Summary
The use of touchscreen applications for the iPad® allows children with disabilities to improve their personal autonomy and quality of life. In light of this emerging literature and our clinical experience with toddlers and children with Fragile X syndrome (FXS), a randomized clinical trial pilot study was conducted of whether an interactive iPad®-based parent training program was efficacious for both individuals with FXS and autism spectrum disorder aged 2-to-12 compared to wait-listed controls. As a second goal, we assessed the difference between direct person-to-person therapy vs. online therapy sessions through telehealth. In this case series report it is presented preliminary results of four individuals with FXS enrolled in the study and described the innovative experience including qualitative and quantitative data analysis. Furthermore, we provide professionals with specific guidelines about the use of touchscreen devices as in-home learning tools and parent training strategies to actively involve families in educational treatments in conjunction with clinical guidance.
Keywords: Touchscreen devices, educational applications, parent training, innovative therapy
1. Introduction
Technology provides an invaluable support for enhancing adaptive skills and learning for individuals with neurodevelopmental disabilities (1). Digital technology can improve communication, support social interaction, enhance learning tasks and personal independence and increase leisure time in daily life (2). However, there is only very little research on the impact of innovative technology, communication devices, touch-screen tablets, and educational applications (3).
In the last ten years, the National Center for Technology Innovation (NCTI) has been following changes in educational and assistive technology (AT), which has shifted into a more portable, networked, customizable, and multitasking approach converging in touch interface devices which, additionally, are widely used by the general population (4). Touch screen devices, such as Apple iPad® emerged in 2010, while not specifically designed for education or developmental interventions, have already proven to be suitable for therapeutic and educational benefits in disorders such as autism (5) and schizophrenia (6). Despite the increased technological research interest in the field of neurodevelopmental disorders and the current application in the clinical practice for education, and promoting communication, there is no research which involves the use of touch-screen devices in children with Fragile X syndrome (FXS). Advances in our understanding of the neurobiological basis of FXS have led to new targeted treatments for the disease (7). However, very little progress has been made regarding educational interventions mainly consisting of speech-language therapy (SLT), occupational therapy (OT) and behavior management therapy (ABA) (8). Many of our families have incidentally found that a variety of learning applications for tablets can be helpful for their children, but there have been no controlled trials or standardized guidelines for their use in FXS so far.
FXS is the most common inherited cause of intellectual disability with a prevalence of 1 in 5,000 males (9) and 1 in 8,000 females (10). It is the most common genetic disorder associated with ASD (11–13). Individuals with FXS show a specific behavioral phenotype of co-occurring conditions including hyperactivity, short attention span, anxiety, social avoidance, difficulty maintaining eye contact, difficulties in sensory processing, lack of reciprocity in relationships, stereotyped behaviors and seizures (14). FXS is caused by expansion of a trinucleotide repeat in the FMR1 gene. The production of FMRP, the FMR1 gene product, is significantly diminished or absent in FXS because of methylation of the CpG island at the 5’ end of FMR1, thus silencing the gene (15). Studies show that approximately 2 to 5% of people with an ASD carry the fragile X mutation, and 60% of those with FXS have ASD (16,17). In general those with FXS plus autism have more anxiety, but more sociability than those with idiopathic autism (18).
The combination of intensive educational support and psychopharmacological interventions has remarkable effects on behavior and cognition in children with FXS (19). The main purpose of any behavioral interventions in FXS is not only to reduce or eliminate the unwanted behavior, but also to teach children socially appropriate behavior to enhance cognitive and social skills that can be generalized to other settings outside the therapeutic or academic environment (8). We believe strongly that technology is increasingly contributing to this generalization in our patient population.
The goal of the present study is to provide information to educate, facilitate, and document the power of touchscreen technology for individuals with FXS, and to describe the best practices in the use of the iPad® for promoting learning and interaction in family settings. This research will provide insights for future professionals (teachers, clinicians, application developers, therapists, researchers, etc.) and families hoping to meaningfully use computer tablets to help children with neurodevelopmental disorders. Devices like the iPad®, have an abundance of available educational and recreational applications (20) that easily support the Universal Design for Learning(UDL) framework for making a curriculum more inclusive for individuals with special needs. Therefore, specialized digital therapies are essential for addressing developmental challenges in those with FXS and for other neurodevelopmental disorders, although there is little research regarding their efficacy. In addition, interventions that showed efficacy for ASD such as Pivotal Response Treatment (PRT), Applied Behavior Analysis (ABA), Early Start Denver Model (ESDM), etc. have not been specifically studied in individuals with FXS.
On the other hand, caring for a child with complex disabilities such as FXS may have negative impact on both the physical and the mental health of the parents and caregivers (21). Parental stress could be child-driven (22); however, interventions that improve the child's functioning and communication may be expected to decrease the parents' stress level. Therefore, the impact of parent-delivered intervention based on an iPad® intervention could go beyond the educational goals and reduce parental stress through an unknown mechanism. However, to our knowledge, no outcome studies have focused on intervention programs for children with FXS that combine parent-delivered one-on-one behavioral iPad®-based intervention along with learning apps. In this sense, support to parents can also be provided remotely by a telehealth approach, a mechanism that enables individuals to receive professional guidance and effective recommendations from a distance (23) and may involve several multimedia platforms from real time video streaming to interactive website and tablet applications that can be effortlessly accessed at any time and location and shared across settings and individuals (24). Current studies implementing telehealth have already demonstrated encouraging results in training professionals and family members in ABA behavior management procedures (25), and successful outcomes in training parents of children with ASD in specific intervention models such as the ESDM (26).
The current series report presents 4 cases, belonging to a larger randomized controlled trial (RCT) cross-over design (n = 18), to describe the challenges and benefits of an innovative in-home iPad®-centered parent-delivered intervention on social interaction skills, language development, and academic gains (early concepts and literacy) in children with FXS. The report also describes qualitative differences between those patients seen at the MIND Institute for weekly outpatient therapy sessions vs an online follow-up modality (telehealth). The underlying processes such as motivation, engagement with technology, parent-child interaction, and parent satisfaction will be also reviewed. This is an effort to provide initial information and data to formulate a systematic guideline of what we believe is an innovative promising intervention for children with FXS and their families.
