Summary
Fragile X-associated tremor/ataxia syndrome (FXTAS) is caused by a premutation CGG-repeat expansion in the 5′UTR of the fragile X mental retardation 1 (FMR1) gene. The classical clinical manifestations include tremor, cerebellar ataxia, cognitive decline and psychiatric disorders. Other less frequent features are peripheral neuropathy and autonomic dysfunction. Cognitive decline, a form of frontal subcortical dementia, memory loss and executive function deficits are also characteristics of this disorder. In this review, we present an expansion of recommendations for genetic testing for adults with suspected premutation disorders and provide an update of the clinical, radiological and molecular research of FXTAS, as well as the current research in the treatment for this intractable complex neurodegenerative genetic disorder.
Keywords: FXTAS, tremor/ataxia, premutation carrier, FMR1, FMR1 mRNA, FMPR, late-onset neurological disorder and neurodegenerative disorder
1. Introduction
The fragile X mental retardation 1 gene (FMR1) which causes fragile X syndrome if fully mutated (more than 200 CGG-repeats in the polymorphic region- 5′UTR), was discovered in 1991. This discovery led to the description of premutation carriers (individuals with smaller alleles, 55–200 CGG repeats) and was followed by a better understanding of the transition propensity, the expansion of the unstable allele of women with the premutation, and a better genetic counseling for risk of offspring with fragile X syndrome (the most common monogenetic form of autism and intellectual disability). Later on an intermediate allele was described (45–54 repeats) which has a variable risk for disorders that are associated with the premutation. Although before the discovery of the FMR1 gene, Cronister and colleagues (1) had reported a much higher incidence of early ovarian failure (before the age of 40 years) in females premutation carriers (PMC), PMC were generally seen as clinically unaffected (2–4). After the description of fragile X-associated primary ovarian insufficiency (FXPOI) in 1991 and FXTAS in 2001, there has been a general recognition that premutation alleles are associated with a wide range of clinical involvement. It is now widely recognized that PMC are at risk to develop a range of mild cognitive and behaviors problems during childhood and neurological, psychiatric and other immune-mediated disorders during adulthood (5). The prevalence of the FMR1 premutation has been described to be 1 in 113 to 259 females and 1 in 260 to 813 males in the general population (6–11). This suggests that about 1 in 3,000 men and about 1 in 6,000 women in the general population have fragile X-associated tremor/ataxia syndrome (FXTAS), which could be a common neurodegenerative disorder among the general population; however, more studies are necessary to define the incidence and prevalence of FXTAS. The clinical recommendations for testing of FMR1 mutation have been expanded after the description of premutation disorders and in this review we provide recommendations of offering testing for adults and will discuss the recent clinical, radiological, molecular and treatment research in FXTAS.
2. Clinical indications for FXS genetic testing in adults
The family history is crucial to determine whether there is an X-linked inheritance pattern of intellectual disability (ID) which would be typical for fragile X syndrome. However clinical suspicion of a premutation disorder should also be a consideration for FMR1 DNA testing. The American Academy of Pediatrics and the American College of Medical Genetics currently recommends FMR1 DNA testing for all children and adults with undiagnosed developmental delay/ID (12) and/or autism (ASD) (13). The American College of Obstetricians and Gynecologist (ACOG) also recommends testing in women with a family history of fragile X-related disorders, such as, unexplained ID/developmental delay, ASD or primary ovarian insufficiency (POI). In order to expand the screening criteria and to capture more premutation carriers the ACOG also recommends offering testing to all women who request fragile X carrier screening regardless of their personal and family history and also recommends to offer prenatal testing by amniocentesis or CVS to a known pregnant PMC (14). We also recommend considering genetic testing when there is personal medical history of unexplained late onset dementia or parkinsonism with any other associated premutation disorder and to consider testing in individuals with family history of a member with unexplained POI and mood/anxiety disorder, fibromyalgia and mood/anxiety disorder, and undiagnosed dementia or parkinsonism and anxiety/mood disorder (Table 1).
Table 1. Guidelines to recommend and offer FXS genetic testing.
| Recommend Genetic Testing | Offer Genetics Testing | |
|---|---|---|
| Women |
|
|
| All Adults |
|
|
3. FXTAS
Although the prevalence of FXTAS in the general population is uncertain, FXTAS occurs in approximately 40–45% of male PMC and 8–16% of female PMC over the age of 50 (15–18). The common features of FXTAS are cognitive decline, autonomic dysfunction, neuropathy, and psychiatric features such as anxiety, depression, and apathy (16,19–21). Impairments in executive function abilities including working memory, inhibitory control and visuospatial processing begin as early as middle adulthood, and progressively worsen with increasing age (22–26). Subsequently dementia develops in approximately 50% of male PMC and autonomic dysfunction which is thought to be a consequence of involvement of the peripheral nervous system in common (27,28). Premutation-associated psychiatric problems are common in adulthood but these problems can worsen before the appearance of tremor and ataxia (20,29–31). The increased lifetime prevalence of mood disorders (65%) and of anxiety disorders (52%) in individuals with FXTAS is greater than in those PMC without the FXTAS (20,31). The age of onset of FXTAS is typically between the ages of 60 and 65 years; the mean age of onset is 62 years (32,33). However, the chance of developing core symptoms of FXTAS (tremor and ataxia) increases with age. From age 50–59 the prevalence of FXTAS in males is 17 percent, from age 60–69 about 38 percent, from age 70–79 about 47 percent, and in males over 80 years old, about 75 percent (32).
Men are more frequently diagnosed with a definite diagnosis of FXTAS compared to women (34). A previous longitudinal study of progression of tremor and ataxia in 55 male PMCs showed that tremor usually occurs first, with median onset of ∼ 60 years of age (35). After the tremor onset, the median onset of ataxia was 2 years later; onset of falls was 6 years later; dependence on a walking aid was 15 years later; and death was 21 years later (35). The rate of progression of FXTAS varies and life expectancy is between 5 to 25 years after the onset of the symptoms (36).
