Summary
A debilitating late-onset disorder of the premutation in the FMR1 gene is the neurodegenerative disorder fragile X-associated tremor ataxia syndrome (FXTAS). We report two patients with FXTAS who have a history of substance abuse (opiates, alcohol, and cocaine) which may have exacerbated their rapid neurological deterioration with FXTAS. There has been no case report regarding the role of substance abuse in onset, progression, and severity of FXTAS symptoms. However, research has shown that substance abuse can have a negative impact on several neurodegenerative diseases, and we propose that in these cases, substance abuse contributed to a faster progression of FXTAS as well as exacerbated white matter disease.
Keywords: Substance abuse, neurological deterioration, FXTAS, premutation, opiates
1. Introduction
Fragile X-associated tremor/ataxia syndrome (FXTAS) (OMIM: 300623) is a late-onset progressive neurodegenerative disorder with core features of intention tremor and gait ataxia affecting carriers of premutation repeat expansions (55–200 CGG repeats) in the 5’ UTR region of the FMR1 gene. This syndrome affects mainly men, and the mean age of onset is 62 years with incomplete penetrance (1,2).
Other conditions such as chronic pain related to neuropathy (3,4), fibromyalgia (5–7) and migraines (8) are commonly reported symptoms by individuals with FXTAS, and in general observation, narcotics are often used to treat the pain symptoms. Moreover, anxiety, depression and other psychiatric disorders are also reported with a higher prevalence in premutation carriers, and self-treatment using addictive substances is increased compared to controls (9,10).
While there has been no comprehensive study regarding the role of addictive substances in onset, progression, and severity of neurological symptoms in FXTAS, it is hypothesized that the clinical symptoms can be triggered by environmental factors. General examples of this phenomenon include alpha-1 antitrypsin, in which smoking can exacerbate lung disease (11), and hemochromatosis in which alcohol intake results in liver disease (12). Some environmental factors, such as chemotherapy, have been reported to exacerbate or induce earlier presentation of clinical symptoms in FXTAS (9). In addition previous studies in the general population have shown that chronic use of addictive substances has negative consequences on cognition, memory and other neurological functions. Therefore, we propose that addictive substances particularly opiates, alcohol and cocaine may contribute to a faster progression of neurological symptoms in FXTAS. Here we present two cases of adults with FXTAS with a long history of addictive substance use, including opiates, alcohol and cocaine.
2. Case reports
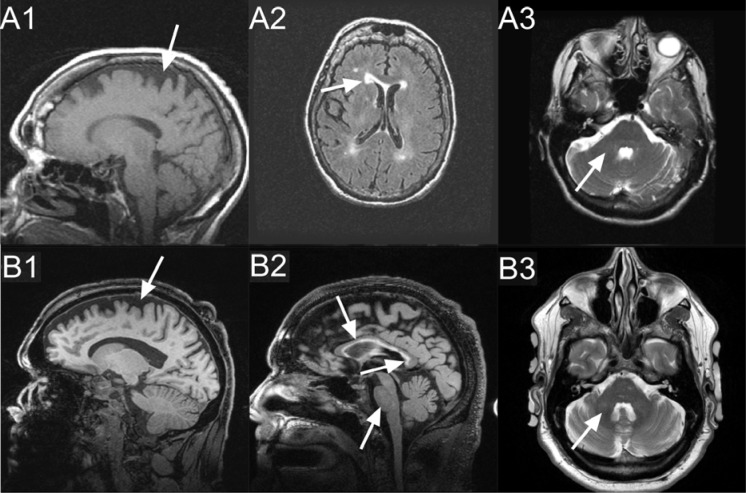
Case 1, a 79 year-old Caucasian woman who was initially evaluated at the UC Davis MIND Institute clinic in 2007, had alleles of 30 and 73 CGG repeats with an X-chromosome activation/inactivation ratio of 0.71 (Figure 1). Her medication history includes codeine with varying doses since age 54. She started on high dose Vicodin (hydrocodone and acetaminophen, 10 mg/300 mg) at age 75, which she was still taking at age 79 (12 tablets a day). She reported the onset of her neurological symptoms at age 54 and she complained of strong intermittent generalized pain which was more severe in her lower extremities and back. She was diagnosed with fibromyalgia at age 58. The patient showed a rapid neurological decline at age 77, when she started to experience handwriting problems, swallowing difficulties, cognitive deficits, frequent falling related to lower extremity weakness and the necessity of using a four pronged cane. Her pain intensified and her weakness worsened to the point she needed to use a walker at age 78. The neurological symptoms continued to progress so that she was unable to even sit and finally spent most of her time in bed (FXTAS stage 6). In bed, she was unable to lift her legs against gravity at age 79. She developed respiratory difficulties that were exacerbated by pneumonia and she died at 79 years of age. Her MRI at age 78 shows periventricular white matter lesions affecting the anterior and posterior horns, bilaterally, and moderate cerebral and mild cerebellar volume loss (Figure 2).
