Abstract
The proposal that separate populations of subicular cells provide the direct hippocampal projections to the mammillary bodies and anterior thalamic nuclei was tested by placing two different fluorescent tracers in these two sites. In spite of varying the injection locations within the mammillary bodies and within the three principal anterior thalamic nuclei and the lateral dorsal thalamic nucleus, the overall pattern of results remained consistent. Neurons projecting to the thalamus were localised to the deepest cell populations within the subiculum while neurons projecting to the mammillary bodies consisted of more superficially placed pyramidal cells within the subiculum. Even when these two cell populations become more intermingled, e.g. in parts of the intermediate subiculum, almost no individual cells were found to project to both diencephalic targets. In adjacent limbic areas, i.e. the retrosplenial cortex, postsubiculum, and entorhinal cortex, populations of cells that project to the anterior thalamic nuclei and mammillary bodies were completely segregated. This segregated pattern included afferents to those nuclei comprising the head-direction system. The sole exception was a handful of double-labelled cells, mainly confined to the ventral subiculum, that were only found after pairs of injections in the anteromedial thalamic nucleus and mammillary bodies. The projections to the anterior thalamic nuclei also had a septal-temporal gradient with relatively fewer cells projecting from the ventral (temporal) subiculum. These limbic projections to the mammillary bodies and anterior thalamus comprise a circuit that is vital for memory, within which the two major components could convey parallel, independent information.
Keywords: entorhinal cortex, fornix, head-direction system, hippocampus, lateral dorsal thalamic nucleus, retrosplenial cortex, subicular cortex
Introduction
The projections from the hippocampal formation to the medial diencephalon appear to be vital for memory. This conclusion follows directly from a consideration of those brain regions implicated in amnesia. Within the temporal lobe, hippocampal formation damage appears necessary for anterograde amnesia (Spiers et al., 2001) while diencephalic amnesia is consistently linked to pathology in the mammillary bodies, the anterior thalamic nuclei, and the mammillothalamic tract (Von Cramon et al., 1985; Dusoir et al., 1990; Harding et al., 2000; Van der Werf et al., 2000, 2003; Tsivilis et al., 2008). Both the mammillary bodies (MB) and the anterior thalamic nuclei (ATN) receive dense hippocampal inputs via the fornix (Meibach and Seigel, 1975; Swanson and Cowan, 1975; Poletti and Cresswell, 1977; Allen and Hopkins, 1989; Kishi et al., 2000; Aggleton et al., 1987, 2005). Furthermore, there is considerable evidence that fornix damage is sufficient to produce anterograde amnesia in people (McMackin et al., 1995; Gaffan et al., 1991; Aggleton et al., 2000; D’Esposito et al., 1995), and this effect has recently been linked to subsequent mammillary body atrophy (Tsivilis et al., 2008). The conclusion, that the hippocampus functions via the fornix in an integrated manner with the mammillary bodies and anterior thalamic nuclei to support memory, receives further support from lesion studies with animals (Parker and Gaffan, 1997a,b; Aggleton and Brown, 1999; Warburton et al., 2000, 2001; Henry et al., 2004).
Why these particular hippocampal projections are so critical for memory is not yet known. The present study, therefore, re-examined these diencephalic inputs with respect to one key issue: whether the projections to the mammillary bodies and the anterior thalamic nuclei arise from the same or separate hippocampal cells. It has long been known that the hippocampal projections to the medial diencephalon actually originate from the subiculum rather than from the CA fields (Rosene and Van Hoesen, 1977; Swanson and Cowan, 1977; Aggleton et al., 1986; Allen and Hopkins, 1989). Furthermore, the subicular efferents to the anterior thalamic nuclei and mammillary bodies appear to arise from different cell populations. While pyramidal cells in the middle subicular layer project to the mammillary bodies, deeper pyramidal cells, as well as polymorphic and fusiform cells from the deepest subicular layers, project to the anterior thalamic nuclei. This seemingly segregated arrangement is found in both the rat (Meibach and Siegel, 1975; Ishizuka, 2001) and monkey (Aggleton et al., 1986; 2005), and so is presumably of functional importance. At the same time, this laminar arrangement appears to be orthogonal to the columnar organization of the subiculum that has been emphasized by recent anatomical studies (Witter, 2006), making it an important target for careful analysis.
The present study had a number of inter-related goals. The first was to determine whether, as supposed, the subicular projections to the mammillary bodies and anterior thalamic nuclei (including the closely related lateral dorsal thalamic nucleus) are completely segregated in the rat brain (Meibach and Siegel, 1975; Sikes et al., 1977; Namura et al., 1994; Allen and Hopkins, 1989; Ishizuka, 2001). Almost all previous studies of these connections have used single tracers, but it is only by injecting different retrograde tracers into the anterior thalamic nuclei and mammillary bodies in the same animals that it becomes possible to compare directly the sources of these afferents. One previous study (Namura et al., 1994) used this double-tracer approach although the findings were from only three cases, a serious limitation as it is now known that the subicular projections to both the mammillary bodies and the anterior thalamic nuclei are topographic (Ishizuka, 2001; Shibata, 1989; Kishi et al., 2000; Allen and Hopkins, 1989). As a consequence, it is necessary to examine multiple combinations of injection placements to confirm this preliminary finding of independence (Namura et al., 1994). In addition, there is a need to broaden the scope of the investigation in order to determine whether there are independent subpopulations that extend beyond the subiculum to include the postsubiculum, parasubiculum, and entorhinal cortex.
A second goal arises from the discovery that some of these connections link key elements of the ‘head-direction system’ (Taube, 2007). The presence of this distinct parallel set of connections (Vann and Aggleton, 2004) raises the possibility that these particular pathways might have different properties. For this reason, the afferents to the anterodorsal and lateral dorsal thalamic nuclei, along with those to the lateral mammillary bodies, were included in the present study (Taube, 2007). A third goal was to extend these analyses dorsally into the retrosplenial cortex, an area with dense projections to the anterior thalamic nuclei but much lighter projections to the mammillary bodies (Van Groen and Wyss, 1990a, 1992, 2003). As a result, the present study directly compared the sources of the projections to the mammillary bodies and anterior thalamic nuclei that arise from the continuous band of limbic cortex extending from the retrosplenial region through to the entorhinal cortex. Finally, a small number of additional cases involved injecting the highly sensitive tracer, wheat germ agglutinin (Horikawa and Powell, 1986), into either the thalamus or the mammillary bodies. The purpose of these experiments was to provide comparisons with the fluorescent tracers and so test for the presence of any connections not revealed by the main method. The wheat germ agglutinin tracer has further advantages as it is rarely picked up by fibers of passage (Steindler, 1982) and the reaction product is stable, i.e. it is not subject to photo-bleaching.
Materials and Methods
The experiments were performed on 67 male Lister Hooded rats weighing 270-300g (Harlan, U.K.). The retrograde fluorescent tracers, Fluorogold (Fluorochrome LLC, Denver, USA) or Fast Blue (Polysciences Inc, Eppelheim, Germany), were used along with Cholera Toxin Subunit B (CTB) conjugated to Alexa Fluor 488 (CTB488) (Invitrogen Ltd, Paisley UK) to allow double fluorescent labelling in the same animal. Additional experiments used single injections of wheat germ agglutinin (WGA) injected into either the mammillary bodies (MB) or the anterior thalamic nuclei (ATN). Animal weight was monitored so that all rats remained at 85% or more of their free feeding weight. All experiments were carried out in accordance with UK Animals (Scientific Procedures) Act, 1986.
General surgical methods
All animals were anesthetised with 6% sodium pentobarbital (Sigma-Aldrich, Gillingham, UK). Chloramphenicol eye ointment (Martindale Pharmaceuticals, Romford, UK) was topically applied to the eyes to protect the cornea. Animals were then placed in a stereotaxic frame (Kopf, Tujunga, CA, USA), with the mouthbar set at +0.5mm. Under aseptic conditions, small openings were made in the skull and dura to allow access for either 1μl Hamilton syringes (Hamilton, Bonaduz Switzerland) for CTB488 and Fast Blue or 0.5μl Hamilton syringes for Fluorogold and WGA.
The injections were clustered around the following coordinates for the mammillary bodies: AP −2.1, ML +/− 0.8, DV −10.4 from bregma, but varied slightly to encompass different subregions. For the anteroventral nucleus, the coordinates for a single injection centered around AP −0.2, ML +/− 1.4, DV −6.7 from bregma. Where two injections were made into the anteroventral nucleus in the same hemisphere, the following coordinates were used for the medial placement: AP −0.25, ML +/− 1.0, DV −6.9 from bregma. For the more lateral location, the coordinates were centered around AP −0.25, ML +/− 1.8, DV −6.2 from bregma. For injections into the anteromedial nucleus, the single target was AP −0.2, ML +/− 1.0, DV −7.5 from bregma, and in cases where the lateral dorsal thalamic nucleus was targeted, a single injection was placed at AP −0.8, ML +/− 2.2, DV −6.1 from bregma.
After surgery, animals received a 5 ml subcutaneous injection of 5% glucose in 0.9% saline (Baxter Healthcare Ltd, Norfolk, UK), and Aureomycin antibiotic powder (Fort Dodge Animal Health Ltd, Southampton, UK) was applied over the closed sutured scalp. Animals were then allowed to recover in a thermostatically controlled container before returning to individual housing with ad lib food and water. Their drinking water contained paracetamol (500mg/L) and sucrose (2%) for 3 days after surgery. Each animal’s health was monitored daily.
Fluorescent tracer injections
A total of 55 animals were injected with Fast Blue and CTB488 or Fluorogold and CTB488 into the mammillary bodies and the anterior thalamic nuclei. In all of these cases, the tracers used for the two targets were varied. An additional 6 animals were injected with only a single tracer into either MB or ATN (Table 2). In 22 of the 55 cases, two injection placements were targeted within the ATN in each hemisphere, i.e. four thalamic injections per animal (Table 2). The goal was to maximise the coverage of the ATN and so ensure the strongest chance of visualising any cells that project to both the MB and ATN, should such cells exist.
TABLE 2.
Injection Locations, Types and Volumes of Fluorescent Tracer.