FXTAS in females was initially reported in 2004 (37). FXTAS is less common and shows a milder presentation in females because they have a normal X chromosome in addition to the FMR1-premutated X-chromosome (34). The proportion of normal FMR1 alleles on the active X chromosome (activation ratio) is thought to modulate the phenotypic severity in females (38); however, double heterozygous female have been reported and they seem to have a similar clinical presentation to heterozygous females (39,40). Dementia was found in 21–50% males with FXTAS (32,36). In females with FXTAS, however, dementia is far less common (36,37) and it has been reported in only a few cases (41–45). Females with FXTAS may also exhibit parkinsonism, although at a lower rate than in males with FXTAS (34). There are associated symptoms in females with FXTAS that usually do not occur in males, including thyroid disorders, fibromyalgia and chronic muscle pain (46,47). Conversion disorder has also been reported in a PMC female (48). Migraine headache were reported in a higher rate in females (54.2%) when compared with males (26.8%) with the permutation (49). Immune mediated disorders have been described at a higher rate in females with FXTAS 72.73% compared with 46.54% female carriers and both rates are higher than published controls (47). Females with FXTAS also have a lower frequency of tremor compared to males with FXTAS (34).
4. Radiological findings
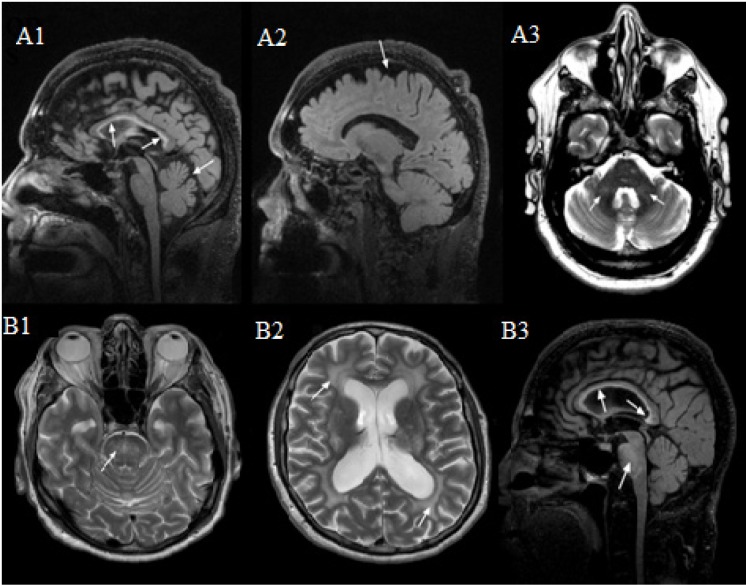
Cerebral magnetic resonance imaging (MRI) in patients with FXTAS shows global brain atrophy, enlargement of ventricular volume, white matter disease and heightened signal intensity with lesions in the middle cerebellar peduncles (50–53) (Figure 1). The middle cerebellar peduncle (MCP) sign presents as white matter hyperintensities in the middle cerebellar peduncles, and it is a cardinal radiological sign for the diagnosis of FXTAS (16). The MCP sign includes fronto-cerebellar tracts connecting to orbitofrontal and dorsolateral prefrontal cortices that are critical for cognitive control (54). Correspondingly, those with FXTAS and the MCP sign are likely to have more severe cognitive deficits and a longer history of symptoms than those without the MCP sign (55). Asymptomatic FMR1 premutation carriers show white matter alterations (demyelination and axonal damage) of the afferent projections of the MCPs and superior cerebellar peduncles (53,56,57), which may be the earliest neuroanatomical marker of the onset of cognitive and motor symptoms associated with FXTAS (58).
Figure 1.
MRI features of FXTAS. (A1) Moderately thin truncus of the corpus callosum with severe increased signal intensity in both the truncus and the splenium, and moderate cerebellar and cerebral (A2) volume loss. (A3) Mild white matter changes in the middle cerebellar peduncles (MCPs). (B1) Severe increased T2 signal intensity in the pons (can also be seen in B3). (B2) Severe diffuse increased T2 signal intensity in the deep white matter of the cerebrum, as well as periventricular. (B3) Moderately thin truncus of the corpus callosum with severe increased T2 signal intensity in both the truncus and the splenium.
Other common neuroimaging signs of FXTAS include white matter hyperintensities in the pons, insula, splenium of the corpus callosum, and periventricular region (59,60). T2-weighted and FLAIR corpus callosum splenium (CCS) hyperintensity was as frequent (68%) as MCP hyperintensities (64%) and it may be a marker of severe disease progression in FXTAS (34). Women with FXTAS have less white matter disease and brain atrophy on MRI, as well as less dementia in late-stages of FXTAS than men with FXTAS (36,50). The MCP sign was demonstrated in 13% of females compared with 58% of males with FXTAS (50). Corpus Callosum Splenium (CCS) hyperintensities were present in 50% of females versus 72% males (34).
5. Diagnosis and clinical severity stage of FXTAS
The FXTAS diagnostic revised criteria are presented in Table 2 (16,17). The clinical severity of FXTAS is estimated by using an empirical staging system, which incorporates the motor signs of FXTAS. The system gives an indication of the impact of motor aspects of the disease on activities of daily living as described in Table 3.
Table 2. Current diagnostic criteria of FXTAS.
| Molecular: FMR1 gray mutation, premutation or full mutation (Mandatory for all categories). | ||
| Diagnostic | Definite | one major clinical + one major radiological or one major clinical + intranuclear inclusions (postmortem) |
| Probable | two major clinical or one minor clinical + one major radiological | |
| Possible | one major clinical + one minor radiological | |
| Clinical | Major | intention tremor; cerebellar ataxia |
| Minor | Parkinsonism; moderate to severe short term or executive function deficits; neuropathy | |
| Radiological | Major | MCPs; MRI white matter lesions in splenium of the corpus callosum (or postmortem intranuclear inclusions) |
| Minor | MRI lesions in the cerebral white matter; moderate to severe generalized atrophy | |
MCPs; white matter lesions in middle cerebellar peduncle sign.
