Summary
The fragile X mental retardation 1 gene (FMR1), which codes for the fragile X mental retardation 1 protein (FMRP), is located at Xp27.3. The normal allele of the FMR1 gene typically has 5 to 40 CGG repeats in the 5′ untranslated region; abnormal alleles of dynamic mutations include the full mutation (> 200 CGG repeats), premutation (55–200 CGG repeats) and the gray zone mutation (45–54 CGG repeats). Premutation carriers are common in the general population with approximately 1 in 130–250 females and 1 in 250–810 males, whereas the full mutation and Fragile X syndrome (FXS) occur in approximately 1 in 4000 to 1 in 7000. FMR1 mutations account for a variety of phenotypes including the most common monogenetic cause of inherited intellectual disability (ID) and autism (FXS), the most common genetic form of ovarian failure, the fragile X-associated primary ovarian insufficiency (FXPOI, premutation); and fragile X-associated tremor/ataxia syndrome (FXTAS, premutation). The premutation can also cause developmental problems including ASD and ADHD especially in boys and psychopathology including anxiety and depression in children and adults. Some premutation carriers can have a deficit of FMRP and some unmethylated full mutation individuals can have elevated FMR1 mRNA that is considered a premutation problem. Therefore the term “Fragile X Spectrum Disorder” (FXSD) should be used to include the wide range of overlapping phenotypes observed in affected individuals with FMR1 mutations. In this review we focus on the phenotypes and genotypes of children with FXSD.
Keywords: Fragile X syndrome, autism spectrum disorder, intellectual disability, developmental delay, premutation
1. Introduction
A variety of disorders are associated with mutations in the fragile X mental retardation 1 (FMR1) gene including fragile X syndrome (FXS) caused by a full mutation (> 200 CGG repeats in the 5′ untranslated region of FMR1 gene) leading to absence or deficiency of the FMR1 protein (FMRP) and premutation (55 to 200 CGG repeats) disorders characterized by elevation of FMR1 mRNA 2 to 8 times normal. Although these 2 types of disorders are distinct in their phenotypes and molecular pathology, recent studies have demonstrated significant overlap that has been fertile areas for research. The term fragile X spectrum disorder (FXSD) has been developed to emphasize the continuity of clinical involvement from the gray zone (45 to 54 repeats) throughout the premutation and into the full mutation range. FMR1 mutations are dynamic in that they usually expand between generations particularly when passed on by a female to her children when it can expand from a premutation to a full mutation (1).
FXS was the first identified disorder in this spectrum and it was discovered in association with the fragile site of the X chromosome in two brothers in 1969 by Lubs and colleagues (2). In retrospect the first X- linked pedigree of intellectual disability (XLID) reported by Martin and Bell in 1949 turned out to be a fragile X pedigree when tested by the FMR1 DNA test that was developed after the discovery of FMR1 in 1991 (3,4). The fragile site was characterized by not only the CGG expansion to > 200 repeats, but also methylation of the cytosine bases leading to silencing of translation and little or no production of FMR1 mRNA and FMRP. Since FMRP is a critical protein for regulation of translation for hundreds of mRNAs into their respective proteins, most of them involved with synaptic plasticity (5), the lack or severe deficiency of FMRP almost always leads to intellectual deficits as seen in males with FXS. In females with FXS the normal X produces FMRP so only 25% will have an IQ below 70 and an additional 50% will have an IQ in the borderline range (6).
Premutation disorders were first identified with the discovery of an increased incidence of early menopause (prior to the age of 40) in female carriers in 1991 (7). This has been confirmed by multiple investigators and has now been named fragile X-associated primary ovarian insufficiency (FXPOI) (8). Approximately 20% of female carriers have FXPOI, although the rate varies in a curvilinear fashion with CGG repeat number; the greatest prevalence of FXPOI is between 70 to 100 CGG repeats (9).
The next premutation disorder identified was the fragile X-associated tremor ataxia syndrome (10,11) seen initially in older male carriers (> 50 years) involving an intention tremor and cerebellar gait ataxia in addition to autonomic dysfunction, Parkinsonism, neuropathy, memory and executive function deficits followed by cognitive decline. This is a neurodegenerative disorder that occurs in approximately 40% of men and 16% of women with the premutation (12,13). FXTAS is hypothesized to be caused by mRNA toxicity from the elevated FMR1 mRNA levels (14) leading to the production of pathognomonic inclusion formation in neurons and astrocytes throughout the CNS, peripheral nervous system and even in some organs such as the adrenals, heart and pancreas (15).
Currently there are numerous additional medical, neurological and psychiatric problems associated with the premutation both with and without FXTAS including depression (16), anxiety (17,18), migraines (19) hypertension (20), immune mediated disorders including fibromyalgia and hypothyroidism (21,22), sleep apnea (23), restless legs syndrome (RLS) (24), and neuropathy (25,26) often associated with chronic pain symptoms. Since the prevalence of the premutation is much higher (1 in 130–250 females and 1 in 250–810 males) (27) than those with the full mutation (1 in 4,000–7,000) the impact of multiple medical and neurological problems in premutation carriers is far more significant in the population than the full mutation (28,29). The association of other disorders in adults with the premutation led to multiple studies in children and here we present a review of the manifestations in children with FXSD.
2. Full mutation - Fragile X syndrome
The FMR1 gene, which codes for the fragile X mental retardation protein (FMRP, a major negative translation regulator), is located at Xp27.3 from base pair 146,993,469 to base pair 147,032,647 (GRCh37/hg19). The FMR1 gene is highly expressed in the brain and testis (30). FXS is associated with a variety of neurological, cognitive and behavioral deficits, and less frequent dysmorphic features. Males with the full mutation and full methylation have little to no FMR1 mRNA and little to no FMRP contributing to the clinical phenotype of FXS. The range of involvement in females is determined by the X-chromosome activation/inactivation ratio (the percentage of cells with active normal X chromosome) because this will determine how much FMRP is produced by the normal X chromosome depending on whether it is active or not.
2.1. Physical findings
The physical phenotype and dysmorphology of FXS include signs of a connective tissue disorder such as a long and narrow face, large and prominent ears, a high arched palate, hyperextensible finger joints, pectus excavatum, flat feet, soft skin and mitral valve prolapse. Other features include low muscle tone, and pubertal macroorchidism (31,32). Noteworthy approximately 30% of young children with FXS will not have obvious dysmorphic features; the physical features are associated with the FMRP deficits. The most evident effects of lower levels of FMRP in both males and females are prominent ears and hypermobility of the metacarpal-phalangeal (MP) joints (33,34). In males FMRP deficits are associated with a narrow face and large ears, while in females the FMRP deficits are associated with increased ear prominence and jaw length (35). In about 5–10% of children with FXS a Prader-Willi phenotype is observed including severe obesity, hyperphagia, hypogonadism and in some cases delayed puberty (36,37) (Figure 1). The reduced expression of the cytoplasmic interacting FMR1 protein gene (CYFIP, located at 15q11-13) is believed to be the cause of this phenotype (37).
Figure 1.
A female adolescent with FXS Prader-Willi-like phenotype.
2.2. Neurological disorders
In a national survey of caregivers of individuals with FXS (1,394 individuals), 14% of males and 6% of females were reported to have seizures (38). The seizures were easily treated, often partial and infrequent; however they were associated with more severe developmental and behavioral problems (38). Remarkably those with seizures are more likely to have ASD. The seizures may add to the severity of the phenotype because animal studies of early life seizures have shown that the FMRP leaves the dendrites and migrates to the perinuclear area during seizures, thereby depleting the dendrites of the regulatory effects of FMRP (39). Hypersensitivity to audiogenic stimuli and hyperarousal are also characteristics of children with FXS. These children have enhanced amplitude to sensory stimuli measured by electrodermal studies and a lack of habituation to repetitive stimuli (35). In addition, MEG studies also demonstrate an enhanced electromagnetic response to stimuli (36).
2.3. Cognition deficits
Male and female individuals with FXS present a wide range of learning disabilities in a context of normal, borderline IQ or mild to severe ID. The average IQ of males with the full mutation is 40 (40). Intellectual and developmental disability occurs in 85% of males and 25% of females. The level of FMRP correlates directly with IQ (41); males with the full mutation with unmethylated or only partially methylated alleles produce more FMRP than those with fully methylated alleles (35). The higher levels of FMRP explain the typically higher IQ (above 70) in high-functioning individuals with FXS. Similarly those individuals with “size-mosaicism” (full mutation plus premutation, gray zone or normal alleles) have a higher IQ than those without mosaicism. Therefore full mutation cells have a deficit of FMRP and the premutation cells produce an excess of FMR1 mRNA, leading to mRNA toxicity but relatively normal levels of FMRP (“dual mutation effects”, pathological involvement from two different mechanisms). Higher rates of psychotic thinking have been observed in individuals with this type of mosaicism leading to dual mutation effects (42). In females with FXS the normal X typically produces 25% to 50% of the normal FMRP level and these females have IQ scores that range from normal to moderate intellectual disability (6). Working and short-term memory (43), executive function (44), visual memory, visual-spatial processing (45) and verbal deficits are common in FXS (verbal comprehension and vocabulary) (46). Almost all males and approximately 30% of females with FXS have impaired speech (47).