| Case | Location |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left MB | Right MB | Left ATN | Right ATN | |||||||||
| location | tracer | volume (μ l) | location | tracer | Volume (μ l) | location | tracer | Volume (μ l) | location | tracer | Volume (μ l) | |
| 18_1 | MMl, LM | FB | 0.05 | SUMl, MMl | FB | 0.05 | AVvl, AVdm | FG | 0.05 | AVdm | FG | 0.05 |
| 18_2 | MMl | FG | 0.05 | MMl | FG | 0.05 | AD, AVdm | FB | 0.05 | AD, fi | FB | 0.05 |
| 18_4 | LM | CTB | 0.05 | MMme | CTB | 0.05 | AVdm, AVvl, AM | FB | 0.05 | AVdm, AD | FB | 0.05 |
| 18_8 | LM | FB | 0.05 | MMl | FB | 0.05 | AM, AVdm, AVvl | CTB | 2 xs 0.04 | AM, AVdm, AVvl | CTB | 2 xs 0.04 |
| 18_10 | MMl | CTB | 0.05 | MMl | CTB | 0.05 | AVdm, AVvl | FG | 0.05 | AVdm, AVvl | FG | 0.05 |
| 18_11 | LM | CTB | 0.05 | SUMl, MMl | CTB | 0.05 | AD, AVvl | FB | 0.05 | AD | FB | 0.05 |
| 18_12 | LM | FB | 0.05 | SUMl, MMl, MMme | FB | 0.05 | AM, AVdm, AVvl | CTB | 2 xs 0.04 | AM, AVdm, AVvl | CTB | 2 xs 0.04 |
| 19_4 | LM, MMl | CTB | 0.05 | MMme, MMl | CTB | 0.05 | LDvl, AVvl, AD | FB | 0.05 | LDvl, fi | FB | 0.05 |
| 19_5 | MMl | CTB | 0.05 | MMl | CTB | 0.05 | AVdm, | FB | 0.05 | AVdm, AVvl | FB | 0.05 |
| 19_12 | MMl | CTB | 0.05 | MMl | CTB | 0.05 | AD, AVdm | FB | 0.05 | AVvl, AM | FB | 0.05 |
| 20_5 | MMl | CTB | 0.05 | MMl | CTB | 0.05 | AD, AVvl, AVdm | FG | 2 xs 0.04 | AM, AVvl, AVdm | FG | 2 xs 0.04 |
| 20_8 | MMl, LM | CTB | 0.05 | MMme, MMl | CTB | 0.05 | AD, AVdm | FB | 0.05 | AD, sm, MoDG | FB | 0.05 |
| 22_4 | LM | CTB | 0.05 | SUMl | CTB | 0.05 | AVdm, AD | FB | 0.05 | * | ||
| 22_12 | LM | FB | 0.05 | LM | FB | 0.05 | * | AM, AVvl, AVdm | CTB | 2 xs 0.05 | ||
| 28_3 | LM | CTB | 0.04 | MMl | CTB | 0.04 | AVvl, AVdm | FB | 0.04 | AVvl, AVdm | FB | 0.04 |
| 28_7 | LM,MMl | CTB | 0.04 | MMl | CTB | 0.04 | AVvl, AVdm, AD | FB | 0.04 | AVvl, AVdm | FB | 0.04 |
| 15_2 | AM, AVdm | FG | 2 xs 0.04 | |||||||||
| 15_5 | SUMl, MMl, LM | FG | 0.04 | |||||||||
| 15_8 | SUMl, MMl, MMme | FG | 0.04 | |||||||||
| 15_11 | AD, MoDG, AVdm | FG | 2 xs 0.04 | |||||||||
| 18_5 | * | * | AVvl, VAL | FB | 0.05 | AVvl, AVdm | FB | 0.05 | ||||
| 18_6 | * | * | AVvl, AVdm | FG | 0.05 | AD, AVdm | FG | 0.05 | ||||
| 18_7 | * | * | AVvl, AVdm | FG | 0.05 | AD, AVdm | FG | 0.05 | ||||
| 18_9 | * | * | AD, AVdm | FG | 0.05 | AD | FG | 0.05 | ||||
| 19_1 | * | * | AD, AVdm | CTB | 0.05 | AD, AVdm | CTB | 0.05 | ||||
| 19 2 | MMl | FG | 0.05 | MMl | FG | 0.05 | * | * | ||||
| 19_6 | * | * | AVvl, AVdm | FG | 0.05 | * | ||||||
| 19_8 | * | * | * | AVvl, AVdm | FB | 0.05 | ||||||
| 19_11 | * | * | AVdm | FB | 0.05 | AM, AVvl | FB | 0.10 | ||||
| 20_2 | * | * | AVvl, VAL | FB | 0.05 | * | ||||||
| 20_10 | MMme, MMl | FG | 0.05 | |||||||||
| 22_3 | LM,MMl | CTB | 0.05 | SUMl | CTB | 0.05 | * | * | ||||
| 22_8 | LM,MMl | FB | 0.05 | LM | FB | 0.05 | * | * | ||||
| 22_9 | AVvl, AVdm | FB | 0.05 | |||||||||
| 24_3 | MMl | FB | 0.05 | MMl | FB | 0.05 | * | * | ||||
| 24_4 | MMl | CTB | 0.05 | MMl | CTB | 0.05 | * | * | ||||
| 26_1 | SUMl, MML | FB | 0.05 | SUMl, MML | FB | 0.05 | * | * | ||||
| 26_6 | MML | FB | 0.05 | * | * | * | ||||||
| 26_10 | SUMl | FB | 0.05 | * | ||||||||
|
| ||||||||||||
| 26_12 | MMl | FB | 0.05 | * | ||||||||
Injection site located to tip of needle, but sometimes falls on a border, as indicated.
CTB488 was made up as a 0.2-0.5% solution in sterile 0.1M phosphate buffered saline (PBS) containing 2mM sodium azide. Fast Blue was made up as a 3% solution in sterile PBS, and Fluorogold was made up as a 4% solution in distilled water. Following pressure injections of 0.04-0.05μl into each site, the syringe was left in place for at least 7 minutes to help limit any tracer travelling back up the syringe tract.
Following a postoperative period of 3-4 days, the animals were deeply anesthetized with sodium pentobarbital (Euthatal, Merial, Harlow, UK). They were then perfused intracardially with 0.1M PBS at room temperature followed by 4% paraformaldehyde in 0.1M PBS at ~4°C. Brains were removed and placed in the dark for 4 hours in paraformaldehyde and then transferred to 25% sucrose solution in 0.1M PBS for 24 hours in the dark to cyroprotect the tissue before cutting. Brains were placed on a freezing platform and 40μm coronal sections were cut on a sledge microtome (Leica 1400). Two, 1-in-3 series of sections were mounted directly onto gelatine-subbed slides, and then allowed to dry in the dark at room temperature. One series was stained with cresyl violet to allow localisation of injection sites, while the second series was rehydrated and cover-slipped with Hydromount (National Diagnostics UK, East Riding, UK) before being viewed with fluorescence microscopy. A Leica DM6000B microscope was used for brightfield and fluorescence microscopy. An attached Leica DFC350FX digital camera and LAS AF image acquisition software (Leica) were used to capture images.
Wheat germ agglutinin tracer experiments
Additional experiments involved single injections of native unconjugated wheat germ agglutinin (WGA) (Vector Labs, Peterborough, UK) into either ATN or MB. The immunohistochemical method employed to localise the transported WGA is highly sensitive (Horikawa and Powell, 1986) and has the advantage that WGA does not appear to be taken up by fibers of passage within the central nervous system (Steindler, 1982). A total of 0.04μl 1% WGA was injected unilaterally into the MB of four animals, and the postoperative period ranged between 12 hours and 1 day. In a further two rats, 0.04μl 1% WGA was injected into the ATN, and the postoperative period varied from 6 hours to 1 day. Animals were perfused with 4% buffered paraformaldehyde and brains cut as already described. From the 1-in-3 section series, one series was then stained with cresyl violet, a Nissl stain, and a second series was used to detect WGA immunohistochemically.
An antiserum directed against the WGA (Vector Labs) was used at a dilution of 1:2000 and incubated at 4°C for 48 hours. The antigen-antibody complex was localised with a standard avidin-biotin process (ABC Elite Kit, Vector Labs). The chromagen diaminobenzidine produced the visualised reaction product. Osmium tetroxide (0.1%) further enhanced visualisation and permanence of the staining (Collins et al., 1991). Reacted sections were then mounted onto gelatine-subbed slides and dehydrated through increasing concentrations of alcohol before being cover-slipped from xylene with DPX (Raymond Lamb, Eastbourne, UK). For the brightfield microscopy, the study used a Leica DMRB microscope equipped with an Olympus DP70 digital camera and AnalySIS image acquisition software (Soft Imaging System, Münster, Germany).
Anatomical nomenclature
Anatomical names and borders follow Swanson (1992), except for the divisions within the retrosplenial cortex and postsubiculum where we employ the terminology of Van Groen and Wyss (2003). The latter authors divide the retrosplenial cortex into a dorsal, dysgranular subregion (Rdg) and two ventral, granular subregions (Rga, Rgb). The principal anterior thalamic nuclei consist of the anterodorsal, anteromedial, anteroventral thalamic nuclei (Sripanidkulchai and Wyss, 1986; Bentivoglio et al., 1993). Some authors, e.g. Swanson (1992), identify a further nucleus at the midline (the interoanteromedial nucleus) although this nucleus is often not recognised in primate brains (e.g. Olszewski, 1952). The lateral dorsal thalamic nucleus is included in the study given its close anatomical (Bentivoglio et al., 1993) and functional links (Taube, 2007; Van Groen and Wyss, 2002) with the anterior thalamic nuclei. As a consequence, it is sometimes treated as an additional anterior thalamic nucleus (e.g. Van Groen and Wyss, 1992b).
The postsubiculum consists of an external lamina subdivided into layers I-III and an internal lamina subdivided into layers IV-VI (Van Groen and Wyss, 2003). Layer IV of the postsubiculum corresponds to the obvious cell free zone, or lamina dissecans.
The borders of the subiculum, presubiculum, parasubiculum and postsubiculum match those described by Swanson et al. (1987), although it should be noted that, while Swanson et al. (1987) describe the rat subiculum as having three laminae, other authors, e.g. Kloosterman et al. (2003), only describe two (i.e. a superficial molecular layer and a deeper thick layer of pyramidal cells). In this paper, we will adopt the latter practice, although it should be added that the cells within this deeper lamina are not entirely homogeneous. We also use the term ‘intermediate subiculum’ (Groenewegen, 1987) to describe that region of the subiculum at the caudal extent of the hippocampal flexure where the dorsal subiculum and ventral subiculum converge.
Following the practice of Swanson (1992) and others, the term postsubiculum is used for that caudal subicular region adjacent to the retrosplenial cortex (Rga). While other authors regard this region in the rat brain as being part of the presubiculum, e.g. Kloosterman et al. (2003), we retain the use of the term postsubiculum based on its connectivity and its distinctive cytoarchitecture (Van Groen and Wyss, 1990b). Similarly, within the mammillary bodies, only the major nuclei (lateral and medial) are distinguished, although finer subdivisions can be described (Rose, 1939).
Results
A large number of cases was required to provide a full array of combinations of injection sites within the thalamic and mammillary body target areas, and sufficient repetition within each combination was needed to establish the reliability of the results. While we can only describe a small number of representative cases in detail (see Table 2 for full list of cases), the patterns observed in the remaining cases were very similar (unless specifically noted).
Fluorescent tracer injections
First, cases #28_3 and #28_7 are described in detail as they highlight the general pattern of results, and their similarity underlines the reproducibility of the findings. Cases #37_12, #18_4 and #37_1 are then described as they refer to injections within different thalamic nuclei. Three features typified the findings in this study. First, in almost all joint thalamic and MB injections, no double labelled cells exist, the only exceptions being those cases with pairs of injections in the anteromedial thalamic nucleus and MB. Here, a few individual cells were found that projected to both injection sites. Second, there was a consistent layer difference, with the thalamic projections arising from deeper cells within the subicular regions. This consistent layer difference extended into adjacent cortical areas. Third, there were dorsal-ventral gradients of afferent projections to the ATN and lateral dorsal nucleus as retrogradely transported label was more frequent in dorsal subicular/retrosplenial regions, and as a consequence, the MB-transported label became predominant in more ventral regions. Having established the general pattern of retrograde label, additional cases are then introduced to provide a more complete picture.
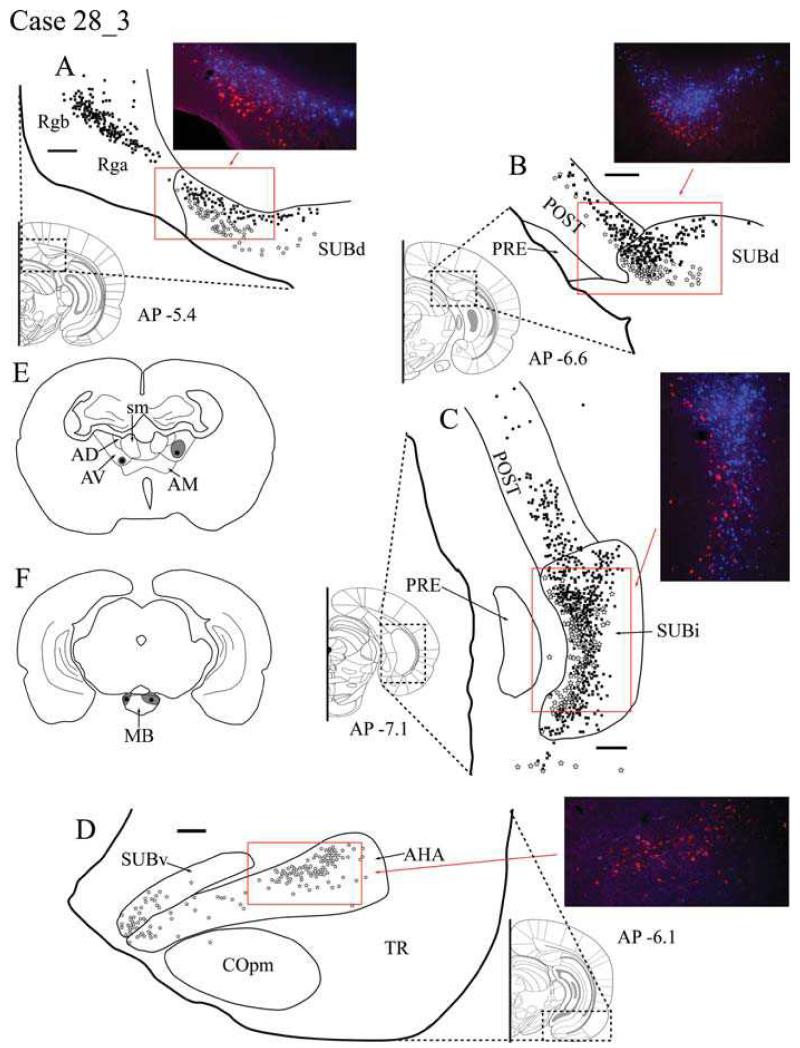
Case #28_3: Anteroventeral thalamic nuclei and MB
Tracer injection locations
Bilateral injections were made in both the MB and ATN, with a single injection in each site in each hemisphere. Cholera toxin B conjugated to Alexa Fluor 488 (CTB488) was injected bilaterally into caudal MB (Fig. 1F). The injection sites were largely confined to the medial mammillary nucleus (MM), resulting in very limited spread into the adjacent lateral mammillary nucleus (LM). Both the left and right ATN injections of Fast Blue involved the dorsal (AVd) and ventral (AVv) regions of AV (Fig. 1E). In the right hemisphere, the Fast Blue injection extended dorsally to reach the AD border. In the left hemisphere, the thalamic injection site appeared much smaller and did not involve AD. As both MB and AV injections were larger in the right hemisphere and the resultant labelling appeared more abundant, this hemisphere is described below as it provides a more sensitive test for any double labelled cells.