Table 3. Clinical staging of FXTAS.
| Stage | Clinical Description |
|---|---|
| 0 | Normal functions |
| 1 | Subtle or questionable signs such as subtle tremor or mild balance problems and no interference with ADLs |
| 2 | Clear tremor and/or balance problems and minor interference with ADLs |
| 3 | Moderate tremor and/or balance problems and occasional falls and significant interference with ADLs |
| 4 | Severe tremor and/or balance problems with at least intermittent use of a cane or a walker |
| 5 | The use of a wheelchair on a daily basis |
| 6 | Bedridden |
ADL: activities of daily living.
6. Molecular mechanisms of FXTAS
PMC were initially described with normal FMRP levels (61–64). However new molecular techniques led Tassone and colleagues (2000) (65) to the identification of increased FMR1 mRNA levels; Kenneson and colleagues (2001) (66) also demonstrated low FMRP levels in PMC. Current research shows that as the premutation increases from 55 to 200, particularly more than 110 CGG repeats, the level of FMR1 mRNA increases and the levels of FMRP start to decline (67,68). The CGG repeat size also correlates with the age of onset and the age of death from FXTAS (38,67). The elevated level of mRNA in PMCs led to the hypothesis of “FMR1 mRNA toxicity” in FXTAS, however the causative mechanism of increase transcription by the CGG repeat remains unclear as well as the mechanism of neuronal toxicity by the accumulation of the FMR1 mRNA. There are a few suggested pathological models including; “RNA toxicity”; a sequestration model which suggests that the RNA expanded CGG repeats are pathogenic by toxic sequestration of crucial transcriptional proteins (DROSHA-DGCR8, hnRNP A2/B1, SAM68, Purα, Rm62, and CUGBP1) (69–72); a non-canonical translation the CGG repeats which may result in the expression of toxic polyglycine products (73,74); and lastly the presence of antisense FMR1 transcription which may lead to toxicity by antisense transcripts products (75).
7. Neuropathology and neurobiology of FXTAS
The neuronal toxicity is thought to be led by the formation of pathognomonic eosinophilic and ubiquitin-positive intranuclear inclusions in neurons and astrocytes throughout the brain, peripheral nervous system and other organs such as the adrenals, thyroid, heart, Leydig cells and pancreas (28,76–78). Other findings include mild brain atrophy and involvement of the cerebellum (MPC sign), loss of Purkinje neuronal cells, spongiosis of the deep cerebellar white matter, Bergman gliosis, and swollen axons (51,77). Neurons of heterozygous female mice with the premutation showed shorter dendritic lengths and fewer branches between 7 and 21 days compared with wild-type (WT) littermates, display lower viability, and express elevated stress protein levels (79). Furthermore altered embryonic neocortical development (80) and abnormal spontaneous clustered calcium bursts (81,82) with glutamate hyper-responsiveness have been described (81); thus suggesting a clear state of neuronal vulnerability.
Mitochondrial abnormalities have also been found in PMC (83,84) and recently a decreased immune responses and immune dysregulation in both humans and mice with the premutation were described (85). It is unknown how the premutation alters mitochondrial and immunological responses, and if these abnormalities contribute to FXTAS and other associations found in PMC, such as, autoimmune and rheumatologic disorders (47).
8. Treatment of FXTAS
There are as yet no effective targeted therapies for the treatment of FXTAS; however there are many medications that have been use to ameliorate some of the symptoms associated to FXTAS. However the use of these medications rely on very few small trials and case studies that showed improvements only in some individuals (86,87). The only clinical targeted trial for FXTAS utilized memantine (NMDA receptor antagonist, FDA approved for treatment of moderate to severe Alzheimer's disease since 2003). Memantine was thought to selectively block the excitotoxic effects associated with abnormal transmission of glutamate while allowing for the physiological transmission associated with normal cell functioning. In this randomized, double-blind, placebo-controlled trail, 94 individuals aged 34–80 years with probable or possible FXTAS diagnosis and clinical stages 1–5 were enrolled for one year. Primary outcome measures were the Behavioral Dyscontrol Scale (BDS) score and CATSYS intention tremor severity. Intention-to-treat analysis showed no improvement with respect to intention tremor severity nor BDS scores (88). However of those (94 participants) 41 completed longitudinal ERP studies (20 placebo/21 memantine group) and the use of this compound showed improvements on cued-recall memory and N400 repetition effect amplitude; thus suggest that the treatment may have benefits on verbal memory (88). More frequent mild adverse events were observed in the placebo group, while more frequent moderate adverse events occurred in the memantine group and these included dizziness, headache and constipation amongst others. As mention before other treatments are directed to symptom reduction. For anxiety and depression selective serotonin and selective norepinephrine reuptake inhibitors are effective (5,86) as well as psychotherapy (31). Atypical antipsychotics are effective in individuals with psychosis and agitation (89). Propranolol and primidone may improve tremor (86,87,90). Deep brain stimulation has shown benefits for tremor and in few cases for ataxia (91); however the general outcome for FXTAS patients was poor (92). As previously mentioned the premutation causes neuronal susceptibility and therefore other treatments for PMC focus on preventive measures, such as, avoidance of toxins including smoking alcohol and some types of anesthesia, healthy diet and vitamins/antioxidants supplementation, exercise and cognitive training, and stress reduction (93).
9. Current research on FXTAS
Phenotype studies aim to determine early signs of disease for early diagnosis and treatment as well as to determine timing and reversibility of the pathological mechanism. A preliminary study shows that oculomotor inhibitory control impairments (measured by eye tracking) might precede FXTAS, and thus indicating elevated risk for motor impairment associated with FXTAS (94). Magnetic resonance imaging is useful for non-invasive testing; functional MRI for brain activation during cognitive tasks, and structural MRI for quantification of volume changes, morphometry, and white/gray matter integrity and connectivity. In fact, verbal working memory in male and female premutation carriers (95) has been associated with reduced activation in the right inferior frontal cortex and left premotor cortex in both asymptomatic premutation carriers and carriers with FXTAS. Reduced activation was found in right premotor/inferior frontal cortex in individuals with FXTAS. Individuals with FXTAS also showed diffuse gray matter loss most prominent in areas important for working memory, including prefrontal cortex, anterior cingulate cortex, and cerebellum (96). Molecular studies aim to determine the early molecular mechanisms that induce neurodegeneration including cellular stress and toxicity. The mechanism for inclusion formation and identification the intranuclear inclusions proteins are also a fertile area of research. Current targeted treatment research focuses on reversing the neurobiological abnormalities in FXTAS with pharmaceutical compounds (e.g. allopregnanolone) and other molecular mechanisms of disease modification (oligonucleotide-based therapies to reduce FMR1 mRNA) as well as designing a mechanism that will allow blood-brain cross-transportation of pharmacological compounds.
