Abstract
Plant viruses may affect the viability and development process of their herbivore vectors. Small brown planthopper (SBPH) is main vector of Rice stripe virus (RSV), which causes serious rice stripe disease. Here, we reported the effects of RSV on SBPH offspring by crossing experiments between viruliferous and non-viruliferous strains. The life parameters of offspring from different cross combinations were compared. The hatchability of F1 progeny from viruliferous parents decreased significantly, and viruliferous rate was completely controlled by viruliferous maternal parent. To better elucidate the underlying biological mechanisms, the morphology of eggs, viral propagation and distribution in the eggs and expression profile of embryonic development genes were investigated. The results indicated that RSV replicated and accumulated in SBPH eggs resulting in developmental stunt or delay of partial eggs; in addition, RSV was only able to infect ovum but not sperm. According to the expression profile, expression of 13 developmental genes was regulated in the eggs from viruliferous parents, in which two important regulatory genes (Ls-Dorsal and Ls-CPO) were most significantly down-regulated. In general, RSV exerts an adverse effect on SBPH, which is unfavourable for the expansion of viruliferous populations. The viewpoint is also supported by systematic monitoring of SBPH viruliferous rate.
Rice stripe virus (RSV) has caused serious disease in rice fields in China over the last few decades1. RSV is transmitted mainly by the small brown planthopper (SBPH) Laodelphax striatellus Fallén in a persistent, circulative-propagative manner2. After invading SBPH, RSV escapes from the midgut, salivary glands and ovary barriers and propagates throughout the body3,4. It has been confirmed that the ribonucleoproteins (RNPs) of RSV exist in follicular cells of the ovarioles and can be transmitted vertically from female adults to their progeny via eggs3,5. Female and male adults and nymphs all can transmit the virus, whereas SBPH nymphs were reported as more efficient vectors than adults, and females as more efficient vectors than males in terms of RSV transmission2. The epidemic and outbreak of rice stripe disease are closely related to the occurrence of viruliferous SBPH populations6. Moreover, the latest research has shown that a few SBPH could also transmit rice stripe disease to overseas rice fields through long-distance migration in East Asian countries7. Therefore, elucidating the interactions between RSV and SBPH is crucial for disease control.
The variation in the relationships between plant viruses and their herbivore vectors may affect the viability and development process of vector offspring. Studying the viral effects on vectors is crucial for a better understanding of RSV's epidemiology and designing control strategies8. Currently, whether RSV has a favourable or adverse effect on SBPH and its offspring remains very unclear. Some previous studies showed that the number and hatchability of eggs from viruliferous SBPH populations decreased9. However, Kisimoto (1965)10 reported that the fecundity and longevity of RSV-infected SBPH were unaffected. To better understand the viral influences on insect offspring, we compared the life parameters of F1 progeny from 4 cross combinations of viruliferous (V) and non-viruliferous (N) parents, including the fecundity, hatchability, developmental phase, survival rate, viruliferous rate (VR) and sex ratio. Thereafter, biological, molecular and field ecological studies were further performed to analyse the results of the crossing experiments.
Results
The life parameters of SBPH in crossing experiments
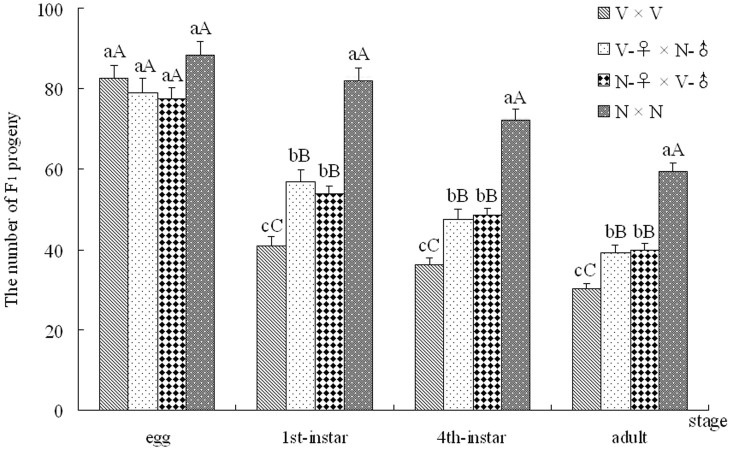
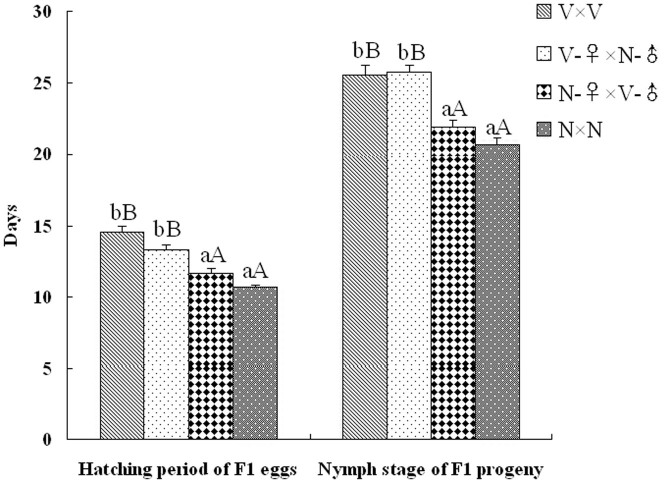
After crosses, the life parameters of SBPH were recorded, including the numbers (eggs, nymphs and adults), the hatching period and the developmental phase of F1 progeny from the four crosses (V × V, V-♀ × N-♂, N-♀ × V-♂ and N × N). The quantities of F1 progeny from each cross group were plotted with SBPH major developmental stages (egg, 1st-instar, 4th-instar and adult stages) as the abscissa (Fig. 1). As is shown in Fig. 1, the number of eggs from each cross was greater than 77 without a significant difference, which suggests that viruliferous and non-viruliferous SBPH parents have similar fecundity on rice plants. After hatching, the hatchabilities of the F1 eggs from different crosses were compared. The number of 1st-instar nymphs from the N × N cross reached 82.0, a 1.44-, 1.53- and 2.0-fold, respectively, higher value than the nymphs from the V-♀ × N-♂, N-♀ × V-♂ and V × V crosses. Compared with the highest hatchability (92.76%) of the F1 eggs from the N × N cross, the hatchabilities of the F1 eggs from the other three crosses were significantly decreased, in which the V × V cross was the lowest (49.70%). The survival rate (SR) of the 4th-instar nymphs was 88.17% (N × N), 83.30% (V-♀ × N-♂), 90.13% (N-♀ × V-♂) and 88.29% (V × V), without significant differences. Similarly, the SR situation of the F1 adults from 4 crosses was basically consistent with that of the 4th-instar nymphs (Fig. 1). In addition, the hatching periods and nymph stages of F1 progeny were also compared after ovipositions. The hatching periods of the F1 eggs from the V × V (14.55 days) and V-♀ × N-♂ (13.30 days) crosses were significantly longer relative to the other two cross groups (10.65 and 11.60 days) (Fig. 2). Similarly, the nymph stages of the F1 progeny from the V × V and V-♀ × N-♂ crosses were 25.55 and 25.70 days, respectively, and were significantly longer compared to the other two crosses (20.70 and 21.85 days) (Fig. 2). Furthermore, there were no significant differences in the sex ratio of the F1 adults from all crosses (Table S2). After statistical analysis, F1 adult individuals were used to detect VR using the DIBA method. The VR of the F1 progeny from the V × V and V-♀ × N-♂ crosses were both 100% (no difference between female and male), and that of the other two crosses were 0% (Table S2), which suggests that the VR of the SBPH offspring is completely controlled by the viruliferous maternal parent.
Figure 1. The numbers of SBPH F1 progeny from the different cross groups on major developmental stages.