Fig. 1.
A-D: Distribution of labelled cells in Case #28_3 following injections of Fast Blue into the anterior thalamic nuclei (primarily in the anteroventral nucleus) and CTB488 into the mammillary bodies. Fast Blue is indicated by black squares on the line drawings and blue labelled cells on the photomicrographs. The CTB488 label is shown by open stars and red label, respectively. Scale bars = 250 μm; AP positions are in reference to bregma. E: Line drawing indicating the injection sites within the anterior thalamic nuclei. F: Line drawing indicating the injection sites within the mammillary bodies. All sections are in the coronal plane. Abbreviations as Table 1.
Retrosplenial cortex
Large numbers of Fast Blue-labelled cells (thalamic injection) were present in both granular subdivisions of the retrosplenial cortex (Rga and Rgb), but very few in Rdg (Fig. 1A). The retrogradely labelled cells in Rga and Rgb that project to the thalamus were always located in the deeper layers (V-VI). In contrast, no CTB488-labelled cells (MB injection) were found in any of the retrosplenial cortex subregions.
Postsubiculum
Following separate injections into AV and MB, labelled cells were present throughout the rostro-caudal extent of the postsubiculum (Fig. 1B). In more rostral postsubiculum, there was a clear separation with Fast Blue-labelled cells (AV) in layers V-VI, while the CTB488 labelling (MB) was more superficial in layer V and appeared to consist of only pyramidal cells. The Fast Blue cells (AV) outnumbered the CTB488 cells, and as the labelling was followed through to more posterior regions of the postsubiculum, the number of MB projecting cells diminished further and eventually all CTB488 staining disappeared, leaving only Fast Blue-labelled cells (Fig. 1C). No double labelled cells were found.
Dorsal subiculum
Anteroventral thalamic afferents (Fast Blue-labelled cells) were found within the deeper part of layer II of the dorsal subiculum throughout the extent of the structure. These cells extended to the very base of the subiculum and thus some were located against the alveus (Fig. 1A). While many of the thalamic afferents arose from pyramidal cells, at the more anterior (septal) regions of the dorsal subiculum, the Fast Blue label was concentrated in the smaller polymorphic cells that occupy the deepest part of layer II. In the anterior region of the dorsal subiculum, the projections to MB (CTB488 label) were confined to larger pyramidal cells in the more superficial parts of layer II. There was rarely any overlap in the cells labelled after injections centered in AV and MB; rather the cells were located one above the other. Once again, no double labelling was observed. Afferents to the AV region were most densely located distal to CA1 in the dorsal subiculum, but also did extend into regions proximal to CA1. MB afferents were more consistently located distal to CA1.
In the more posterior regions of the dorsal subiculum, a limited amount of intermixing between the two cell populations occurred, although the cells projecting to the ATN were typically deep to those projecting to the MB (Fig. 1B). Here, the corresponding Nissl-stained sections revealed no obvious differences in the morphology of the cells projecting to the thalamus or mammillary bodies.
Intermediate subiculum
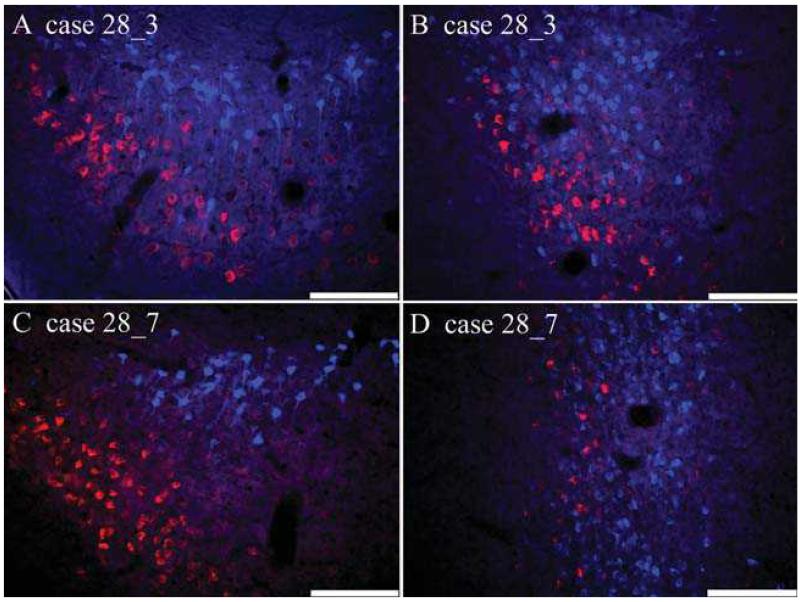
Labelling in SUBi was particularly common for the tracer (Fast Blue) placed in AV (Fig 1C). Numerous pyramidal-like cells containing Fast Blue were distributed throughout the region, while cells labelled from the MB injection were considerably less numerous and tended to be localised to the border area closest to the presubiculum and parasubiculum. In contrast to the previously described regions, the two cell populations appeared more intermingled in SUBi, although there was still a clear tendency for the thalamic projections to originate from deeper cells (Fig. 1C). Despite this increased overlap between cell populations, there were still no examples of double labelling (Fig. 2B).
Fig. 2.
Photomicrographs of coronal sections showing subicular fluorescent label following anterior thalamic (Fast Blue - blue cells) and mammillary body (CTB488 - red cells) injections. A, the dorsal subiculum (Case #28_3); B, the intermediate subiculum (Case #28_3); C, the dorsal subiculum (Case #28_7); D, the intermediate subiculum (Case #28_7). Scale bar = 100μm.
Ventral subiculum
In the rostral SUBv, the only labelled cells came from the MB injection. These labelled cells were most frequently located at the distal (close to presubiculum) region of SUBv, becoming more diffuse throughout the proximal (close to CA1) region (Fig. 1D). They were also found in the deepest area of the pyramidal cell layer. An additional area of retrograde label was located in the amygdalohippocampal area (Pitkanen, 2000). At the most caudal region of SUBv as it progresses dorsally, labelled cells projecting to MB and the AV were diffusely intermingled, but again there was no evidence of any double labelled cells.
Presubiculum and parasubiculum
No labelling from either the Fast Blue or CTB488 injections was present in the presubiculum or parasubiculum.
Entorhinal cortex
CTB488-labelled cells (MB injection) were observed in the medial entorhinal cortex (ENTm), immediately posterior to SUBi. The medial entorhinal cortex displayed a very diffuse pattern of labelled cells in layers II and III from the MB injection, but no labelling was seen from the AV injection. No labelled cells were present in the lateral entorhinal cortex (ENTl).
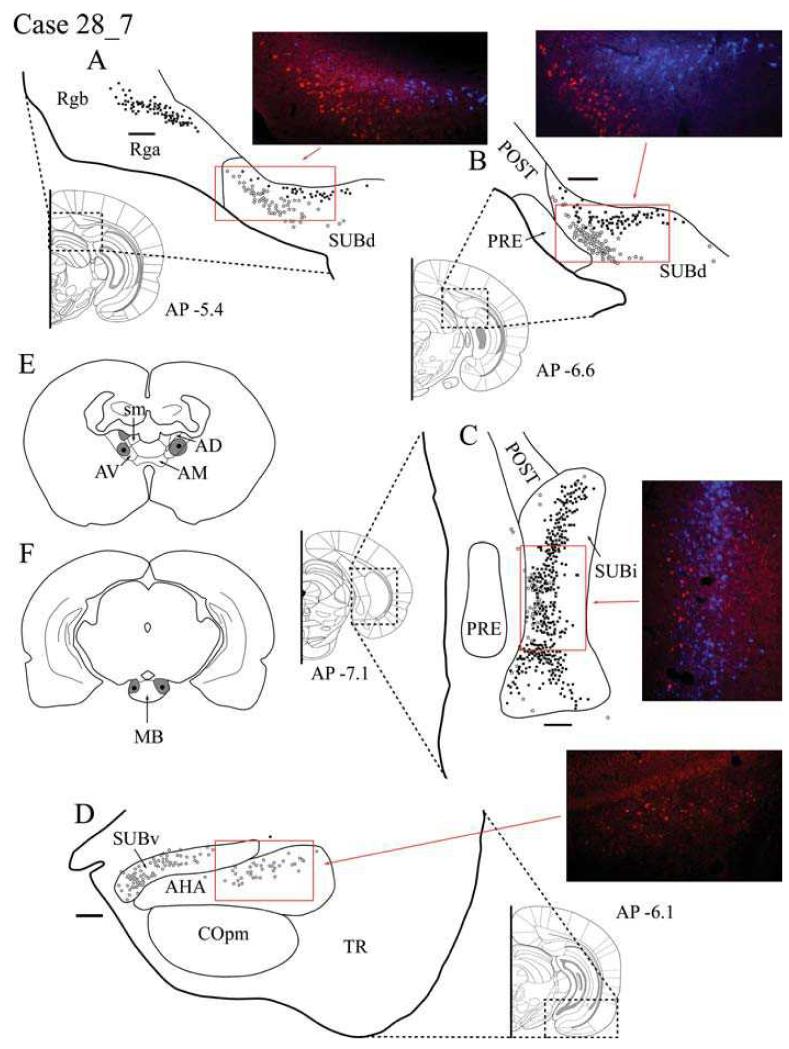
Case #28_7: Anteroventeral thalamic nucleus and MB
Tracer injection locations
The injection locations were similar to the previous case with single injections of Fast Blue (0.04 μl) centred in AV combined with injections of CTB488 (0.04 μl) into the posterior MB of both hemispheres. The MB injections appeared to fill much of the posterior part of the medial mammillary nucleus (Fig. 3F). The ATN injections were largely confined to AVv and AVd (Fig. 3E), although in the left hemisphere some tracer appears to have spread back up the injection tract into AD. The label in the right hemisphere, following an injection more confined to AV, will be described, i.e. the injection was more discrete than the previous case, with little or no AD involvement. This case is presented in detail to highlight the reliability of the major findings described for Case #28_3.
Fig. 3.
A-D: Distribution of labelled cells in Case #28_7 following injections of Fast Blue into the anteroventral thalamic nucleus and CTB488 into the mammillary bodies. Fast Blue is indicated by black squares on the line drawings and blue labelled cells on the photomicrographs. The CTB488 label is shown by open stars and red label, respectively. Scale bars = 250 μm; AP positions are in reference to bregma. E: Line drawing of the injection sites within the anterior thalamic nuclei. F: Line drawing of the injection sites within the mammillary bodies. All sections are in the coronal plane. Abbreviations as Table 1.
Retrosplenial cortex
The Fast Blue injections into AV produced extensive labelling within both granular subdivisions of the retrosplenial cortex (Rga and Rgb), but the label in the dysgranular subdivision (Rdg) was restricted to that portion rostral to the splenium (Fig. 3A). Retrogradely labelled cells from AV were situated in the deepest layers (V and VI) of Rga and Rgb. Unlike Case #28_3, there was an extremely small number (a maximum of four cells per section) of retrogradely labelled cells from the injection of CTB488 into the MB. These cells were confined to the ventral Rgb (layer V) and located immediately dorsal to the corpus callosum.