2. Methods
2.1. Participants
The 4 cases with a fragile X full mutation were selected from alarger randomized clinical trial study (crossover RCT) (n = 18), MIND APPs, for an iPad®-based therapeutic parent training for enhancing language development, social interaction skills, and learning in children with FXS and ASD. Of the 4 cases 2 individuals are female and 2 are male, the mean age is 6.2 years old (SD 3.05 years) with a mean IQ of 73.5 (SD 18.8). The current aforementioned RCT (Díez-Juan et al. in preparation) was conducted at the Fragile X Research and Treatment Center at the University of California Davis MIND Institute and it was monitored for safety and ethics by the UC Davis Institutional Review Board (IRB). All participants and their caregivers have signed informed consent and were informed about the characteristics of the study with the option of conclude their participation at any point before the end of the study. The subjects had existing molecular results about their genetic status and were administered a series of behavior and cognitive assessments. The four participants' characteristics are shown in Table 1 with information about their genotypic and phenotypic profile and MIND APPs study characteristics. Families were eligible to participate if: i) Child was between the ages of 2.0 and 12.0 at the time of enrollment, ii) Child had a molecular diagnosis of FXS (with or without ASD) or an ASD diagnosis by a clinical team with the results of the Autism Diagnostic Observation Schedule (ADOS) (27), iii) Child had a reliable parent or caregiver able to perform a guided iPad® in-home interactive intervention for 32 weeks, iv) No serious co-morbid medical condition affecting brain function and/or behavior was present, including uncontrolled seizures, and v) Child was not participating in a pharmacological trial simultaneously, although subject could be under stable medication treatment and any kind of behavioral intervention or school condition. Other community care such as behavioral interventions, education program and other therapies are included in Table 1.
Table 1. Genotypic and Phenotypic features for each patient.
| Cases | Category | CGG repeats | % Meth. | AR | FMR1 mRNA levels | Std Error | Ethnicity | IQ/Level Language | ADOS CSS | VABS II Total COMP | Dysmorphic features | Medications | Behavioral interventions | Treat. Modality | iPad® Total Hours/16w |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1♂ (2.9 y) | Full mutation | 6,40 1,160 (light UM smear)* | > 98% | - | 0.14 | 0.01 | Latino/Hispanic | 55/Non verbal | 4 | 75 | Prominent and large ears | Sertraline Minocycline Melatonin |
- ESDM** - Speech Therapy - ABA*** - Preschool aid |
Online | 24.90 |
| Case 2♀ (5 y) | Full mutation | 33, 200, 595, 711, 848 | - | 0.24 | 0.24 | 0.01 | White | 97/Verbal | 4 | 72 | Prominent yaw and forehead | Sertraline Minocycline Melatonin |
-Speech Therapy - OT**** - School support |
Online | 53.63 |
| Case 3♂ (6.9 y) | Meth. mosaic | 360 (light UM smear)* | > 95% | - | 1.32 | 0.08 | White | 62/Verbal | 2 | 72 | Prominent ears and epicanthal folds | Sertraline Minocycline |
-OT - Speech Therapy - School aid |
Online | 65.15 |
| Case 4♀ (10.1 y) | Full mutation | 29, 113, 303, 373, 476, 642 | - | 0.83 | 0.68 | 0.06 | White | 80/Verbal | 6 | 70 | Hydantoin Syndrome features | Vitamins Allergy pills |
- IEP***** at school | Local | 50.92 |
UM= Unmethylated from normal/pre to full mutation,
ESDM = Early Start Denver Model,
ABA = Applied Behavior Analysis,
OT = Occupational Therapy,
IEP = Individualized Education Plan.
Participants were randomly assigned to the active treatment or wait-list group after baseline assessments. The four cases presented in the current report were all assigned to the first active treatment group receiving the training one time per week in 1-hour sessions during 16 weeks. All four families were instructed to continue with their child's usual treatments and to report every in-home iPad® session through Care Circles® application tracking system.
2.2. Genotypic measures
Genomic DNA was isolated from peripheral blood leukocytes by standard methods (Qiagen, Valencia, CA). CGG size and methylation status were determined using Southern Blot and PCR analyses as previously described in Tassone et al. (28,29). Total RNA was isolated using Tempus tubes (Applied Biosystems, Foster City, CA). cDNA synthesis and qRT-PCR used to quantify FMR1 mRNA levels were performed as described in Tassone et al. (30)
2.3. Phenotypic measures
2.3.1. Baseline measures
The cognitive baseline assessments described in the current report, depending on age, included standardized IQ tests such as Stanford-Binet Intelligence Scales, 5th edition (SB-5) (31); non-verbal IQ and verbal IQ are assessed and combined to a full-scale IQ score (M = 100; SD = 15); or the Mullen Scales of Early Learning (32) developmentally integrated scales for toddlers (M = 50; SD = 10). To quantify the severity symptoms of autism also in the fragile X population, we used the Autism Diagnostic Observation Scale, ADOS (27), which has been broadly used in other studies of FXS (16,33). ADOS autism calibrated severity score (CSS) was determined using the procedures described by Gotham et al. (34) in which a higher severity score indicates more severe autism features (ADOS classification: 1–3 no symptoms; 4–5 ASD symptoms; 6–10 Autism symptoms). Finally, the Vineland Adaptive Behavior Scales, 2nd edition (VABS-II) (35) were used to determine adaptive functioning, like daily life routines, and to identify strengths and weaknesses (M = 100, SD = 15).
2.3.2. Outcome Measures
The outcome measures consisted of a battery of standardized assessments administered at three time points across the duration of the study (baseline, follow up 1 after 16 weeks, and follow up 2 after 32 weeks). The measures included the Expressive Vocabulary Test, Second Edition (EVT2) (36), measuring expressive vocabulary and word retrieval (M = 100, SD = 15), Preschool Language Scales, Fifth Edition (PLS-5) (37), an interactive assessment of developmental language skills based on auditory comprehension and expressive communication standard scores (M = 100, SD = 15), and the Process Assessment of the Learner, Second Edition (PAL-II) (38), measuring a variety of reading and writing processes for children in Kindergarten to Grade 6 (K-6). PAL-II subtests and composite scaled scores are derived from normative data and have a mean of 10 and a standard deviation of 3.