Animal models for the fragile X premutation have been developed to understand the molecular mechanism of FXTAS (97). Mice models have shown increased FMR1 mRNA levels, decreased FMRP levels and ubiquitin-positive intranuclear inclusions (98). In addition, mice models showed neurocognitive deficits in spatial and temporal memory processes, impaired motor performance, and anxiety traits (99). In order to determine timing and reversibility of disease and their associate molecular mechanism, a doxycycline-inducible premutation mouse has been created (R. Hukema, Abstracts of the 1st Premutation Meeting, Perugia, Italy, 2013). Animal models are crucial in the testing of preclinical therapies, for instance the acute administration of the neurosteroid allopregnanolone mitigated cluster burst firing in mouse hippocampal premutation-neurons and identified allopregnanolone as a potential targeted treatment for premutation disorders (100).
10. FXTAS
The identification of the FMR1 gene has led to characterization of risk alleles and recently there are a variety of disorders associated with the premutation in children and adults. Although FXTAS is described to occur in premutation carriers only, recent reports identified FXTAS in individuals with grey zone/intermediate alleles (101,102), as well as in individuals with unmethylated full-mutation alleles (103) and in a few patients with full-mutation/premutation mosaicism (104). These findings increase the number of patients that are at risk for FXTAS with elevated FMR1 mRNA besides only those with the premutation. The description of FXTAS as an intractable disorder, has led to expansion of recommendations for genetic testing in adults which in turn have caused ethical concerns for the identification of individuals at risk of FXTAS. These is a concern especially in males with the suspicion of the premutation because males do not have increased risk of having children with fragile X syndrome, but have about a 40% chances to develop FXTAS, if they are determined to be premutation carriers. However, the documentation of the premutation is helpful for both males and females because these individuals can be treated for many of the childhood and adult problems related to the premutation such as anxiety, depression, ADHD, hypertension, hypothyroidism, fibromyalgia, sleep apnea, and can be counseled to avoid toxicity from the environment that has the potential to bring on FXTAS at an earlier age. The identification of radiological signs of FXTAS is used by clinicians to make a clinical diagnosis of FXTAS; however, the phenotypic variability and progression of FXTAS should be taken in consideration as many adults will not meet all clinical criteria until advanced age, particularly females. There are also radiological and clinical gender variations, while males are more prone to develop dementia, females are more likely to develop other autoimmune-related disorders. The phenotypic variability of the premutation is partially explain by CGG expansion size, FMR1 mRNA levels, decrease FMRP and mosaicism; however, other mechanisms are now being consider including protein synthesis alterations, non-AUG translation, and antisense transcription, as well as, additional genomic variants and environmental exposures (5). Further genotype to phenotype studies are necessary to determine the relative contribution of these pathological processes in this complex disorder. Many FDA approved medications have shown to improve some of the symptoms of FXTAS; however there are limited clinical trials and none that can prove the efficacy of these treatments. It is crucial to undertake further clinical trials of drugs that anecdotally have shown positive results in individuals with FXTAS. There has been only one targeted clinical trial for FXTAS and there is an urgent need to identify more compounds that target the pathogenesis of FXTAS, which in theory may reverse, treat or prevent the development of FXTAS.
Acknowledgements
This work was supported by the National Institute on Aging of the National Institutes of Health (P30AG043097) and NICHD diversity supplement (HD036071). RL and ZM declare no competing financial interests.
References
- 1. Cronister A, Schreiner R, Wittenberger M, Amiri K, Harris K, Hagerman RJ. Heterozygous fragile X female: Historical, physical, cognitive, and cytogenetic features. Am J Med Genet. 1991; 38:269-274. [DOI] [PubMed] [Google Scholar]
- 2. Mazzocco MM, Pennington BF, Hagerman RJ. The neurocognitive phenotype of female carriers of fragile X: Additional evidence for specificity. J Dev Behav Pediatr. 1993; 14:328-335. [PubMed] [Google Scholar]
- 3. Reiss AL, Freund L, Abrams MT, Boehm C, Kazazian H. Neurobehavioral effects of the fragile X premutation in adult women: A controlled study. Am J Hum Genet. 1993; 52:884-894. [PMC free article] [PubMed] [Google Scholar]
- 4. Rousseau F, Heitz D, Tarleton J, et al. A multicenter study on genotype-phenotype correlations in the fragile X syndrome, using direct diagnosis with probe StB12.3: The first 2,253 cases. Am J Med Genet. 1994; 55:225-237. [PMC free article] [PubMed] [Google Scholar]
- 5. Hagerman R, Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013; 12:786-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dombrowski C, Levesque S, Morel ML, Rouillard P, Morgan K, Rousseau F. Premutation and intermediate-size FMR1 alleles in 10572 males from the general population: Loss of an AGG interruption is a late event in the generation of fragile X syndrome alleles. Hum Mol Genet. 2002; 11:371-378. [DOI] [PubMed] [Google Scholar]