Postsubiculum
This case contained far fewer labelled cells in the postsubiculum from the thalamic injection than the previous case (#28_3), consistent with the lack of AD involvement in the right hemisphere injection site. Cells projecting to AV were found in layers V and VI of the postsubiculum while cells projecting to MB were found in layer V, with the label from the thalamic injection consistently located more deeply than the MB label. At more posterior regions of the postsubiculum, labelled cells projecting to AV eventually disappeared, leaving only a few CTB488-labelled (MB injection) cells (Fig. 3C).
Dorsal subiculum
Projections to AV were located within the deeper region of the dorsal subiculum throughout the extent of the structure. In the more anterior dorsal subiculum, the labelling from the thalamic injection was concentrated within the region of smaller and more densely packed cell bodies rather than the more superficial pyramidal cells that comprise most of layer II. Projections to MB originated from the larger (pyramidal) cells in the more superficial layers of rostral, dorsal subiculum (Fig. 3A). Once again, these two populations of labelled cells remained separate, with very little overlap. Both populations of cells projecting to MB and AV were concentrated more distal to CA1 in the dorsal subiculum, with the MB-labelled projection being located directly above those to AV.
In the more posterior dorsal subiculum, there was increased overlap between the two cell populations, although overall the cells providing the AV projection (Fast Blue) were always positioned deep to those giving rise to the MB projection (Fig. 3B). Consequently, the pattern of labelled cells appeared continuous, proceeding from the dorsal subiculum to the postsubiculum. No double labelled cells were observed in any part of the dorsal subiculum (Fig. 2C).
Intermediate subiculum
This region contained numerous Fast Blue-labelled cells (AV). These cells were distributed throughout much of SUBi, and contrasted with the far less numerous CTB488-positive (MB) cells (Fig. 3C). This MB label was largely localised close to the border with the presubiculum and parasubiculum. While there was appreciably more intermingling of labelled cells than in the dorsal subiculum, the thalamic cells tended to be located more deeply, and no double labelled cells were observed (Fig. 2D).
Ventral subiculum
The label in SUBv was very similar to that seen in the preceding Case #28_3. Only labelled cells projecting from the MB were located in the rostral SUBv. These cells were found in the pyramidal cell layer, being most densely distributed at the distal region of SUBv and becoming more diffuse throughout the proximal portion (Fig. 3D). The highest concentration of labelled cells occurred in deeper regions of the pyramidal cell layer, but addtional scattered cells were located superficially. CTB488 labelling was also present in the nearby amygdalohippocampal area. At the most posterior location of SUBv, before the transition into SUBi, the MB projecting cells became less numerous, while there was an increase in the numbers of labelled cells from the thalamic injection.
Presubiculum and parasubiculum
No retrograde label was observed in either region.
Entorhinal cortex
Only labelled cells projecting to the MB were found in the entorhinal cortex. In the ENTm, cells projecting to the MB formed a dense grouping of CTB488-labelled cells located in layers II and III in a location posterior to the SUBi.
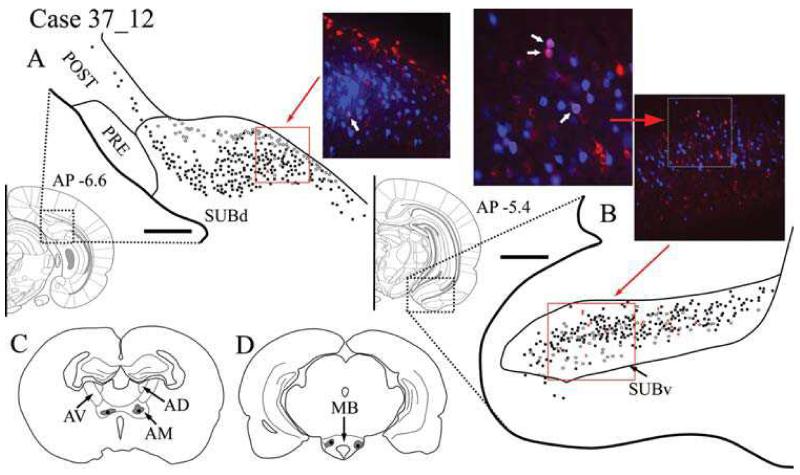
Case #37_12: Anteromedial thalamic nucleus and MB
Tracer injection locations
Bilateral injections were made in both MB and ATN, with a single injection in each site in each hemisphere. The ATN injection, made with CTB488 (0.04 μl), was confined to AM in both hemispheres (Fig. 4D). Injections of Fast Blue (0.04 μl) were made into MB. Ipsilateral to the hemisphere that was examined for labelling, the injection site involved both the medial and lateral mammillary nuclei; contralaterally, the injection site was in the medial mammillary nucleus (Fig. 4C).
Fig. 4.
A-B: Distribution of labelled cells in Case #37_12 following injections of Fast Blue into the mammillary bodies and CTB488 into the anteriomedial thalamic nucleus. Fast Blue is indicated by black squares on the line drawings and blue labelled cells on the photomicrographs. The CTB488 label is shown by open stars and red label, respectively. Double labelled cells of both CTB488 and Fast Blue are indicated by red crosses on the line drawings and appear as purple labelled cells on the photomicrographs. Scale bars = 250 μm, AP positions are in reference to bregma. C: Line drawing of the injection sites within the anterior thalamic nuclei. D: Line drawing of the injection sites within the mammillary bodies. All sections are in the coronal plane. Abbreviations as Table 1.
Retrosplenial cortex
CTB488-labelled cells from the AM injection were clearly present in the Rga and Rgb subdivisions of the retrosplenial cortex, while only a limited number of labelled cells were found in Rdg. This CTB488-labelling projecting to the thalamus was located in the deeper layers (V-VI). No Fast Blue label was present in Rdg, and only a few superficially labelled cells from the MB injection were found in layer V of Rga and Rgb. No double labelling was present in the retrosplenial cortex.
Postsubiculum
Fast Blue-labelled cells from the MB injections were present in the postsubiculum (Fig. 4A), while the CTB488 injection into AM produced very few labelled cells. The Fast Blue labelling occurred in pyramidal cells within layer V, while the cells labelled with CTB488 originated in layers V-VI and consisted of pyramidal and smaller polymorphic cell bodies. The thalamic projections were most densely located on the border with the SUBd. There was no intermingling between these two populations of labelled cells, and this separation was displayed throughout the postsubiculum. No double labelled cells were found. No retrograde label was observed in either the presubiculum or the parasubiculum in this case.
Dorsal subiculum
Fast Blue-labelled cells were distributed throughout the pyramidal cells in the superficial region of layer II of the entire dorsal subiculum (Fig. 4A). The CTB488 labelling from the AM injection was less abundant and more confined to the deepest regions of layer II. Unlike other patterns of labelling resulting from injections into the ATN, the CTB488 labelling from these AM injections was more densely localized proximal to CA1 (Fig. 4A). Going further caudally within SUBd, the two populations of labelled cells became increasingly intermingled. Unlike all other cases examined, injections of different tracers into AM and MB produced a very small number (typically 1 cell per section) of double labelled cells in the SUBd (indicated with white arrow in photomicrograph Fig. 4A). Double labelled cells appeared pyramidal in nature.
Intermediate subiculum
Both Fast Blue- and CTB488-labelled cells were located in the SUBi. Fast Blue labelling (MB injection) in the SUBi was predominantly located in the superficial region of layer II. CTB488-labelled cells (AM injection) were distributed deeper in layer II, in the most lateral region of SUBi. All labelled cells were pyramidal, and those double labelled cells (14 cells in a typical section, i.e. 2.6% of the total population) were scattered across SUBi, with the largest occurrence in more ventral regions.
Ventral subiculum
Labelled afferents from both AM and MB were intermixed in layer II of the SUBv. While this region displayed the greatest proportion of double labelled cells (Fig 4B), they remained scarce (13 cells in a typical section, i.e. 4.7% of the total population), and there was no apparent pattern to their distribution.
Entorhinal cortex
Separate labelled cells projecting to the MB and AM were found in both layers II and III of the ENTm. The Fast Blue-labelled cells (MB injection) were more numerous than the CTB488-labelled cells (AM injection). No labelled cells were located in the ENTl.
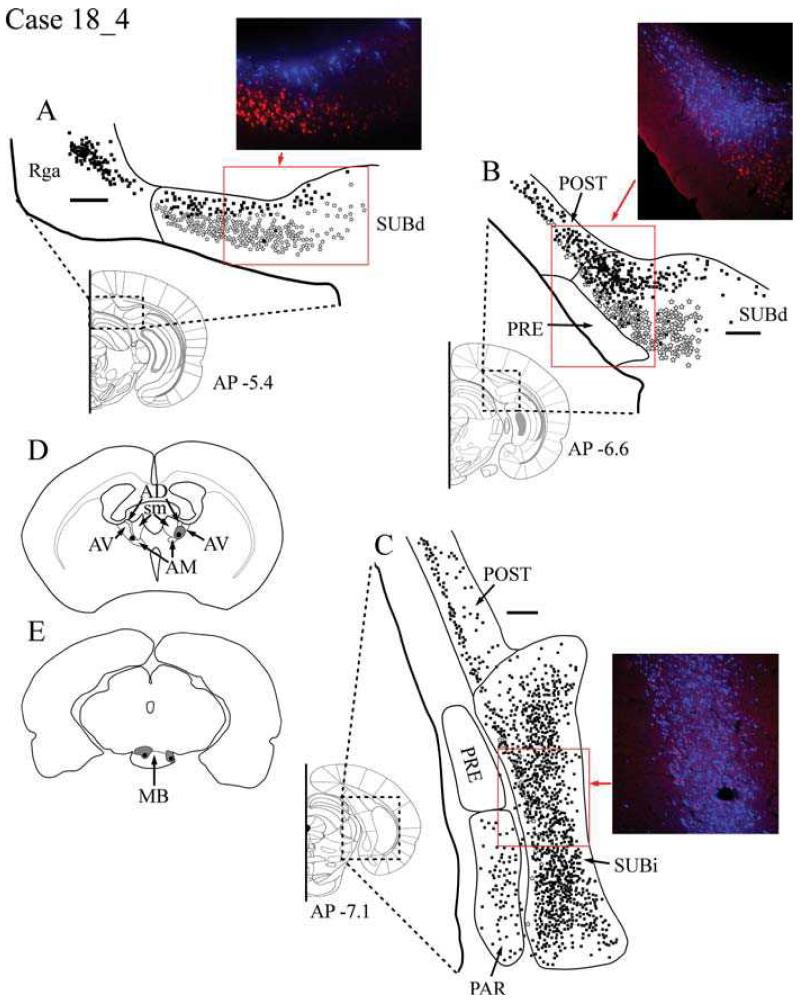
Case #18_4: Anterodorsal thalamic nucleus and lateral mammillary body nucleus
This case was selected because both the thalamic and the mammillary body injections in the right hemisphere involved the head-direction system (Taube, 2007). The connectivity of the head-direction system is distinctly different from that of adjacent nuclei (Taube, 2007), raising the possibility of a different frequency of double labelled cells.
Tracer injection locations
The bilateral, single injections of Fast Blue into anterior thalamus were more dorsal and more anterior than in the two preceding cases. Consequently the tracer occupied much of AD as well as AV (Fig. 5D). The larger thalamic injection (FB, 0.05μl) in the right hemisphere is described. In the same hemisphere, the CTB488 injection (0.05 μl) was centered in the lateral mammillary nucleus (Fig. 5E).
Fig. 5.
A-C: Distribution of labelled cells in Case #18_4 following injections of Fast Blue involving the anterodorsal thalamic nucleus and CTB488 into the lateral mammillary nucleus. Fast Blue is indicated by black squares on the line drawings and blue labelled cells on the photomicrographs. The CTB488 label is shown by open stars and red label, respectively. Scale bars = 250 μm; AP positions are in reference to bregma. D: Line drawing indicating the injection sites within the anterior thalamic nuclei. E: Line drawing indicating the injection sites within the mammillary bodies. All sections are in the coronal plane. Abbreviations as Table 1.
Retrosplenial cortex
Numerous Fast Blue-labelled cells (thalamic injection) were found in both granular subregions of the retrosplenial cortex (Rga and Rgb) (Fig. 5A). A small quantity of labelling was also evident in Rdg. These retrogradely labelled cells from the injection involving AD were situated in the deepest layers (V-VI) of Rga and Rgb. As in Case #28_7, the present case contained very small numbers of labelled cells (CTB488) in the retrosplenial cortex following the injection into lateral MB. The CTB488 injection resulted in a very light scattering of retrogradely labelled cells in layer V of the granular subregion, which were located immediately dorsal to the corpus callosum. No CTB488-labelled cells were present in more caudal Rga in this case, and no double labelled cells were observed anywhere in the retrosplenial cortex.