In general, overall IQ declines with age in those with FXS because of the deficits in abstract reasoning which cannot keep up with the intellectual growth seen in typical children and adolescents (48). The adaptive skills also decline in FXS from adolescence into adulthood (49). This emphasizes the importance of early intervention with intensive behavioral/cognitive programs and targeted treatments early in life to improve or prevent cognitive decline.
2.4. Behavioral phenotype
FXS accounts for approximately 2–5% of all individuals diagnosed with FXS accounts for approximately 2–5% of all individuals diagnosed with ASD (50) . In FXS about 60% of males have an ASD (51,52). About 80% of males and 30% of females with FXS have symptoms of attention deficit hyperactivity disorder (ADHD) (53). Sleep disturbances, such as difficulty falling asleep and/or interrupted sleep are also characteristic of individuals with FXS (54). Altered sleep patterns and dysregulated melatonin profiles were found in 13 boys with fragile X when compare with age-matched normal controls (55). Results showed greater variability in total sleep time, difficulty in sleep maintenance, and significantly greater nocturnal melatonin production in the boys with FXS.
A hallmark feature of FXS that can also occur in some premutation carriers is social anxiety. This behavior leads to the characteristic “Fragile X handshake”; where the individuals may shake the interviewer's hand or acknowledge his/her presence but will avoid eye contact until the interviewer looks away (56). Additional behavioral features include stereotypies such as hand-flapping and hand-biting, shyness, perseveration, mood instability, aggression and impaired speech (52). Cross-sectional analyses suggest that dimensions of problem behavior, anxiety, and hyperactivity are age-related; thus, age should serve as an important control variable in behavioral studies in FXS. Measures of anxiety, attention, and hyperactivity are highly associated with other behavior problems (29). There is evidence that autism scores decreased with time, particularly in communication and social aspects of adaptive behavior (57). However, emotional symptoms, behavioral difficulties, problems with peers and social behaviors may remain relatively stable over time (58). These trajectories may be associated with variations of FMRP, which in turn can be related to epigenetic changes, but there have been no large longitudinal studies that assess the molecular variations and behavior/cognitive correlations. Further longitudinal studies are necessary to assess the developmental trajectories of FXS across the lifetime and relate the outcomes to molecular and environmental factors.
2.5. Genotypes
The unstable dynamic FMR1 mutation can result in “size-mosaicism”, but cells of individuals who have only one size allele may also show different patterns of methylation (none, partial, and full methylation) referred as “methylation mosaicism”. Some individuals may have the presence of three or more populations of cells with different size-alleles and methylation-patterns. Therefore, the complex molecular mechanism and multiple possibilities of genotypes results in the wide variety of clinical characteristics of individuals with FXS and may also relate to different responses to standard and targeted treatments but this has not been well studied (59).
2.6. Neurobiology
At the cellular level, FXS is associated with immature dendritic spine morphology (60,61). FMRP is an essential protein for synaptic development and plasticity because it is a key negative regulator mRNA translation and subsequent protein synthesis that can down-regulate and/or up-regulate their targets at the synapse (62). FMRP inhibits protein synthesis that is needed for internalizing the AMPA receptors leading to long term depression (LTD); thus without FMRP there is enhanced LTD in the hippocampus (63). The Fmr1-KO mouse shows enhanced protein translation and protein synthesis in the hippocampus (64), LTD is significantly increased and this leads to deficits in synaptic plasticity and weakening of synaptic connections (65). Protein synthesis promotes synaptic plasticity activation, which is thought to be mainly coordinated by the action of metabotropic glutamate receptors (mGluRs) (66). This is the basis of the “mGluR theory of fragile X syndrome” (63). The neurobiology and several symptoms of FXS were rescued when the mGluR heterozygous mouse was crossed with the Fmr1-KO mouse (63,67).
Currently there are many other pathophysiological mechanisms described that are thought to be the result of absence or low FMRP. The lack of FMRP can also up-regulate PI3K, an important signaling molecule downstream of the activation of mGluR (31). Recently Matic et al. (2014), showed a global down-regulation of the MAPK/ERK pathway and decrease in phosphorylation level of ERK1/2 in the murine Fmr1 KO. However, others show an increase in this system in patient fibroblasts (68). A differential expression of many proteins involved in the p53 pathway, Wnt and calcium signaling was also found and led to postulate that calcium imbalance is part of pathophysiology of FXS (69). Although FMRP is mainly a negative regulator, there is evidence that it can up-regulate the translation of some mRNAs, such as those encoding GABAA receptor subunits (α1, α3, α4, β1, β2, ɤ1, ɤ2, and δ), which were significantly reduced in neocortex and cerebellum of the Fmr1-KO mice (70). Other proteins required for GABA synthesis (Glutamate decarboxylase, GAD), transport (GABA transporter, GAT) and catabolism (GABA transaminase, GABA succinic semialdehyde) were also found to be reduced (71). A balanced GABA system is required for neuronal activation, network oscillations, neuronal synchrony and facilitation of movement and integration of information in many brain regions (72). The imbalance between the GABA and Glutamate systems is believed to contribute to the cognitive impairments, anxiety, hyperarousal, ASD, and epilepsy in children with FXS (73).
A novel FMRP target mRNA is the neuronal nitric oxide synthase (NOS1 or nNOS) in mid-fetal human neocortex. FMRP was found to be a positive regulator of NOS1 translation, controlling NOS1 protein levels in a dose-dependent manner in vitro and in vivo (74), and the NOS1 was severely reduced in the fetal and post-natal developing neocortex of FXS patients (74). The evidence of the multiple roles of nitric oxide (NO) in multiple neural processes such as synaptic developmental, retrograde signaling and synaptic plasticity (75–79) led to the hypothesis that the decrease expression of NOS1 and secondary depletion of NO in the developing FXS brain may contribute to the neuropathology of FXS (80).
The absence of FMRP also affects the Brain Derived Neurotropic Factor (BDNF) levels in early and late development in the murine hippocampus. In early development of the KO mouse brain, hippocampal expression of BDNF is increased compared to wild type (WT) (81,82), whereas by age 3–4 months, BDNF expression is reduced compared to the WT (82,83). The mechanism of regulation of BDNF remains to be described, but this evidence suggests dual FMRP effects in BDNF expression during brain development. FMRP may also positively regulate many other mRNAs including SOD1, ASCL1, Kcnd2, and DLG4 (84–86). It is estimated that FMRP regulates the translation of about 4% of brain mRNAs (87,88). We have discussed the mechanisms of pathogenesis mediated by the absence of FMRP; however, the mechanism that causes the silencing of the FMR1 gene by the full mutation remains uncertain. There are many targeted treatments that focus on these pathways to reestablish the normal neurobiology in the KO mouse and these have led to clinical trials of targeted treatments in patients with FXS.
2.7. FMR1 silencing mechanism of the full mutation
It is intriguing that the premutation can lead to enhanced expression of the gene, whereas the full mutation leads to suppression of transcription. There are mechanisms that could explain the reduced transcription of the FMR1 gene in the full mutation; these mechanisms can be divided in two groups: DNA-mediated and RNA-mediated (89). A model in which hairpin aggregation by the CGG repeats results in the DeNovo methylation has been suggested because tridimensional CGG-structures can trigger their own methylation by DNA methyltransferases in vitro (90); another suggested DNA-mediated model involves repeat-binding transcription factors which in turn can aggregate other proteins and prevent transcription. This model was hypothesized from the existing evidence of a similar mechanism in mice where the pericentromic repeats in mice are silenced by Pax3 and Pax9 hybridization and recruitment of H3K9 trimethylase and Suv39h1 (91) that finally inactivate these regions. The FMR1 mRNA products are a variety of transcripts of different sizes and reverted sequences that result from a number of splicing sites and the transcription of both, the sense and anti-sense strands. Colak et al. (2014), suggested an RNA mediated mechanism of silencing, in which the FMR1 gene is silenced through a hybridization of the complementary CGG-repeat track of the FMR1 mRNA (92). Other RNA-mediated mechanisms have been suggested to involve the formation of RNA hairpins subtracts of the enzyme Dicer, RNA-DNA hybrids for chromatin compaction and promoter antisense-transcripts (89). The silencing mechanisms of FMR1 are potential targets for drug therapy. Since the FMRP is a key transcription regulator of many neurobiological pathways, in theory targeted treatments to prevent the inactivation of the FMR1 gene may lead to more normal FMRP levels and reestablish the function of many neurobiological systems. Therefore silencing gene modifiers could be more efficient, although more difficult to translate into patients than specific-system treatments, such as the mGluR5 antagonist and GABAA agonists.