Major developmental stages included the egg, 1st-instar, 4th-instar and adult stages. V and N are viruliferous and non-viruliferous strains, respectively. All date are shown as the mean (± SE), and the different letters above the error bars indicate significant difference with a statistical analysis system (SAS) followed by Tukey's honest significant difference (HSD) test (P < 0.01 and P < 0.05).
Figure 2. The hatching period and nymph stage of the F1 progeny from the 4 cross groups.
All date are shown as the mean (± SE), and the different letters above the error bars indicate significant difference with a statistical analysis system (SAS) followed by Tukey's honest significant difference (HSD) test (P < 0.01 and P < 0.05).
Morphology of delayed eggs
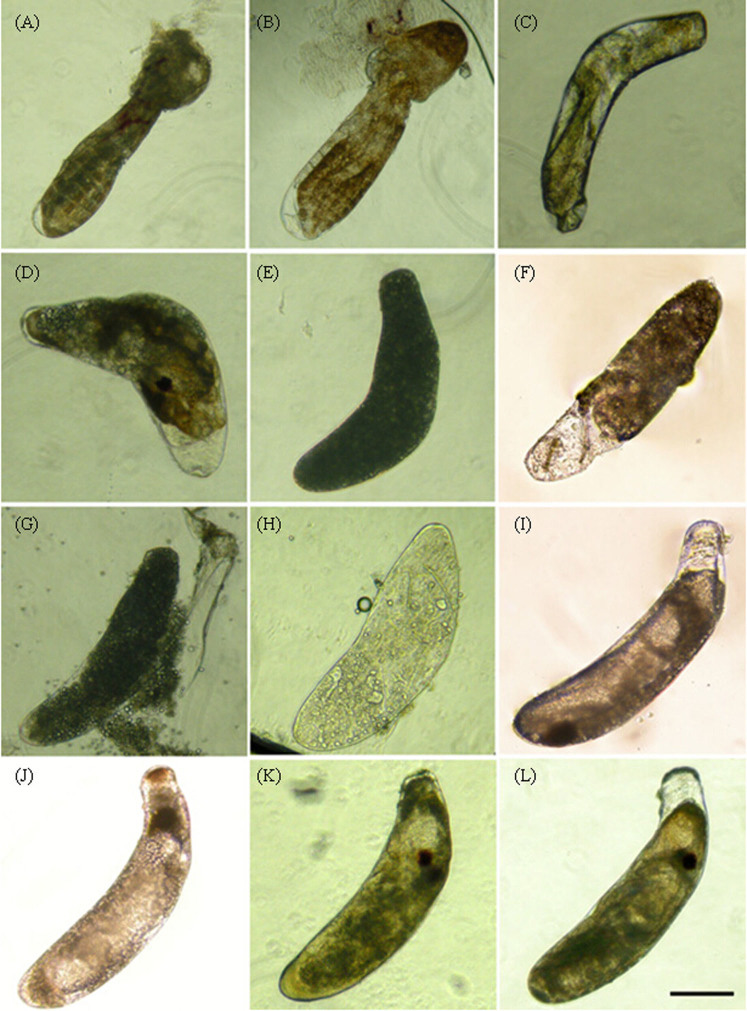
The significant decline in the hatchability of offspring from viruliferous SBPH parents was observed through cross experiments. To explain this phenomenon, microscopy of eggs from viruliferous parents was performed. The results showed that a number of eggs (approximately 25%) were developmentally stunted or impaired in rice sheaths at the time when most 1st-instar nymphs from non-viruliferous SBPH had already hatched. As shown in Fig. 3, some eggs became malformations or wizened (Fig. 3A–D), and some were already dead and rotten (blackening) (Fig. 3E–H). Moreover, the majority of the eggs (approximately 75%) were developmentally delayed without morphological abnormalities; for example, their egg-eyes had not yet formed until the 10th day after spawning (Fig. 3I), whereas normal eggs only required 4–5 days to reach this stage (Fig. 3K). After microscopy, RNA from the delayed and stunted eggs was used to detect the RSV RNP gene via qPCR, and the result of the amplification was positive indicating that these abnormal eggs were infected by RSV.
Figure 3. The morphology of delayed and stunted eggs.
A–I: The stunted or delayed eggs from viruliferous SBPH parents. Some eggs became malformations (A, B and D) or wizened (C), some were already dead, rotten and blackening (E–H), and some eggs were developmentally delayed that their egg-eyes had not yet formed until the 10th day after spawning (I); J–L: non-viruliferous normal eggs in the 2nd (J), 5th (K) and 7th (L) day after spawning. Scale bars: 200 μm.
Analysis of RSV propagation in the eggs
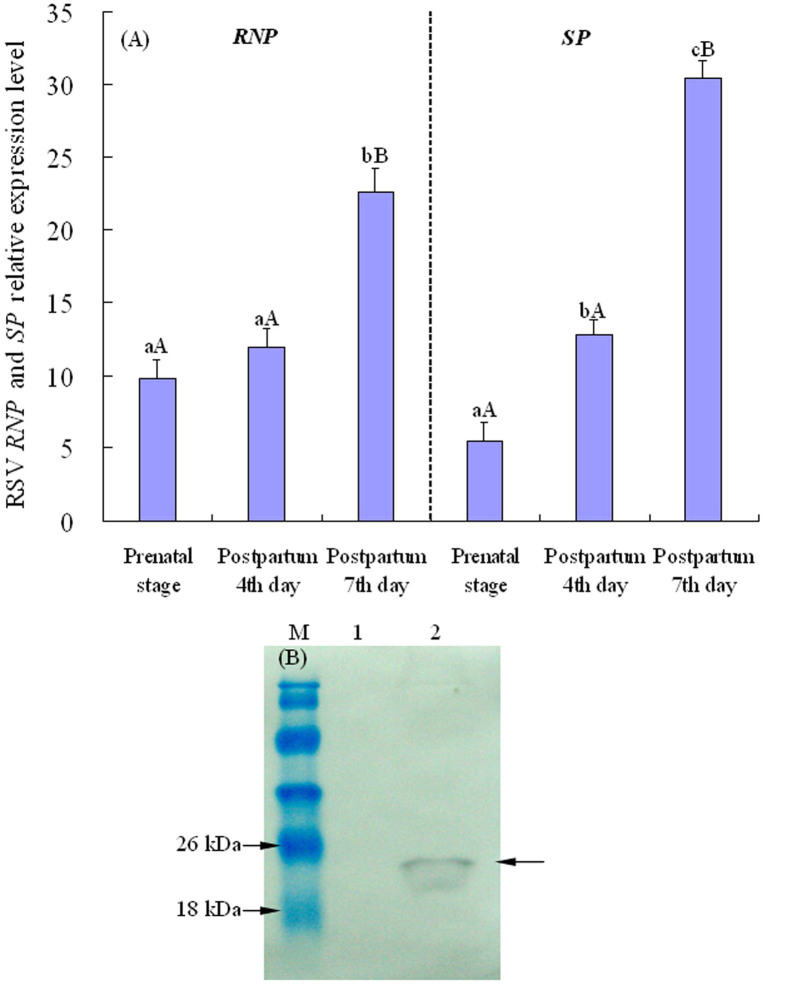
The variations of virus transcript levels in the viruliferous eggs were examined by qPCR. As demonstrated in Fig. 4A, the relative expression quantity of RNP and SP genes increased gradually together with egg development. Furthermore, the important nonstructural protein SP of RSV as a symbol target was also detected for analysing viral propagation. The results demonstrated that SP protein of RSV was detected in viruliferous eggs, with the expected size of 21 kDa (Fig. 4B). The increased viral transcripts and expression of SP sufficiently indicates that processes of virus replication and accumulation occur in SBPH eggs, which is the first report of RSV propagation in the eggs.
Figure 4. RSV propagation in the eggs.