Postsubiculum
Numerous labelled cells were observed in the postsubiculum following injections in both AD and the lateral MB, although the very large majority appeared to project to the thalamus. Throughout the postsubiculum, the thalamic label was located in the internal lamina in layers V-VI and consisted of pyramidal and polymorphic cell types, while the MB labelling was more superficial in layer V and appeared in pyramidal cell types only. As the labelling was followed through to more posterior regions of the postsubiculum, the thalamic projections remained numerous and formed a distinct band in layer V, while only a few MB-projecting cells were labelled at this level (Fig. 5C).
Dorsal subiculum
Once again, projections to the thalamus were located within the deepest regions of the pyramidal cell layer (layer II) throughout the extent of the SUBd. In the more anterior (septal) SUBd, the label from the AD/AV injection was located within a region of smaller and more densely distributed cell bodies. These cells were located immediately adjacent to the alveus of the hippocampus and consisted of both pyramidal and smaller polymorphic cell types. Projections to MB were again confined to larger (pyramidal) cells in the more superficial levels of layer II (Fig. 5A). The distribution of these two separate populations of cells showed very little overlap and no double labelling was observed. Consequently, this pattern of cell labelling remained constant across the border between the dorsal subiculum and the postsubiculum. In more posterior SUBd, the thalamic projection remained mostly in the deeper layer II, but there was also an increased number of thalamic projections scattered amongst the more superficially located group of MB-projecting cells (Fig. 5B).
Intermediate subiculum
A great many Fast Blue-positive (thalamic injection) cells were distributed throughout the area. Label associated with the lateral MB injection was more sparse and tended to be concentrated near the border with the presubiculum and parasubiculum (Fig. 5C). Even so, cells projecting to AD/AV and MB, respectively, were more intermingled than in the dorsal subiculum.
Ventral subiculum
There was an abundance of Fast Blue-labelled cells in the SUBv following the thalamic injection. These cells were located throughout layer II, yet there were other cases where injections involving AD produced little or no retrograde label in the ventral subiculum (Cases #18_2, #18_11, #20_5 and #20_8). A few CTB488-labelled cells, projecting to the MB, were also present in deeper parts of layer II. Both populations of labelled cells were pyramidal and were highly intermingled.
Presubiculum and parasubiculum
No labelled cells were observed in the presubiculum or parasubiculum following the injection of CTB488 into the lateral MB. Numerous Fast Blue-labelled cells were scattered in deeper layer V and layer VI of the parasubiculum, all of which appeared to be pyramidal (Fig. 5C).
Entorhinal cortex
No CTB488-labelled cells from the lateral MB injection were present in the entorhinal cortex. While a small number of Fast Blue-labelled cells (AD/AV injection) were present in layers II/III of ENTl and ENTm, no comparable label was found in the other 10 cases with injections involving AD or even AV (Table 2).
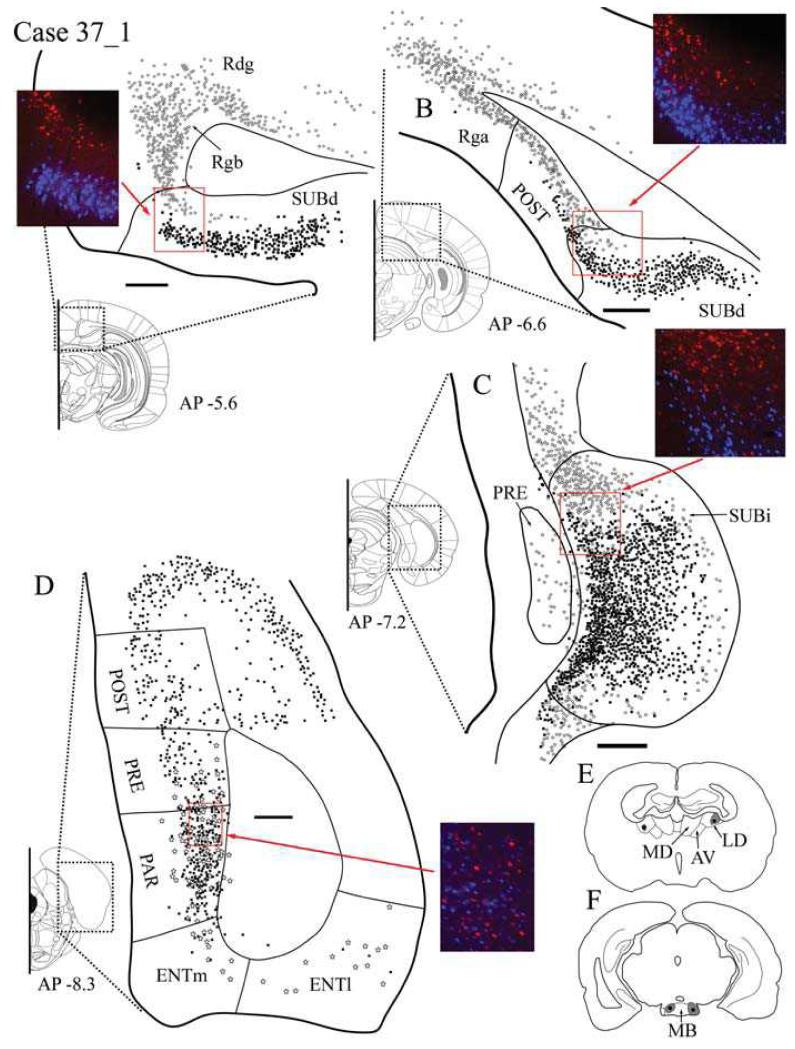
Case #37_1: Lateral dorsal thalamic nucleus and lateral MB
Tracer injection locations
Bilateral injections were made in both MB and the lateral dorsal thalamic nucleus, with a single injection in each site in each hemisphere. In both hemispheres, the thalamic injection was confined within the boundaries of the lateral dorsal thalamic nucleus. Fast Blue (0.04 μl) was injected bilaterally into MB (Fig. 6F). In the left hemisphere, the injection site was confined to the medial mammillary nucleus. On the right, the injection site covered the medial mammillary nucleus but also spread throughout the adjacent lateral mammillary nucleus. The right hemisphere is described in detail (Fig. 6E) as both injections included parts of the head-direction system (LD and LM).
Fig. 6.
A-D: Distribution of labelled cells in Case #37_1 following injections of Fast Blue into the mammillary bodies and CTB488 into the lateral dorsal thalamic nucleus. Fast Blue is indicated by black squares on the line drawings and blue labelled cells on the photomicrographs. The CTB488 label is shown by open stars and red label, respectively. Scale bars = 250 μm; AP positions are in reference to bregma. E: Line drawing of the injection sites within the anterior thalamic nuclei. F: Line drawing of the injection sites within the mammillary bodies. All sections are in the coronal plane. Abbreviations as Table 1.
Retrosplenial cortex
Extensive CTB488 labelling of cells (thalamic injection) was present in all subdivisions of the retrosplenial cortex (Rga, Rgb and Rdg) (Fig. 6A). This CTB488 labelling projecting to the thalamus was located in the deeper layers (V-VI). A very few Fast Blue-labelled cells from the MB injection were found superficially in Rga and Rgb.
Postsubiculum
Labelled cells from both the lateral dorsal thalamic nucleus and MB injections were present in the postsubiculum (Fig. 6B). Fast Blue labelling was restricted to pyramidal cells within layer V, while the more abundant CTB488 labelling was located more deeply in layers V-VI and consisted of both pyramidal and smaller polymorphic cell bodies. There was very little intermingling between these two populations projecting to the lateral dorsal thalamic nucleus or MB, and this separation was displayed throughout the postsubiculum. No double labelled cells were found.
Dorsal subiculum
The mammillary body afferents labelled with Fast Blue were distributed evenly throughout the entire dorsal subiculum (Fig. 6A and B). This labelling consisted of the pyramidal cells in the superficial region of layer II. CTB488-labelled cells following the injection into LD were considerably less numerous and confined to the deepest regions of layer II. In the rostral dorsal subiculum, the CTB488 labelling was mostly localized distal to CA1, and was characterized by a shallow band of labelled cells situated against the alveus (Fig. 6A). Rostrally these two populations remained largely separate, with a few intermingled cells. In more caudal regions of SUBd, the majority of CTB488 labelling was still occurring distal to CA1 and continuous with labelling within the postsubiculum (Fig. 6B). Once again, no double labelling was observed. At the most caudal locations, the labelled thalamic afferents extended proximal to CA1, and become more intermixed with the larger number of Fast Blue-labelled cells.
Intermediate subiculum
As with the previously described SUBd, the Fast Blue (MB injection) labelling in the SUBi tended to be located most commonly in the superficial region of layer II (Fig 6C). Fast Blue labelling was also more abundant in this part of the subiculum, and while the CTB488 labelling from the lateral dorsal thalamic nucleus injection was concentrated where SUBd and SUBv merge into SUBi, far fewer CTB488-labelled cells were lightly scattered throughout the SUBi proper. Some of these labelled mammillary body afferents occurred deeply in layer II and on the most lateral parts of SUBi. All labelled cells were pyramidal, but no double labelled cells were seen.
Ventral subiculum
Unlike SUBd, only a few labelled cells were localized in SUBv. Labelled afferents from both the lateral dorsal thalamic nucleus and MB were present, but those projecting to the mammillary bodies (Fast Blue) were more common. Both tracers were intermingled in rostral areas, and labelled cells became more numerous in the caudal SUBv, with the thalamic projection cells (CTB488) occurring more deeply than those projecting to the MB. Again, no double labelling was present.
Presubiculum and parasubiculum
Labelled cells from the injections of Fast Blue into MB and CTB488 into the lateral dorsal thalamus were both present in the deep layers V/VI of the presubiculum and parasubiculum (Fig. 6D) along the border with the most caudal region of SUBi. The labelling in the parasubiculum was the most numerous, but no double labelled cells were present.
Entorhinal cortex
Following the injection of CTB488 into the lateral dorsal thalamic nucleus, labelled cells were diffusely spread throughout layers II/III and V of the ENTl and continued into layers V/VI of the ENTm. Fast Blue-labelled cells (MB injection) were also observed in layers V-VI of the ENTm, but the very few labelled cells seen in the ENTl were scattered over layers II/III and V.
Additional Cases
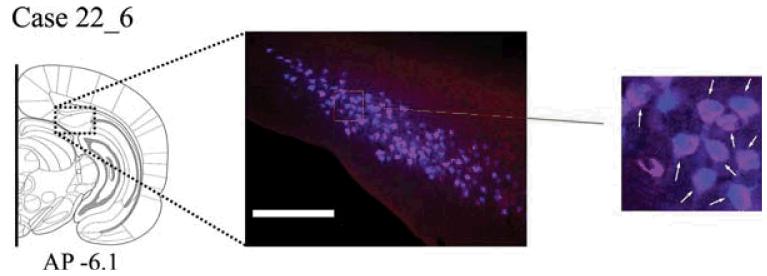
Case #22_6 The rarity of double labelled cells prompted the following test of the sensitivity of the method. Both tracers were placed in the mammillary bodies in the same hemisphere, with CTB488 (0.05 μl) centered in the medial MB and Fast Blue (0.05 μl) centered in the more lateral MB. In both placements, the injection spread into the other injection site. In this case, numerous double labelled cells were evident in SUBd where they appeared purple (Fig. 7). The Fast Blue label completely filled the cells, while the CTB488 label was absent in the cell nucleus. This case helps to confirm that the methodology can reveal double labelled cells should they be present.
Fig. 7.
A: Case #22_6. Photomicrograph of the dorsal subiculum following injections of Fast Blue and CTB488 into overlapping portions of the mammillary bodies. This case confirms that the two tracers can double label the same cell (examples of double labelled cells are indicated by arrows in the magnified region of the photomicrograph shown on the right). Scale bar = 250 μm. Section is in the coronal plane. Abbreviations as Table 1.
Wheat-germ agglutinin (WGA) injections
Six rats received single injections of WGA directed at either the ATN or MB. One typical case involving each of these target sites is described. Both of these WGA cases had a short survival period (24 hour) to limit the extent of any transneuronal retrograde labelling (Collins et al., 1991). These injections are described because: 1) the injections were unilateral, making it possible to describe any contralateral projections, 2) the method is thought to be highly sensitive (Horikawa and Powell, 1986), and 3) the tracer is less prone to uptake by fibers of passage (Steindler, 1982). Finally, the immunoreaction product for WGA is permanent while fluorescent labels fade over time from photo-bleaching.