3. Premutation allele
As previously mentioned in adults the premutation is associated with FXTAS, FXPOI and a variety of other medical/psychiatric problems. Recently the studies of children with the premutation have demonstrated that some carriers can demonstrate limited physical features of FXS in addition to psychological or developmental problems whereas most carriers do not show any symptoms.
3.1. Physical findings
Premutation carries can present with facial dysmorphic features and the most common finding is prominent ears (89,90). Recently a study of premutation carriers found that 33% of postpubertal carrier males had macroorchidism (93). Those with macroorchidism had a lower verbal and full scale IQ and increased FMR1 mRNA levels compared to those without macroorchidism (93). This suggests that about one third of individuals with the premutation have significantly lowered FMRP leading to their macroorchidism and mildly lowered cognitive abilities. Premutation carriers can also have joint-laxity and smooth skin typical of those with FXS (94,95).
3.2. Neurological disorders
Chonchaiya et al. (2011) studied boys with the premutation and found an association between seizures, ASD, and ID. These problems are more common in premutation boys who present clinically compared to those who are identified through cascade testing. FXS children of premutation mothers with autoimmune disorders were found to have increased epilepsy and tics compared to children whose mothers did not have autoimmune problems (96).
3.3. Cognitive and behavioral phenotype
The cognitive effects of the premutation show variable results depending on the age of the carrier and whether they present as the proband or were identified through cascade testing. Not clinically referred children typically do not show differences compared to controls, particularly in girls (97). Probands who presented clinically usually have cognitive deficits compared to controls (97,98). ADHD is increased in carriers compared to controls (97) and in adulthood these symptoms can persist or present as executive function deficits (34,99,100). Myers et al. (2001), in a small study of 14 children with the premutation found a trend towards lower performance IQ (101). Boys with the premutation have higher rates of ADHD symptoms, shyness, social deficits, autism spectrum disorder (98,102) and, less commonly, intellectual disability (ID) compared to controls. Many case reports of premutation involvement and ASD have been published. Clifford et al. (2007) reported seven males with the premutation; two were probands, and one of these had ASD (104). Goodlin-Jones et al. (2004), reported four premutation boys and two girls with ASD, and their levels of FMRP were significantly lower than normal (103). In the Farzin et al. (2006) study, there were 14 boys with the premutation whose parents sought medical attention for their sons' behavior problems (probands), 13 boys with the premutation diagnosed by cascade testing (non-probands), and 16 boys who were siblings without the premutation (controls). They found that 93% (13 of 14) of probands, 38% (6 of 13) of the non-probands and 13% (2 of 16) of the controls had ADHD. In addition 71% of probands (10 of 14) and 8% of non-probands (1 of 13) had ASD. In a screening study of individuals from families with FXS, about 14% of boys and 5% of girls with the premutation met diagnostic criteria for ASD (104). A web questionnaire of more than 1,000 families demonstrated a prevalence of autism or ASD of 13% in boys with the premutation and 1% in girls with the premutation (105).
Recently, the Rivera group at the MIND Institute (106) using a contrast-detection task found low-level visual processing deficits in infants with deficits in infants with FXS and with the premutation. In both groups of infants the contrast levels needed for detection of motion were significantly greater than those of typically developing infants. They concluded that early in life premutation infants can show visual or perhaps other deficits that are also observed in children with FXS.
Psychiatric problems in adults, including depression and anxiety, occur in about 40% of premutation carriers (14). Although initial studies of psychiatric disorders in premutation carriers hypothesized that the mood disorders found were associated with the difficulties of caring for a child with FXS, these problems can occur independently from having an affected child (17). In the life-time of individuals with FXTAS, 65% met the clinical criteria for a mood disorder according to the DSM-IV, remarkably for anxiety in 52% of the cases (17). It has been found that adult females have more problems with attention, hyperactivity (105), sleep problems (23), autistic behaviors such as rigidity (107), perseverance and aloofness (108) and language dysfunction (109) compared to controls.
3.4. Neurobiology
Hippocampal neurons with the premutation in culture (in vitro) showed reduced dendritic maturity with shorter dendritic lengths and fewer branches between 7 and 21 days compared with WT neurons (110). The premutation neurons had elevations of stress proteins and their mRNAs, including heat shock proteins (Hsp27 and Hsp70) and αB-crystallin. In addition premutation neuronal cultures die more easily in culture by 21 days compared with WT type neurons (110,111). Furthermore, altered embryonic neocortical development in the premutation mouse compared to WT has been reported (112). At 12 weeks early deficits in learning were observed in KO mice, the premutation mouse was unable to detect a change in the distance between two objects; and at 48 weeks, they could not detect a transposition of objects (113). This suggests that the premutation leads to a clear neuronal susceptibility that in addition to other genetic hits (93) or environmental toxicity (114) can result in a pathogenic neurobiology. Further studies are necessary to determine the neurobiology of affected individuals with the premutation.
3.5. Premutation genotypes
Initially FMR1 premutation carriers were thought to have normal FMRP levels, however recent research findings suggest that carriers have elevated levels of mRNA due to increased transcription, but decreased level of FMRP because the translation is less efficient (95,103). As the premutation increases from 55 to 200, the level of FMR1 mRNA increases and the levels of FMRP begin to decline (115,116). Reduced FMR1 translation is observed in adult individuals with large size premutation alleles (> 110 CGG repeats) and these individuals can have cognitive deficits. Also recent animal studies of the premutation mouse demonstrate lowered levels of FMRP in addition to elevated FMR1-mRNA in many brain areas, particularly the amygdala, hippocampus, and cortex, when compared with controls without the premutation (117).
The causative molecular mechanism of cognitive deficits and neurodevelopmental problems were thought to be related to silencing of the FMR1 gene (“loss of function”) and decreased amount of FMRP while the mechanisms involved in FXTAS and FXPOI are thought to be associated with abnormally increased levels of FMR1 RNA (“gain of function”) and RNA-toxicity. However recent evidence supports that both the FMRP deficits and elevated FMR1 RNA in carriers are associated with amygdala dysfunction, which causes cognitive deficits, anxiety, autism spectrum disorders, social avoidance, and aggressive behavior.
There are at least 3 mechanisms that could explain the elevation of FMR1 mRNA (89). One suggests that the observed increase of acetylated histones at the FMR1 promoter (118) could increase the FMR1 gene transcription. Second, the long tracts of CGG-repeats have been shown to exclude nucleosomes in vitro (119) and if this occurs in vivo it may increase the accessibility of transcription factors to the promoter. Third, the R-loops formed by the CGG-repeats (120,121) may lead to chromatin decondensation (122). The mechanism of FMR1 mRNA-toxicity remains to be established, and there are at least 3 models proposed. The “sequestration” model which proposes that the RNA expanded CGG repeats are pathogenic by sequestrating proteins, including Purα, Rm62, CUGBP1, hnRNP A2/B1, SAM68, and DROSHA-DGCR8 (123–127) that in turn alter the transcription of many other proteins. A second model, “RAN translation”, represents non-canonical translation that results in expression of toxic polyglycine- and polyalanine-containing products (128,129). A third model, “antisense FMR1 (ASFMR1) toxicity”, involves the expression of antisense transcripts products (130). Mitochondrial abnormalities have also been found in FXS and premutation carriers. The mechanism of mitochondrial dysfunction is unknown but this mechanism is another cause of premutation and full mutation involvement (131,132).
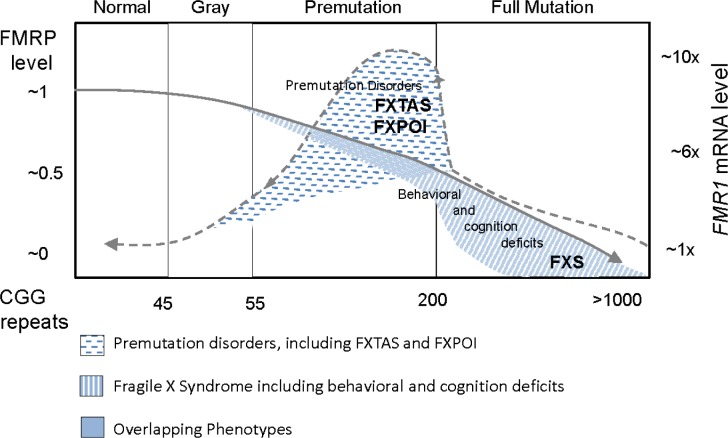
4. Overlapping phenotypes, FMR1 spectrum disorders
The overlap between premutation disorders and full mutation disorders occurs when the full mutation is partially or completely unmethylated or there is a high level of mosaicism in FXS. This puts those with FXS at risk for FXTAS and other premutation problems. In fact there have been a handful of individuals with FXS who have developed FXTAS and these individuals are high functioning and have unmethylated alleles or mosaicism (133–136). Even in the midrange of CGG repeats in premutation carriers there may be mild deficits of FMRP leading to behavioral problems or psychiatric phenotypes (137).