(A) The variations of RSV transcripts in the viruliferous SBPH eggs during the egg development. The expression levels of RNP and SP genes were normalized relative to β-actin transcript, and the resulting 2−ΔCt values were used to plot with the developmental stages as the abscissa, including the prenatal (forthcoming) stage, postpartum 4th and 7th day. Each histogram bar represents the mean (± SE) from four repeats, and the different letters above the error bars indicate significant difference by Tukey's honest significant difference (HSD) test (P < 0.01 and P < 0.05). (B) Western-blot analysis of SP protein. Proteins from SBPH eggs were probed with the polyclonal antibody against SP. Protein markers were indicated on the left, and the detected bands were indicated by arrows. Lane M: relative molecular weight markers; lane 1: proteins from RSV-free SBPH eggs; lane 2: proteins from viruliferous SBPH eggs.
Distribution of RSV in germ cells
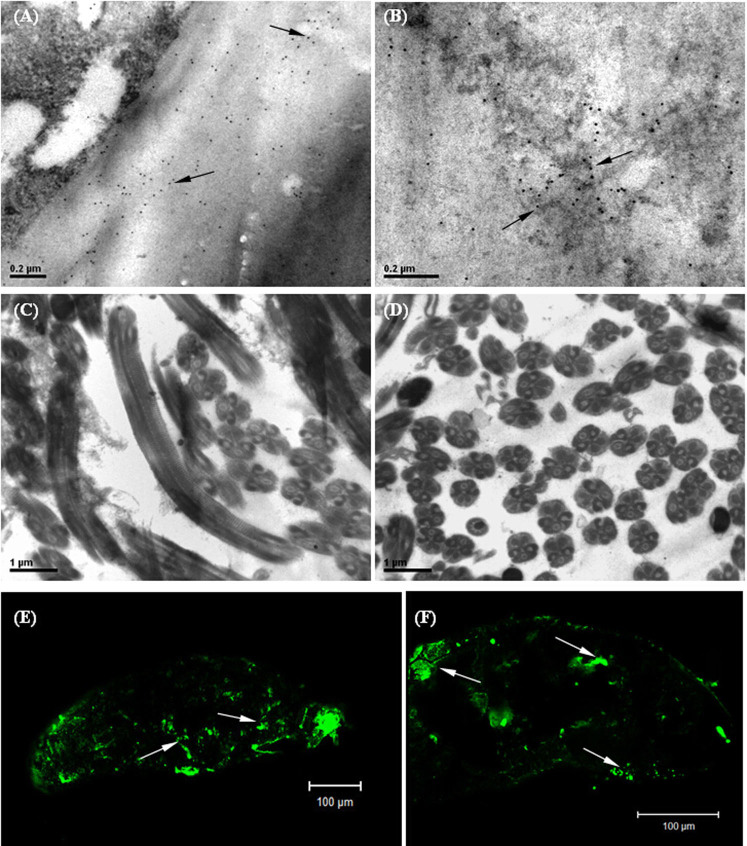
Immuno-gold labelling and transmission electron microscopy were used to investigate the distribution of RSV in SBPH female and male germ cells. As shown in Fig. 5A and B, a large number of colloidal gold nanoparticles (virus RNPs) uniformly distributed on the eggshell surface and inner ovum in the ovary of viruliferous SBPH, which suggests that RSV not only adheres to the eggshell but also enters the interior of the ovum, which is the site of embryonic development. Large numbers of sperm were observed in the seminal vesicle, but colloidal gold nanoparticles were barely found in the sperm and testes of viruliferous SBPH (Fig. 5C and D). Therefore, RSV was only able to infect the ovum and did not infect the SBPH sperm. In addition, the viruliferous eggs in the sheaths were examined via immunofluorescence labelling on the 3rd day after spawning, and the staining of FITC accumulation was observed on an extensive interior area of the eggs (Fig. 5E and F), which is basically consistent with the results of the immuno-gold labelling.
Figure 5. Immunolabelling micrographs showing the distribution of RSV RNP particles in SBPH germ cells.
A–D are transmission electron micrographs, and E and F are confocal laser micrographs. A: RSV RNP particles on the eggshell; B: RSV RNPs in the interior of ovum; C and D: the sperms of viruliferous SBPH without virus infection; the transverse section of sperms was approximately circular, and the longitudinal section was cylindrical shape; E and F: RSV RNPs in the interior of eggs after spawning. Virus RNPs were indicated by arrows. Scale bars: 0.2 μm (A, B), 1 μm (C, D) and 100 μm (E, F).
Expression profile of embryonic development genes in SBPH eggs
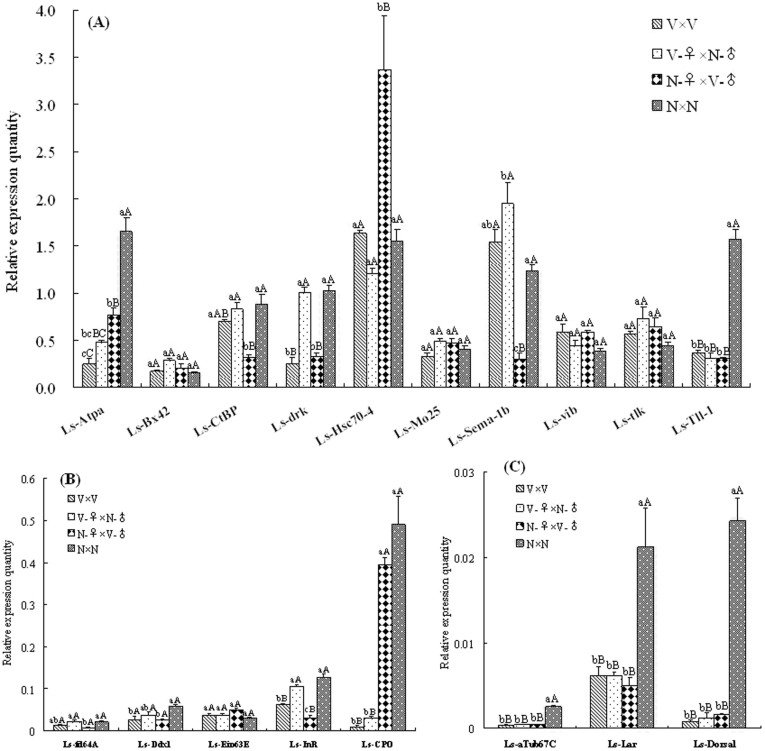
To reveal the viral adverse effects on egg development at the molecular level, the SBPH transcriptome database was constructed based on Newbler assembly of the qualified 454 sequencing reads. After assembly, a total of 14,611 contigs were obtained from the SBPH datasets, with the average and maximum lengths of 352.8 bp and 2,811 bp, respectively. After 115 Drosophila embryonic development genes were subjected to Blastx similarity search against the SBPH database, 18 SBPH genes (E-value < 10−10) were found to be potentially involved in embryonic development (Table 1). For gene expression analysis, the information on the expression level of SBPH embryonic development genes in the F1 eggs from four crosses (V × V, V-♀ × N-♂, N-♀ × V-♂ and N × N) was collected by qPCR. As shown in Fig. 6, the expression level of 13 embryonic development genes changed significantly in the F1 eggs from different crosses except 5 genes (Ls-Bx42, Ls-Eip63E, Ls-Mo25, Ls-tlk and Ls-vib). In the 13 genes, the expression of Ls-Atpα, Ls-αTub67C, Ls-Dorsal, Ls-Lar and Ls-Tll-1 decreased significantly in the F1 eggs from viruliferous maternal and paternal parents or either one, in which the level of Ls-Dorsal was the most notable decline, a 96.7% decreasing in the F1 eggs from the V × V cross than the N × N cross. In addition, the expression of 7 genes (Ls-CtBP, Ls-Ddx1, Ls-drk, Ls-fd64A, Ls-Hsc70-4, Ls-Sema-1b and Ls-InR) was merely affected by viruliferous paternal parent. Independent of whether the paternal parent was carrying RSV, the expression of Ls-Chorion peroxidase (Ls-CPO) decreased in the F1 eggs from viruliferous maternal parents, and compared with the N × N cross, it decreased by 97.9% in the eggs from the V × V cross.
