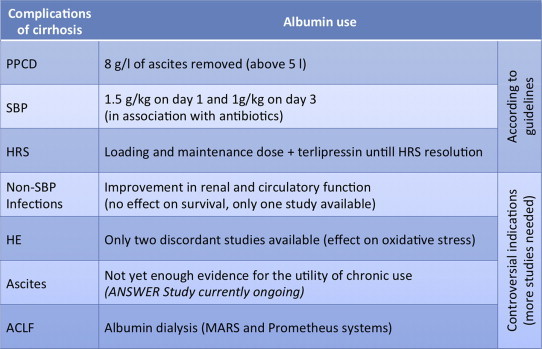
Summary table of the current uses of albumin in hepatology, according to the main international guidelines and looking at future perspectives (PPCD: post-paracentesis circulatory dysfunction; SBP: spontaneous bacterial peritonitis; HRS: hepatorenal syndrome; HE: hepatic encephalopathy; ACLF: acute-on-chronic liver failure).
Prevention of Post-Paracentesis Circulatory Dysfunction
Ascites is the most frequent complication of cirrhosis, and is associated with a poor prognosis, the 5-year survival being as low as 40% after its development.
Current guidelines indicate therapeutic paracentesis as the first line of treatment for patients with tense and refractory ascites. It has to be outlined, however, that the removal of large ascitic fluid volumes leads to a sharp drop in intra-abdominal pressure, boosting venous return to the heart and, therefore, cardiac output. However, because of an excessive drop in peripheral vascular resistance, effective volume further declines. Thus, large volume paracentesis can ultimately lead to arterial hypotension, renal impairment, dilutional hyponatremia, hepatic encephalopathy and reduced survival (Figure 2). Such a condition, named post-paracentesis circulatory dysfunction (PPCD), is defined by a rise of more than 50% in the basal plasma renin activity 4–6 days after paracentesis, indicating a worsening of effective volemia.18
Several randomized controlled studies have shown that PPCD can be prevented by albumin administration after the completion of paracentesis, and that albumin is more effective in doing so than artificial plasma expanders, especially with large volume paracentesis exceeding 5 L.19–22 Importantly, a recent meta-analysis has shown that albumin not only reduces the occurrence of PPCD more efficiently than any other plasma expander, but is also superior than either artificial plasma expanders or vasoconstrictors in lowering the incidence of hyponatremia and mortality.23 Thus, the use of albumin after large volume paracentesis appears to be more cost-effective than cheaper, but less efficient, plasma expenders. Therefore, the administration of 8 g of albumin per L of ascites removed is the treatment of choice for preventing PPCD in patients undergoing the withdrawal of more than 5 L of ascites.
Prevention of Renal Dysfunction Induced by Spontaneous Bacterial Peritonitis
Patients with cirrhosis are highly susceptible to bacterial infections, due to enhanced bacterial translocation from the gut and impaired defense mechanisms against bacteria. The most common bacterial infection in advanced cirrhosis is represented by spontaneous bacterial peritonitis (SBP). It is diagnosed by finding a neutrophil count, greater than 250/mm3 in ascitic fluid. Its in-hospital mortality approximates 20%, despite the fact that infection resolution occurs in the vast majority of patients.24 Such a high mortality is linked to the impact of pro-inflammatory cytokines on the cardiovascular system, ultimately leading to renal and, sometimes, multiorgan failure (Figure 2). Therefore, early diagnosis and treatment are of paramount importance to prevent the deterioration of hemodynamics.7,17