- 7. Toledano-Alhadef H, Basel-Vanagaite L, Magal N, Davidov B, Ehrlich S, Drasinover V, Taub E, Halpern GJ, Ginott N, Shohat M. Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am J Hum Genet. 2001; 69:351-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dawson AJ, Chodirker BN, Chudley AE. Frequency of FMR1 premutations in a consecutive newborn population by PCR screening of Guthrie blood spots. Biochem Mol Med. 1995; 56:63-69. [DOI] [PubMed] [Google Scholar]
- 9. Rousseau F, Rouillard P, Morel ML, Khandjian EW, Morgan K. Prevalence of carriers of premutation-size alleles of the FMRI gene - and implications for the population genetics of the fragile X syndrome. Am J Med Genet. 1995; 57:1006-1018. [PMC free article] [PubMed] [Google Scholar]
- 10. Fernandez-Carvajal I, Walichiewicz P, Xiaosen X, Pan R, Hagerman PJ, Tassone F. Screening for expanded alleles of the FMR1 gene in blood spots from newborn males in a Spanish population. J Mol Diagn. 2009; 11:324-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hagerman PJ. The fragile X prevalence paradox. J Med Genet. 2008; 45:498-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Monaghan KG, Lyon E, Spector EB. ACMG Standards and Guidelines for fragile X testing: A revision to the disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics and Genomics. Genet Med. 2013; 15:575-586. [DOI] [PubMed] [Google Scholar]
- 13. Schaefer GB, Mendelsohn NJ. Clinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisions. Genet Med. 2013; 15:399-407. [DOI] [PubMed] [Google Scholar]
- 14. ACOG Committee Opinion No. 469: Carrier screening for fragile X syndrome. Obstet Gynecol. 2010; 116:1008-1010. [DOI] [PubMed] [Google Scholar]
- 15. Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001; 57:127-130. [DOI] [PubMed] [Google Scholar]
- 16. Jacquemont S, Hagerman RJ, Leehey M, et al. Fragile X premutation tremor/ataxia syndrome: Molecular, clinical, and neuroimaging correlates. Am J Med Genet. 2003; 72:869-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berry-Kravis E, Abrams L, Coffey SM, et al. Fragile X-associated tremor/ataxia syndrome: Clinical features, genetics, and testing guidelines. Mov Disord. 2007; 22:2018-2030, quiz 2140. [DOI] [PubMed] [Google Scholar]
- 18. Tassone F, Adams J, Berry-Kravis EM, Cohen SS, Brusco A, Leehey MA, Li L, Hagerman RJ, Hagerman PJ. CGG repeat length correlates with age of onset of motor signs of the fragile X-associated tremor/ataxia syndrome (FXTAS). Am J Med Genet B Neuropsychiatr Genet. 2007; 144B:566-569. [DOI] [PubMed] [Google Scholar]
- 19. Amiri K, Hagerman RJ, Hagerman PJ. Fragile X-associated tremor/ataxia syndrome: An aging face of the fragile X gene. Arch Neurol. 2008; 65:19-25. [DOI] [PubMed] [Google Scholar]
- 20. Bourgeois JA, Seritan AL, Casillas EM, Hessl D, Schneider A, Yang Y, Kaur I, Cogswell JB, Nguyen DV, Hagerman RJ. Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. J Clin Psychiatry. 2011; 72:175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sevin M, Kutalik Z, Bergman S, Vercelletto M, Renou P, Lamy E, Vingerhoets FJ, Di Virgilio G, Boisseau P, Bezieau S, Pasquier L, Rival JM, Beckmann JS, Damier P, Jacquemont S. Penetrance of marked cognitive impairment in older male carriers of the FMR1 gene premutation. J Med Genet. 2009; 46:818-824. [DOI] [PubMed] [Google Scholar]
- 22. Cornish KM, Li L, Kogan CS, Jacquemont S, Turk J, Dalton A, Hagerman RJ, Hagerman PJ. Age-dependent cognitive changes in carriers of the fragile X syndrome. Cortex. 2008; 44:628-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cornish KM, Kogan CS, Li L, Turk J, Jacquemont S, Hagerman RJ. Lifespan changes in working memory in fragile X premutation males. Brain Cogn. 2009; 69:551-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cornish KM, Hocking DR, Moss SA, Kogan CS. Selective executive markers of at-risk profiles associated with the fragile X premutation. Neurology. 2011; 77:618-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hocking DR, Kogan CS, Cornish KM. Selective spatial processing deficits in an at-risk subgroup of the fragile X premutation. Brain Cogn. 2012; 79:39-44. [DOI] [PubMed] [Google Scholar]
- 26. Grigsby J, Brega AG, Engle K, Leehey MA, Hagerman RJ, Tassone F, Hessl D, Hagerman PJ, Cogswell JB, Bennett RE, Cook K, Hall DA, Bounds LS, Paulich MJ, Reynolds A. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008; 22:48-60. [DOI] [PubMed] [Google Scholar]
- 27. Gokden M, Al-Hinti JT, Harik SI. Peripheral nervous system pathology in fragile X tremor/ataxia syndrome (FXTAS). Neuropathology. 2009; 29:280-284. [DOI] [PubMed] [Google Scholar]
- 28. Hunsaker MR, Greco CM, Spath MA, Smits AP, Navarro CS, Tassone F, Kros JM, Severijnen LA, Berry-Kravis EM, Berman RF, Hagerman PJ, Willemsen R, Hagerman RJ, Hukema RK. Widespread non-central nervous system organ pathology in fragile X premutation carriers with fragile X-associated tremor/ataxia syndrome and CGG knock-in mice. Acta Neuropathol. 2011; 122:467-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roberts JE, Bailey DB, Jr, Mankowski J, Ford A, Sideris J, Weisenfeld LA, Heath TM, Golden RN. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B Neuropsychiatr Genet. 2009; 150B:130-139. [DOI] [PubMed] [Google Scholar]
- 30. Seritan AL, Bourgeois JA, Schneider A, Mu Y, Hagerman RJ, Nguyen DV. Ages of Onset of Mood and Anxiety Disorders in Fragile X Premutation Carriers. Curr Psychiatry Rev. 2013; 9:65-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seritan AL, Ortigas M, Seritan S, Bourgeois JA, Hagerman RJ. Psychiatric Disorders Associated with FXTAS. Curr Psychiatry Rev. 2013; 9:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jacquemont S, Hagerman RJ, Leehey MA, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004; 291:460-469. [DOI] [PubMed] [Google Scholar]