Table 1. Retrieved SBPH genes that might be involved in embryonic development by the BlastX search.
| Retrieved genes in SBPH | Lengths (bp) | Code of ESTs (e-value) | Corresponding genes in D. melanogaster | Symbol | Annotation ID in FlyBase |
|---|---|---|---|---|---|
| Ls-Atpα | 1325 | Contig09158 (0.0) | Na pump α subunit | Atpα | CG5670 |
| Ls-αTub67C | 1457 | Contig03312 (0.0) | α-Tubulin at 67C | αTub67C | CG8308 |
| Ls-Bx42 | 1617 | Contig11057 (0.0) | Bx42 | Bx42 | CG8264 |
| Ls-CPO | 1506 | Contig06909 (0.0) | Chorion peroxidase | CPO | CG5873 |
| Ls-CtBP | 722 | Contig03706 (7e-145) | C-terminal Binding Protein | CtBP | CG7583 |
| Ls-Ddx1 | 1496 | Contig08425 (0.0) | Dead-box-1 | Ddx1 | CG9054 |
| Ls-Dorsal | 982 | Contig08131 (4e-139) | Embryonic polarity protein Dorsal | Dorsal | CG6667 |
| Ls-drk | 629 | Contig07136 (1e-139) | Downstream of receptor kinase | drk | CG6033 |
| Ls-Eip63E | 663 | Contig09723 (2e-96) | Ecdysone-induced protein 63E | Eip63E | CG10579 |
| Ls-fd64A | 715 | Contig12748 (1e-31) | Forkhead domain 64A | fd64A | CG1132 |
| Ls-Hsc70-4 | 2223 | Contig00880 (0.0) | Heat shock protein cognate 4 | Hsc70-4 | CG4264 |
| Ls-InR | 928 | Contig10933 (1e-103) | Insulin-like receptor | InR | CG18402 |
| Ls-Lar | 1954 | Contig04055 (0.0) | Leukocyte-antigen-related-like | Lar | CG10443 |
| Ls-Mo25 | 639 | Contig04692 (2e-122) | Mo25 | Mo25 | CG4083 |
| Ls-Sema-1b | 1262 | Contig07906 (8e-84) | Semaphorin-1b | Sema-1b | CG6446 |
| Ls-tlk | 1698 | Contig04217 (0.0) | Tousled-like kinase | tlk | CG34412 |
| Ls-Tll-1 | 975 | Contig13491 (2e-175) | Dorsal-ventral patterning tolloid-like protein 1 | Tll-1 | CG34370 |
| Ls-vib | 984 | Contig03520 (2e-158) | Vibrator | vib | CG5269 |
Detailed sequence information can be obtained from the Appendix S1.
Figure 6. Expression profile of the embryonic development genes in SBPH eggs from different crosses.
The expression levels of target genes were normalized relative to β-actin transcript. Each histogram bar represents the mean (± SE) from triplicate repeats, and the different letters above the error bars indicate significant difference within the same gene by Tukey's honest significant difference (HSD) test (P < 0.01 and P < 0.05). A: relative expression quantity of the 10 genes (Ls-Atpα, Ls-Bx42, Ls-CtBP, Ls-drk, Ls-Hsc70-4, Ls-Mo25, Ls-Sema-1b, Ls-vib, Ls-tlk and Ls-Tll-1); B: relative expression quantity of the 5 genes (Ls-fd64A, Ls-Ddx1, Ls-Eip63E, Ls-InR and Ls-CPO); C: relative expression quantity of Ls-αTub67C, Ls-Lar and Ls-Dorsal genes.
The dynamics of the viruliferous rate of SBPH natural populations in Jiangsu
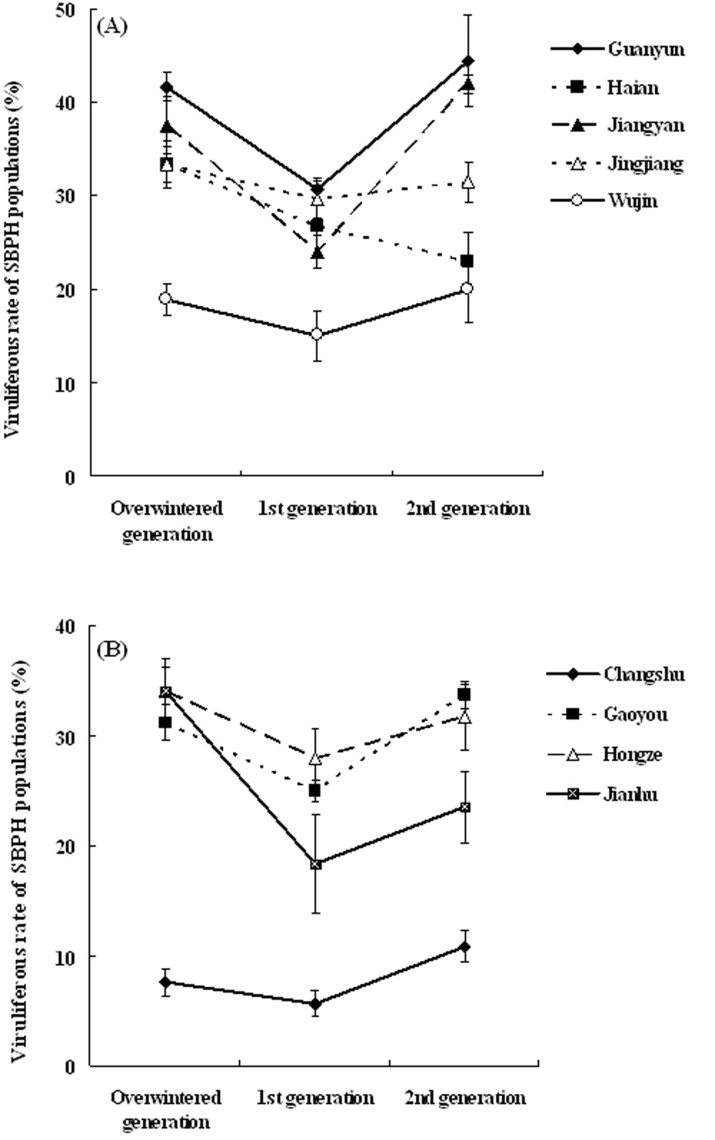
The VR of SBPH field populations in Jiangsu province was surveyed and monitored systematically over the past 13 years. The resulting VR of the natural SBPH populations throughout each of the years from 2001–2013 is shown in Fig. 7. The dynamics of SBPH's VR can be divided into three stages, including the rapid rise phase (2001–2003), the stationary phase with high VR (2003–2007) and the slow decline phase (2007–2013). Since 2007, the VR of SBPH decreased year by year. Currently, the VR of SBPH in the farmland ecosystem is very low, and rice stripe disease has also become a minor threat to rice production in Jiangsu. In addition, the monitoring results of VR generation fluctuations of SBPH from 9 counties (Guanyun, Haian, Jiangyan, Jingjiang and Wujin in 2003; Changshu, Gaoyou, Hongze and Jianhu in 2004) over 2 years showed that the VR displayed a “V”-shaped variation in the overwintering, 1st and 2nd generation SBPH, except in Haian county (Fig. 8). In general, independent of a high or low VR level, the VR of the 1st generation SBPH is lower than the previous overwintering generation, whereas the VR of the 2nd generation insects increased again. Although Haian does not show a V-shape variation, it is worth noticing that no county showed an increase in VR from overwinter to 1st generation.
Figure 7. The dynamics of the viruliferous rate of SBPH natural populations in Jiangsu between 2001–2013.