Albumin should be administered at a dose of 1.5 g/kg body weight on day 1 and then 1 g/kg on day 3. This therapeutic scheme was able to decrease the incidence of renal failure and reduce in-hospital and three-month mortality in a randomized controlled trial, compared with antibiotic therapy alone.25
Whether the prophylactic administration of albumin should be given to all patients developing SBP or be limited to specific settings has not been clearly established as yet. Available data suggest that the most striking effects are obtained in patients with severe liver failure, as defined by serum bilirubin greater than 4 mg/dl, and renal dysfunction, as defined by serum creatinine greater than 1 mg/dl, at the time of diagnosis. Thus, prophylactic albumin administration could be avoided in patients not fulfilling these features, who are characterized by a very low incidence of SBP-induced renal failure and low mortality.26 However, until more information is available, the Clinical Practice Guidelines of the European Association for the Study of the Liver (EASL) recommend that all patients who develop SBP should be treated with broad spectrum antibiotics and intravenous albumin.25
Management of Hepatorenal Syndrome
Hepatorenal syndrome (HRS) is a serious complication of advanced cirrhosis. As reported above, cirrhosis with portal hypertension is characterized by a reduction in the effective arterial blood volume as a result of two main mechanisms: splanchnic arterial vasodilation and cardiac dysfunction (cirrhotic cardiomyopathy). The RAAS, the SNS and arginine-vasopressin, activated as a compensatory response to this abnormality, cause, over time, severe intrarenal vasoconstriction, renal hypoperfusion and, ultimately, renal failure. In this context, HRS can develop (Figure 2).
HRS is a functional renal failure, unresponsive to plasma volume expansion that occurs in patients with end stage liver diseases.25,27 Two types of HRS can be identified: type 1, which is a rapid deterioration of renal function, defined as an increase in serum creatinine to a value twice the baseline and >2.5 mg/dl within 2 weeks; type 2, which is a slow and gradual deterioration of renal function with serum creatinine above 1.5 mg/dl. While the first condition is usually associated with an acute event, frequently represented by bacterial infections, the second often parallels the worsening of cirrhosis, representing the substrate for refractory ascites and hyponatremia.25,28
The current therapeutic approach to hepatorenal syndrome includes the administration of both vasoconstrictors and human albumin with the aim of improving effective volemia. Several vasoconstrictors have been used in the management of type 1 HRS, but terlipressin, which mainly acts on the splanchnic vascular bed, is the most studied and widely used. As recently shown by a systematic review of randomized studies, terlipressin may reduce mortality and improve renal function in patients with type 1 HRS.29 The recommended doses of terlipressin and albumin for treating HRS are not unanimously indicated as yet; suitable examples are shown in Figure 3.30,31
Figure 3.
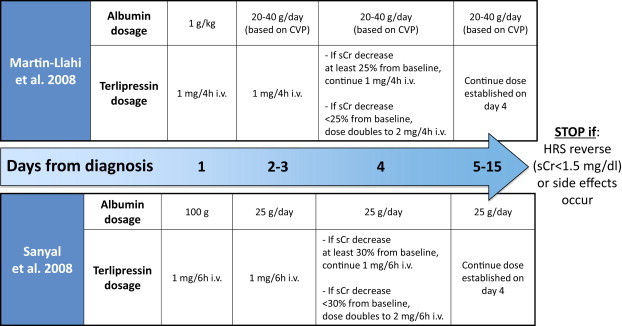
Dose schedules of terlipressin and albumin derived from the two main controlled clinical trials devoted to the treatment of patients with cirrhosis and hepatorenal syndrome (i.v.: intravenous; CVP: central venous pressure; sCr: serum creatinine; HRS: hepatorenal syndrome).