- 33. Li Y, Jin P. RNA-mediated neurodegeneration in fragile X-associated tremor/ataxia syndrome. Brain Res. 2012; 1462:112-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Apartis E, Blancher A, Meissner WG, et al. FXTAS: New insights and the need for revised diagnostic criteria. Neurology. 2012; 79:1898-1907. [DOI] [PubMed] [Google Scholar]
- 35. Leehey MA, Berry-Kravis E, Min SJ, et al. Progression of tremor and ataxia in male carriers of the FMR1 premutation. Mov Disord. 2007; 22:203-206. [DOI] [PubMed] [Google Scholar]
- 36. Seritan AL, Nguyen DV, Farias ST, Hinton L, Grigsby J, Bourgeois JA, Hagerman RJ. Dementia in fragile X-associated tremor/ataxia syndrome (FXTAS): Comparison with Alzheimer's disease. Am J Med Genet B Neuropsychiatr Genet. 2008; 147B:1138-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hagerman RJ, Leavitt BR, Farzin F. Fragile X-associated tremor/ataxia syndrome (FXTAS) in females with the FMR1 premutation. Am J Hum Genet. 2004; 74:1051-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leehey MA, Berry-Kravis E, Goetz CG, et al. FMR1 CGG repeat length predicts motor dysfunction in premutation carriers. Neurology. 2008; 70:1397-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sorensen PL, Basuta K, Mendoza-Morales G, Gane LW, Schneider A, Hagerman R, Tassone F. A fragile X sibship from a consanguineous family with a compound heterozygous female and partially methylated full mutation male. Am J Med Genet A. 2012; 158A:1221-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Basuta K, Lozano R, Schneider A, Yrigollen CM, Hessl D, Hagerman RJ, Tassone F. A family with two female siblings with compound heterozygous FMR1 premutation alleles. Clinical genetics. 2014; 85:458-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, Xuncla M, Badenas C, Kulisevsky J, Gomez B, Mila M. Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur J Hum Genet. 2009; 17:1359-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Al-Hinti JT, Nagan N, Harik SI. Fragile X premutation in a woman with cognitive impairment, tremor, and history of premature ovarian failure. Alzheimer Dis Assoc Disord. 2007; 21:262-264. [DOI] [PubMed] [Google Scholar]
- 43. Karmon Y, Gadoth N. Fragile X associated tremor/ataxia syndrome (FXTAS) with dementia in a female harbouring FMR1 premutation. J Neurol Neurosurg Psychiatry. 2008; 79:738-739. [DOI] [PubMed] [Google Scholar]
- 44. Yachnis AT, Roth HL, Heilman KM. Fragile X dementia Parkinsonism Syndrome (FXDPS). Cogn Behav Neurol. 2010; 23:39-43. [DOI] [PubMed] [Google Scholar]
- 45. Tassone F, Greco CM, Hunsaker MR, Seritan AL, Berman RF, Gane LW, Jacquemont S, Basuta K, Jin LW, Hagerman PJ, Hagerman RJ. Neuropathological, clinical and molecular pathology in female fragile X premutation carriers with and without FXTAS. Genes Brain Behav. 2012; 11:577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Coffey SM, Cook K, Tartaglia N, Tassone F, Nguyen DV, Pan R, Bronsky HE, Yuhas J, Borodyanskaya M, Grigsby J, Doerflinger M, Hagerman PJ, Hagerman RJ. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A. 2008; 146A:1009-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Winarni TI, Chonchaiya W, Sumekar TA, Ashwood P, Morales GM, Tassone F, Nguyen DV, Faradz SM, Van de Water J, Cook K, Hamlin A, Mu Y, Hagerman PJ, Hagerman RJ. Immune-mediated disorders among women carriers of fragile X premutation alleles. Am J Med Genet A. 2012; 158A:2473-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seritan AL, Schneider A, Olichney JM, Leehey MA, Akins RS, Hagerman RJ. Conversion disorder in women with the FMR1 premutation. Am J Med Genet A. 2009; 149A:2501-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Au J, Akins RS, Berkowitz-Sutherland L, Tang HT, Chen Y, Boyd A, Tassone F, Nguyen DV, Hagerman R. Prevalence and risk of migraine headaches in adult fragile X premutation carriers. Clin Genet. 2013; 84:546-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Adams JS, Adams PE, Nguyen D, Brunberg JA, Tassone F, Zhang W, Koldewyn K, Rivera SM, Grigsby J, Zhang L, DeCarli C, Hagerman PJ, Hagerman RJ. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS). Neurology. 2007; 69:851-859. [DOI] [PubMed] [Google Scholar]
- 51. Brunberg JA, Jacquemont S, Hagerman RJ, Berry-Kravis EM, Grigsby J, Leehey MA, Tassone F, Brown WT, Greco CM, Hagerman PJ. Fragile X premutation carriers: Characteristic MR imaging findings of adult male patients with progressive cerebellar and cognitive dysfunction. AJNR Am J Neuroradiol. 2002; 23:1757-1766. [PMC free article] [PubMed] [Google Scholar]
- 52. Peters N, Kamm C, Asmus F, Holinski-Feder E, Kraft E, Dichgans M, Bruning R, Gasser T, Botzel K. Intrafamilial variability in fragile X-associated tremor/ataxia syndrome. Mov Disord. 2006; 21:98-102. [DOI] [PubMed] [Google Scholar]
- 53. Hashimoto R, Srivastava S, Tassone F, Hagerman RJ, Rivera SM. Diffusion tensor imaging in male premutation carriers of the fragile X mental retardation gene. Mov Disord. 2011; 26:1329-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kamali A, Kramer LA, Frye RE, Butler IJ, Hasan KM. Diffusion tensor tractography of the human brain cortico-ponto-cerebellar pathways: A quantitative preliminary study. J Magn Reson Imaging. 2010; 32:809-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Juncos JL, Lazarus JT, Graves-Allen E, Shubeck L, Rusin M, Novak G, Hamilton D, Rohr J, Sherman SL. New clinical findings in the fragile X-associated tremor ataxia syndrome (FXTAS). Neurogenetics. 2011; 12:123-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Battistella G, Niederhauser J, Fornari E, Hippolyte L, Gronchi Perrin A, Lesca G, Forzano F, Hagmann P, Vingerhoets FJ, Draganski B, Maeder P, Jacquemont S. Brain structure in asymptomatic FMR1 premutation carriers at risk for fragile X-associated tremor/ataxia syndrome. Neurobiol Aging. 2013; 34:1700-1707. [DOI] [PubMed] [Google Scholar]