Another area of overlap occurs in the gray zone (45–54 CGG repeats). The rate of FMR1 gray zone expansions in the general population is variable, but large population studies report rates of 0.8% to 3.0% for repeat sizes between 41 and 54 (138–140). In 2006, it was recognized that gray zone expansion carriers can also present with premature ovarian insufficiency at a higher rate that in the general population (141,142). In a screening study in 2011 a higher rate of Parkinsonism was found in the gray zone mutation carriers compared to controls without the gray zone. There have also been reports of FXTAS in those with a gray zone (143,144) because elevated FMR1 mRNA can also occur in this range (145). Other clinical associations with the gray zone in adults include anxiety (146) and cognitive decline (147). However other studies did not show this association (147–151). Pertinent to children, in 2000, a 5-year survey of boys who required special education showed an excess of gray zone expansions (152), however, this result has not been replicated (153). Further studies are necessary to study the association of the gray zone mutation and the mechanisms of disease in adults and children.
5. Conclusion
Clinicians need to know that those with an FMR1 mutation are at risk for a wide range of neurovelopmental and/or psychological disorders/neurological disease, referred as Fragile X Spectrum Disorders (Figure 2). It is also important to have a holistic model of understanding on how the phenotype is related to the number of CGG repeats and/or size-mosaicism, including epigenetic changes or methylation status (partial and full, as well as methylation mosaicism), genetic background (gene modifiers and second genetic hits which can be protective or pathogenic) and environmental exposures (environmental changes, exposures to toxins, and social interactions “socionome” among other factors).
Figure 2.
Overlapping phenotypes between FXS and premutation disorders. Dotted-line indicates FMR1 mRNA levels and solid-line indicates FMRP expression levels.
Our understanding of FMRP deficits in the FXSD has been hampered by the limited technology available to assess quantitative FMRP levels. Although the immunocytochemical methodology demonstrated a strong correlation with IQ in those with a fragile X mutation (35,154), it was not sufficiently quantitative to show the remarkable variation that exists even in the normal population. This variation has been demonstrated by ELISA technology but the technique is difficult to replicate in subsequent samples (155). Newer techniques including the immunoassay utilizing time-resolved Forster's resonance energy transfer (156) and also the Luminex immunoassay (157). These techniques will lead to a new understanding of FMRP deficits not only in FXSD, but also in other neurodevelopmental/neuropsychiatric disorders. The recent publication of FMRP deficits in the brains of individuals with bipolar disorder, schizophrenia, depression and autism (156–158) has opened our eyes to the importance of FMRP outside of the FXSD population. Even more remarkable is the finding that the age of onset and overall IQ in those with schizophrenia is correlated with FMRP deficits in peripheral blood (159). The advances in treatments for FXS may also be helpful for premutation carriers with low FMRP and perhaps in other disorders with low FMRP such as ASD.
An area of overlap that is in need of research is the aging process in FXS because many patients experience cognitive decline and the cause is not known, although occult mosaicism leading to a FXTAS-like picture is possible (160). Older patients with FXS also have a high risk for Parkinson's disease and it is uncertain if this is also related to occult mosaicism (161). These are important considerations for children with FXS because they are raised by mothers with the premutation who may experience a premutation disorder that could influence the development of their offspring. These intergenerational influences require more study. Certainly the development of effective targeted treatments aim to have a significant effect on the ultimate outcome for those with FXSD.
Acknowledgements
This work was supported by the National Institute On Aging of the National Institutes of Health (P30AG043097) and NIH diversity supplement for the NICHD (HD036071).
References
- 1. Yrigollen CM, Martorell L, Durbin-Johnson B, Naudo M, Genoves J, Murgia A, Polli R, Zhou L, Barbouth D, Rupchock A, Finucane B, Latham GJ, Hadd A, Berry-Kravis E, Tassone F. AGG interruptions and maternal age affect FMR1 CGG repeat allele stability during transmission. J Neurodev Disord. 2014; 6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lubs HA. A marker X chromosome. Am J Hum Genet. 1969; 21:231-244. [PMC free article] [PubMed] [Google Scholar]
- 3. Verkerk AJ, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991; 65:905-914. [DOI] [PubMed] [Google Scholar]
- 4. Oberle I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boue J, Bertheas MF, Mandel JL. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991; 252:1097-1102. [DOI] [PubMed] [Google Scholar]
- 5. Darnell JC, Richter JD. Cytoplasmic RNA-binding proteins and the control of complex brain function. Cold Spring Harb Perspect Biol. 2012; 4:a012344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Vries BB, Wiegers AM, Smits AP, Mohkamsing S, Duivenvoorden HJ, Fryns JP, Curfs LM, Halley DJ, Oostra BA, van den Ouweland AM, Niermeijer MF. Mental status of females with an FMR1 gene full mutation. Am J Hum Genet. 1996; 58:1025-1032. [PMC free article] [PubMed] [Google Scholar]
- 7. Cronister A, Schreiner R, Wittenberger M, Amiri K, Harris K, Hagerman RJ. Heterozygous fragile X female: Historical, physical, cognitive, and cytogenetic features. Am J Med Genet. 1991; 38:269-274. [DOI] [PubMed] [Google Scholar]
- 8. Sullivan SD, Welt C, Sherman S. FMR1 and the continuum of primary ovarian insufficiency. Semin Reprod Med. 2011; 29:299-307. [DOI] [PubMed] [Google Scholar]
- 9. Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, Yadav-Shah M, Sherman SL. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005; 20:402-412. [DOI] [PubMed] [Google Scholar]
- 10. Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001; 57:127-130. [DOI] [PubMed] [Google Scholar]
- 11. Jacquemont S, Hagerman RJ, Leehey M, et al. Fragile X premutation tremor/ataxia syndrome: Molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003; 72:869-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacquemont S, Farzin F, Hall D, Leehey M, Tassone F, Gane L, Zhang L, Grigsby J, Jardini T, Lewin F, Berry-Kravis E, Hagerman PJ, Hagerman RJ. Aging in individuals with the FMR1 mutation. Am J Ment Retard. 2004; 109:154-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodriguez-Revenga L, Madrigal I, Badenas C, Xunclà M, Jiménez L, Milà M. Premature ovarian failure and fragile X female premutation carriers: No evidence for a skewed X-chromosome inactivation pattern. Menopause. 2009; 16:944-949. [DOI] [PubMed] [Google Scholar]
- 14. Hagerman P. Fragile X-associated tremor/ataxia syndrome (FXTAS): Pathology and mechanisms. Acta Neuropathologica. 2013; 126:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hunsaker MR, Greco CM, Spath MA, Smits AP, Navarro CS, Tassone F, Kros JM, Severijnen LA, Berry-Kravis EM, Berman RF, Hagerman PJ, Willemsen R, Hagerman RJ, Hukema RK. Widespread non-central nervous system organ pathology in fragile X premutation carriers with fragile X-associated tremor/ataxia syndrome and CGG knock-in mice. Acta Neuropathol. 2011; 122:467-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roberts JE, Bailey DB, Mankowski J, Ford A, Sideris J, Weisenfeld LA, Heath TM, Golden RN. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B Neuropsychiatr Genet. 2009; 150:130-139. [DOI] [PubMed] [Google Scholar]