The efficacy of this therapy derives from the vasoconstriction induced by terlipressin, or other vasoconstrictors, on the splanchnic vascular district that improves effective hypovolemia, thus restoring renal perfusion. Interestingly, the simultaneous administration of albumin appears to be crucial, as the association between terlipressin and albumin is more effective than terlipressin alone.32
Use of albumin in patients with cirrhosis: Controversial indications (Figure 4)
Bacterial Infections Other Than Spontaneous Bacterial Peritonitis
While the use of albumin in preventing renal dysfunction occurring in patients with SBP, the indication to the prophylactic use of albumin in patients developing bacterial infections other than SBP has not been established as yet, as very few data are available. Indeed, a high incidence of renal dysfunction has been reported in this context.33 However, it should be outlined that stable or progressive renal failure, which are closely linked to short-term mortality, prevalently occur with sub-diaphragmatic infections, while the transient form prevails with infection located in other sites.34
A recent randomized controlled trial showed that treatment with albumin together with antibiotics, as compared with standard antibiotic therapy alone, had some beneficial effects on the renal and circulatory function and showed a potential survival benefit. However, the improvements in both the incidence of progressive renal failure and mortality were not statistically significant.35 The relatively low sample size likely and, possibly, patient selection prevented firmer conclusions from being reached. Indeed, sub-diaphragmatic infections and severe sepsis from bacteremias only accounted for about one third of cases. Thus, a relatively low incidence of progressive renal failure could be expected in the remainder. At present, a clear recommendation on the use of albumin in this setting cannot be given.
Hepatic Encephalopathy
Hepatic encephalopathy (HE) is a neuropsychiatric syndrome complicating acute and chronic liver failure and characterized by a wide range of manifestations, in absence of other brain disease.36 HE is very frequent in course of cirrhosis and even mild forms involve a great additional burden on patients, their families and health care resources.37
The pathophysiology of HE has been connected to several substances (mostly ammonia) produced in the gut and normally metabolized by the liver. More recently, other factors, such as inflammation driven by bacterial translocation and oxidative stress have thought to play an important role.38
The management of HE includes early diagnosis and prompt treatment of precipitating factors (infection, gastrointestinal bleeding, electrolyte disturbances, dehydration, hypotension, use of benzodiazepines, psychoactive drugs, and/or alcohol). Current treatment is based on reducing gut ammonia production with nonabsorbable disaccharides (lactulose or lactitol)39 and/or Rifaximin.40
The effect of albumin administration in patients with HE has been investigated in two clinical trials. The first enrolled fifteen patients with alcoholic cirrhosis and diuretic-induced HE of Grade II or more.41 The effect of volume expansion with albumin was compared to that induced by colloids. While a significant and sustained improvement of HE Grade was only seen in the group treated with albumin, significant improvements in plasma ammonia concentration, plasma renin activity and angiotensin II, which are markers of effective volemia, mean arterial pressure, renal plasma flow and urinary ammonia excretion were detected in both groups. Interestingly, however, increased plasma malondialdehyde concentration, a marker of oxidative stress, only declined in patients treated with albumin.
The beneficial effects of albumin on HE were not confirmed by a more recent multicenter trial, in which fifty-six patients with cirrhosis and HE grade II–IV were randomly assigned to receive albumin or saline.42 Even though albumin administration reduced 3-month mortality, it was not superior to saline in improving HE grade during hospitalization. Thus, further studies to explore the potential of such a therapeutic approach are needed.
Chronic Administration of Albumin for the Treatment of Ascites
The chronic and prolonged use of albumin to treat ascites is still debated, due to the lack of both the definitive evidence of a clinical benefit and an adequate assessment of the cost/effectiveness ratio.
So far, the effect of the combination of albumin and diuretics in resolving ascites and in reducing hospital stay has only been assessed in two controlled clinical trials conducted in Italy and involving a relatively small number of patients.43,44 In the first study, two groups of patients with cirrhosis and ascites were randomized to receive diuretics associated or not with the weekly infusion of 12.5 g of albumin.43 The treatment with diuretics plus albumin was overall more effective than diuretics alone in resolving ascites and reducing the length of the hospital stay. However, further data analysis showed that these results were only achieved in the subset of patients receiving the lowest dose of potassium-kanrenoate and furosemide, while a difference between the two groups was not seen when higher diuretic doses were needed. Moreover, as often occurs for studies characterized by a rigorous methodology designed to obtain clear-cut results, it could be difficult to transfer these results to every-day clinical practice, as patients were treated in hospital, while, at present, ascites is usually managed in the outpatient setting. Moreover, treatment duration was relatively short, as it lasted about three weeks. In the second study, carried out by the same group, a cohort of patients received either standard treatment or standard treatment plus albumin (25 g/week for the first year, then 25 g every 2 weeks) long-term (median follow up 84 months).44 Patients receiving albumin had a lower ascites recurrence rate and a better survival. The relatively low sample size prevented, however, to reach definitive conclusions.