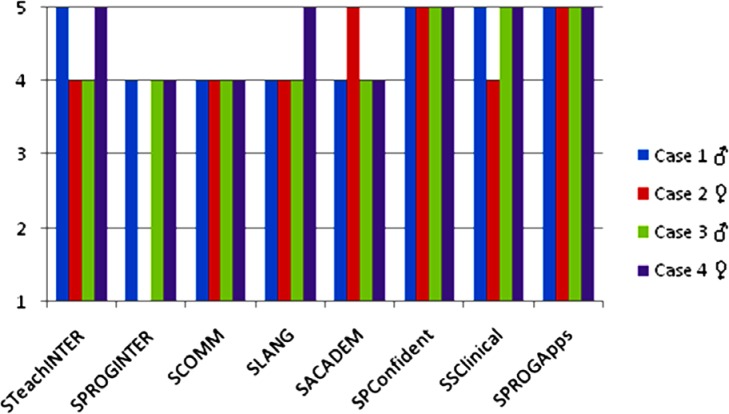
A Likert-scaled Parent Satisfaction Survey (scores 1–5) specifically designed for the purposes of the study was used to measure the level of parent satisfaction towards particular components of the iPad®-based training program at the end of the intervention. Higher scores mean better satisfaction levels referring to eight particular treatment domains: i) STeachIntervention, the study helps you to better teach your child using the iPad® for interaction with you, ii) SProgInter, level of progress in shared interactions you observed, iii) SComm, level of progress in communication, iv) SLang, progress in language (expressive and receptive), v) SAcadem, progress in academic learning, vi) SPConfident, parent's confidence about helping the child with the iPad® for educational purpose and interaction, vii) SSClinical, satisfaction with the clinical guidance, and viii) SProgApps, satisfaction with the program of educational applications provided. Figure 1 summarizes the parent's satisfaction scores for each scale after the active treatment.
Figure 1.
Parent Satisfaction Survey (Likert Scale 1–5). STeachIntervention = the study helps you to better teach your child using the iPad® for interaction with you; SProgInter = level of progress in shared interactions you observed; SComm = level of progress in communication; Slang = progress in language (expressive and receptive); SAcadem = progress in academic learning; SPConfident = parent's confidence about helping the child with the iPad® for educational purpose and interaction; SSClinical = satisfaction with the clinical guidance; SProgApps = satisfaction with the program of educational applications provided.
2.4. Procedures and timeline
The intervention program for the individuals in the present case series consisted of 2 periods of an iPad®-based intervention program. The first period is guided intervention with a therapist and the second is a maintenance period without the therapist. The initial active period consisted of a 16-week long, low-intensity (1-hour/week of therapist/clinical guidance and parent-delivered intervention (3-hour/week in-home sessions) with an estimated average of 64 hours of iPad® intervention during the 16 weeks. During the maintenance period no clinical guidance was provided and only the parent-delivered 3-hour/week of in-home intervention was administered by following the guidelines learned in the previous period.
The clinical guidance and supervision across the 16 weeks was provided on-line or on-site according to participants' preferences and consisted of:
General iPad® management orientation to parents and child (depending on age): common terms of use, multitasking gestures, accessibility, guided access, Apple Store® operation, code redeeming, applications downloading, updating and deleting (Week 1–4).
Care Circles platform application installation and creation of family profile to track in-home iPad® sessions and initiate daily parents-professional interactive journal (Week 1–4).
Weekly review and explanation of educational applications and parent-child customized guidance for interactive, communicational and learning purposes (Week 1–16).
Establishment of individual family goals regarding communication, social interaction, learning and behavior through iPad® use (Guidance and supervision of goals from week 1–16).
Share progress and handle behavior or learning difficulties during iPad® sessions at home (Week 1–16).
Closure and review of training principles and applications, thus parents could continue intervention during maintenance period afterwards (Week 14–16).
2.5. iPad®-based training program
The iPad® pilot study's primary aim was to evaluate the effects of a comprehensive educative program in which parents are receiving individual coaching about the use of the iPad® as a learning device and as an interactive therapeutic tool for their children.
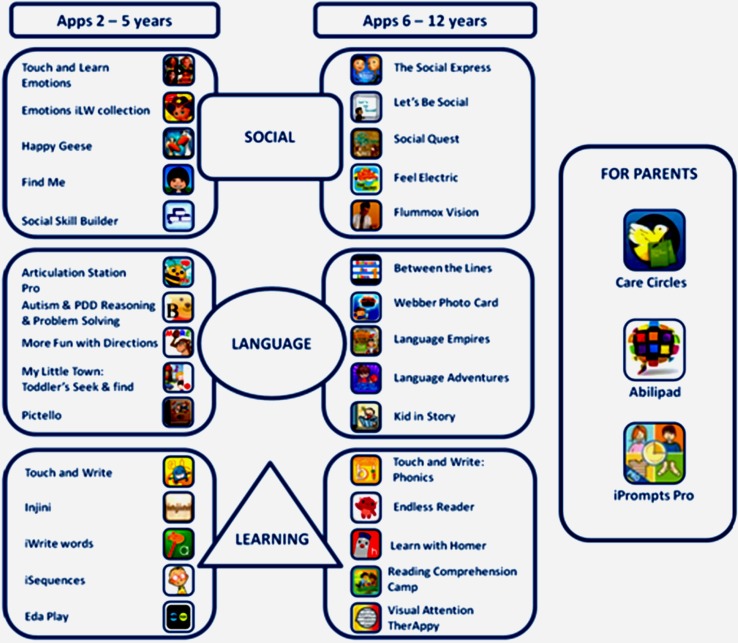
The iPad®-program was based on: i) A comprehensive selection of Apple store educational applications, previously reviewed and analyzed by experts in the field, which were distributed according to three developmental domains (language, social interaction and academic learning); ii) Individually-tailored treatment objectives to the child's individual learning profile, dominant interests and family educational values; iii) A set of psychoeducational strategies substantiated on the principles of broad spectrum applied behavior analysis (ABA), cognitive techniques about theory of mind and emotions management programs, naturalistic learning through interpersonal interaction, and meaningful teaching approaches. Figure 2 includes the main applications that were used during the intervention periods according to developmental stages and skill domains.
Figure 2.
MIND APPs applications program.
2.6. Adherence to iPad® intervention
Care Circles® application from the Apple Store® was implemented as a digital platform to follow on adherence to intervention and to measure the time of in-home applied intervention, level of motivation, attention and frustration during the parent-child interaction at home. An interactive journal was used for the family to professional everyday communication.