- 17. Bourgeois JA, Seritan AL, Casillas EM, Hessl D, Schneider A, Yang Y, Kaur I, Cogswell JB, Nguyen DV, Hagerman RJ. Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. J Clin Psychiatry. 2011; 72:175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Besterman AD, Wilke SA, Mulligan TE, Allison SC, Hagerman R, Seritan AL, Bourgeois JA. Towards an understanding of neuropsychiatric manifestations in fragile X premutation carriers. Future Neurol. 2014; 9:227-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Au J, Akins RS, Berkowitz-Sutherland L, Tang HT, Chen Y, Boyd A, Tassone F, Nguyen DV, Hagerman R. Prevalence and risk of migraine headaches in adult fragile X premutation carriers. Clin Genet. 2013; 84:546-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamlin AA, Sukharev D, Campos L, Mu Y, Tassone F, Hessl D, Nguyen DV, Loesch D, Hagerman RJ. Hypertension in FMR1 premutation males with and without fragile X-associated tremor/ataxia syndrome (FXTAS). Am J Med Genet A. 2012; 158A:1304-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coffey SM, Cook K, Tartaglia N, Tassone F, Nguyen DV, Pan R, Bronsky HE, Yuhas J, Borodyanskaya M, Grigsby J, Doerflinger M, Hagerman PJ, Hagerman RJ. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A. 2008; 146:1009-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Winarni TI, Chonchaiya W, Sumekar TA, Ashwood P, Morales GM, Tassone F, Nguyen DV, Faradz SM, Van de Water J, Cook K, Hamlin A, Mu Y, Hagerman PJ, Hagerman RJ. Immune-mediated disorders among women carriers of fragile X premutation alleles. Am J Med Genet A. 2012; 158A:2473-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamlin A, Liu Y, Nguyen DV, Tassone F, Zhang L, Hagerman RJ. Sleep apnea in fragile X premutation carriers with and without FXTAS. Am J Med Genet B Neuropsychiatr Genet. 2011; 156B:923-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Summers SM, Cogswell J, Goodrich JE, Mu Y, Nguyen DV, Brass SD, Hagerman RJ. Prevalence of restless legs syndrome and sleep quality in carriers of the fragile X premutation. Clin Genet. 2014; 86:181-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soontarapornchai K, Maselli R, Fenton-Farrell G, Tassone F, Hagerman PJ, Hessl D. Abnormal nerve conduction features in fragile X premutation carriers. Arch Neurol. 2008; 65:495-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Apartis E, Blancher A, Meissner WG, et al. FXTAS: New insights and the need for revised diagnostic criteria. Neurology. 2012; 79:1898-1907. [DOI] [PubMed] [Google Scholar]
- 27. Tassone F, Iong KP, Tong TH, Lo J, Gane LW, Berry-Kravis E, Nguyen D, Mu LY, Laffin J, Bailey DB, Hagerman RJ. FMR1 CGG allele size and prevalence ascertained through newborn screening in the united states. Genome Med. 2012; 4:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hagerman R, Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013; 12:786-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wheeler A, Raspa M, Bann C, Bishop E, Hessl D, Sacco P, Bailey DB., Jr Anxiety, attention problems, hyperactivity, and the aberrant behavior checklist in fragile X syndrome. Am J Med Genet A. 2014; 164A:141-155. [DOI] [PubMed] [Google Scholar]
- 30. Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet. 1993; 4:335-340. [DOI] [PubMed] [Google Scholar]
- 31. Davids JR, Hagerman RJ, Eilert RE. Orthopaedic aspects of fragile-X syndrome. J Bone Joint Surg Am. 1990; 72:889-896. [PubMed] [Google Scholar]
- 32. Pyeritz RE, Stamberg J, Thomas GH, Bell BB, Zahka KG, Bernhardt BA. The marker Xq28 syndrome (“fragile-X SYndrome”) in a retarded man with mitral valve prolapse. Johns Hopkins Med J. 1982; 151:231-237. [PubMed] [Google Scholar]
- 33. Loesch DZ, Huggins R, Hay DA, Gedeon AK, Mulley JC, Sutherland GR. Genotype-phenotype relationships in fragile X syndrome: A family study. Am J Hum Genet. 1993; 53:1064-1073. [PMC free article] [PubMed] [Google Scholar]
- 34. Loesch DZ, Huggins RM, Bui QM, Taylor AK, Hagerman RJ. Relationship of deficits of FMR1 gene specific protein with physical phenotype of fragile X males and females in pedigrees: A new perspective. Am J Med Genet A. 2003; 118A:127-134. [DOI] [PubMed] [Google Scholar]
- 35. Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Ment Retard Dev Disabil Res Rev. 2004; 10:31-41. [DOI] [PubMed] [Google Scholar]
- 36. McLennan Y, Polussa J, Tassone F, Hagerman R. Fragile x syndrome. Curr Genomics. 2011; 12:216-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nowicki ST, Tassone F, Ono MY, Ferranti J, Croquette MF, Goodlin-Jones B, Hagerman RJ. The prader-willi phenotype of fragile X syndrome. J Dev Behav Pediatr. 2007; 28:133-138. [DOI] [PubMed] [Google Scholar]
- 38. Berry-Kravis E, Raspa M, Loggin-Hester L, Bishop E, Holiday D, Bailey DB. Seizures in fragile X syndrome: Characteristics and comorbid diagnoses. Am J Intellect Dev Disabil. 2010; 115:461-472. [DOI] [PubMed] [Google Scholar]
- 39. Bernard PB, Castano AM, O'Leary H, Simpson K, Browning MD, Benke TA. Phosphorylation of FMRP and alterations of FMRP complex underlie enhanced mLTD in adult rats triggered by early life seizures. Neurobiol Dis. 2013; 59:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garber KB, Visootsak J, Warren ST. Fragile X syndrome. Eur J Hum Genet. 2008; 16:666-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaufmann WE, Abrams MT, Chen W, Reiss AL. Genotype, molecular phenotype, and cognitive phenotype: Correlations in fragile X syndrome. Am J Med Genet. 1999; 83:286-295. [PubMed] [Google Scholar]
- 42. Schneider A, Seritan A, Tassone F, Rivera SM, Hagerman R, Hessl D. Psychiatric features in high-functioning adult brothers with fragile X spectrum disorders. Prim Care Companion CNS Disord. 2013; 15 pii: PCC.12l01492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Munir F, Cornish KM, Wilding J. Nature of the working memory deficit in fragile-X syndrome. Brain Cogn. 2000; 44:387-401. [DOI] [PubMed] [Google Scholar]
- 44. Wilding J, Cornish K, Munir F. Further delineation of the executive deficit in males with fragile-X syndrome. Neuropsychologia. 2002; 40:1343-1349. [DOI] [PubMed] [Google Scholar]
- 45. Cornish KM, Munir F, Cross G. Spatial cognition in males with fragile-X syndrome: Evidence for a neuropsychological phenotype. Cortex. 1999; 35:263-271. [DOI] [PubMed] [Google Scholar]
- 46. Hall SS, Burns DD, Lightbody AA, Reiss AL. Longitudinal changes in intellectual development in children with fragile X syndrome. J Abnorm Child Psychol. 2008; 36:927-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Finestack LH, Richmond EK, Abbeduto L. Language development in individuals with fragile X syndrome. Top Lang Disord. 2009; 29:133-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bennetto L PB. Neuropsychology. In: Fragile X syndrome: Diagnosis, treatment, and research. 3rd ed., The Johns Hopkins University Press, Baltimore, Maryland, USA, 2002. [Google Scholar]
- 49. Klaiman C, Quintin EM, Jo B, Lightbody AA, Hazlett HC, Piven J, Hall SS, Reiss AL. Longitudinal profiles of adaptive behavior in fragile X syndrome. Pediatrics. 2014; 134:315-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schaefer GB, Mendelsohn NJ. Genetics evaluation for the etiologic diagnosis of autism spectrum disorders. Gen Med. 2008; 10:4-12. [DOI] [PubMed] [Google Scholar]
- 51. Hatton DD, Sideris J, Skinner M, Mankowski J, bailey DBJ, Roberts J, Mirrett P. Autistic behavior in children with fragile X syndrome: Prevalence, stability, and the impact of FMRP. Am J Med Genet A. 2006; 140A:1804-1813. [DOI] [PubMed] [Google Scholar]
- 52. Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, Tassone F, Hagerman PJ, Herman H, Hagerman RJ. Autism profiles of males with fragile X syndrome. Am J Ment Retard. 2008; 113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hagerman RJ, Hagerman PJ. Fragile X syndrome: Diagnosis, treatment, and research. 3rd ed., The Johns Hopkins University Press, Baltimore, Maryland, USA, 2002; p.540. [Google Scholar]
- 54. Wirojanan J, Jacquemont S, Diaz R, Bacalman S, Anders TF, Hagerman RJ, Goodlin-Jones BL. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. J Clin Sleep Med. 2009; 5:145-150. [PMC free article] [PubMed] [Google Scholar]
- 55. Gould EL, Loesch DZ, Martin MJ, Hagerman RJ, Armstrong SM, Huggins RM. Melatonin profiles and sleep characteristics in boys with fragile X syndrome: A preliminary study. Am J Med Genet. 2000; 95:307-315. [PubMed] [Google Scholar]