In order to fill the gap of knowledge deriving from the lack of studies evaluating long-term albumin administration for the treatment of ascites, an open-label, multicentre, randomized clinical trial (NCT 01288794, www.clinicaltrials.gov, the “ANSWER Study”) that will enroll more than 400 patients is currently ongoing in Italy. This study has the potential to assess the effectiveness of long-term administration of albumin in reducing the occurrence of complications of ascites and improving the survival of patients.
Effects of exogenous albumin administration unrelated to its colloid osmotic properties
Even though it is likely that its non-oncotic properties contribute to the effects of exogenous albumin administration, to which extent this occurs is far from being defined. However, some evidence has emerged from available studies. Indeed, just to mention a few examples, the beneficial effects of albumin administration to patients with spontaneous bacterial peritonitis are not only amenable to plasma volume expansion, but also to an increase in peripheral vascular resistance and cardiac work. These effects are likely due to albumin ability to bind reactive oxygen species and inhibit pro-inflammatory cytokines production, thus attenuating endothelial dysfunction and vasodilation (Figure 2) as witnessed by a reduced production of nitric oxide and Von Willebrand-related antigen. Moreover, from the experimental point of view, albumin administration to cirrhotic rats with ascites restores heart contractility by counteracting reactive oxygen species and TNF-α.45 The non-oncotic properties of albumin should likely be taken into account to explain the beneficial effects on hepatic encephalopathy (HE), whose complex pathophysiology includes inflammation, vascular disturbances and ROS. Indeed, patients with diuretic-induced HE underwent a more significant improvement than patients treated with colloids, and showed a reduction in markers of oxidative stress.38,41
The detoxifying functions of albumin may be artificially and partly substituted by systems based upon the principles of albumin dialysis. Two systems have been subjected to extensive studies: the Molecular Adsorbent Recirculating Systems (MARS) and the Fractionated Plasma Separation and Adsorption (Prometheus), both based on removing albumin-bound and water-soluble substances.46,47 They associate a conventional dialysis membrane with a second dialysis circuit filled with a circulating 20% albumin solution (in the case of MARS) or with a second filter containing albumin (in the case of Prometheus). Both have been tested in patients with acute liver failure or patients with previously stable chronic liver disease (ACLF). They showed a good impact on systemic hemodynamics, severe HE, and removal of toxic molecules, even though no substantial effects on major outcomes, such as survival, were seen. Further studies are obviously needed, mainly aimed at identifying subgroups of patients who would actually benefit from these detoxification systems, as suggested by the improvement in survival observed in patients with severe cirrhosis (MELD score > 30) and type 1 HRS treated with the Prometheus system.7
Pharmacoeconomic considerations
The high cost of albumin certainly represents a major limitation to its use in clinical practice: indeed, besides inappropriate prescription, this has often prevented the widespread application of emerging indications. In the last few years, there have been several studies aiming at clarifying the correct indications of albumin administration and supporting the formulation of international guidelines. Thanks to these studies, the use of albumin in the clinical setting of liver cirrhosis and its complications is currently supported by evidence arising from prospective randomized trials and meta-analyses. However, the great amount of albumin required to fulfill current guidelines often raises concerns in regulatory Authorities because of its cost. Interestingly, in our University hospital, a huge hospital with 91 units, more than 1700 beds and 72,000 admissions per year, with a program for liver transplantation, albumin consumption and costs had been relentlessly increasing over the years. In this scenario, the use of albumin in liver patients according to the current guidelines was seen with apprehension. For these reasons, internal practice guidelines for the use of albumin were implemented in 2007, identifying conditions where albumin was formally not recommended and others where albumin had to be seen as a second line treatment. All the current indications for albumin administration in cirrhosis were accepted. The enforcement of these guidelines led to a sudden drop in albumin consumption by about 20%, which remained steady over the following years despite the fact that Internal Medicine and Gastroenterology units involved in the liver transplantation program actually increased their consumption.48 We think that our experience clearly shows that albumin use should not be uncritically restricted: the enforcement and application of in-hospital clinical practice guidelines allows the appropriate treatment of patients and keeps health care expenditure under control.