Figure 1.
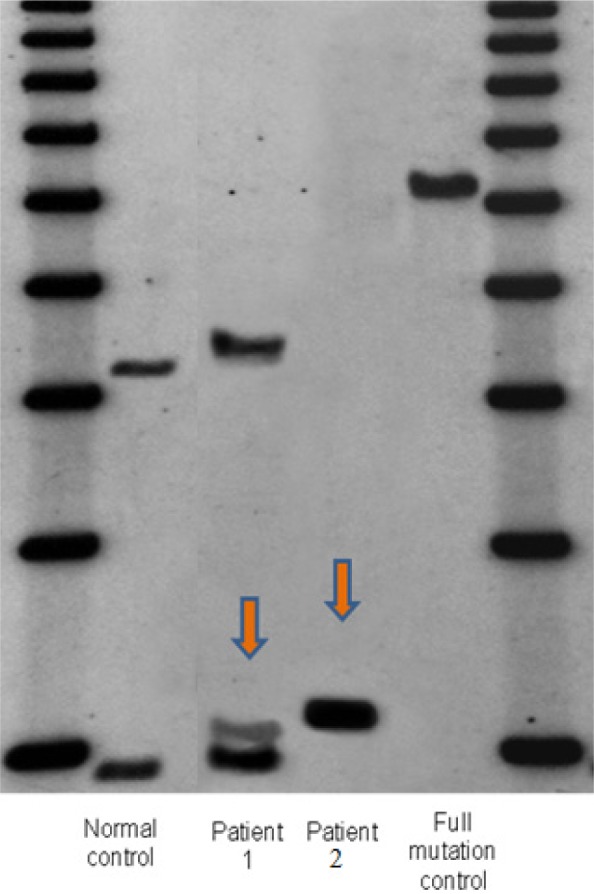
Southern blot results of two patients. 1Kb ladder size marker: lane 1 and 6. Lane 2 and 5: normal female control and full mutation male, respectively. Lane 3 and 4 showing the presence of a premutation allele in patient 1 and 2 respectively. CGG repeat sizes were measured by PCR analysis.
Figure 2.
Tesla MRI (1.5): T1 (A1), T2 FLAIR (A2), T2 (A3). 3 Tesla MRI: MPRAGE (B1), T2-FLAIR (B2), T2-TSE (B3); shown by arrows: Case 1: Moderate cerebral (A1) and mild cerebellar (not shown) volume loss, periventricular white matter lesions affecting anterior and posterior horns, bilaterally (A2). No white matter changes in the middle cerebellar peduncles (A3). Case 2: Moderate cerebral (B1), mild increased white matter changes in the middle cerebellar peduncles (B3) and pons (B2), moderately thin truncus of the corpus callosum (B2), with severe increased T2 signal intensity in both the truncus and the splenium of the corpus callosum (B2).
Case 2, a 55-year-old Caucasian male, presented at the UC Davis MIND Institute clinic in 2010. He had 100 CGG repeats (Figure 1). He has a long history of significant alcohol and cocaine abuse. He started to smoke marijuana daily in 1998, to help him with chronic pain. He used Vicodin (hydrocodone and acetaminophen, 10 mg/300 mg) three times a day on and off since the age of 35. He has a long psychiatric history including severe anxiety, depression, and post traumatic stress disorder (PTSD), particularly after he was in a vehicle accident at age 36. His neurological symptoms at age 35 included numbness, tingling, and pain in his lower and upper extremities. He had hand writing problems since about age 45. He has a history of intention tremor, beginning at age 51, and occasional resting tremor, as well as balance problems at age 51, and swallowing and choking problems since about age 53. Head tremor was observed at age 54. His MRI at age 55 showed moderate cerebellar and cerebral volume loss, mild increased T2 signal intensity in the middle cerebellar peduncles (MCPs), a moderately thin truncus of the corpus callosum, with severely increased T2 signal intensity in both the truncus and the splenium of the corpus callosum (Figure 2).