- 56. Cornish K, Turk J, Hagerman R. The fragile X continuum: New advances and perspectives. J Intellect Disabil Res. 2008; 52:469-482. [DOI] [PubMed] [Google Scholar]
- 57. Roberts JE, Mankowski JB, Sideris J, Goldman BD, Hatton DD, Mirrett PL, Baranek GT, Reznick JS, Long AC, Bailey DB., Jr Trajectories and predictors of the development of very young boys with fragile X syndrome. J Pediatr Psychol. 2009; 34:827-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Frolli A, Piscopo S, Conson M. Developmental changes in cognitive and behavioural functioning of adolescents with fragile-X syndrome. J Intellect Disabil Res. 2014. 10.1111/jir.12165. [DOI] [PubMed] [Google Scholar]
- 59. Hare EB, Hagerman RJ, Lozano R. Targeted treatments in fragile X syndrome. Expert Opin Orphan Drugs. 2014; 2:531-543. [Google Scholar]
- 60. Braun K, Segal M. FMRP involvement in formation of synapses among cultured hippocampal neurons. Cereb Cortex. 2000; 10:1045-1052. [DOI] [PubMed] [Google Scholar]
- 61. Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000; 10:1038-1044. [DOI] [PubMed] [Google Scholar]
- 62. Brown V, Jin P, Ceman S, Darnell JC, O'Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001; 107:477-487. [DOI] [PubMed] [Google Scholar]
- 63. Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004; 27:370-377. [DOI] [PubMed] [Google Scholar]
- 64. Qin M, Kang J, Burlin TV, Jiang C, Smith CB. Postadolescent changes in regional cerebral protein synthesis: An in vivo study in the FMR1 null mouse. J Neurosci. 2005; 25:5087-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bagni C, Tassone F, Neri G, Hagerman R. Fragile X syndrome: Causes, diagnosis, mechanisms, and therapeutics. J Clin Invest. 2012; 122:4314-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Massey PV, Bashir ZI. Long-term depression: Multiple forms and implications for brain function. Trends Neurosci. 2007; 30:176-184. [DOI] [PubMed] [Google Scholar]
- 67. Dölen G, Carpenter RL, Ocain TD, Bear MF. Mechanism-based approaches to treating fragile X. Pharmacol Ther. 2010; 127:78-93. [DOI] [PubMed] [Google Scholar]
- 68. Kumar D, Bhattacharya A, Nadel J, Moulton K, Zeak NM, Glicksman A, Dobkin C, Brick DJ, Schwartz PH, Smith CB, Klann E, Usdin K. Identification of fragile X syndrome-specific molecular markers in human fibroblasts: A useful model to test the efficacy of therapeutic drugs. Hum Mutat. 2014; 35:1485-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Matic K, Eninger T, Bardoni B, Davidovic L, Macek B. Quantitative phosphoproteomics of murine Fmr1-KO cell lines provides new insights into FMRP-dependent signal transduction mechanisms. J Proteome Res. 2014; 13:4388-4397. [DOI] [PubMed] [Google Scholar]
- 70. Rubenstein JL, Merzenich MM. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003; 2:255-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gatto CL, Broadie K. Genetic controls balancing excitatory and inhibitory synaptogenesis in neurodevelopmental disorder models. Front Synaptic Neurosci. 2010; 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lozano R, Hare EB, Hagerman RJ. Modulation of the GABAergic pathway for the treatment of fragile X syndrome. Neuropsychiatr Dis Treat. 2014; 10:1769-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cordeiro L, Ballinger E, Hagerman R, Hessl D. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: Prevalence and characterization. J Neurodev Disord. 2011; 3:57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kwan KY, Lam MM, Johnson MB, et al. Species-dependent posttranscriptional regulation of NOS1 by FMRP in the developing cerebral cortex. Cell. 2012; 149:899-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996; 84:757-767. [DOI] [PubMed] [Google Scholar]
- 76. Hölscher C. Nitric oxide, the enigmatic neuronal messenger: Its role in synaptic plasticity. Trends Neurosci. 1997; 20:298-303. [DOI] [PubMed] [Google Scholar]
- 77. Contestabile A. Roles of NMDA receptor activity and nitric oxide production in brain development. Brain Res Brain Res Rev. 2000; 32:476-509. [DOI] [PubMed] [Google Scholar]
- 78. Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AMG. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007; 8:766-775. [DOI] [PubMed] [Google Scholar]
- 79. Steinert JR, Chernova T, Forsythe ID. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist. 2010; 16:435-452. [DOI] [PubMed] [Google Scholar]
- 80. Colvin SM, Kwan KY. Dysregulated nitric oxide signaling as a candidate mechanism of fragile X syndrome and other neuropsychiatric disorders. Front Genet. 2014; 5:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Louhivuori V, Vicario A, Uutela M, Rantamäki T, Louhivuori LM, Castrén E, Tongiorgi E, Akerman KE, Castrén ML. BDNF and TrkB in neuronal differentiation of Fmr1-knockout mouse. Neurobiol Dis. 2011; 41:469-480. [DOI] [PubMed] [Google Scholar]
- 82. Uutela M, Lindholm J, Louhivuori V, Wei H, Louhivuori LM, Pertovaara A, Akerman K, Castrén E, Castrén ML. Reduction of BDNF expression in Fmr1 knockout mice worsens cognitive deficits but improves hyperactivity and sensorimotor deficits. Genes Brain Behav. 2012; 11:513-523. [DOI] [PubMed] [Google Scholar]
- 83. Uutela M, Lindholm J, Rantamäki T, Umemori J, Hunter K, Võikar V, Castrén ML. Distinctive behavioral and cellular responses to fluoxetine in the mouse model for fragile X syndrome. Front Cell Neurosci. 2014; 8:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Todd PK, Mack KJ, Malter JS. The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95. Proc Natl Acad Sci U S A. 2003; 100:14374-14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bechara EG, Didiot MC, Melko M, Davidovic L, Bensaid M, Martin P. A novel function for fragile X mental retardation protein in translational activation. PLoS Biol. 2009; 7:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fähling M, Mrowka R, Steege A, Kirschner KM, Benko E, Förstera B, Persson PB, Thiele BJ, Meier JC, Scholz H. Translational regulation of the human achaete-scute homologue-1 by fragile X mental retardation protein. J Biol Chem. 2009; 284:4255-4266. [DOI] [PubMed] [Google Scholar]
- 87. Ashley CT, Wilkinson KD, Reines D, Warren ST. FMR1 protein: Conserved RNP family domains and selective RNA binding. Science. 1993; 262:563-566. [DOI] [PubMed] [Google Scholar]
- 88. Bassell GJ, Warren ST. Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron. 2008; 60:201-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Usdin K, Hayward BE, Kumari D, Lokanga RA, Sciascia N, Zhao XN. Repeat-mediated genetic and epigenetic changes at the FMR1 locus in the fragile X-related disorders. Front Genet. 2014; 5:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Smith SS, Laayoun A, Lingeman RG, Baker DJ, Riley J. Hypermethylation of telomere-like foldbacks at codon 12 of the human c-ha-ras gene and the trinucleotide repeat of the FMR-1 gene of fragile X. J Mol Biol. 1994; 243:143-151. [DOI] [PubMed] [Google Scholar]
- 91. Bulut-Karslioglu A, Perrera V, Scaranaro M, de la Rosa-Velazquez IA, van de Nobelen S, Shukeir N, Popow J, Gerle B, Opravil S, Pagani M, Meidhof S, Brabletz T, Manke T, Lachner M, Jenuwein T. A transcription factor-based mechanism for mouse heterochromatin formation. Nat Struct Mol Biol. 2012; 19:1023-1030. [DOI] [PubMed] [Google Scholar]
- 92. Colak D, Zaninovic N, Cohen MS, Rosenwaks Z, Yang WY, Gerhardt J, Disney MD, Jaffrey SR. Promoter-bound trinucleotide repeat mRNA drives epigenetic silencing in fragile X syndrome. Science. 2014; 343:1002-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lozano R, Hagerman RJ, Duyzend M, Budimirovic DB, Eichler EE, Tassone F. Genomic studies in fragile X premutation carriers. J Neurodev Disord. 2014; 6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Aziz M, Stathopulu E, Callias M, Taylor C, Turk J, Oostra B, Willemsen R, Patton M. Clinical features of boys with fragile X premutations and intermediate alleles. Am J Med Genet B Neuropsychiatr Genet. 2003; 121B:119-127. [DOI] [PubMed] [Google Scholar]
- 95. Tassone F, Hagerman RJ, Taylor AK, Mills JB, Harris SW, Gane LW, Hagerman PJ. Clinical involvement and protein expression in individuals with the FMR1 premutation. Am J Med Genet. 2000; 91:144-152. [DOI] [PubMed] [Google Scholar]