Conflicts of interest
Dr. Giacomo Zaccherini and Dr. Carmen Serena Ricci declare no conflicts of interest.
Prof. Mauro Bernardi declares grants from CLS Behring GmbH (as Consultant and Speaker), from PPTA Europe (as Speaker) and from Baxter Healthcare C (as Consultant and Speaker).
References
- 1.He X.M., Carter D.C. Atomic structure and chemistry of human serum albumin. Nature. 1992;358:209–215. doi: 10.1038/358209a0. [DOI] [PubMed] [Google Scholar]
- 2.Carter D.C., He X.M., Munson S.H. Three-dimensional structure of human serum albumin. Science. 1989;244:1195–1198. doi: 10.1126/science.2727704. [DOI] [PubMed] [Google Scholar]
- 3.Kragh-Hansen U. Structure and ligand binding properties of human serum albumin. Dan Med Bull. 1990;37:57–84. [PubMed] [Google Scholar]
- 4.Quinlan G.J., Martin G.S., Evans T.W. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41:1211–1219. doi: 10.1002/hep.20720. [DOI] [PubMed] [Google Scholar]
- 5.Chen W., Gassner B., Börner S. Atrial natriuretic peptide enhances microvascular albumin permeability by the caveolae-mediated transcellular pathway. Cardiovasc Res. 2012;93:141–151. doi: 10.1093/cvr/cvr279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henricksen J.H., Siemssen O., Krintel J.J., Malchow-Møller A., Bendtsen F., Ring-Larsen H. Dynamics of albumin in plasma and ascitic fluid in patients with cirrhosis. J Hepatol. 2001;34:53–60. doi: 10.1016/s0168-8278(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Martinez R., Caraceni P., Bernardi M., Gines P., Arroyo V., Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58:1836–1846. doi: 10.1002/hep.26338. [DOI] [PubMed] [Google Scholar]
- 8.Oettl K., Stauber R.E. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br J Pharmacol. 2007;151:580–590. doi: 10.1038/sj.bjp.0707251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruschi M., Candiano G., Santucci L., Ghiggeri G.M. Oxidized albumin. The long way of a protein of uncertain function. Biochim Biophys Acta. 2013;1830:5473–5479. doi: 10.1016/j.bbagen.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Bernardi M., Predieri S. Disturbances of acid-base balance in cirrhosis: a neglected issue warranting further insights. Liver Int. 2005;2:463–466. doi: 10.1111/j.1478-3231.2005.01115.x. [DOI] [PubMed] [Google Scholar]
- 11.Oettl K., Stadlbauer V., Petter F. Oxidative damage of albumin in advanced liver disease. Biochim Biophys Acta. 2008;1782:469–473. doi: 10.1016/j.bbadis.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Jalan R., Schnurr K., Mookerjee R.P. Alterations in the functional capacity of albumin in patients with decompensated cirrhosis is associated with increased mortality. Hepatology. 2009;50:555–564. doi: 10.1002/hep.22913. [DOI] [PubMed] [Google Scholar]
- 13.Domenicali M., Baldassarre M., Giannone F.A. Post-transcriptional changes of serum albumin: clinical and prognostic significance in hospitalized patients with cirrhosis. Hepatology. 2014 doi: 10.1002/hep.27322. [DOI] [PubMed] [Google Scholar]
- 14.Giannone F.A., Domenicali M., Baldassarre M. Abstract, The 64th Annual Meeting of the American Association for the Study of Liver Diseases: The Liver Meeting. 2013. Ischemia modified albumin (IMA) as a novel diagnostic marker of bacterial infection in hospitalized patients with cirrhosis. [DOI] [PubMed] [Google Scholar]