3. Discussion
Premutation carries have a susceptibility to develop a variety of symptoms such as neurodevelopmental disorders (13–16), psychiatric involvement (17–19), immune dysregulation (5–7,20–23), and neurological problems including FXTAS (1,9). We recently proposed that the phenotypic variability of premutation carriers could be explained by genetic background effects, including second “genetic hits” and environmental exposures including substance or medication abuse (9,24).
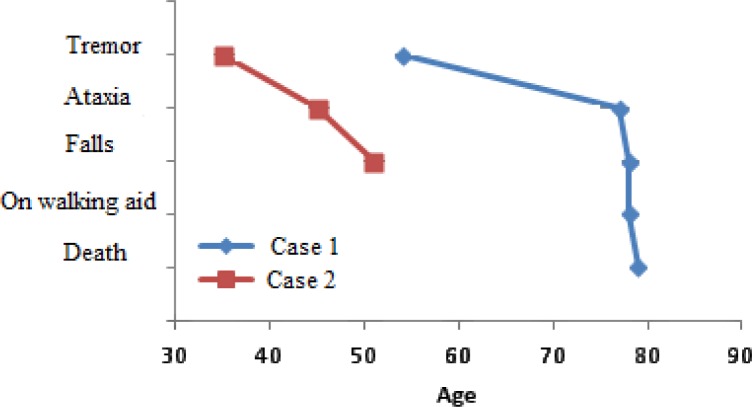
In a study of premutation carriers with FXTAS, median onset of tremor was 60 years of age (25). Median delay of onset, from the onset of initial motor deficits, for ataxia, falls, dependence upon a walking aid, and death was 2, 6, 15, and 21 years, respectively (25). The life expectancy is within the range of 5 to 25 years after the onset of symptoms (25). In case 1, the age of onset of pain symptoms was age 54. Tremor, ataxia and falls started at age 77. Dependency on a walker occurred at age 78, with death at age 79. In Case 2, the age of onset of pain symptoms was 35 years. Onset of tremor was age 45. Onset of ataxia and falls was age 51 (Figure 3).
Figure 3.
Median onset of pain symptoms, tremor, ataxia, falls, dependence on walking aid and death in two patients with FXTAS and addictive substance abuse.
A long history of codeine and hydrocodone use in Case 1 that was intensified at age 75 may be related to a more rapid decline in her late 70s. Her early course was prolonged but this is typical for a female with the premutation and FXTAS. The very early onset of neurological symptoms in Case 2 could have been exacerbated by the long history of alcohol, cocaine, marijuana, and hydrocodone use. Premutation carriers may be more vulnerable to the toxicity of substances of abuse because the premutation neuron dies more easily in culture compared to control neurons (26).
Addiction to opiates was associated with decreases in white matter integrity in a diffusion tensor imaging (DTI) study, suggesting widespread axonal injury in the brain (27). Another DTI study reported that males with alcohol, cocaine and amphetamine abuse have reduced white matter integrity in the corpus callosum (28). Furthermore, reduced white matter integrity in the corpus callosum is an important marker of gait instability in the elderly (29), and corpus callosum splenium (CCS) hyperintensity on T2 MRI is a marker of the severity of disease progression in FXTAS (30). The above-mentioned MRI findings are in accordance with MRI findings and clinical progressions in our patients.
In our clinical experience, substance abuse by patients with FXTAS occurs either to treat pain symptoms or as a self-treatment of depression and anxiety. Since opiates are associated with white matter disease, in general, we recommend that opiates should be avoided if possible in premutation carriers with neurological symptoms or FXTAS. If opiates are absolutely necessary for pain control, their use should be closely monitored and tapered as soon as possible. Alternative treatments for pain include acupuncture, gabapentin, pregabalin, duloxetine, venlafaxine, tricyclics, omega 3s, and psychological approaches with cognitive behavioral therapy.