- 96. Chonchaiya W, Tassone F, Ashwood P, Hessl D, Schneider A, Campos L, Nguyen DV, Hagerman RJ. Autoimmune disease in mothers with the FMR1 premutation is associated with seizures in their children with fragile X syndrome. Hum Genet. 2010; 128:539-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Seritan A, Cogswell J, Grigsby J. Cognitive dysfunction in FMR1 premutation carriers. Curr Psychiatr Rev. 2012, 9:78-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chonchaiya W, Au J, Schneider A, Hessl D, Harris SW, Laird M, Mu Y, Tassone F, Nguyen DV, Hagerman RJ. Increased prevalence of seizures in boys who were probands with the FMR1 premutation and co-morbid autism spectrum disorder. Hum Genet. 2012; 131:581-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Grigsby J, Cornish K, Hocking D, Kraan C, Olichney JM, Rivera SM, Schneider A, Sherman S, Wang JY, Yang JC. The cognitive neuropsychological phenotype of carriers of the FMR1 premutation. J Neurodev Disord. 2014; 6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cornish KM, Hocking DR, Moss SA, Kogan CS. Selective executive markers of at-risk profiles associated with the fragile X premutation. Neurology. 2011; 77:618-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Myers GF, Mazzocco MM, Maddalena A, Reiss AL. No widespread psychological effect of the fragile X premutation in childhood: Evidence from a preliminary controlled study. J Dev Behav Pediatr. 2001; 22:353-359. [DOI] [PubMed] [Google Scholar]
- 102. Farzin F, Perry H, Hessl D, Loesch D, Cohen J, Bacalman S, Gane L, Tassone F, Hagerman P, Hagerman R. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006; 27(Suppl 2):137-144. [DOI] [PubMed] [Google Scholar]
- 103. Goodlin-Jones BL, Tassone F, Gane LW, Hagerman RJ. Autistic spectrum disorder and the fragile X premutation. J Dev Behav Pediatr. 2004; 25:392-398. [DOI] [PubMed] [Google Scholar]
- 104. Clifford S, Dissanayake C, Bui QM, Huggins R, Taylor AK, Loesch DZ. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. J Autism Dev Disord. 2007; 37:738-747. [DOI] [PubMed] [Google Scholar]
- 105. Bailey DB, Jr., Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. Am J Med Genet A. 2008; 146A:2060-2069. [DOI] [PubMed] [Google Scholar]
- 106. Gallego PK, Burris JL, Rivera SM. Visual motion processing deficits in infants with the fragile X premutation. J Neurodev Disord. 2014; 6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Franke P, Leboyer M, Gänsicke M, Weiffenbach O, Biancalana V, Cornillet-Lefebre P, Croquette MF, Froster U, Schwab SG, Poustka F, Hautzinger M, Maier W. Genotype-phenotype relationship in female carriers of the premutation and full mutation of FMR-1. Psychiatry Res. 1998; 80:113-127. [DOI] [PubMed] [Google Scholar]
- 108. Hessl D, Wang JM, Schneider A, Koldewyn K, Le L, Iwahashi C, Cheung K, Tassone F, Hagerman PJ, Rivera SM. Decreased fragile X mental retardation protein expression underlies amygdala dysfunction in carriers of the fragile X premutation. Biol psychiatry. 2011; 70:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Losh M, Klusek J, Martin GE, Sideris J, Parlier M, Piven J. Defining genetically meaningful language and personality traits in relatives of individuals with fragile X syndrome and relatives of individuals with autism. Am J Med Genet B Neuropsychiatr Genet. 2012; 159B:660-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chen Y, Tassone F, Berman RF, Hagerman PJ, Hagerman RJ, Willemsen R, Pessah IN. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet. 2010; 19:196-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010; 19(R1):83-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cunningham CL, Martínez Cerdeño V, Navarro Porras E, Prakash AN, Angelastro JM, Willemsen R, Hagerman PJ, Pessah IN, Berman RF, Noctor SC. Premutation CGG-repeat expansion of the Fmr1 gene impairs mouse neocortical development. Hum Mol Genet. 2011; 20:64-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hunsaker MR, Goodrich-Hunsaker NJ, Willemsen R, Berman RF. Temporal ordering deficits in female CGG KI mice heterozygous for the fragile X premutation. Behav Brain Res. 2010; 213:263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Paul R, Pessah IN, Gane L, Ono M, Hagerman PJ, Brunberg JA, Tassone F, Bourgeois JA, Adams PE, Nguyen DV, Hagerman R. Early onset of neurological symptoms in fragile X premutation carriers exposed to neurotoxins. Neurotoxicology. 2010; 31:399-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ludwig AL, Espinal GM, Pretto DI, Jamal AL, Arque G, Tassone F, Berman RF, Hagerman PJ. CNS expression of murine fragile X protein (FMRP) as a function of CGG-repeat size. Hum Mol Genet. 2014; 23:3228-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Pretto DI, Mendoza-Morales G, Lo J, Cao R, Hadd A, Latham GJ, Durbin-Johnson B, Hagerman R, Tassone F. CGG allele size somatic mosaicism and methylation in FMR1 premutation alleles. J Med Genet. 2014; 51:309-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Qin M, Entezam A, Usdin K, Huang T, Liu ZH, Hoffman GE, Smith CB. A mouse model of the fragile X premutation: Effects on behavior, dendrite morphology, and regional rates of cerebral protein synthesis. Neurobiol Dis. 2011; 42:85-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Todd PK, Oh SY, Krans A, Pandey UB, Di Prospero NA, Min K, Taylor JP, Paulson HL. Histone deacetylases suppress CGG repeat-induced neurodegeneration via transcriptional silencing in models of fragile X tremor ataxia syndrome. PLoS Genet. 2010; 6:e1001240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wang YH, Gellibolian R, Shimizu M, Wells RD, Griffith J. Long CCG triplet repeat blocks exclude nucleosomes: A possible mechanism for the nature of fragile sites in chromosomes. J Mol Biol. 1996; 263:511-516. [DOI] [PubMed] [Google Scholar]
- 120. Groh M, Lufino MM, Wade-Martins R, Gromak N. R-loops associated with triplet repeat expansions promote gene silencing in friedreich ataxia and fragile X syndrome. PLoS Genet. 2014; 10:e1004318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Loomis EW, Sanz LA, Chedin F, Hagerman PJ. Transcription-associated R-loop formation across the human FMR1 CGG-repeat region. PLoS Genet. 2014; 10:e1004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Powell WT, Coulson RL, Gonzales ML, Crary FK, Wong SS, Adams S, Ach RA, Tsang P, Yamada NA, Yasui DH, Chédin F, LaSalle JM. R-loop formation at Snord116 mediates topotecan inhibition of Ube3a-antisense and allele-specific chromatin decondensation. Proc Natl Acad Sci U S A. 2013; 110:13938-13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Iwahashi CK, Yasui DH, An HJ, Greco CM, Tassone F, Nannen K, Babineau B, Lebrilla CB, Hagerman RJ, Hagerman PJ. Protein composition of the intranuclear inclusions of FXTAS. Brain. 2006; 129:256-271. [DOI] [PubMed] [Google Scholar]
- 124. Sofola OA, Jin P, Qin Y, Duan R, Liu H, de Haro M, Nelson DL, Botas J. RNA-binding proteins hnRNP A2/B1 and CUGBP1 suppress fragile X CGG premutation repeat-induced neurodegeneration in a drosophila model of FXTAS. Neuron. 2007; 55:565-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jin P, Duan R, Qurashi A, Qin Y, Tian D, Rosser TC, Liu H, Feng Y, Warren ST. Pur alpha binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a drosophila model of fragile X tremor/ataxia syndrome. Neuron. 2007; 55:556-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sellier C, Freyermuth F, Tabet R, et al. Sequestration of DROSHA and DGCR8 by expanded CGG RNA repeats alters microRNA processing in fragile X-associated tremor/ataxia syndrome. Cell Rep. 2013; 3:869-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Qurashi A, Li W, Zhou J, Peng J, Jin P. Nuclear accumulation of stress response mRNAs contributes to the neurodegeneration caused by fragile X premutation rCGG repeats. PLoS Genet. 2011; 7:e1002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Reddy K, Pearson CE. RAN translation: Fragile X in the running. Neuron. 2013; 778:405-408. [DOI] [PubMed] [Google Scholar]