3. Results
3.1. Case 1 (FXS, boy 2. 9 y)
3.1.1. Personal background
Case 1 is an almost 3-year-old boy diagnosed with FXS shortly after birth through cord blood due to positive family history (mother, maternal aunt and maternal grand-father with the premutation). He did not present with hypotonia, had fairly good eye contact, and a high energy level, which eventually led to meltdowns involving throwing himself to the floor to demonstrate frustration. He also presented with attentional problems and perseverative behaviors, such as spinning and flipping through book pages impulsively. He did not have staring spells or seizures, although he showed shivering episodes once or twice a day. He had prominent ears with ear pinnae cupping bilaterally, epicanthal folds and flat feet with mild degree of pronation. He showed psychomotor delay (Baseline MSEL- Early Learning Composite 55) started walking after 15 months, and his overall Adaptive Behavior Composite was 75 on the Vineland (VABS-II). He presented with a number of autistic behaviors including lack of joint attention and language difficulties. At the time of the study he also met criteria for developmental speech and language disorder (no words at the age of 35 months) in addition to the ASD features (ADOS CSS 4).
3.1.2. MIND APPs study involvement, outcomes and challenges
At the in-take interview for the iPad® study he had developed a sleep disturbance where he would awaken two to three times a night. Melatonin was used to treat these symptoms together with applied behavior analysis techniques (ABA). Parents were applying Early Start Denver Model (ESDM) (39) principles at home for developmental purposes learned through a telehealth study about early intervention in children with FXS (40), and he was receiving speech therapy at preschool where he also had special support personnel. He was on a stable treatment with sertraline and minocycline before and throughout the study.
Parents recently purchased an iPad®, and they had limited experience with it before the study. The patient was completely unable to actively use the iPad®, and the parents mainly used the touch-screen device for playing games and entertainment. Family had not used educational apps before the study, nor had they received any training in using iPad® for promoting parent-child interaction and learning. The child and parents were highly motivated to participate in the study. Case 1 was trained online through telehealth. The family successfully completed the 16-week training, and after-treatment assessment, nevertheless it was impossible to obtain the last follow up due to travelling distance to the MIND Institute, and family issues at that time point. Right after the active treatment period, parents felt better prepared to use the iPad® as an educational tool and observed specific areas of improvement such as: increased vocabulary, improved language (expressive and receptive) and also more precise fine motor skills. The patient improved his abilities to sort objects, trace lines and solve puzzles. He also enjoyed and learned letters, basic counting, shapes and colors. Parents reported that “clinical guidance was of key importance” in the sense of receiving individualized professional guidelines when introducing a new app, and handling behavioral challenges during the interaction as reflected in the satisfaction survey.
Numerous behavioral challenges needed to be addressed during the iPad® training for Case 1. He presented with low flexibility and concurrent repetitive behaviors toward the device and it was not his preference to share during the activities with his caregivers. Establishing the iPad® time routine together was an elaborate process; although once it was part of his schedule he accepted it and looked forward to it with appropriate requesting of the tasks from the parents. Apps of his interest were related to matching numbers, letters, shapes, colorful images, music, and later on tracing letters and numbers. Caregivers felt highly discouraged during the first 4 weeks of the training given that Case 1 did not want to collaborate and share the iPad® time together. Clinical guidance was critical in order to support parents and enhance their skills and confidence as essential providers and supporters of their child's progress.
Figure 3 demonstrates an example for a high quality parent-child iPad® interactive situation in which joint attention, positive social reinforcement and shared learning experience can be observed.
Figure 3.
Case 1♂- Parent-child interactive iPad® time.
3.2. Case 2 (FXS, girl 5 y)
3.2.1. Personal background
Case 2 is a 5-year-old girl with the full mutation who has participated in targeted treatments since early in life, including sertraline and minocycline trials. She showed significant improvement with minocycline and sertraline and a good response to early interventions, such as occupational therapy, physical therapy and language intervention in an enriched in-home environment since the mother has an educational background in special needs. She showed adequate developmental milestones, walking at 13 months, saying first words at 17 months and simple sentences after 20 months with a mild delay in later language development. She had a very short attention span, and it was difficult for her to sit through a whole story and follow verbal prompts. She had a sleep disturbance, picky eating and anxious behavior towards new situations and animals. On early examinations, she had a mildly prominent forehead, epicanthal folds, hyperextensiblefinger joints and overall normal motor tone. She was hyperactive and inattentive throughout the study and she met criteria for ADHD. She was taking sertraline, minocycline, folic acid and melatonin during her participation in the iPad® clinical trial. At school she is receiving speech and occupational therapy (OT), but no assistive technology was implemented in her individualized education plan (IEP).
3.2.2. MIND APPs study involvement, outcomes and challenges
Baseline assessments confirmed intellectual ability in the normal range (IQ 97), no ASD diagnosis (ADOS CSS 4), and low adaptive skills (VABS II 72). She had been using the iPad® since she was eighteen months old when the first device emerged on the market. She used it mainly by herself for watching cartoons, playing fun educational games, and reading stories. She knew how to handle the device and she could even create folders in the screen herself, which is an advanced skill. Parents played together with her about 3 to 4 times a week and they knew a great variety of educational apps, nevertheless, they never received a parent-based training in the use of technology for interaction targeting language, literacy and social development. Parents were motivated to complete the 16-week study, and they reported all their iPad® sessions through Care Circles® app and completed all the follow-up assessments. The patient went through several reactive behaviors when the caregiver was trying to share the screen together and it took some weeks for her to get used to the new “we-work-iPad® -together” routine. Once the digital task was part of her daily schedule, she became smoothly involved in the interactive dynamic with parents and even started to ask them to play together. Parents reported moderate satisfaction to the psychoeducational program since they also expressed concern about the new routine being time consuming and overwhelming at particular points. Tracking sessions and reporting data was not always convenient for them and they wish it could have been addressed through a more practical modality. Otherwise they identified progress in their child mainly related to an enhancement of speech fluency, fine motor skills and tracing letters ability.