In conclusion, the two cases support our previous hypothesis that environmental exposures (in this case addictive substances) may have exacerbated the neurological symptoms of FXTAS, including tremor, ataxia and cognitive decline. We recommend avoiding the overuse of opiates for pain management and also early treatment of depression and anxiety in patients with the fragile X premutation, and especially with FXTAS.
Acknowledgements
This work was supported by the National Institute on Aging of the National Institutes Of Health (P30AG043097) and NIH diversity supplement for the NICHD (HD036071). We thank the patients for their participation as well as their families. We also appreciate the help of Kylee Cook and Antonia Boyd for data collection.
References
- 1. Jacquemont S, Hagerman RJ, Leehey MA, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004; 291:460-469. [DOI] [PubMed] [Google Scholar]
- 2. Li Y, Jin P. RNA-mediated neurodegeneration in fragile X-associated tremor/ataxia syndrome. Brain Res. 2012; 1462:112-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berry-Kravis E, Hall D, Leehey M, Hagerman R. Treatment and management of FXTAS. In: Tassone F, Berry-Kravis E, eds. The fragile X-associated tremor ataxia syndrome (FXTAS). New York: Springer, 2011: 137-154. [Google Scholar]
- 4. Leehey M, Hagerman P. Fragile X-associated Tremor/Ataxia Syndrome (FXTAS). Subramoney SH, Durr A, editors. Ataxic Disorders 1: Handbook of Clinical Neurology, 3rd Series. Edinburgh, Scotland: Elsevier; In press. [Google Scholar]
- 5. Leehey M, Legg W, Tassone F, Hagerman R. Fibromyalgia in fragile X mental retardation 1 gene premutation carriers. Rheumatology (Oxford). 2011; 50:2233-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Winarni TI, Chonchaiya W, Sumekar TA, Ashwood P, Morales GM, Tassone F, Nguyen DV, Faradz SM, Van de Water J, Cook K, Hamlin A, Mu Y, Hagerman PJ, Hagerman RJ. Immune-mediated disorders among women carriers of fragile X premutation alleles. Am J Med Genet A. 2012; 158A:2473-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coffey SM, Cook K, Tartaglia N, Tassone F, Nguyen DV, Pan R, Bronsky HE, Yuhas J, Borodyanskaya M, Grigsby J, Doerflinger M, Hagerman PJ, Hagerman RJ. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A. 2008;146A:1009-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Au J, Akins RS, Berkowitz-Sutherland L, Tang HT, Chen Y, Boyd A, Tassone F, Nguyen DV, Hagerman R. Prevalence and risk of migraine headaches in adult fragile X premutation carriers. Clin Genet. 2013;84:546-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hagerman R, Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013; 12:786-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kogan CS, Turk J, Hagerman RJ, Cornish KM. Impact of the Fragile X mental retardation 1 (FMR1) gene premutation on neuropsychiatric functioning in adult males without fragile X-associated Tremor/Ataxia syndrome: A controlled study. Am J Med Genet B Neuropsychiatr Genet. 2008; 147B:859-872. [DOI] [PubMed] [Google Scholar]
- 11. Kumar V, Abbas AK, Fausto N, ed. Robbins and Cotrans. Pathological basis of disease (7th ed.). Elsevier/Saunders; 2005; 911-2 ISBN 0-7216-0187-1 [Google Scholar]
- 12. Fletcher LM, Powell LW. Hemochromatosis and alcoholic liver disease. Alcohol (Fayetteville, NY). 2003; 30:131-136. [DOI] [PubMed] [Google Scholar]
- 13. Farzin F, Perry H, Hessl D, Loesch D, Cohen J, Bacalman S, Gane L, Tassone F, Hagerman P, Hagerman R. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006; 27:137-144. [DOI] [PubMed] [Google Scholar]
- 14. Chonchaiya W, Au J, Schneider A, Hessl D, Harris SW, Laird M, Mu Y, Tassone F, Nguyen DV, Hagerman RJ. Increased prevalence of seizures in boys who were probands with the FMR1 premutation and co-morbid autism spectrum disorder. Hum Genet. 2012; 131:581-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tassone F, Hagerman RJ, Taylor AK, Mills JB, Harris SW, Gane LW, Hagerman PJ. Clinical involvement and protein expression in individuals with the FMR1 premutation. Am J Med Genet. 2000; 91:144-152. [DOI] [PubMed] [Google Scholar]