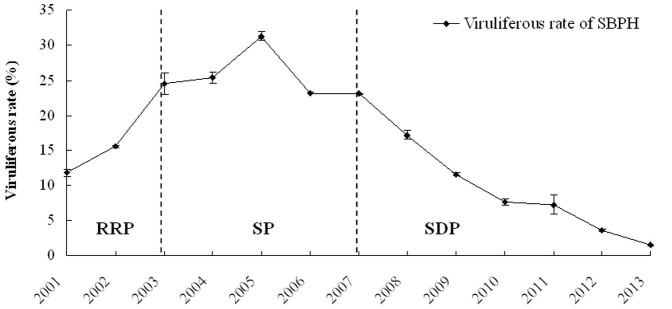
All date are shown as the mean values (± SD). The dynamics of SBPH's VR can be divided into three stages, including the rapid rise phase (RRP, 2001–2003), the stationary phase with high VR (SP, 2003–2007) and the slow decline phase (SDP, 2007–2013).
Figure 8. The monitoring of VR generation fluctuations of SBPH natural populations in Jiangsu.
All date are shown as the mean values (± SD). A: the monitoring VR results of SBPH from Guanyun, Haian, Jiangyan, Jingjiang and Wujin counties in 2003; B: VR fluctuations of SBPH from Changshu, Gaoyou, Hongze and Jianhu counties in 2004.
Discussion
In recent years, the virus–vector–plant relationships, particularly the interactions between viruses and insect vectors, have received increasing attention. During the last few decades, RSV has caused serious disease outbreaks in rice fields in China. Understanding the interactions between RSV and SBPH can help to forecast, control and manage rice stripe disease. In addition, as one of the few plant viruses that replicate in their insect vectors, the RSV–planthopper interactions can serve as an ideal model system to study relationships between propagating plant viruses and their vectors. In this study, RSV's effects on SBPH offspring were investigated via genetic cross experiments. Additionally, the morphology of eggs, viral propagation in the eggs, distribution of RSV in germ cells, expression profile of embryonic development genes and VR monitoring were further analysed to support the biological experiments.
The results of the cross experiments showed that all F1 progeny from the N × N and N-♀ × V-♂ crosses were non-viruliferous; in contrast, the VR of offspring from the other two crosses (V × V and V-♀ × N-♂) were 100%, which indicates that viruliferous maternal parent completely controls whether SBPH offspring become infected with RSV. Therefore, RSV is transmitted vertically in SBPH populations through maternal inheritance. If the maternal parent is viruliferous, the offspring are definitely viruliferous, independent of the infection status of the paternal parent, and this conclusion is also supported by RSV localization in the germ cells. Based on immuno-gold labelling, RSV only infects insect ovum but does not infect sperm. Suzuki et al. (1992)3 and Deng et al. (2013)5 confirmed that RSV RNPs existed in follicular cells of SBPH ovarioles and could be transmitted from female adults to their progeny via eggs. Wu et al. (2001)11 failed to identify viral SP protein in insect sperm. Our results were basically consistent with these previous studies. It has been confirmed that some transovarial transmission viruses are transmitted by maternal inheritance in through vectors, such as San Angelo virus in Aedes albopictus12.
The number of eggs from viruliferous parents was not affected by RSV infection, which suggests that the reproductive capacity of viruliferous and non-viruliferous SBPH is similar on rice plants. Guo et al. (2006)13 showed that the fecundity of Ae. albopictus was also unaffected by the vertical transmission Dengue virus. Of course, the interactions of virus–vector relationships are complex. Some viruses can reduce the fecundities of viruliferous vectors. Tomato yellow leaf curl virus (TYLCV) infected vectors Bemisia tabaci exhibit a reduced fecundity of approximately 40–50%14. Similarly, the white-backed planthopper (Sogatella furcifera), the vector of Southern rice black-streaked dwarf virus (SRBSDV), dramatically reduces their oviposition when the parents are infected with SRBSDV15,16. However, the vector green leafhopper (Nephotettix cincticeps) increased their fecundity when they fed on Rice dwarf virus (RDV)-infected rice plants17.
A previous study on the relationship between RSV and SBPH indicated that the hatchability of F1 progeny from viruliferous SBPH decreased, and more than half of the 1st- and 2nd-instar nymphs died9. Our study suggests that when both SBPH parents or either SBPH parent are infected with RSV, there is an adverse effect on the hatchability of F1 eggs, and the apparent reason for this effect is that a number of eggs from viruliferous parents become developmentally stunted or impaired. The increased viral titre and the expression of nonstructural SP protein demonstrates that RSV can propagate and accumulate in SBPH eggs, therefore it was considered that RSV infection caused a degradation of the quality of the eggs. Expression profile of the embryonic development genes in SBPH eggs, to a certain extent, revealed the viral adverse effect on egg development at the molecular level. After oviposition, viral accumulations indeed interfered with the expression of development genes in the developing embryos. In the 13 differentially expressed genes, the expression of two important developmental regulatory genes (Ls-Dorsal and Ls-CPO) was the most significantly down-regulated in the F1 eggs from viruliferous parents. Dorsal is a sequence-specific transcription factor that is distributed in a broad nuclear gradient in the precellular embryo18,19, and it can initiate dorsal-ventral (DV) patterning of Drosophila embryo20. The Dorsal nuclear gradient determines the differentiation of prospective mesoderm, neuroectoderm and ectoderm by activating or repressing zygotic target gene expression in a concentration-dependent manner21,22. The chorion of insect eggs is a protective structure and undergoes a hardening process either before or right after oviposition23, and it is involved in the response to developmental signals of cracking the eggshell24. It has been shown that chorion peroxidase (CPO)-catalyzed chorion protein crosslinking is one of the major mechanisms that contribute to the formation of rigid and insoluble chorion in Drosophila and Aedes aegypti25,26,27. Therefore, it was considered that abnormal expression of Ls-Dorsal affected the morphogenesis of SBPH embryos, which might result in the developmental abnormalities of partial eggs (Fig. 3A, B and D); the reduced level of Ls-CPO reduced the hardness of the chorion and its ability to protect the developing embryo against potential environmental insults, leading to desiccation and microbial infection of the eggs (Fig. 3C, E–H). According to the above findings, the suppressed expression of Ls-Dorsal and Ls-CPO genes by RSV infection might be a major underlying molecular mechanism of decreased hatchability of SBPH eggs.
Because SBPH offspring are all viruliferous through maternal parent vertical transmission, the suggested cause of the adverse effects on the hatchability from female parents was that RSV infection directly worsened the normal development of the embryos. Similarly, we found that the hatching period and nymph stage of F1 progeny from the V × V and V-♀ × N-♂ parents were increased in length compared with other crosses, and these delayed eggs and nymphs were all viruliferous. The embryonic and nymphal developmental delay was also considered to be a direct negative effect of RSV. Tu et al. (2013)15 showed that SRBSDV prolonged the nymph stages of white-backed planthopper populations. Moreover, it was demonstrated that the larvae of A. aegypti infected by the Yellow fever virus and Ae. albopictus infected by Kunjin virus also experienced a similar developmental delay28,29.
It is interesting that the hatchability of eggs from the N-♀ × V-♂ cross also decreased. Based on our immuno-gold labelling investigation, the sperm of viruliferous SBPH males is not infected by RSV; thus, the virus cannot directly enter the embryo along with sperm to affect the quality of the eggs, suggesting that the mechanisms of the negative effects from viruliferous male parents might be different from that of viruliferous female parents. It was hypothesised that the cause of the adverse effects on the hatchability from viruliferous male SBPH was that sperm motility and quality might be decreased during the process of spermatogenesis by RSV, resulting in subsequent abnormal expression of some development genes in the embryo. The differential regulation of developmental genes also suggested that the cause of the negative effect from viruliferous female and male parents was different. According to the expression profile analysis, there were 5 down-regulated genes (Ls-Atpα, Ls-αTub67C, Ls-Dorsal, Ls-Lar and Ls-Tll-1) in the eggs from both viruliferous parents or either one, and the down-regulated expression of Ls-CPO was mostly controlled by viruliferous maternal parents. In contrast, the expression of 7 genes (Ls-CtBP, Ls-Ddx1, Ls-drk, Ls-fd64A, Ls-Hsc70-4, Ls-Sema-1b and Ls-InR) was merely affected by viruliferous paternal parent. The detailed mechanisms remain to be further elucidated in the future.