- 129. Todd PK, Oh SY, Krans A, et al. CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron. 2013; 78:440-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ladd PD, Smith LE, Rabaia NA, Moore JM, Georges SA, Hansen RS, Hagerman RJ, Tassone F, Tapscott SJ, Filippova GN. An antisense transcript spanning the CGG repeat region of FMR1 is upregulated in premutation carriers but silenced in full mutation individuals. Hum Mol Genet. 2007; 16:3174-3187. [DOI] [PubMed] [Google Scholar]
- 131. Napoli E, Ross-Inta C, Wong S, Omanska-Klusek A, Barrow C, Iwahashi C, Garcia-Arocena D, Sakaguchi D, Berry-Kravis E, Hagerman R, Hagerman PJ, Giulivi C. Altered zinc transport disrupts mitochondrial protein processing/import in fragile X-associated tremor/ataxia syndrome. Hum Mol Genet. 2011; 20:3079-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ross-Inta C, Omanska-Klusek A, Wong S, Barrow C, Garcia-Arocena D, Iwahashi C, Berry-Kravis E, Hagerman RJ, Hagerman PJ, Giulivi C. Evidence of mitochondrial dysfunction in fragile X-associated tremor/ataxia syndrome. Biochem J. 2010; 429:545-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Loesch DZ, Sherwell S, Kinsella G, Tassone F, Taylor A, Amor D, Sung S, Evans A. Fragile X-associated tremor/ataxia phenotype in a male carrier of unmethylated full mutation in the FMR1 gene. Clin Genet. 2012; 82:88-92. [DOI] [PubMed] [Google Scholar]
- 134. Santa María L, Pugin A, Alliende MA, Aliaga S, Curotto B, Aravena T, Tang HT, Mendoza-Morales G, Hagerman R, Tassone F. FXTAS in an unmethylated mosaic male with fragile X syndrome from chile. Clin Genet. 2014; 86:378-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Pretto DI, Hunsaker MR, Cunningham CL, Greco CM, Hagerman RJ, Noctor SC, Hall DA, Hagerman PJ, Tassone F. Intranuclear inclusions in a fragile X mosaic male. Transl Neurodegener. 2013; 2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hall D, Tassone F, Klepitskaya O, Leehey M. Fragile X-associated tremor ataxia syndrome in FMR1 gray zone allele carriers. Mov Disord. 2012; 27:296-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hessl D, Rivera S, Koldewyn K, Cordeiro L, Adams J, Tassone F, Hagerman PJ, Hagerman RJ. Amygdala dysfunction in men with the fragile X premutation. Brain. 2007; 130:404-416. [DOI] [PubMed] [Google Scholar]
- 138. Tassone F, Iong KP, Tong TH, Lo J, Gane LW, Berry-Kravis E, Nguyen D, Mu LY, Laffin J, Bailey DB, Hagerman RJ. FMR1 CGG allele size and prevalence ascertained through newborn screening in the united states. Genome Med. 2012; 4:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Seltzer MM, Barker ET, Greenberg JS, Hong J, Coe C, Almeida D. Differential sensitivity to life stress in FMR1 premutation carrier mothers of children with fragile X syndrome. Health Psychol. 2012; 31:612-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Peñagarikano O, Gil A, Télez M, Ortega B, Flores P, Veiga I, Peixoto A, Criado B, Arrieta I. A new insight into fragile X syndrome among basque population. Am J Med Genet A. 2004; 128A:250-255. [DOI] [PubMed] [Google Scholar]
- 141. Bodega B, Bione S, Dalprà L, Toniolo D, Ornaghi F, Vegetti W, Ginelli E, Marozzi A. Influence of intermediate and uninterrupted FMR1 CGG expansions in premature ovarian failure manifestation. Hum Reprod. 2006; 21:952-957. [DOI] [PubMed] [Google Scholar]
- 142. Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet. 2005; 117:376-382. [DOI] [PubMed] [Google Scholar]
- 143. Liu Y, Winarni TI, Zhang L, Tassone F, Hagerman RJ. Fragile X-associated tremor/ataxia syndrome (FXTAS) in grey zone carriers. Clin Genet. 2013; 84:74-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Hall DA, Berry-Kravis E, Zhang W, Tassone F, Spector E, Zerbe G, Hagerman PJ, Ouyang B, Leehey MA. FMR1 gray-zone alleles: Association with parkinson's disease in women? Mov Disord. 2011; 26:1900-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Loesch DZ, Bui QM, Huggins RM, Mitchell RJ, Hagerman RJ, Tassone F. Transcript levels of the intermediate size or grey zone fragile X mental retardation 1 alleles are raised, and correlate with the number of CGG repeats. J Med Genet. 2007; 44:200-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kenna HA, Tartter M, Hall SS, Lightbody AA, Nguyen Q, de los Angeles CP, Reiss AL, Rasgon NL. High rates of comorbid depressive and anxiety disorders among women with premutation of the FMR1 gene. American J Med Genet B. 2013; 142b:872-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kurz MW, Schlitter AM, Klenk Y, Mueller T, Larsen JP, Aarsland D, Dekomien G. FMR1 alleles in parkinson's disease: Relation to cognitive decline and hallucinations, a longitudinal study. J Geriatr Psychiatry Neurol. 2007; 20:89-92. [DOI] [PubMed] [Google Scholar]
- 148. Kraff J, Tang HT, Cilia R, Canesi M, Pezzoli G, Goldwurm S, Hagerman PJ, Tassone F. Screen for excess FMR1 premutation alleles among males with parkinsonism. Arch Neurol. 2007; 64:1002-1006. [DOI] [PubMed] [Google Scholar]
- 149. Cilia R, Kraff J, Canesi M, Pezzoli G, Goldwurm S, Amiri K, Tang HT, Pan R, Hagerman PJ, Tassone F. Screening for the presence of FMR1 premutation alleles in women with parkinsonism. Arch Neurol. 2009; 66:244-249. [DOI] [PubMed] [Google Scholar]
- 150. Tan EK, Zhao Y, Puong KY, Law HY, Chan LL, Yew K, Shen H, Chandran VR, Yuen Y, Pavanni R, Wong MC, Ng IS. Expanded FMR1 alleles are rare in idiopathic parkinson's disease. Neurogenetics. 2005; 6:51-52. [DOI] [PubMed] [Google Scholar]
- 151. Toft M, Aasly J, Bisceglio G, Adler CH, Uitti RJ, Krygowska-Wajs A, Lynch T, Wszolek ZK, Farrer MJ. Parkinsonism, FXTAS, and FMR1 premutations. Mov Disord. 2005; 20:230-233. [DOI] [PubMed] [Google Scholar]
- 152. Youings SA, Murray A, Dennis N, Ennis S, Lewis C, McKechnie N, Pound M, Sharrock A, Jacobs P. FRAXA and FRAXE: The results of a five year survey. J Med Genet. 2000; 37:415-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Crawford DC, Meadows KL, Newman JL, Taft LF, Pettay DL, Gold LB, Hersey SJ, Hinkle EF, Stanfield ML, Holmgreen P, Yeargin-Allsopp M, Boyle C, Sherman SL. Prevalence and phenotype consequence of FRAXA and FRAXE alleles in a large, ethnically diverse, special education-needs population. Am J Hum Genet. 1999; 64:495-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Tassone F, Hagerman RJ, Iklé DN, Dyer PN, Lampe M, Willemsen R, Oostra BA, Taylor AK. FMRP expression as a potential prognostic indicator in fragile X syndrome. Am J Med Genet. 1999; 84:250-261. [PubMed] [Google Scholar]
- 155. Iwahashi C, Hagerman PJ. Isolation of pathology-associated intranuclear inclusions. Methods Mol Biol. 2008; 463:181-190. [DOI] [PubMed] [Google Scholar]
- 156. Schutzius G, Bleckmann D, Kapps-Fouthier S, di Giorgio F, Gerhartz B, Weiss A. A quantitative homogeneous assay for fragile X mental retardation 1 protein. J Neurodev Disord. 2013; 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. LaFauci G, Adayev T, Kascsak R, Kascsak R, Nolin S, Mehta P, Brown WT, Dobkin C. Fragile X screening by quantification of FMRP in dried blood spots by a luminex immunoassay. J Mol Diagn. 2013; 15:508-517. [DOI] [PubMed] [Google Scholar]
- 158. Kovács T, Kelemen O, Kéri S. Decreased fragile X mental retardation protein (FMRP) is associated with lower IQ and earlier illness onset in patients with schizophrenia. Psychiatry Res. 2013; 210:690-693. [DOI] [PubMed] [Google Scholar]
- 159. Kelemen O, Kovács T, Kéri S. Contrast, motion, perceptual integration, and neurocognition in schizophrenia: the role of fragile-X related mechanisms. Prog Neuropsychopharmacol Biol Psychiatry. 2013; 46:92-97. [DOI] [PubMed] [Google Scholar]
- 160. Hunsaker MR, Greco CM, Tassone F, Berman RF, Willemsen R, Hagerman RJ, Hagerman PJ. Rare intranuclear inclusions in the brains of 3 older adult males with fragile X syndrome: Implications for the spectrum of fragile X-associated disorders. J Neuropathol Exp Neurol. 2011; 70:462-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Utari A, Adams E, Berry-Kravis E, Chavez A, Scaggs F, Ngotran L, Boyd A, Hessl D, Gane LW, Tassone F, Tartaglia N, Leehey MA, Hagerman RJ. Aging in fragile X syndrome. J Neurodev Disord. 2010; 2:70-76. [DOI] [PMC free article] [PubMed] [Google Scholar]