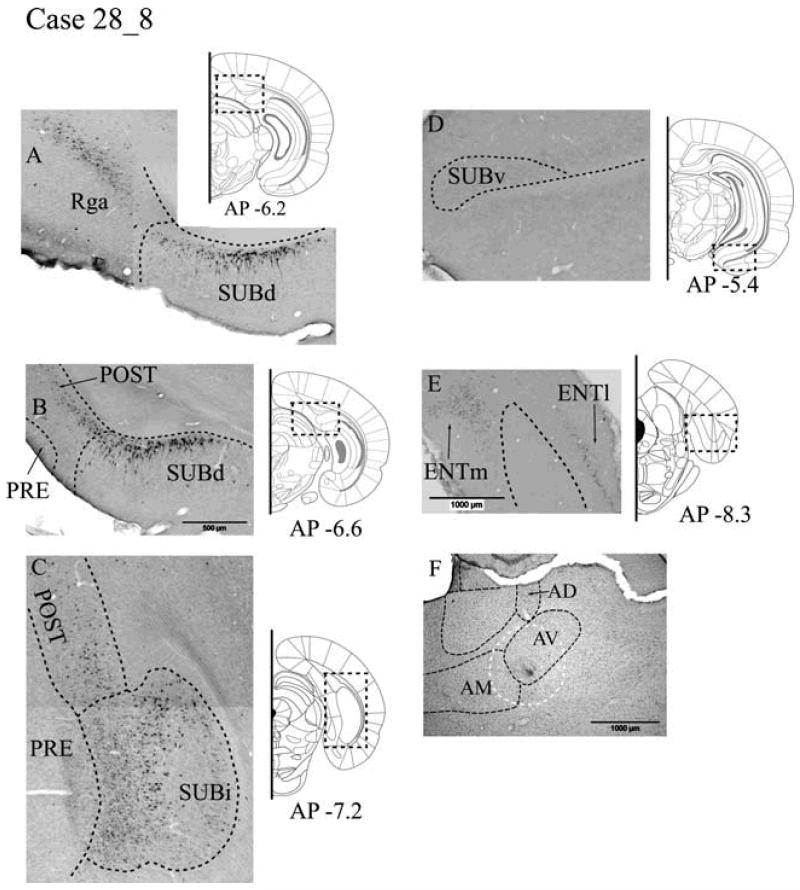
WGA Case #28_8 - anterior thalamic nuclei
Tracer injection locations
The single, unilateral injection of 1% WGA was centered where the dorsal (AVd) and ventral (AVv) regions of AV meet (Fig. 8F). The injection may have also reached the most lateral part of AM (Fig. 8F).
Fig. 8.
A-E: Distribution of labelled cells in Case #28_8 following a unilateral injection of wheat germ agglutinin (WGA) into the anterior thalamic nucleus (primarily the anteroventral nucleus). WGA-positive cells are revealed by immunohistochemistry. Scale bar in B (500 μm) is appropriate for A-E. F: Location of the injection site, with potential spread (white dashes), is shown in the Nissl-stained section of the anterior thalamic region. Scale bar = 1000 μm. All sections are in the coronal plane. Abbreviations as Table 1.
Retrosplenial cortex
Large numbers of WGA-labelled cells were present in both the granular and dysgranular subdivisions (Rdg, Rga and Rgb) of the retrosplenial cortex (Fig. 8A). These WGA-labelled cells were located in the deeper layers (V-VI). While most retrosplenial label was ipsilateral to the injection, numerous labelled cells were also located on the contralateral side.
Postsubiculum
Numerous labelled cells were present throughout the rostro-caudal extent of the postsubiculum, with the majority near the border with the subiculum (Fig. 8B, 8C). These cells were located deep within the internal lamina of the postsubiculum in layers V-VI and appeared to consist of smaller polymorphic cells. Far fewer labelled cells were found in the contralateral postsubiculum.
Subiculum
Anterior thalamic afferents were found within the deepest part of layer II of the dorsal subiculum throughout the structure. These cells extended to the very base of the subiculum, with some located against the alveus (Fig. 8A). This label continued caudally so that numerous pyramidal-like cells containing WGA were distributed throughout the intermediate subiculum (Fig. 8C). In contrast, no WGA-labelled cells were found in the ventral (temporal) SUBv (Fig. 8D).
The labelled cells in the dorsal subiculum were most numerous distal to the CA1 field of the hippocampus. While many of the labelled cells were large pyramidal cells, at the more anterior (septal) levels of the dorsal subiculum, there were also smaller labelled polymorphic cells that occupied the deepest part of layer II. In more posterior regions of the dorsal subiculum, only the larger pyramidal cell type was labelled. Label was present in both hemispheres in the dorsal and intermediate subiculum, although this was primarily in the ipsilateral hemisphere.
Presubiculum and parasubiculum
No retrograde label was observed in the presubiculum or parasubiculum.
Entorhinal projections
The ENTl contained WGA-labelled cells in layer II throughout the rostro-caudal extent of the structure. Most, but not all, labelled cells in the ENTl were located ipsilaterally (Fig. 8E). A deeper scattering of labelled cells appeared in layers IV/V of the ENTm. There was considerable entorhinal label (ENTl and ENTm) on the ipsilateral side but only very few labelled cells in the contralateral hemisphere.
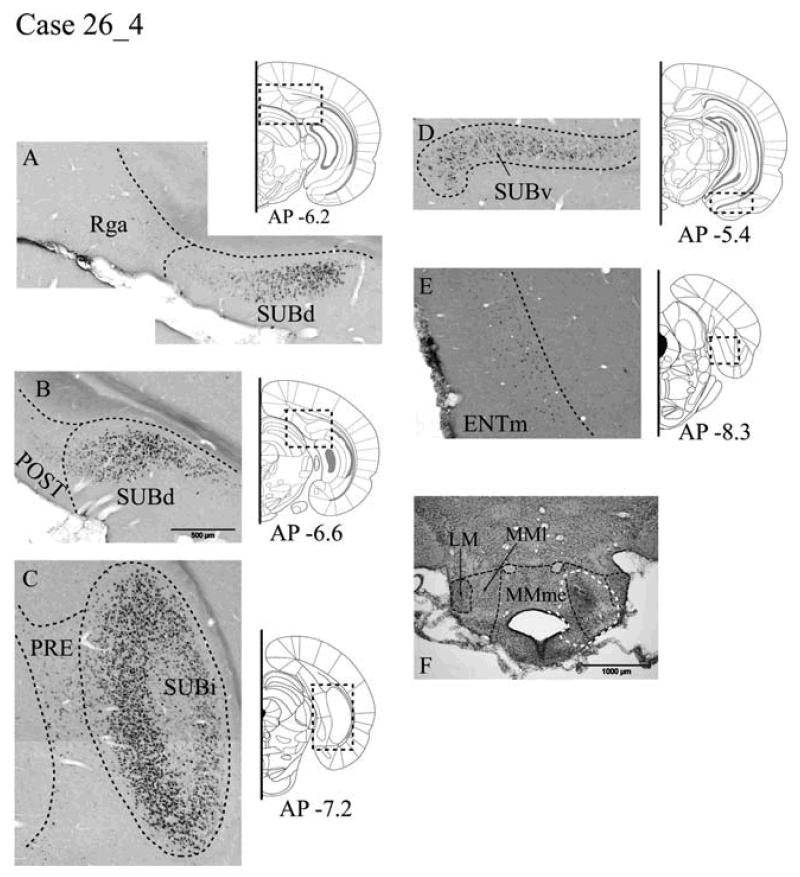
WGA Case #26_4 - mammillary bodies
Tracer injection locations
The single unilateral injection of 1% WGA was centered in the lateral part of the medial mammillary nucleus (Fig. 9F), but may have spread into the neighboring medial portion of the medial nucleus as well as just reaching the lateral mammillary nucleus.
Fig. 9.
A-E: Distribution of labelled cells in Case #26_4 following a unilateral injection of wheat germ agglutinin (WGA) into the mammillary bodies. WGA-positive cells revealed by immunohistochemistry. Scale bar in B (500 μm) is appropriate for A-E. F: Location of the injection site (white arrow) is shown in the Nissl-stained section of the mammillary body region. Scale bar = 1000 μm. All sections are in the coronal plane. Abbreviations as Table 1.
Retrosplenial cortex
In Case #26_4, a handful of labelled cells was found in Rgb, immediately dorsal of the corpus callosum. These cells were located in layer V and appeared in both hemispheres, but were predominantly ipsilateral. Posterior to the splenium, only a few labelled cells were seen in Rga (layer V).
Postsubiculum
The relatively few WGA-labelled cells were almost exclusively located in the ipsilateral postsubiculum. This MB label was more superficially located in layer V than in Case #28_8, and consisted of polymorphic type cells.
Subiculum
Labelled cells were located in a more superficial part of layer II (Fig. 9A, 9B) than in the thalamic WGA case (#28_8). There was a relatively even distribution of this ipsilateral label in the dorsal, intermediate and ventral subiculum (Fig. 9D) that extended from distal regions, adjacent to the postsubiculum, to proximal regions next to the CA1 field. The labelled cells consisted exclusively of large pyramidal cells and appeared particularly numerous in the intermediate subiculum (Fig. 9C). Appreciable label was also present on the contralateral side, which in the dorsal subiculum was close to the CA1 field.
Presubiculum and parasubiculum
A diffuse band of WGA-labelled cells was observed in both the presubiculum and parasubiculum at the level of the SUBi (Fig. 9C). These labelled cells, projecting to MB, were located within layer V of the internal lamina and appeared to be small polymorphic type cells. Labelling in the parasubiculum appeared bilateral, while labelling in the presubiculum was confined to the ipsilateral hemisphere.
Entorhinal projections
No WGA-labelled cells were located in the rostral ENTl, and only a very small number of cells were labelled in the most caudal ENTl. These few cells were confined to the superficial layer II and appeared pyramidal. A scattering of labelled cells was present in posterior ENTm, and this population of small pyramidal cells was located in layers II and III (Fig. 9E). Labelled cells were only present in the ipsilateral entorhinal cortex.
Discussion
Renewed interest in the projections from the hippocampal formation to the anterior thalamic nuclei and mammillary bodies has been prompted by a series of recent clinical studies that underline the importance of these diencephalic sites for episodic memory (Van der Werf et al., 2003; Gold and Squire, 2006; Carlesimo et al., 2007; Tsivilis et al., 2008; Vann et al., 2009). The present results can be summarised succinctly as they demonstrate two independent populations of neurons that project from the subiculum to the mammillary bodies and anterior thalamic nuclei, respectively. While previous studies have suggested that these subicular projections arise from adjacent cell groups (Ishizuka, 2001), the intermingling of the projecting neurons within the intermediate subiculum highlights the need to use multiple tracers to test this assumption. This approach confirmed that the two cell populations remain almost completely separate throughout the subiculum, with the sole exception of a very small minority of cells that innervate both the MB and the anteromedial thalamic nucleus. It was also possible to demonstrate that this segregated property of the subiculum extends dorsally into the retrosplenial cortex and ventrally into the entorhinal cortex, as well as to the postsubiculum, presubiculum, and parasubiculum. As a consequence, the present study both confirmed and extended previous findings (Namura et al., 1994). The discovery that all of these limbic inputs to the anteroventral, anterodorsal, lateral dorsal thalamic nuclei and mammillary bodies have separate sources is all the more intriguing given that there are massive projections from the mammillary bodies to the anterior thalamus (Seki and Katuya, 1984). Indeed, it is thought that every mammillary body neuron projects to the anterior thalamic nuclei (Hopkins, 2005; Vann et al., 2007). As a consequence, these parallel subicular pathways ultimately converge in the anterior thalamic nuclei, one via a direct route, the other via an indirect route.