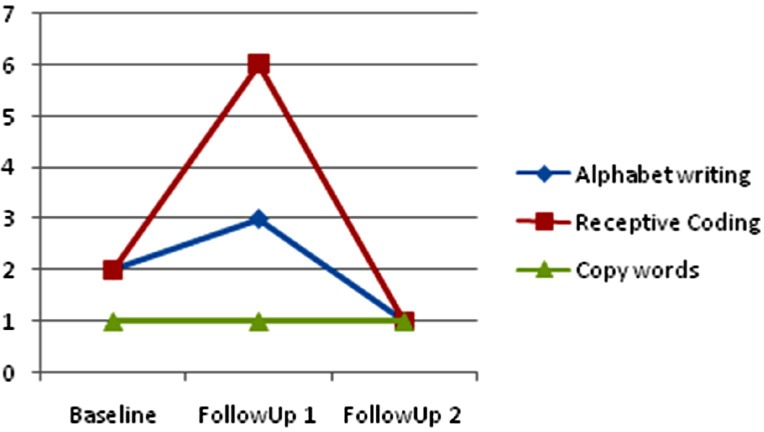
Case 2 presented an impressive advance in tracing and basic writing skills, being able to actually trace alphabet letter and copy short full words both on screen and on paper at the end of the second follow-up after the maintenance period. Her main progress turned up clearly after 32 weeks of low intensity iPad®-based intervention. See Figure 4 regarding the progress at baseline, follow-up 1 and follow-up 2 assessments through PAL-II, Alphabet Writing task in which the child is asked to print the alphabet in lowercase in alphabetic order as quickly and accurately as possible. Case 2 was only 5 years old at the beginning of the study so her scores fell out of PAL-II normative data even at the last follow-up assessment (5y 8m). Although her writing is performed in uppercase, it is possible to observe the improvement of her copy skills regarding legibility and accuracy. The main applications implemented to practice literacy abilities are listed in Figure 2 (Learning apps 2–5 years).
Figure 4.
Case 2 ♀- PAL-II Alphabet Writing Task (Baseline, Follow-up 1 & Follow-up 2).
3.3. Case 3 (FXS, boy 6 y)
3.3.1. Personal background
Case 3 is a 6 years and 10 months old boy with FXS and high levels of anxiety in crowded situations. He also presents global developmental delay (IQ 62), is highly inattentive and hyperactive especially in academic settings or in a larger group, and his adaptive skills are below average (VABS-II 72). He receives OT and SLT at school and has a special aid in class. Just before enrolling in the study he stopped ABA therapy. He was on minocycline and sertraline before and throughout the study. Family owned a device for less than a year before the study enrollment and usually used it as a reward tool after successfully completing homework or chores. Usually he used it to watch cartoons or play games. No educational applications were used before the trial and he used to play by himself. His mother was highly motivated to conduct the treatment at home and they properly completed the whole sequence of clinical sessions and follow-ups.
Case 3 presented with a history of motor and speech delay; he walked at 19 months and said first words at15 months and combined words around 30 months. On previous exam he had a slightly long and narrow face, mildly prominent ears, hyperextensible finger joints and flat feet bilaterally. Behaviorally he did not meet an autism diagnosis (ADOS CSS 2) but he had intermittent poor eye contact and hand flapping. Under pressure he can show some self-injurious behaviors, like biting, and has calluses on his right hand, he rocks his whole body at times and sucks his thumb when stressed. Eventually he can get aggressive and may kick or push others.
3.3.2. MIND APPs study involvement, outcomes and challenges
During the study he demonstrated progress in language fluency, being able to narrate tales, and also creating social stories about his daily life activities and social events. The family demonstrated progress in parent-child interactions, and implementing the iPad®-time rules, and the parents saw moderate improvement in tracing letters and spelling short words. They commented that clinical guidance was “extremely valuable during the study as they were able to use the acquired skills in everyday activities outside of the iPad® too”.
Primary caregiver in the training was the mother, a premutation carrier who was also implementing the iPad® program with her daughter, a full mutation 5-year-old girl, in the second active treatment period. Because the burden of active intervention maintained over 8 months, the mother experienced high levels of stress, expressing the push to complete the study as it involved a great family and educational effort. However, parents reported the program contained extremely suitable applications and that clinical guidelines were useful and even went beyond the interactive iPad®-time itself. Figure 1 shows the results in the Parent's Satisfaction Survey (Likert Scale 0–5), in which higher scores relate to higher levels of satisfaction. Case 3 showed the highest scores (5) in Parent's Self-Confidence, Satisfaction to Clinical Guidance and Satisfaction to Program of Applications.
3.4. Case 4 (FXS, girl 10 y)
3.4.1. Personal background
Case 4 is an almost 11-year-old girl who has the full mutation of FXS that was diagnosed in utero. Family pedigree reveals her great-grandfather died from fragile X-associated tremor ataxia syndrome (FXTAS). Her mother has FXS with normal intellectual abilities, but she took phenytoin during pregnancy due to a seizure disorder. Thus Case 4 is not only affected by FXS, but shows additional features of fetal hydantoin syndrome, as a second hit, identified by mild bowing of the upper lip in addition to the broad and low nasal bridge. Her early development included sitting at 8 months, crawling at 1 year, walking at 18 months, and delays in receptive and expressive language. Her behavior included hand flapping, finger biting and inconsistent eye contact. She had appropriate join attention and good social skills, although she had severe shyness, social anxiety and learning difficulties. She also underwent developmental testing in childhood with adaptive behavior problems and mild motor delay. She met criteria for selective mutism, anxiety disorder and borderline intellectual functioning prior to the beginning of our study.
3.4.2. MIND APPs study involvement, outcomes and challenges
When she joined the RCT she was 10 years 6 months old and parents already owned an iPad®, which was used mainly for entertainment. At that time she was receiving neither psychosocial nor medical treatment, but had an Individualized Educational Plan (IEP) at school (5th grade) where she used the computer for learning purposes. She could properly manage the iPad® and parents played with her by sharing games and educational applications. They had never received an iPad®-based training program and were highly motivated to be involved in the therapy. Case 4 fully completed the three timeline assessments. During the study she was not taking any medications apart from allergy pills and inhalant for asthma symptoms. We assessed autistic behavior as part of the baseline measures and she met criteria for moderate ASD (ADOS CSS 6). She had a low average cognitive level (IQ 80) and below average adaptive skills (VABS-II 70).