- 15.Henriksen J.H., Stage J.G., Schlichting P., Winkler K. Intraperitoneal pressure: ascitic fluid and splanchnic vascular pressures, and their role in prevention and formation of ascites. Scand J Clin Lab Invest. 1980;40:493–501. doi: 10.3109/00365518009091956. [DOI] [PubMed] [Google Scholar]
- 16.Arroyo V., Fernandez J. Pathophysiological basis of albumin use in cirrhosis. Ann Hepatol. 2011;10:S6–S14. [PubMed] [Google Scholar]
- 17.Bernardi M., Maggioli C., Zaccherini G. Human albumin in the management of complications of liver cirrhosis. Crit Care. 2012;16:211. doi: 10.1186/cc11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruiz-del-Arbol L., Monescillo A., Jimenéz W., Garcia-Plaza A., Arroyo V., Rodés J. Paracentesis-induced circulatory dysfunction: mechanism and effect on hepatic hemodynamics in cirrhosis. Gastroenterology. 1997;113:579–586. doi: 10.1053/gast.1997.v113.pm9247479. [DOI] [PubMed] [Google Scholar]
- 19.Ginès A., Fernández-Esparrach G., Monescillo A. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. 1996;111:1002–1010. doi: 10.1016/s0016-5085(96)70068-9. [DOI] [PubMed] [Google Scholar]
- 20.Ginès P., Titó L., Arroyo V. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology. 1988;94:1493–1502. doi: 10.1016/0016-5085(88)90691-9. [DOI] [PubMed] [Google Scholar]
- 21.Sola-Vera J., Mi-ana J., Ricart E. Randomized trial comparing albumin and saline in the prevention of paracentesis-induced circulatory dysfunction in cirrhotic patients with ascites. Hepatology. 2003;37:1147–1153. doi: 10.1053/jhep.2003.50169. [DOI] [PubMed] [Google Scholar]
- 22.Moreau R., Valla D.-C., Durand-Zaleski I. Comparison of outcome in patients with cirrhosis and ascites following treatment with albumin or a synthetic colloid: a randomised controlled pilot trial. Liver Int. 2006;26:46–54. doi: 10.1111/j.1478-3231.2005.01188.x. [DOI] [PubMed] [Google Scholar]
- 23.Bernardi M., Caraceni P., Navickis R.J., Wilkes M.M. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172–1181. doi: 10.1002/hep.24786. [DOI] [PubMed] [Google Scholar]
- 24.Rimola A., García-Tsao G., Navasa M. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32:142–153. doi: 10.1016/s0168-8278(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 25.European Association for the Study of the Liver EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Fernández J., Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56:S1–S12. doi: 10.1016/S0168-8278(12)60002-6. [DOI] [PubMed] [Google Scholar]
- 27.Salerno F., Gerbes A., Ginès P., Wong F., Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salerno F., Monti V. Hepatorenal syndrome type 1 and bacterial infection: a catastrophic association in patients with cirrhosis. Hepatology. 2014;59:1239–1241. doi: 10.1002/hep.27015. [DOI] [PubMed] [Google Scholar]
- 29.Gluud L.L., Christensen K., Christensen E., Krag A. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology. 2010;51:576–584. doi: 10.1002/hep.23286. [DOI] [PubMed] [Google Scholar]
- 30.Martín-Llahí M., Pépin M.N., Guevara M. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352–1359. doi: 10.1053/j.gastro.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Sanyal A.J., Boyer T., Garcia-Tsao G. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360–1368. doi: 10.1053/j.gastro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortega R., Ginès P., Uriz J. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology. 2002;36:941–948. doi: 10.1053/jhep.2002.35819. [DOI] [PubMed] [Google Scholar]