There already exist numerous descriptions of the projections from the hippocampal formation to the mammillary bodies and anterior thalamus (e.g. Meibach and Siegel, 1975, 1977a; Swanson and Cowan, 1977; Allen and Hopkins, 1989; Shibata, 1989; van Groen and Wyss, 1990b,c; Canteras and Swanson, 1992; Kishi et al., 2000; Ishizuka, 2001). In agreement with these reports, the present study found no evidence of hippocampal projections from within the CA fields. Instead, the projections principally arose from the subiculum and postsubiculum. The populations of cells in the subiculum projecting to the two sites showed a difference in their relationship to the CA1 border, as previously noted (e.g. Sikes et al., 1977; Ishizuka, 2001). The cells in the dorsal subiculum projecting to the anterodorsal or anteroventral thalamic nuclei and also the lateral dorsal thalamic nucleus were concentrated more distal to CA1 but did extend into proximal regions in fewer numbers. Medial mammillary body projections were more evenly distributed across the dorsal subiculum, and the lateral mammillary body projections were concentrated more distally to CA1. Cells in the subiculum projecting to the anteromedial thalamic nucleus were situated proximal to CA1. Further projections to both of the target diencephalic sites arose from the parasubiculum and presubiculum, although these projections were only noted in the minority of cases involving injections into AD and AV. The sporadic nature of theprojection from the presubiculum to the anterior thalamus is consistent with evidence that the presubiculum preferentially innervates the lateral dorsal thalamic nucleus, with only light projections restricted to dorsolateral part of the anteroventral nucleus along with the anterodorsal thalamic nucleus (Van Groen and Wyss, 1990c; Ishizuka, 2001). For those cases where the subicular projections to the anteroventral nucleus were described in detail (cases #28_3 and #28_7), the injection sites were located in ventral AV and, hence, below the site of termination of the presubicular projections to this thalamic nucleus (Van Groen and Wyss, 1990c). Support for this explanation comes from the presence of sparse label in the presubiculum (and parasubiculum) in other cases (Table 2) with more dorsal injections involving AV and AD. For all mammillary body injections, presubiculum and parasubiculum label was much more prominent in the more sensitive WGA case, with far fewer labeled cells present in the same sites only in a limited number of cases involving fluorescent tracer injections in the mammillary bodies. Previous anterograde tracing studies also indicate that both the presubiculum and parasubiculum project lightly to the mammillary bodies (Swanson and Cowan, 1977; Van Groen and Wyss, 1990c), but terminate in different regions within the lateral part of the medial mammillary nucleus (Van Groen and Wyss, 1990c).
Numerous projections to the anteroventral, anterodorsal and anteromedial nuclei arose from the granular a and b retrosplenial subregions, along with lighter inputs from the dysgranular retrosplenial cortex. A similar pattern of projections from the lateral dorsal thalamic nucleus occurred, but with far more cells of origin in the dysgranular retrosplenial cortex. This distribution of efferents across the retrosplenial subregions closely matches the known pattern of projections to the anterior thalamic nuclei and the lateral dorsal nucleus (Berger et al., 1980; Van Groen and Wyss, 1990a, 1992a, 2003; Shibata, 1998). In addition, there was evidence of very light projections to the mammillary bodies from the granular a retrosplenial cortex and from the most ventral portion of the granular b retrosplenial cortex, again consistent with previous reports (Van Groen and Wyss, 1990a, 2003).
In many of the experiments, injections into MB produced retrograde labeling of cells in layers II/III of ENTm. In a subset of these cases lightly scattered label was also present in layers II/III of ENTl. That the large majority of these inputs arose from the medial entorhinal cortex is largely consistent with previous studies that have also emphasized the importance of ENTm rather than ENTl as the source of these projections (Shibata, 1988). Surprisingly, few labeled cells were found in the entorhinal cortex after injections into the anterior thalamic nuclei, most being observed in ENTm after injections into the anteromedial nuclei. Evidence of a more substantial entorhinal input (principally from ENTm) to the lateral dorsal thalamic nucleus matched previous observations (Shibata, 1996). There was also an appreciable input to the mammillary bodies from the caudal amygdalohippocampal area (Pitkanen, 2000).
Within the subiculum, there was evidence of a septal/temporal gradient as the thalamic inputs lessened very appreciably in the ventral (temporal) subiculum. A similar pattern has been described (Meibach and Siegel 1977b; Sikes et al., 1977; Swanson and Cowan 1977; Canteras and Swanson, 1992; Namura et al., 1994) as all of these studies reported few if any inputs to the anterior thalamic nuclei from the ventral (temporal) subiculum. A rather different conclusion is provided by Ishizuka (2001) who reported that all parts of the septo-temporal axis of the subiculum project to the anterior thalamic nuclei, but that the caudal anterior thalamic nuclei receive most inputs from the septal subiculum while the rostral anterior thalamic nuclei receive most inputs from temporal subiculum. This conclusion by Ishizuka (2001) does not appear to match our results, nor does it agree with those anatomical studies that have placed anterograde tracers in the ventral subiculum and reported few if any projections to the anterior thalamic nuclei (Meibach and Siegel, 1977b; Swanson and Cowan, 1977; Canteras and Swanson, 1992). Unlike the anterior thalamic projections, all parts of the septal-temporal axis of the subiculum gave rise to mammillary body projections, although these projections again have a topographic arrangement (Shibata, 1989; Allen and Hopkins, 1989; Kishi et al., 2000).
Particularly striking was the consistent separation between the cells of origin of these two major pathways. Not only was the separation found in the subiculum, as strongly suggested by previous studies (Namura et al., 1994; Ishizuka, 2001), but it extended to all other regions under investigation. This same independent pattern of innervation was found for the head-direction system, i.e. when injections involved the anterodorsal or lateral dorsal thalamic nuclei and the lateral mammillary nucleus in the same animal. These three nuclei, along with the postsubiculum, are distinguished by the fact that they contain ‘head-direction’ cells, presumed to be important for navigation (Wiener and Taube, 2005). Consequently, these nuclei can be separated from the other anterior thalamic and mammillary nuclei by virtue of their different anatomical and electrophysiological properties (Vann and Aggleton, 2004; Hopkins, 2005). Even so, all of these diencephalic regions share the common feature of having independent inputs from the hippocampus and parahippocampal regions.
Other evidence suggests that the present finding (that the subpopulations of subicular projections to the mammillary bodies and anterior thalamus arise from different neurons) can be extended to the subicular projections to other subcortical sites, such as nucleus accumbens, ventromedial hypothalamus, and the lateral septum (Namura et al., 1994; Naber and Witter, 1998; Ishizuka, 2001). Two of these studies (Namura et al., 1994; Naber and Witter, 1998) used pairs of fluorescent retrograde tracers. Both studies found only a small percentage (1-4%) of double labelled cells for the subicular projections to the various pairing of these sites. In the present study, only one combination of injections (anteromedial thalamic nucleus and medial mammillary bodies) produced double labeled cells, and these only reached levels around 4% where they were most numerous (ventral subiculum). A rather different picture was found, however, when comparing the subicular efferents to the hypothalamus (e.g. mammillary bodies or ventromedial hypothalamus) with the projections to a cortical (entorhinal cortex) site (Donovan and Wyss, 1983). Now, the source cells in the subiculum that project to these sites were closely intermingled, with as many as one third of the cells projecting to both targets. Similarly, about one third of the dorsal subicular cells that project to the septum are also thought to project to the entorhinal cortex (Swanson et al., 1981). In contrast, the study by Naber and Witter (1998) examined many more combinations of cortical and subcortical sites but found far lower percentages of double labeled cells. The conclusion is that subicular cells can give rise to collateral projections to more than one site, but that these bifurcations are typically rare and that, for the numerous projections to the mammillary bodies and anterior thalamic nuclei, such collateral projections are almost completely absent.
One consistent finding concerning the laminar organization of the subiculum was that different cell layers projected to different structures, as previously noted by other studies (Ishizuka, 2001). An alternative subicular organization has been highlighted by other anatomists who have reported that many subicular projections have a topography based upon their proximity with the CA1 border (Kloosterman et al., 2001; Witter, 2006). This pattern gives rise to columnar modules within the subiculum (Witter, 2006) that are orthogonal to the lamina differences in the origins of the projections to the mammillary bodies and anterior thalamic. The implication from the present findings is that the emerging idea of the subiculum as a complex matrix of columns with subtly changing patterns of afferents and efferents (Witter, 2006) will need to accommodate the laminar gradients of some of their major efferents. The final point relates to the importance for memory of the connections under investigation. The conclusion from the present study is that the hippocampal formation projections via the fornix to the mammillary bodies and anterior thalamic nuclei are potentially capable of providing independent information, despite the strong likelihood of an ultimate convergence within the anterior thalamic nuclei. To this pattern, we can add the retrosplenial cortex, which is also important for memory (Vann et al., 2009). This conclusion concerning the convergence of independent information on the anterior thalamic and lateral dorsal nuclei rests on the assumption that the different populations of subicular, entorhinal and retrosplenial cells have their own distinct patterns of afferent information, a potentially important issue that remains to be resolved.
Table 1.
Table of Abbreviations Used in Figures and Tables.
| AD | anterior dorsal nucleus thalamus | AHA | amydalohippocampal area |
| AM | anteromedial thalamic nucleus | ATN | anterior thalamic nucleus |
| AV | anteroventral nucleus thalamus | AVvl | anteroventral thalamic nucleus (ventrolateral) |
| AVdm | anteroventral thalamic nucleus (dorsomedial) | COApm | cortical nucleus amygdala, (posterior medial) |
| ENTl | entorhinal area (lateral part) | ENTm | entorhinal area (medial part) |
| fi | fimbria | LDvl | latero dorsal nucleus thalamus (ventrolateral) |
| MB | mammillary bodies | MDl | mediodorsal nucleus thalamus (lateral part) |
| MDm | mediodorsal nucleus thalamus (medial part) | MM | medial mammillary nucleus |
| LM | lateral mammillary nucleus | MMme | medial mammillary nucleus (median part) |
| MMl | medial mammillary nucleus (lateral part) | MoDG | molecular layer of the dentate gyrus |
| PAR | parasubiculum | POST | postsubiculum |
| PRE | presubiculum | sm | stria medullaris of the thalamus |
| Rga | retrosplenial granular a cortex | Rgb | retrosplenial granular a cortex |
| SUBd | subiculum (dorsal part) | SUBi | subiculum (intermediate part) |
| SUBv | subiculum (ventral part) | SUMl | supramammillary nucleus (lateral part) |
| SUMm | supramammillary nucleus (median part) | TR | postpiriform transition area |
| VAL | ventral anterior-lateral complex thalamus |
Acknowledgments
Grant Sponsor: Wellcome Trust; Grant number #081075
Literature Cited
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–489. [PubMed] [Google Scholar]
- Aggleton JP, Desimone R, Mishkin M. The origin, course, and termination of the hippocampothalamic projections in the macaque. J Comp Neurol. 1986;243:409–421. doi: 10.1002/cne.902430310. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Friedman DP, Mishkin M. A comparison between the connections of the amygdala and hippocampus with the basal forebrain in the macaque. Exp Brain Res. 1987;67:556–568. doi: 10.1007/BF00247288. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, McMackin D, Carpenter K, Hornak J, Kapur N, Halpin S, Wiles CM, Kamel H, Brennan P, Carton S, Gaffan D. Differential cognitive effects of colloid cysts in the third ventricle that spare or compromise the fornix. Brain. 2000;123:800–815. doi: 10.1093/brain/123.4.800. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Vann SD, Saunders RC. Projections from the hippocampal region to the mammillary bodies in macaque monkeys. Eur J Neurosci. 2005;22:2519–2530. doi: 10.1111/j.1460-9568.2005.04450.x. [DOI] [PubMed] [Google Scholar]