Case 4 completed the 16-week iPad®-centered training together with her parents and they noted mild progress in academic learning and moderate improvement in expressive language and social comprehension. Her program followed educational apps with a particular emphasis in applications for enhancing literacy, expressive language and social skills, such as The Social Express®. She was followed locally so the family came to the MIND Institute clinic once a week and also tracked the online data through the Care Circles® platform application.
Her mother needed to stop being the primary therapist in the pilot study due to overwhelming feelings of anxiety and a high level of stress. Case 4's father and grandmother needed to step in for the iPad®-based sessions at home one month before the end of the active period.
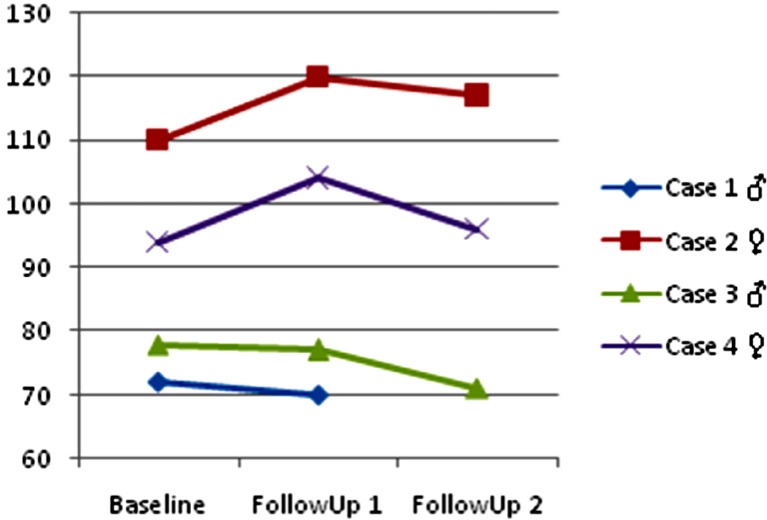
Figure 5 shows Case 4's PAL-II Reading and Writing Profile in which literacy tasks progress can be seen across the study timeline. The Receptive Coding task consisted of identifying single letters from a word. The patient presents maximum improvement in this task, and dramatically decreases the scores during the maintenance period. Alphabet Writing, in which the child is asked to print the alphabet in lowercase as quickly and accurately as possible presents a mild improvement, nevertheless a loss can be seen in the follow-up 2. Finally, Copying Task A, in which the child is asked to copy a sentence as quickly and accurately as possible shows a flat scoring across the 3 time points. We believe this is due to the complexity of the assignment where the low-intensity iPad®-based intervention cannot impact on complex literacy performance. Overall Figure 5 indicates that the active intervention was positively affecting learning in simple literacy tasks while during the maintenance period performance decreases, maybe caused by the lack of practice.
Figure 5.
Case 4 ♀- PAL-II Writing Profile.
4. Discussion
The search for touchscreen-based intervention procedures that are efficient, family and socially relevant and therapeutically viable is essential to the improvement of the services provided to children with FXS and their families. However, the present case series report is the first of its kind, and indicates that there is still a need for more controlled studies, with a larger number of participants, involving school setting and a multidisciplinary team, and more appropriate standardized tools to assess the outcomes of technology-based educational treatments.
The great majority of existing literature reveals that touch-screen devices can be successfully utilized within educational programs targeting academic skills, communication, employment, and recreational activities for individuals with developmental and intellectual disabilities (3). Success relies on the use of well-established instructional procedures based on the principles of ABA, early intervention models, psychosocial approaches, or other specific models integrated in the community, as well as the school and in-home setting. Therefore, ownership of a tablet alone does not guarantee parental engagement in supporting their child for using this technology for learning (41) and that the presence of these mainstream devices does not automatically lead to a meaningful implementation for therapeutic interventions.
The current case series explored an innovative psychoeducational intervention for children with FXS and their families to help them to acquire new skills regarding touchscreen technology and its use for learning purposes. By the end of the iPad®-based training parents reported having a better understanding and appreciation for assisting their child on managing the iPad® for interaction, communication and learning at home. Parents felt more confident in providing their child with educational guidelines and sharing social time together using technology as a learning tool. They also described weekly clinical interaction, both locally and on-line, as the most valuable resource for supporting their progress in the apps comprehension and behavioral strategies acquisition and administration. The telehealth modality was rated as effective as traditional one-on-one guidance sessions and parents attending the on-line training did not feel the need to see the therapist since clinical orientations followed the same structure, but based on a multimedia platform (video conference). Video conferencing with the therapist was highly important to understand how to apply the iPad®-based program in their family routine. However, the delivery of the intervention in a different format could affect the effectiveness of the treatment; so further research on a larger sample is needed out.
The iPad®, as well as other touchscreen devices, have the capacity to be used with learners of different ability levels and ages if educational applications are selected appropriately, and subjects are given equal teaching opportunity to access this type of technology for communication and/or learning purposes. As we described before, even 2-year-old toddlers with FXS are not too young or low-functioning (review Case 1) to start a comprehensive parent-delivered iPad® intervention at home. However, it is important to follow an age-appropriate structured program, based on available applications at the Apple Store, such as Injini® (Child Development Game Suite's) which provides excellent and engaging learning opportunities to young children with developmental delays. When parents are provided with behavior management techniques and a previous explanation of the app, they can perform high quality teaching sessions facilitating learning through a social and interactive parent-child exchange.
In addition to these caveats, it is difficult to quantitatively show improvement on standardized measures. Case 1 displayed a relevant improvement in his iPad® management and learning skills, such as fine motor abilities, audio-visual processing, matching, sorting and tracing but our outcome measures did not document significant gains after the 16-week active treatment period. Table 2 shows a summary of the principal outcomes of the participants in 6 clinical categories.