- 57. Wang JY, Hessl DH, Hagerman RJ, Tassone F, Rivera SM. Age-dependent structural connectivity effects in fragile X premutation. Arch Neurol. 2012; 69:482-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kraan CM, Hocking DR, Georgiou-Karistianis N, Metcalfe SA, Archibald AD, Fielding J, Trollor J, Bradshaw JL, Cohen J, Cornish KM. Cognitive-motor interference during postural control indicates at-risk cerebellar profiles in females with the FMR1 premutation. Behav Brain Res. 2013; 253:329-336. [DOI] [PubMed] [Google Scholar]
- 59. Loesch DZ, Kotschet K, Trost N, Greco CM, Kinsella G, Slater HR, Venn A, Horne M. White matter changes in basis pontis in small expansion FMR1 allele carriers with parkinsonism. Am J Med Genet B Neuropsychiatr Genet. 2011; 156B:502-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Moris G, Arias M, Lopez MV, Alvarez V. Hyperintensity in the basis pontis: A typical neuroradiological findings in a woman with FXTAS. Mov Disord. 2010; 25:649-650. [DOI] [PubMed] [Google Scholar]
- 61. Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet. 1993; 4:335-340. [DOI] [PubMed] [Google Scholar]
- 62. Feng Y, Lakkis L, Devys D, Warren ST. Quantitative comparison of FMR1 gene expression in normal and premutation alleles. American journal of human genetics. 1995; 56:106-113. [PMC free article] [PubMed] [Google Scholar]
- 63. Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991; 66:817-822. [DOI] [PubMed] [Google Scholar]
- 64. Siomi H, Siomi MC, Nussbaum RL, Dreyfuss G. The protein product of the fragile X gene, FMR1, has characteristics of an RNA-binding protein. Cell. 1993; 74:291-298. [DOI] [PubMed] [Google Scholar]
- 65. Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: A new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000; 66:6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. 2001; 10:1449-1454. [DOI] [PubMed] [Google Scholar]
- 67. Ludwig AL, Espinal GM, Pretto DI, Jamal AL, Arque G, Tassone F, Berman RF, Hagerman PJ. CNS expression of murine fragile X protein (FMRP) as a function of CGG-repeat size. Hum Mol Genet. 2014; 23:3228-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pretto DI, Mendoza-Morales G, Lo J, Cao R, Hadd A, Latham GJ, Durbin-Johnson B, Hagerman R, Tassone F. CGG allele size somatic mosaicism and methylation in FMR1 premutation alleles. J Med Genet. 2014; 51:309-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sofola OA, Jin P, Qin Y, Duan R, Liu H, de Haro M, Nelson DL, Botas J. RNA-binding proteins hnRNP A2/B1 and CUGBP1 suppress fragile X CGG premutation repeat-induced neurodegeneration in a Drosophila model of FXTAS. Neuron. 2007; 55:565-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jin P, Duan R, Qurashi A, Qin Y, Tian D, Rosser TC, Liu H, Feng Y, Warren ST. Pur alpha binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a Drosophila model of fragile X tremor/ataxia syndrome. Neuron. 2007; 55:556-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sellier C, Freyermuth F, Tabet R, et al. Sequestration of DROSHA and DGCR8 by expanded CGG RNA repeats alters microRNA processing in fragile X-associated tremor/ataxia syndrome. Cell rep. 2013; 3:869-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Qurashi A, Li W, Zhou JY, Peng J, Jin P. Nuclear accumulation of stress response mRNAs contributes to the neurodegeneration caused by Fragile X premutation rCGG repeats. PLoS genetics. 2011; 7:e1002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reddy K, Pearson CE. RAN translation: Fragile X in the running. Neuron. 2013; 78:405-408. [DOI] [PubMed] [Google Scholar]
- 74. Todd PK, Oh SY, Krans A, et al. CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron. 2013; 78:440-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ladd PD, Smith LE, Rabaia NA, Moore JM, Georges SA, Hansen RS, Hagerman RJ, Tassone F, Tapscott SJ, Filippova GN. An antisense transcript spanning the CGG repeat region of FMR1 is upregulated in premutation carriers but silenced in full mutation individuals. Hum Mol Genet. 2007; 16:3174-3187. [DOI] [PubMed] [Google Scholar]
- 76. Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, Jacquemont S, Leehey M, Hagerman PJ. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002; 125:1760-1771. [DOI] [PubMed] [Google Scholar]
- 77. Greco CM, Berman RF, Martin RM, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS). Brain. 2006; 129:243-255. [DOI] [PubMed] [Google Scholar]
- 78. Greco CM, Soontrapornchai K, Wirojanan J, Gould JE, Hagerman PJ, Hagerman RJ. Testicular and pituitary inclusion formation in fragile X associated tremor/ataxia syndrome. J Urol. 2007; 177:1434-1437. [DOI] [PubMed] [Google Scholar]
- 79. Chen Y, Tassone F, Berman RF, Hagerman PJ, Hagerman RJ, Willemsen R, Pessah IN. Murine hippocampal neurons expressing FMR1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet. 2010; 19:196-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cunningham CL, Martinez Cerdeno V, Navarro Porras E, Prakash AN, Angelastro JM, Willemsen R, Hagerman PJ, Pessah IN, Berman RF, Noctor SC. Premutation CGG-repeat expansion of the FMR1 gene impairs mouse neocortical development. Hum Mol Genet. 2011; 20:64-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liu J, Koscielska KA, Cao Z, Hulsizer S, Grace N, Mitchell G, Nacey C, Githinji J, McGee J, Garcia-Arocena D, Hagerman RJ, Nolta J, Pessah IN, Hagerman PJ. Signaling defects in iPSC-derived fragile X premutation neurons. Hum Mol Genet. 