During the long-term evolutionary process, virus interactions with insect vectors became complex and diverse, and their relationships might be beneficial, neutral, or antagonistic, depending on the involved species. Some evidence indicates that the survival and longevity of vectors exposed to viruses are reduced; for example, Impatiens necrotic spot virus in Frankliniella occidentalis30, Cotton leaf curl virus in B. tabaci31 and Aedes densovirus in A. aegypti32, suggesting that these interaction relationships between virus and vector are antagonistic. However, there are also many mutualistic relationships between viruses and vectors, such as Tobacco curly shoot virus and TYLCV in B. tabaci B biotype33, and Tomato spotted wilt virus in F. occidentalis34. The decreasing hatchability and the prolonged development period of F1 offspring from viruliferous SBPH parents (or either one) was observed, which suggests that RSV generally exerts a potential adverse effect on SBPH populations. The adverse effect facilitates a decrease of SBPH viruliferous populations, which was also supported by the results of VR monitoring in Jiangsu. When rice stripe disease was epidemic, wide cultivation of susceptible cultivars increased the possibility of SBPH acquiring virus; thus, there was a rapid increase or high levels of VR in the SBPH field populations (Fig. 7). Since 2007, RSV-resistant rice cultivars were widely cultivated for controlling disease35,36, which reduced the opportunity of SBPH to acquire virus. In addition, RSV's unfavourable effect on the hatchability also diminished the quantity of viruliferous insects, which resulted in non-viruliferous SBPH increasing year by year and developing the dominant population. Furthermore, SBPH natural populations are mixed populations with different affinities for RSV, and the low-affinity populations also potentially contribute to the decrease in VR. Currently, VR of SBPH in the farmland ecosystem is very low, and rice stripe disease also becomes a minor threat to rice in Jiangsu.
Similarly, the concept of the adverse effect was also supported by VR “V”-shaped fluctuations in the SBPH generations. In general, the incidence of rice stripe disease is low in wheat fields, which is the primary habitat of 1st generation SBPH in Jiangsu, and together with the low air temperatures, the virus acquisition was affected; thus, virus acquisition of the 1st generation insects is blocked in the spring. In this case, without an effective supplementary virus source, the adverse effects of RSV decrease the VR of 1st generation SBPH. When 2nd generation insects move into paddy fields (with susceptible cultivars) in the summer (with warm temperatures), the virus acquisition opportunity of SBPH increases; thus, the VR of SBPH increases once again compared with that of the 1st generation (Fig. 8). In the plant–virus–vector disease systems, RSV depends on SBPH for its transmission, and the resistance of cultivars determines the quantity of the virus source, which are two important factors in the disease epidemic. As long as RSV-resistant varieties are planted widely, the VR of SBPH will decrease, and the disease will not be widely epidemic. Thus, the variations in rice varieties and vector's VR deserve attention in disease prevention and control.
Methods
Insects and virus
The SBPH populations used in this study were collected from Haian, Jiangsu Province, China (32.57°N, 120.45°E with an elevation of 5 m a.s.l.) in 2004. More than 50 high-viruliferous (V) and non-viruliferous (N) strains (more than 1000 insects each strain) were screened using the single-female spawning method and reared in the laboratory. The strains were re-screened every 2 years to avoid the inbreeding depression of the populations. Rice plants (cultivar Wuyujing No.3), as the insects' diet, were grown in soil at 25°C with a photoperiod of 16 hrs/8 hrs (light/dark) in a growth incubator. After the insects were introduced into a glass beaker that contained rice seedlings (2–3 cm high), the beaker was covered with a piece of nylon mesh. The planthoppers were transferred to fresh seedlings every 10 days to ensure sufficient nutrition. The viruliferous rate (VR) of the V strain was detected via a dot immunobinding assay (DIBA) using monoclonal antibody (MAb) against RSV-RNPs according to method described by Wang et al. (2004)37, and the VR was above 80%. MAb against RSV and viral protein polyclonal antibodies (PAbs) were prepared and conserved by the author's laboratory.
Genetic crosses
To analyse RSV's effects on SBPH offspring, 4 groups of experimental crosses were performed. Female and male 5th-instar nymphs from different strains were maintained individually until imaginal moulting, and then virgin adults were introduced into glass beakers (7 cm diameter, 14 cm height) with 30 rice seedlings growing under the same conditions as mentioned above. Four beakers were prepared for the following groups: V × V, N × N and reciprocal crosses (female V × male N and female N × male V) to produce F1 hybrids; each beaker contained a pair of female and male adults. Ten repeats of each cross group were independently performed. After mating for 7 days, to ensure the accuracy of experiment, it was determined whether the adults were viruliferous or not using the DIBA method. Then, to compare the fecundity of the 4 crosses, eggs in rice basal sheaths were observed and counted by gently stripping the sheaths under a binocular stereomicroscope. From the 9th day, 1st-instar nymphs as F1 hybrids appeared and were assessed every day, including their quantities and emergence dates. Viral replication in seedlings might impact the performance of insects. To avoid such effects, after hatching, the nymphs were transferred into new rice seedlings every 5 days until adults. The survival rate (SR) of the 4th-instar nymphs was calculated as follows: SR = S4/S1 × 100%, where S1 and S4 were, respectively the numbers of 1st- and 4th-instar nymphs. When developing into adults, the total number, sex ratio (♀/♂), SR and VR of F1 adults were assessed. The SR of the F1 adults was calculated using the same method with 4th-instar nymphs. All data were expressed as the mean ± SE. Multiple comparisons of the means were conducted based on the Tukey's honest significant difference (HSD) test using a statistical analysis system (SAS).
Morphological observation of delayed eggs
To determine the reasons for the decreased hatchability of offspring from viruliferous SBPH parents on the morphological level, optical microscopy was used. The V × V and N × N crosses were prepared to produce eggs in the beakers as described above. On the 10th day after oviposition, when most of the 1st-instar nymphs appeared, all sheaths were gently stripped, and the unhatched eggs were observed with an optical microscope (Nikon, Japan). After microscopy, RNA from the unhatched eggs was extracted and used to detect RSV.
Investigation of RSV propagation in the eggs
As a circulative-propagative virus, RSV can propagate in an insect body, but whether RSV replicates in vector eggs is still unclear. To this end, two experiments were conducted. One was the examination of viral transcripts variations in the viruliferous eggs during egg development with real-time quantitative PCR (qPCR). The V × V cross was prepared to produce eggs as the described above, and the eggs were dissected out of the parturient female insects and the sheaths on postpartum 4th and 7th day, respectively. Total RNA from different samples (each with 200 eggs) were extracted following the standard TRIzol® reagent protocol (Invitrogen, USA). The concentration and quality of each RNA sample were determined with a NanoDrop 2000C spectrophotometer (Thermo Scientific, USA). The 1st strand cDNA was synthesized using PrimeScript™ RT Master Mix Kit (Takara, China) according to the manufacturer's protocols. The RSV RNP and SP genes were the target genes, and the SBPH β-actin gene was used as an internal standard. The primers used for the qPCR analysis were designed using Primer3 (http://primer3.ut.ee/) and are listed in Table S1. The qPCR analysis was performed using an IQ5 Real-Time PCR System (Bio-Rad, USA) as described by Li et al. (2012)38. After amplification, the melting curve analysis was performed to eliminate the production of non-specific products. The CT (threshold of cycle) values from four independent biological samples were obtained, and the relative expression of RNP and SP genes was calculated according to the ΔCT algorithm39.