- 33.Terra C., Guevara M., Torre A. Renal failure in patients with cirrhosis and sepsis unrelated to spontaneous bacterial peritonitis: value of MELD score. Gastroenterology. 2005;129:1944–1953. doi: 10.1053/j.gastro.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Fasolato S., Angeli P., Dallagnese L. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223–229. doi: 10.1002/hep.21443. [DOI] [PubMed] [Google Scholar]
- 35.Guevara M., Terra C., Nazar A. Albumin for bacterial infections other than spontaneous bacterial peritonitis in cirrhosis. A randomized, controlled study. J Hepatol. 2012;57:759–765. doi: 10.1016/j.jhep.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Khungar V., Poordad F. Hepatic encephalopathy. Clin Liver Dis. 2012;16:301–320. doi: 10.1016/j.cld.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Bajaj J.S., Wade J.B., Gibson D.P. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106:1646–1653. doi: 10.1038/ajg.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.García-Martínez R., Córdoba J. Acute-on-chronic liver failure: the brain. Curr Opin Crit Care. 2011;17:177–183. doi: 10.1097/MCC.0b013e328344b37e. [DOI] [PubMed] [Google Scholar]
- 39.Als-Nielsen B., Gluud L., Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004:CD003044. doi: 10.1002/14651858.CD003044.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Bass N., Mullen K. Rifaximin treatment in hepatic encephalopathy. N Eng J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 41.Jalan R., Kapoor D. Reversal of diuretic-induced hepatic encephalopathy with infusion of albumin but not colloid. Clin Sci. 2004;106:467–474. doi: 10.1042/CS20030357. [DOI] [PubMed] [Google Scholar]
- 42.Simón-Talero M., García-Martínez R., Torrens M. Effects of intravenous albumin in patients with cirrhosis and episodic hepatic encephalopathy: a randomized double-blind study. J Hepatol. 2013;59:1184–1192. doi: 10.1016/j.jhep.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 43.Gentilini P., Casini-Raggi V., Di Fiore G. Albumin improves the response to diuretics in patients with cirrhosis and ascites: results of a randomized, controlled trial. J Hepatol. 1999;30:639–645. doi: 10.1016/s0168-8278(99)80194-9. [DOI] [PubMed] [Google Scholar]
- 44.Romanelli R.G., La Villa G., Barletta G. Long-term albumin infusion improves survival in patients with cirrhosis and ascites: an unblinded randomized trial. World J Gastroenterol. 2006;12:1403–1407. doi: 10.3748/wjg.v12.i9.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bortoluzzi A., Ceolotto G., Gola E. Positive cardiac inotropic effect of albumin infusion in rodents with cirrhosis and ascites: molecular mechanisms. Hepatology. 2013;57:266–276. doi: 10.1002/hep.26021. [DOI] [PubMed] [Google Scholar]
- 46.Bañares R., Nevens F., Larsen F.S. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57:1153–1162. doi: 10.1002/hep.26185. [DOI] [PubMed] [Google Scholar]
- 47.Dethloff T., Tofteng F., Frederiksen H.J., Hojskov M., Hansen B.A., Larsen F.S. Effect of prometheus liver assist system on systemic hemodynamics in patients with cirrhosis: a randomized controlled study. World J Gastroenterol. 2008;14:2065–2071. doi: 10.3748/wjg.14.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirici-Cappa F., Caraceni P., Domenicali M. How albumin administration for cirrhosis impacts on hospital albumin consumption and expenditure. World J Gastroenterol. 2011;17:3479–3486. doi: 10.3748/wjg.v17.i30.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]