Table 2. Principal clinical outcomes (Parent report).
| Cases | Previous iPad® Knowledge/Interactive use | LANGUAGE GAINS | SOCIAL SKILLS ACQUISITION | ACADEMIC LEARNING PROGRESS | BEHAVIORAL OUTCOMES | PARENT SATISFACTION |
|---|---|---|---|---|---|---|
| Case 1♂ (2.9 y) | Low/Low | Vocabulary acquisition | Turn taking and waiting skills | Fine motor skills and early concepts | Proper use of the device for waiting time periods | Very Satisfied |
| Case 2♀ (5 y) | High/Medium | Language fluency | Sharing the screen and accepting others while playing | Tracing letters and words | Increase of self-regulation towards the interactive games | Moderately Satisfied |
| Case 3♂ (6.9 y) | Medium/Low | Increase of utterances in sentences | Accept losing in cooperative games (*apps) with adult and siblings | Motivation for tracing letters and initial reading stage | Acceptance of iPad® time as a reward for a particular amount of time | Very satisfied |
| Case 4♀ (10.1 y) | High/Medium | Expressive language fluency | Communication and social reciprocal skills | Tracing and written expression improvement | Use of the iPad® as a coping tool when she is upset and anxious | Very satisfied |
Applications
On the other hand, a more high-intensive intervention approach focused on a specific developmental skill may be more likely to show a significant improvement using more reliable objective data collection in an ongoing touchscreen-based therapy.
Enjoyment when using the iPad® across the 16-week period was highly regarded according to parent report, and overall, this type of technology was perceived to have the potential to promote more engagement in the learning process at home.
The interactive technology intervention was well accepted by the children and their parents. However, families also reported increased levels of stress at the end of the active treatment period. In the last weeks of the intervention training, some caregivers were exhausted by the iPad® tasks at home and they needed a reduction of the training rhythm and even a break from their educative duties. In Case 4 we described how the primary study iPad® caregiver, a mother with the full mutation and significant anxiety herself, needed to be exchanged with another family member because of the anxiety of performing the sustained interactive sessions at home in addition to other daily life routines. Clinicians must be sensitive to the parent's needs and careful about not to further increase the stress personal levels and family burden.
In general, in the presented study from the 3 hours/week of recommended usage by families, iPad® time was lowered to an average of 1.5 hours/week during a 4-month period which is minimal input for therapy purposes (See Table 1). We highly recommend longer treatment duration and intense periods facilitated by greater professional involvement and incorporation to school setting by educators so that the burden on the families remains manageable.
We believe better standardized outcomes measures need to be designed since the ones implemented in the pilot study were not sensitive enough to quantify improvements over time, for example including video analysis tools for follow-ups could improve the progress tracking throughout treatment. Additionally, the study's design included a wide spectrum of applications targeting different skill domains with a low intensity and specificity in various areas proves to be difficult to measure improvement. We believe a more targeted approach to a particular domain and more intensive iPad® intervention duration will lead to more successful intervention results. Also, newer combinations of treatments will be needed, particularly those that tie this innovative intervention with pharmacological treatments and other educational and social approaches from a multidisciplinary point of view.
Optimal efficacy on a group level was not statistically documented in the preliminary analysis; nevertheless we can qualitatively describe a better performance in the 2 girls in the present report rather than for the boys, probably due to the higher IQ and expressive language levels in girls with FXS. Figure 6 presents the 2 girls' and 2 boys' expressive language profile measured by EVT2 and PLS-EC, depending on the individuals' age, in which we can observe a clear higher trend in girls than in boys. In general, all the participants decrease scores during the maintenance period with no clinical guidance. The hyperactivity was much more severe in the boys than girls interfering with the behavior management and learning progress. A combination of ADHD medication with iPad®-based interventions should be considered in the future.
Figure 6.
Expressive Language EVT2/PLS-EC Profile for all cases.
Touchscreen tablets and educational application programs can be modified to fit particular needs and goals of each individual with neurodevelopmental disorders, particularly with FXS, and are designed to facilitate a more natural use of technology and diminish stigmatization. The emerging research and clinical experience described in these four cases offer a promising vision of the use of technology in children with FXS, particularly in a convenient in-home setting, and a deep understanding of how therapists can implement an individualized touchscreen-based program, and assist families in the best use of computer tablets for support and interaction.
Acknowledgements
This research was originated in the Fragile X Research and Treatment Center at the University of California Davis, MIND Institute. Financial support for the Principal Investigator (first author) was provided by the “Mas Casadevall- La Caixa” scholarship (Barcelona, Spain), and research activity was developed within the postdoctoral Autism Research Training Program (ARTP- MIND Institute).
The authors thank all the children and their families who greatly participated in this research, the MIND Institute professionals supporting the study and are also grateful to Claire Campbell and Mary Zagaynov for their work as outstanding research assistants.
References
- 1. Ramdoss S, Machalicek W, Rispoli M, Mulloy A, Lang R, O'Reilly M. Computer-based interventions to improve social and emotional skills in individuals with autism spectrum disorders: A systematic review. Dev Neurorehabil. 2012; 15:119-135. [DOI] [PubMed] [Google Scholar]
- 2. Kagohara DM, van der Meer L, Ramdoss S, O'Reilly MF, Lancioni GE, Davis TN, Rispoli M, Lang R, Marschik PB, Sutherland D, Green VA, Sigafoos J. Using iPods® and iPads® in teaching programs for individuals with developmental disabilities: A systematic review. Res Dev Disabil. 2013; 34:147-156. [DOI] [PubMed] [Google Scholar]
- 3. Stephenson J, Limbrick L. A review of the use of touch-screen mobile devices by people with developmental disabilities. J Autism Dev Disord. 2013; 10.1007/s10803-013-1878-8. [DOI] [PubMed] [Google Scholar]
- 4. Gray T, Silver-Pacuilla H, Brann A, Overton C, Reynolds R. Converging Trends in Educational and Assistive Technology. In: Breakthrough Teaching and Learning. (Gray T, Silver-Pacuilla H, eds.). Springer; 2011; pp. 5-24. [Google Scholar]