- 16. Aziz M, Stathopulu E, Callias M, Taylor C, Turk J, Oostra B, Willemsen R, Patton M. Clinical features of boys with fragile X premutations and intermediate alleles. Am J Med Genet B Neuropsychiatr Genet. 2003; 121B:119-127. [DOI] [PubMed] [Google Scholar]
- 17. Hessl D, Wang JM, Schneider A, Koldewyn K, Le L, Iwahashi C, Cheung K, Tassone F, Hagerman PJ, Rivera SM. Decreased fragile X mental retardation protein expression underlies amygdala dysfunction in carriers of the fragile X premutation. Biol Psychiatry. 2011; 70:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seritan A, Ortigas M, Seritan S, A. Bourgeois J, J. Hagerman R. Psychiatric disorders associated with FXTAS. Curr Psychiatr Rev. 2013; 9:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bacalman S, Farzin F, Bourgeois JA, Cogswell J, Goodlin-Jones BL, Gane LW, Grigsby J, Leehey MA, Tassone F, Hagerman RJ. Psychiatric phenotype of the fragile X-associated tremor/ataxia syndrome (FXTAS) in males: Newly described fronto-subcortical dementia. J Clin Psychiatry. 2006; 67:87-94. [DOI] [PubMed] [Google Scholar]
- 20. Tassone F, Greco CM, Hunsaker MR, Seritan AL, Berman RF, Gane LW, Jacquemont S, Basuta K, Jin LW, Hagerman PJ, Hagerman RJ. Neuropathological, clinical and molecular pathology in female fragile X premutation carriers with and without FXTAS. Genes Brain Behav. 2012; 11:577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang L, Coffey S, Lua LL, Greco CM, Schafer JA, Brunberg J, Borodyanskaya M, Agius MA, Apperson M, Leehey M, Tartaglia N, Tassone F, Hagerman PJ, Hagerman RJ. FMR1 premutation in females diagnosed with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009; 80:812-814. [DOI] [PubMed] [Google Scholar]
- 22. Greco CM, Tassone F, Garcia-Arocena D, Tartaglia N, Coffey SM, Vartanian TK, Brunberg JA, Hagerman PJ, Hagerman RJ. Clinical and neuropathologic findings in a woman with the FMR1 premutation and multiple sclerosis. Arch Neurol. 2008; 65:1114-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, Xunclà M, Badenas C, Kulisevsky J, Gomez B, Milà M. Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur J Hum Genet. 2009; 17:1359-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lozano R, Hagerman RJ, Duyzend M, Budimirovic DB, Eichler EE, Tassone F. Genomic studies in fragile X premutation carriers. J Neurodev Disord. 2014; 6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leehey MA, Berry-Kravis E, Min SJ, et al. Progression of tremor and ataxia in male carriers of the FMR1 premutation. Mov Disord. 2007; 22:203-206. [DOI] [PubMed] [Google Scholar]
- 26. Chen Y, Tassone F, Berman RF, Hagerman PJ, Hagerman RJ, Willemsen R, Pessah IN. Murine hippocampal neurons expressing FMR1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet. 2010; 19:196-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bora E, Yucel M, Fornito A, Pantelis C, Harrison BJ, Cocchi L, Pell G, Lubman DI. White matter microstructure in opiate addiction. Addict Biol. 2012; 17:141-148. [DOI] [PubMed] [Google Scholar]
- 28. Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT. Corpus callosum damage in heavy marijuana use: Preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuromage. 2008; 41:1067-1074. [DOI] [PubMed] [Google Scholar]
- 29. Bhadelia RA, Price LL, Tedesco KL, Scott T, Qiu WQ, Patz S, Folstein M, Rosenberg I, Caplan LR, Bergethon P. Diffusion tensor imaging, white matter lesions, the corpus callosum, and gait in the elderly. Stroke. 2009; 40:3816-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Apartis E, Blancher A, Meissner WG, et al. FXTAS: New insights and the need for revised diagnostic criteria. Neurology. 2012; 79:1898-1907. [DOI] [PubMed] [Google Scholar]