- Allen GV, Hopkins DA. Mamillary body in the rat: topography and synaptology of projections from the subicular complex, prefrontal cortex, and midbrain tegmentum. J Comp Neurol. 1989;286:311–336. doi: 10.1002/cne.902860303. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M, Kultas-Ilinsky K, Ilinsky I. Limbic thalamus: structure, intrinsic organization, and connections. In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus: A comprehensive handbook. Birkhauser; Boston: 1993. pp. 71–122. [Google Scholar]
- Berger TW, Milner TA, Swanson GW, Lynch GS, Thompson RF. Reciprocal anatomical connections between anterior thalamus and cingulate-retrosplenial cortex in the rabbit. Brain Res. 1980;201:411–417. doi: 10.1016/0006-8993(80)91044-6. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Serra L, Fadda L, Cherubini A, Bozzali M, Caltagirone C. Bilateral damage to the mammillo-thalamic tract impairs recollection but not familiarity in the recognition process: a single case investigation. Neuropsychologia. 2007;45:2467–2479. doi: 10.1016/j.neuropsychologia.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Collins WF, III, Erichsen JT, Rose RD. Pudendal motor and premotor neurons in the male rat: a WGA transneuronal study. J Comp Neurol. 1991;308:28–41. doi: 10.1002/cne.903080104. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Verfaellie M, Alexander MP, Katz DI. Amnesia following traumatic bilateral fornix transection. Neurology. 1995;45:1546–1550. doi: 10.1212/wnl.45.8.1546. [DOI] [PubMed] [Google Scholar]
- Donovan MK, Wyss JM. Evidence for some collateralization between cortical and diencephalic efferent axons of the rat subicular cortex. Brain Res. 1983;259:181–192. doi: 10.1016/0006-8993(83)91249-0. [DOI] [PubMed] [Google Scholar]
- Dusoir H, Kapur N, Byrnes DP, McKinstry S, Hoare RD. The role of diencephalic pathology in human memory disorder. Evidence from a penetrating paranasal brain injury. Brain. 1990;113:1695–1706. doi: 10.1093/brain/113.6.1695. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Gaffan EA. Amnesia in man following transection of the fornix. A review. Brain. 1991;114:2611–2618. doi: 10.1093/brain/114.6.2611. [DOI] [PubMed] [Google Scholar]
- Gold JJ, Squire LR. The anatomy of amnesia: neurohistological analysis of three new cases. Learn Mem. 2006;13:699–710. doi: 10.1101/lm.357406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, Te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain. 2000;123:141–154. doi: 10.1093/brain/123.1.141. [DOI] [PubMed] [Google Scholar]
- Henry J, Petrides M, St-Laurent M, Sziklas V. Spatial conditional associative learning: effects of thalamo-hippocampal disconnection in rats. Neuroreport. 2004;15:2427–2431. doi: 10.1097/00001756-200410250-00025. [DOI] [PubMed] [Google Scholar]
- Hopkins DA. Neuroanatomy of head direction cell circuits. In: Wiener SI, Taube JS, editors. Head direction cells and the neural mechanisms of spatial orientation. MIT Press; Cambridge, MA: 2005. pp. pp17–44. [Google Scholar]
- Horikawa K, Powell EW. Comparison of techniques for retrograde labeling using the rat’s facial nucleus. J Neurosci Methods. 1986;17:287–296. doi: 10.1016/0165-0270(86)90129-9. [DOI] [PubMed] [Google Scholar]
- Ishizuka N. Laminar organization of the pyramidal cell layer of the subiculum in the rat. J Comp Neurol. 2001;435:89–110. doi: 10.1002/cne.1195. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Ono K, Yokota S, Ishino H, Yasui Y. Topographical organization of projections from the subiculum to the hypothalamus in the rat. J Comp Neurol. 2000;419:205–222. doi: 10.1002/(sici)1096-9861(20000403)419:2<205::aid-cne5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Kloosterman F, Peloquin P, Leung LS. Apical and basal orthodromic population spikes in hippocampal CA1 in vivo show different origins and patterns of propagation. J Neurophysiol. 2001;86:2435–2444. doi: 10.1152/jn.2001.86.5.2435. [DOI] [PubMed] [Google Scholar]
- Kloosterman F, Witter MP, Van Haeften T. Topographical and laminar organization of subicular projections to the parahippocampal region of the rat. J Comp Neurol. 2003;455:156–171. doi: 10.1002/cne.10472. [DOI] [PubMed] [Google Scholar]
- McMackin D, Cockburn J, Anslow P, Gaffan D. Correlation of fornix damage with memory impairment in six cases of colloid cyst removal. Acta Neurochir. 1995;135:12–18. doi: 10.1007/BF02307408. [DOI] [PubMed] [Google Scholar]
- Meibach RC, Siegel A. The origin of fornix fibers which project to the mammillary bodies in the rat: a horseradish peroxidase study. Brain Res. 1975;88:508–512. doi: 10.1016/0006-8993(75)90662-9. [DOI] [PubMed] [Google Scholar]
- Meibach RC, Siegel A. Efferent connections of the hippocampal formation in the rat. Brain Res. 1977a;124:197–224. doi: 10.1016/0006-8993(77)90880-0. [DOI] [PubMed] [Google Scholar]
- Meibach RC, Siegel A. Thalamic projections of the hippocampal formation: evidence for an alternate pathway involving the internal capsule. Brain Res. 1977b;134:1–12. doi: 10.1016/0006-8993(77)90921-0. [DOI] [PubMed] [Google Scholar]
- Naber PA, Witter MP. Subicular efferents are organized mostly as parallel projections: a double-labeling, retrograde-tracing study in the rat. J Comp Neurol. 1998;393:284–297. [PubMed] [Google Scholar]
- Namura S, Takada M, Kikuchi H, Mizuno N. Topographical organization of subicular neurons projecting to subcortical regions. Brain Res Bull. 1994;35:221–231. doi: 10.1016/0361-9230(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Olszewski I. The thalamus of Macaca mulatta. S.Karger; Basel: 1952. [Google Scholar]
- Parker A, Eacott MJ, Gaffan D. The recognition memory deficit caused by mediodorsal thalamic lesion in non-human primates: a comparison with rhinal cortex lesion. Eur J Neurosci. 1997a;9:2423–2431. doi: 10.1111/j.1460-9568.1997.tb01659.x. [DOI] [PubMed] [Google Scholar]
- Parker A, Gaffan D. The effect of anterior thalamic and cingulate cortex lesions on object-in-place memory in monkeys. Neuropsychologia. 1997b;35:1093–1102. doi: 10.1016/s0028-3932(97)00042-0. [DOI] [PubMed] [Google Scholar]
- Pitkanen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The amygdala: A functional analysis. Oxford University Press; Oxford: 2000. pp. 31–115. [Google Scholar]
- Poletti CE, Creswell G. Fornix system efferent projections in the squirrel monkey: an experimental degeneration study. J Comp Neurol. 1977;175:101–128. doi: 10.1002/cne.901750107. [DOI] [PubMed] [Google Scholar]
- Rose J. The cell structure of the mamillary body in the mammals and in man. J Anat. 1939;74:91–115. [PMC free article] [PubMed] [Google Scholar]
- Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Seki M, Zyo K. Anterior thalamic afferents from the mamillary body and the limbic cortex in the rat. J Comp Neurol. 1984;229:242–256. doi: 10.1002/cne.902290209. [DOI] [PubMed] [Google Scholar]
- Shibata H. A direct projection from the entorhinal cortex to the mammillary nuclei in the rat. Neurosci Lett. 1988;90:6–10. doi: 10.1016/0304-3940(88)90777-x. [DOI] [PubMed] [Google Scholar]
- Shibata H. Descending projections to the mammillary nuclei in the rat, as studied by retrograde and anterograde transport of wheat germ agglutinin-horseradish peroxidase. J Comp Neurol. 1989;285:436–452. doi: 10.1002/cne.902850403. [DOI] [PubMed] [Google Scholar]
- Shibata H. Direct projections from the entorhinal area to the anteroventral and laterodorsal thalamic nuclei in the rat. Neurosci Res. 1996;26:83–87. doi: 10.1016/0168-0102(96)01083-8. [DOI] [PubMed] [Google Scholar]
- Shibata H. Organization of projections of rat retrosplenial cortex to the anterior thalamic nuclei. Eur J Neurosci. 1998;10:3210–3219. doi: 10.1046/j.1460-9568.1998.00328.x. [DOI] [PubMed] [Google Scholar]
- Sikes RW, Chronister RB, White LE., Jr. Origin of the direct hippocampus--anterior thalamic bundle in the rat: a combined horseradish peroxidase--Golgi analysis. Exp Neurol. 1977;57:379–395. doi: 10.1016/0014-4886(77)90074-7. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Hartley T, Vargha-Khadem F, O’Keefe J. Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus. 2001;11:715–725. doi: 10.1002/hipo.1087. [DOI] [PubMed] [Google Scholar]
- Sripanidkulchai K, Wyss JM. Thalamic projections to retrosplenial cortex in the rat. J Comp Neurol. 1986;254:143–165. doi: 10.1002/cne.902540202. [DOI] [PubMed] [Google Scholar]
- Steindler DA. Differences in the labeling of axons of passage by wheat germ agglutinin after uptake by cut peripheral nerve versus injections within the central nervous system. Brain Res. 1982;250:159–167. doi: 10.1016/0006-8993(82)90963-5. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. Hippocampo-hypothalamic connections: origin in subicular cortex, not ammon’s horn. Science. 1975;189:303–304. doi: 10.1126/science.49928. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Cowan WM. Evidence for collateral projections by neurons in Ammon’s horn, the dentate gyrus, and the subiculum: a multiple retrograde labeling study in the rat. J Neurosci. 1981;1:548–559. doi: 10.1523/JNEUROSCI.01-05-00548.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Kohler C, Bjorklund A. The limbic region. I: The septohippocampal system. In: Bjorklund A, Hokfelt T, Swanson LW, editors. The handbook of chemical neuroanatomy. Vol. 5: Integrated systems of the CNC, Part 1. Elsevier Science; Amsterdam: 1987. pp. pp125–277. [Google Scholar]
- Swanson LW. Brain Maps. Elsevier; Amsterdam: 1992. Structure of the Rat Brain. [Google Scholar]
- Taube JS. The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- Tsivilis D, Vann SD, Denby C, Roberts N, Mayes AR, Montaldi D, Aggleton JP. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci. 2008;11:834–842. doi: 10.1038/nn.2149. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Uylings HB, Jolles J. Neuropsychology of infarctions in the thalamus: a review. Neuropsychologia. 2000;38:613–627. doi: 10.1016/s0028-3932(99)00104-9. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Jolles J, Witter MP, Uylings HB. Contributions of thalamic nuclei to declarative memory functioning. Cortex. 2003;39:1047–1062. doi: 10.1016/s0010-9452(08)70877-3. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. Connections of the retrosplenial granular a cortex in the rat. J Comp Neurol. 1990a;300:593–606. doi: 10.1002/cne.903000412. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. The postsubicular cortex in the rat: characterization of the fourth region of the subicular cortex and its connections. Brain Res. 1990b;529:165–177. doi: 10.1016/0006-8993(90)90824-u. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. The connections of presubiculum and parasubiculum in the rat. Brain Res. 1990c;518:227–243. doi: 10.1016/0006-8993(90)90976-i. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. Connections of the retrosplenial dysgranular cortex in the rat. J Comp Neurol. 1992a;315:200–216. doi: 10.1002/cne.903150207. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. Projections from the laterodorsal nucleus of the thalamus to the limbic and visual cortices in the rat. J Comp Neurol. 1992b;324:427–448. doi: 10.1002/cne.903240310. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Kadish I, Wyss JM. The role of the laterodorsal nucleus of the thalamus in spatial learning and memory in the rat. Behav Brain Res. 2002;136:329–337. doi: 10.1016/s0166-4328(02)00199-7. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol. 2003;463:249–263. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- Von Cramon DY, Hebel N, Schuri U. A contribution to the anatomical basis of thalamic amnesia. Brain. 1985;108:993–1008. doi: 10.1093/brain/108.4.993. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one? Nat Rev Neurosci. 2004;5:35–44. doi: 10.1038/nrn1299. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Vann SD, Saunders RC, Aggleton JP. Distinct, parallel pathways link the medial mammillary bodies to the anterior thalamus in macaque monkeys. Eur J Neurosci. 2007;26:1575–1586. doi: 10.1111/j.1460-9568.2007.05773.x. [DOI] [PubMed] [Google Scholar]
- Vann SD, Tsivilis D, Denby CE, Quamme J, Yonelinas AP, Aggleton JP, Montaldi M, Mayes AR. Impaired recollection but spared familiarity in patients with extended hippocampal system damage: convergence across three methods. Proc Natl Acad Sci (USA) 2009;106:5442–5447. doi: 10.1073/pnas.0812097106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, Baird AL, Morgan A, Muir JL, Aggleton JP. The conjoint importance of the hippocampus and anterior thalamic nuclei for allocentric spatial learning: evidence from a disconnection study in the rat. J Neurosci. 2001;21:7323–7330. doi: 10.1523/JNEUROSCI.21-18-07323.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, Baird AL, Morgan A, Muir JL, Aggleton JP. Disconnecting hippocampal projections to the anterior thalamus produces deficits on tests of spatial memory in rats. Eur J Neurosci. 2000;12:1714–1726. doi: 10.1046/j.1460-9568.2000.00039.x. [DOI] [PubMed] [Google Scholar]
- Weiner SI, Taube JS. Head direction cells and the neural mechanisms of spatial orientation. MIT Press; Cambridge, MA: 2005. [Google Scholar]
- Witter MP. Connections of the subiculum of the rat: topography in relation to columnar and laminar organization. Behav Brain Res. 2006;174:251–264. doi: 10.1016/j.bbr.2006.06.022. [DOI] [PubMed] [Google Scholar]