2012; 21:3795-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cao Z, Hulsizer S, Cui Y, Pretto DL, Kim KH, Hagerman PJ, Tassone F, Pessah IN. Enhanced asynchronous Ca2+ oscillations associated with impaired glutamate transport in cortical astrocytes expressing FMR1 gene premutation expansion. J Biol Chem. 2013; 288:13831-13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ross-Inta C, Omanska-Klusek A, Wong S, Barrow C, Garcia-Arocena D, Iwahashi C, Berry-Kravis E, Hagerman RJ, Hagerman PJ, Giulivi C. Evidence of mitochondrial dysfunction in fragile X-associated tremor/ataxia syndrome. Biochem J. 2010; 429:545-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Napoli E, Ross-Inta C, Wong S, Omanska-Klusek A, Barrow C, Iwahashi C, Garcia-Arocena D, Sakaguchi D, Berry-Kravis E, Hagerman R, Hagerman PJ, Giulivi C. Altered zinc transport disrupts mitochondrial protein processing/import in fragile X-associated tremor/ataxia syndrome. Hum Mol Genet. 2011; 20:3079-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Careaga M, Rose D, Tassone F, Berman RF, Hagerman R, Ashwood P. Immune dysregulation as a cause of autoinflammation in fragile X premutation carriers: Link between FMRI CGG repeat number and decreased cytokine responses. PLoS One. 2014; 9:e94475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hagerman RJ, Hall DA, Coffey S, et al. Treatment of fragile X-associated tremor ataxia syndrome (FXTAS) and related neurological problems. Clin Interv Aging. 2008; 3:251-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Leehey MA. Fragile X-associated tremor/ataxia syndrome: Clinical phenotype, diagnosis, and treatment. J Investig Med. 2009; 57:830-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yang JC, Niu YQ, Simon C, Seritan AL, Chen L, Schneider A, Moghaddam ST, Hagerman PJ, Hagerman RJ. Memantine Effects on verbal memory in fragile X-associated tremor/ataxia syndrome (FXTAS): A double-blind brain potential study. Neuropsychopharmacology. 2014; 39:2760-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bourgeois J, Coffey S, Rivera SM, Hessl D, Gane LW, Tassone F, Greco C, Finucane B, Nelson L, Berry-Kravis E, Grigsby J, Hagerman PJ, Hagerman RJ. Fragile X premutation disorders — Expanding the psychiatric perspective. J Clin Psychiatry. 2009; 70:852-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hall DA, Berry-Kravis E, Hagerman RJ, Hagerman PJ, Rice CD, Leehey MA. Symptomatic treatment in the fragile X-associated tremor/ataxia syndrome. Mov Disord. 2006; 21:1741-1744. [DOI] [PubMed] [Google Scholar]
- 91. Hagerman J, Jamie SP, Ortigas M, Olichney J, Frysinger R, Harrison M, Cornman E, Danuta ZL, Richard GB, Peppard R, Zhang L, Shahlaie K. Case Series: Deep brain stimulation in patients with FXTAS. Brain Disord Ther. 2012; 1:2 [Google Scholar]
- 92. Oyama G, Thompson A, Foote KD, Limotai N, Abd-El-Barr M, Maling N, Malaty IA, Rodriguez RL, Subramony SH, Ashizawa T, Okun MS. Deep brain stimulation for tremor associated with underlying ataxia syndromes: A case series and discussion of issues. Tremor Other Hyperkinet Mov (N Y). 2014; 4:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Polussa J, Schneider A, Hagerman R. Molecular advances leading to treatment implications for fragile X premutation carriers. Brain Disord Ther. 2014; 3:1000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wong LM, Goodrich-Hunsaker NJ, McLennan Y, Tassone F, Zhang M, Rivera SM, Simon TJ. Eye movements reveal impaired inhibitory control in adult male fragile X premutation carriers asymptomatic for FXTAS. Neuropsychology. 2014; 28:571-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hashimoto R, Backer KC, Tassone F, Hagerman RJ, Rivera SM. An fMRI study of the prefrontal activity during the performance of a working memory task in premutation carriers of the fragile X mental retardation 1 gene with and without fragile X-associated tremor/ataxia syndrome (FXTAS). J Psychiatr Res. 2011; 45:36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hashimoto R, Javan AK, Tassone F, Hagerman RJ, Rivera SM. A voxel-based morphometry study of grey matter loss in fragile X-associated tremor/ataxia syndrome. Brain. 2011; 134:863-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Berman RF, Buijsen RA, Usdin K, Pintado E, Kooy F, Pretto D, Pessah IN, Nelson DL, Zalewski Z, Charlet-Bergeurand N, Willemsen R, Hukema RK. Mouse models of the fragile X premutation and fragile X-associated tremor/ataxia syndrome. J Neurodev Disord. 2014; 6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Berman RF, Willemsen R. Mouse models of fragile X-associated tremor ataxia. J Investig Med. 2009; 57:837-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hunsaker MR, Wenzel HJ, Willemsen R, Berman RF. Progressive spatial processing deficits in a mouse model of the fragile X premutation. Behav Neurosci. 2009; 123:1315-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cao Z, Hulsizer S, Tassone F, Tang HT, Hagerman RJ, Rogawski MA, Hagerman PJ, Pessah IN. Clustered burst firing in FMR1 premutation hippocampal neurons: Amelioration with allopregnanolone. Hum Mol Genet. 2012; 21:2923-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hall D, Tassone F, Klepitskaya O, Leehey M. Fragile X-associated tremor ataxia syndrome in FMR1 gray zone allele carriers. Mov Disord. 2012; 27:296-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Liu Y, Winarni TI, Zhang L, Tassone F, Hagerman RJ. Fragile X-associated tremor/ataxia syndrome (FXTAS) in grey zone carriers. Clin Genet. 2013; 84:74-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Loesch DZ, Sherwell S, Kinsella G, Tassone F, Taylor A, Amor D, Sung S, Evans A. Fragile X-associated tremor/ataxia phenotype in a male carrier of unmethylated full mutation in the FMR1 gene. Clin Genet. 2012; 82:88-92. [DOI] [PubMed] [Google Scholar]
- 104. Pretto DI, Hunsaker MR, Cunningham CL, Greco CM, Hagerman RJ, Noctor SC, Hall DA, Hagerman PJ, Tassone F. Intranuclear inclusions in a fragile X mosaic male. Transl Neurodegener. 2013; 2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]