As a general rule, the expression of viral nonstructural proteins is an important sign of occurrence of virus replication and propagation in the vector40. Here, to investigate whether RSV accumulates in SBPH eggs after the eggs are laid in the basal sheaths, the nonstructural disease-specific protein (SP) was selected as the symbol target to detect RSV propagation. The second experiment was also an effective method for analysing viral propagation. Approximately 400 eggs from the V strain were dissected out from the basal rice sheaths for protein extraction. Protein extraction and Western blot were performed as described previously by Li et al. (2011)41. In the Western blot, egg proteins separated by 15% SDS-PAGE were blotted onto nitrocellulose membranes (Pall, USA) and probed with SP-specific PAb. The secondary antibody was horseradish peroxidase (HRP)-conjugated IgG. Egg proteins from N strain were used as a negative control.
Immuno-gold labelling of viruses in insect germ cells
To investigate the distribution of RSV in female and male germ cells, immuno-gold labelling was performed as described by Liang et al. (2005)4 and Deng et al. (2013)5, with some modifications. Briefly, the ovaries and testes of the SBPH adults from the V strain were carefully dissected out, and tissues were fixed in 4% paraformaldehyde and 1% glutaraldehyde for 3 h at 4°C. After washing and dehydration in a graded ethanol series, samples were embedded in Lowicryl K4M low temperature resin with infiltration for 1 week at −20°C before ultraviolet light (360 nm) irradiation for 72 h at −20°C and 24 h at room temperature. Thereafter, ultrathin sections (70 nm) were prepared with an Ultracut E ultramicrotome (Reichart-Jung) and collected with copper grids. The ultrathin sections were subsequently incubated with PAb against RNP (1:400 dilution) in blocking buffer followed by a colloidal gold-conjugated goat anti-mouse IgG (Sigma-Aldrich, USA). After a series of washes, sections were stained with uranyl acetate followed by lead citrate for 15 min and examined using a JEM-1200EX transmission electron microscope (JEOL, Japan) with an acceleration voltage of 60 kV. To ensure the specificity of the immuno-labelling, a control experiment was conducted after the omission of the primary incubation with RNP antiserum, and the other control labelling was also performed on N strain samples to provide a substrate control.
Localization of RSV in SBPH eggs by immunofluorescence
The immunofluorescence microscopy was also used to reveal the distribution of RSV in SBPH eggs. Immunofluorescence labelling was performed as described by Chen et al. (2011)42. Briefly, the eggs in the basal sheaths from the V strain were dissected, fixed in 4% paraformaldehyde, incubated with PAb against RNP, and stained with FITC-conjugated IgG (secondary antibody). Samples were examined with a LSM 710 confocal microscope (Carl Zeiss, Germany). Two control experiments were set up. One was omission of the primary incubation with RNP-specific PAb, and the other labelling was performed using non-viruliferous eggs.
Expression profile of embryonic development genes by qPCR
The expressed sequence tag (EST) SBPH databases have been generated by 454-FLX high-throughput pyrosequencing43. The raw sequencing data were obtained from the Sequence Read Archive (SRA) of the National Center for Biotechnology Information (NCBI) with accession numbers of SRX016333 and SRX016334. An SBPH transcriptome database was constructed through assembly of the two libraries using Newbler software RunAssemble program44. After assembly, gene annotation was completed through a Blastx similarity search against the NCBI-NR protein database, as previously described43. Thereafter, a local BLAST database (SBPH) was created with the transcriptome database and Bioedit software45. Gene information related to SBPH embryonic development is quite limited; thus, we referred to the important model insect Drosophila melanogaster, which has been intensively studied in the field of developmental biology46. The genes involved in Drosophila embryonic development were strictly searched in the FlyBase (http://flybase.org/)47, and as a result, 115 related genes were acquired. Then, the 115 Drosophila genes were subjected to Blastx similarity search against the SBPH local BLAST database with Bioedit software Blastx tool. The retrieved gene sequences (E-value < 10−10) were considered to be reliable and were used to design primers for qPCR analysis with Primer3 (primers listed in Table S1). The preparation of eggs from the four crosses, RNA extraction and qPCR analysis were performed according to the above-mentioned methods.
The monitoring of the viruliferous rate of SBPH in Jiangsu
To evaluate the effects of RSV on SBPH in the farmland ecosystem, an intensive survey and systematic monitoring of VR of SBPH natural populations in Jiangsu province was performed by the Provincial Plant Protection Station and the author's laboratory during 2001–2013. Every year, SBPH field populations (overwintering generation) from approximately 30 counties were collected, and at least three rice fields and 2000 insects were sampled in each county with chessboard sampling method. The VR of the SBPH populations was detected using the DIBA method, and the VR in Jiangsu province was calculated using the weighted average algorithm according to the data from each county. In addition, VR variations in SBPH different generations were also investigated. Continuous detection of the VR of overwintering and the 1st and 2nd generation SBPH from 9 counties in Jiangsu was conducted between 2003 and 2004 using the sampling and detection methods described above.
Author Contributions
S.L. and Y.J.Z. designed the research. S.L., S.J.W., X.W., X.L.L., J.Y.Z., S.S.G. and J.H.D. performed most experiments. Y.J.Z., Z.B.C. and T.Z. monitored the viruliferous rate of SBPH. S.L., Y.H.J., S.M.W. and Y.J.Z. analysed the data. S.L. wrote and finalized the paper.
Supplementary Material
Supplementary information
Acknowledgments
This research was supported by the National Key Basic Research and Development Program (973 Program) of China (grant no. 2014CB138403), the National Natural Science Foundation of China (grant no. 31170142, 31470256 and 31100366), the Jiangsu Agricultural Scientific Self-innovation Fund (grant no. CX[14]2030) and the Special Fund for Agro-scientific Research in the Public Interest of China (grant no. 201003031).
References
- Zhou Y. J., Li S., Cheng Z. B., Zhou T. & Fan Y. J. Research advances in rice stripe disease in China. Jiangsu J. of Agr. Sci. 28, 1007–1015 (2012). [Google Scholar]
- Falk B. W. & Tsai J. H. Biology and molecular biology of viruses in the genus Tenuivirus. Annu. Rev. Phytopathol. 36, 139–163 (1998). [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Fuji S., Takahashi Y. & Kojima M. Immunogold localization of rice stripe virus particle antigen in thin sections of insect host cells. Ann. Phytopathol. Soc. Jpn. 58, 480–484 (1992). [Google Scholar]
- Liang D., Qu Z., Ma X. & Hull R. Detection and localization of Rice stripe virus gene products in vivo. Virus Genes 31, 211–221 (2005). [DOI] [PubMed] [Google Scholar]
- Deng J. H., Li S., Hong J., Ji Y. H. & Zhou Y. J. Investigation on subcellular localization of Rice stripe virus in its vector small brown planthopper by electron microscopy. Virol. J. 10, 310 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H. Biology and epidemiology of rice viruses. Annu. Rev. Phytopathol. 34, 249–274 (1996). [DOI] [PubMed] [Google Scholar]
- Otuka A. et al. Prediction of overseas migration of the small brown planthopper, Laodelphax striatellus (Hemiptera: Delphacidae) in East Asia. Appl. Entomol. Zool. 47, 379–388 (2012). [Google Scholar]
- Colvin J. et al. Host-plant viral infection effects on arthropod-vector population growth, development and behaviour: management and epidemiological implications. Adv. Virus Res. 67, 419–452 (2006). [DOI] [PubMed] [Google Scholar]