- 5. Kagohara DM, Sigafoos J, Achmadi D, O'Reilly M, Lancioni G. Teaching children with autism spectrum disorders to check the spelling of words. Research in Autism Spectrum Disorders, 2012. 6: pp. 304-310. [Google Scholar]
- 6. Dang J, Zhang J, Guo Z, Lu W, Cai J, Shi Z, Zhang C. A pilot study of iPad-assisted cognitive training for schizophrenia. Arch Psychiatr Nurs. 2014; 28:197-199. [DOI] [PubMed] [Google Scholar]
- 7. Hagerman RJ, Des-Portes V, Gasparini F, Jacquemont S, Gomez-Mancilla B. Translating molecular advances in fragile X syndrome into therapy: A review. J Clin Psychiatry. 2014; 75:e294-e307. [DOI] [PubMed] [Google Scholar]
- 8. Martin GE, Ausderau KK, Raspa M, Bishop E, Mallya U, Bailey DB., Jr Therapy service use among individuals with fragile X syndrome: Findings from a US parent survey. J Intellect Disabil Res. 2013; 57:837-849. [DOI] [PubMed] [Google Scholar]
- 9. Coffee B, Keith K, Albizua I, Malone T, Mowrey J, Sherman SL, Warren ST. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am J Hum Genet. 2009; 85:503-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sherman S. Epidemiology in Fragile X Syndrome: Diagnosis, Treatment and Research, 3rd edition (Hagerman RJ, Hagerman PJ, eds.). The Johns Hopkins University Press: Baltimore: 2000; pp. 136-168. [Google Scholar]
- 11. Garber KB, Visootsak J, Warren ST. Fragile X syndrome. Eur J Hum Genet. 2008; 16:666-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. García-Nonell C, Ratera ER, Harris S, Hessl D, Ono MY, Tartaglia N, Marvin E, Tassone F, Hagerman RJ. Secondary medical diagnosis in fragile X syndrome with and without autism spectrum disorder. Am J Med Genet A. 2008; 146A:1911-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, Tassone F, Hagerman PJ, Herman H, Hagerman RJ. Autism profiles of males with fragile X syndrome. Am J Ment Retard. 2008; 113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cornish K, Turk J, Hagerman R. The fragile X continuum: New advances and perspectives. J Intellect Disabil Res. 2008; 52:469-482. [DOI] [PubMed] [Google Scholar]
- 15. Sutcliffe JS, Nelson DL, Zhang F, Pieretti M, Caskey CT, Saxe D, Warren ST. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992; 1:397-400. [DOI] [PubMed] [Google Scholar]
- 16. Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, Tassone F, Hagerman PJ, Herman H, Hagerman RJ. Autism profiles of males with fragile X syndrome. Am J Ment Retard. 2008; 113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: Symptoms of autism in very young childrenwith fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr. 2001; 22:409-417. [DOI] [PubMed] [Google Scholar]
- 18. Thurman AJ, McDuffie A, Hagerman R, Abbeduto L. Psychiatric symptoms in boys with fragile X syndrome: A comparison withnonsyndromic autism spectrum disorder. Res Dev Disabil. 2014; 35:1072-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Kronk R, Delahunty C, Hessl D, Visootsak J, Picker J, Gane L, Tranfaglia M. Advances in the treatment of fragile X syndrome. Pediatrics. 2009; 123:378-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schutzius G, Bleckmann D, Kapps-Fouthier S, di Giorgio F, Gerhartz B, Weiss A. A quantitative homogeneous assay for fragile X mental retardation 1 protein. J Neurodev Disord. 2013; 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iosif AM, Sciolla AF, Brahmbhatt K, Seritan AL. Caregiver burden in fragile X families. Curr Psychiatry Rev. 2013; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keogh BK, Garnier HE, Bernheimer LP, Gallimore R. Models of child-family interactions for children with developmental delays: Child-driven or transactional? Am J Ment Retard. 2000; 105:32-46. [DOI] [PubMed] [Google Scholar]
- 23. Vismara LA, McCormick C, Young GS, Nadhan A, Monlux K. Preliminary findings of a telehealth approach to parent training in autism. J Autism Dev Disord. 2013; 43:2953-2969. [DOI] [PubMed] [Google Scholar]
- 24. Feil EG, Baggett KM, Davis B, Sheeber L, Landry S, Carta JJ, Buzhardt J. Expanding the reach of preventive interventions: Development of an internet-based training for parents of infants. Child Maltreat. 2008; 13:334-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamad CD, Serna RW, Morrison L, Fleming R. Extending the reach of early intervention training for practitioners: A preliminary investigation of an online curriculum for teaching behavioral intervention knowledge in autism to families and service providers. Infants Young Child. 2010; 23:195-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vismara LA, Young GS, Rogers SJ. Telehealth for expanding the reach of early autism training to parents. Autism Res Treat. 2012; 2012:121878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Western Psychological Services, LA, USA, 1999. [Google Scholar]
- 28. Tassone F, Pan R, Amiri K, Taylor AK, Hagerman PJ. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J Mol Diagn. 2008; 10:43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tassone F, Iong KP, Tong TH, Lo J, Gane LW, Berry-Kravis E, Nguyen D, Mu LY, Laffin J, Bailey DB, Hagerman RJ. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med. 2012; 4:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: A new mechanism of involvementin the fragile-X syndrome. Am J Hum Genet. 2000; 66:6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Becker KA. Stanford-Binet Intelligence Scales, 5th, ed,. Riverside Publishing, Itasca. USA, 2003. [Google Scholar]
- 32. Mullen EM. Mullen Scales of Early Learning. American Guidance Service, WA, USA, 1995. [Google Scholar]
- 33. Farzin F, Perry H, Hessl D, Loesch D, Cohen J, Bacalman S, Gane L, Tassone F, Hagerman P, Hagerman R. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006; 27(Suppl):S137-144. [DOI] [PubMed] [Google Scholar]
- 34. Gotham K, Risi S, Pickles A, Lord C. The autism diagnostic observation schedule: Revised algorithms for improveddiagnostic validity. J Autism Dev Disord. 2007; 37:613-627. [DOI] [PubMed] [Google Scholar]
- 35. Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales, 2nd ed., American Geographical Society, WA, USA, 2005. [Google Scholar]
- 36. Williams KT. Expressive Vocabulary Test. 1997, Greenville: Super Duper Publications. [Google Scholar]
- 37. Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scales, 5th, ed,. Pearson, SA, USA, 2011. [Google Scholar]
- 38. Berninger V. Process Assessment of the Learner. Process Assessment of the Learner, 2nd, ed,. Pearson, SA, USA, 2007. [Google Scholar]
- 39. Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Donaldson A, Varley J. Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics. 2010; 125:e17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rogers SJ, Estes A, Lord C, Vismara L, Winter J, Fitzpatrick A, Guo M, Dawson G. Effects of a brief Early Start Denver model (ESDM)-based parent intervention ontoddlers at risk for autism spectrum disorders: A randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2012; 51:1052-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Plowman L, Stevenson O, Stephen C, McPake J. Preschool children's learning with technology at home. Computers & Education, 2012. 59: pp. 30-37 [Google Scholar]