- Nasu S. Studies on some leafhoppers and planthoppers which transmit virus diseases of rice plant in Japan. Bull. Kyushu Agr. Exp. Stat. 8, 153 (1963). [Google Scholar]
- Kisimoto R. On the transovarial passage of the rice stripe virus through the small brown planthopper, Laodelphax striatelllus Fallén. In:: Conference on relationships between arthropods and plant-pathogenic viruses pp. 73–90, Conference organizer, Tokyo (1965). [Google Scholar]
- Wu A. Z. et al. Subcellular localization of the stripe disease-specific protein encoded by rice stripe virus (RSV) in its vector, the small brown planthopper, Laodelphax striatellus. Chin. Sci. Bull. 46, 1819–1822 (2001). [Google Scholar]
- Tesh R. B. & Shroyer D. A. The mechanism of arbovirus transovarial transmission in mosquitoes: San Angelo virus in Aedes albopictus. Am. J. Trop. Med. Hyg. 29, 1394–1404 (1980). [DOI] [PubMed] [Google Scholar]
- Guo X. X., Zhao T. Y. & Dong Y. D. Impact of dengue virus transovarial infection on diapausing eggs hatching and reproductive capacity of F1 adult Aedes albopictus. Acta Parasitol. Med. Entomol. Sin. 13, 36–40 (2006). [Google Scholar]
- Rubinstein G. & Czosnek H. Long-term association of tomato yellow leaf curl virus with its whitefly vector Bemisia tabaci: effect on the insect transmission capacity, longevity and fecundity. J. Gen. Virol. 78, 2683–2689 (1997). [DOI] [PubMed] [Google Scholar]
- Tu Z., Ling B., Xu D. L., Zhang M. X. & Zhou G. H. Effects of Southern rice black-streaked dwarf virus on the development and fecundity of its vector, Sogatella furcifera. Virol. J. 10, 145 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. X. et al. Southern rice black-streaked dwarf virus (SRBSDV) directly affects the feeding and reproduction behavior of its vector, Sogatella furcifera (Horváth) (Hemiptera: Delphacidae). Virol. J. 11, 55 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L. et al. Effects of rice dwarf virus on ovarian development and fecundity of the green leafhopper, Nephotettix cincticeps (Fabricius). Acta Phytophy. Sin. 37, 375–376 (2010). [Google Scholar]
- Steward R. Dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogene, c-rel. Science 238, 692–694 (1987). [DOI] [PubMed] [Google Scholar]
- Belvin M. P. & Anderson K. V. A conserved signaling pathway: the Drosophila toll-dorsal pathway. Annu. Rev. Cell Dev. Bi. 12, 393–416 (1996). [DOI] [PubMed] [Google Scholar]
- Stathopoulos A., Van Drenth M., Erives A., Markstein M. & Levine M. Whole-genome analysis of dorsal-ventral patterning in the Drosophila embryo. Cell 111, 687–701 (2002). [DOI] [PubMed] [Google Scholar]
- Rusch J. & Levine M. Threshold responses to the dorsal regulatory gradient and the subdivision of primary tissue territories in the Drosophila embryo. Curr. Opin. Genet. Dev. 6, 416–423 (1996). [DOI] [PubMed] [Google Scholar]
- Biemar F. et al. Comprehensive identification of Drosophila dorsal-ventral patterning genes using a whole-genome tiling array. Proc. Natl. Acad. Sci. U. S. A. 103, 12763–12768 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trougakos I. P. & Margaritis L. H. Immunolocalization of the temporally “early” secreted major structural chorion proteins, Dvs38 and Dvs36, in the eggshell layers and regions of Drosophila virilis. J. Struct. Biol. 23, 111–123 (1998). [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L. Drosophila chorion genes: cracking the eggshell's secrets. Bioessays. 13, 97–105 (1991). [DOI] [PubMed] [Google Scholar]
- Mindrinos M. N., Petri W. H., Galanopoulos V. K., Lombard M. F. & Margaritis L. H. Cross linking of the Drosophila melanogaster chorion involves a peroxidase. Roux's Arch. Dev. Biol. 189, 87–196 (1980). [DOI] [PubMed] [Google Scholar]
- Keramaris K. E., Margaritis L. H., Zografou E. N. & Tsiropoulos G. J. Egg laying suppression in Drosophila melanogaster (Diptera: Drosophilidae) and Dacus (Bactrocera) oleae (Diptera: Tephritidae) by phloroglucinol, a peroxidase inhibitor. Bull. Entomol. Res. 86, 369–375 (1996). [Google Scholar]
- Li J., Hodgeman B. A. & Christensen B. M. Involvement of peroxidase in chorion hardening in Aedes aegypti. Insect Biochem. Molec. 26, 309–317 (1996). [DOI] [PubMed] [Google Scholar]
- Beaty B. J., Tesh R. B. & Aitken T. H. Transovarial transmission of yellow fever virus in Stegomyia mosquitoes. Am. J. Trop. Med. Hyg. 29, 125–132 (1980). [DOI] [PubMed] [Google Scholar]
- Tesh R. B. Experimental studies on the transovarial transmission of Kunjin and San Angelo viruses in mosquitoes. Am. J. Trop. Med. Hyg. 29, 657–666 (1980). [DOI] [PubMed] [Google Scholar]
- Deangelis J. D., Sether D. M. & Rossignol P. A. Survival, development, and reproduction in western flower thrips (Thysanoptera: Thripidae) exposed to Impatiens necrotic spot virus. Environ. Entomol. 22, 1308–1312 (1993). [Google Scholar]
- Sidhu J. S., Mann R. S. & Butter N. S. Deleterious effects of Cotton leaf curl virus on longevity and fecundity of whitefly, Bemisia tabaci (Gennadius). J. Entomol. 6, 62–66 (2009). [Google Scholar]
- Suchman E. L. et al. Effects of AeDNV infection on Aedes aegypti lifespan and reproduction. Biol. Control 39, 465–473 (2006). [Google Scholar]
- Jiu M. et al. Vector-virus mutualism accelerates population increase of an invasive whitefly. PLoS One 2, e182 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris P. C., Joosten N. N., Goldbach R. W. & Peters D. Tomato spotted wilt virus infection improves host suitability for its vector, Frankliniella occidentalis. Phytopathology 94, 706–711 (2004). [DOI] [PubMed] [Google Scholar]
- Zhang Y. X. et al. Fine mapping of qSTV11 (KAS), a major QTL for rice stripe disease resistance. Theor. Appl. Genet. 122, 1591–1604 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. et al. Fine mapping of qSTV11TQ, a major gene conferring resistance to rice stripe disease. Theor. Appl. Genet. 122, 915–923 (2011). [DOI] [PubMed] [Google Scholar]
- Wang G. Z., Zhou Y. J., Chen Z. X. & Zhou X. P. Production of monoclonal antibodies to rice stripe virus and application in virus detection. Acta Phytopathol. Sin. 34, 302–306 (2004). [Google Scholar]
- Li S., Li X., Sun L. J. & Zhou Y. J. Analysis of rice stripe virus whole-gene expression in rice and in the small brown planthopper by real-time quantitative PCR. Acta Virol. 56, 75–79 (2012). [DOI] [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- Ullman D. E., German T. L., Sherwood J. L., Westcot D. M. & Cantone F. A. Tospovirus replication in insect vector cells: Immunocytochemical evidence that the nonstructural protein encoded by the S RNA of tomato spotted wilt tospovirus is present in thrips vector cells. Phytopathology 83, 456–463 (1993). [Google Scholar]
- Li S., Xiong R. Y., Wang X. F. & Zhou Y. J. Five proteins of Laodelphax striatellus are potentially involved in the interactions between rice stripe virus and vector. PLoS One 6, e26585 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. Y., Chen Q., Omura T., Uehara-Ichiki T. & Wei T. Y. Sequential infection of Rice dwarf virus in the internal organs of its insect vector after ingestion of virus. Virus Res. 160, 389–394 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang F. J. et al. Massively parallel pyrosequencing-based transcriptome analyses of small brown planthopper (Laodelphax striatellus), a vector insect transmitting rice stripe virus (RSV). BMC Genomics 11, 303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature 437, 376–380 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 41, 95–98 (1999). [Google Scholar]
- Brody T. The interactive fly: gene networks, development and the internet. Trends Genet. 15, 333–334 (1999). [DOI] [PubMed] [Google Scholar]
- St. Pierre S. E., Ponting L., Stefancsik R., McQuilton P. & the FlyBase Consortium. FlyBase 102-advanced approaches to interrogating FlyBase. Nucleic Acids Res. 42, D780–D788 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information