Abstract
Hearing is one of our main sensory systems and having a hearing disorder can have a significant impact in an individual's quality of life. Sensory neural hearing loss (SNHL) is the most common form of hearing loss; it results from the degeneration of inner ear sensory hair cells and auditory neurons in the cochlea, cells that are terminally differentiated. Stem cell–and gene delivery–based strategies provide an opportunity for the replacement of these cells. In recent years, there has been an increasing interest in gene delivery to mesenchymal stem cells. In this study, we evaluated the potential of human umbilical cord mesenchymal stromal cells (hUCMSCs) as a possible source for regenerating inner ear hair cells. The expression of Atoh1 induced the differentiation of hUCMSCs into cells that resembled inner ear hair cells morphologically and immunocytochemically, evidenced by the expression of hair cell–specific markers. The results demonstrated for the first time that hUCMSCs can differentiate into hair cell–like cells, thus introducing a new potential tissue engineering and cell transplantation approach for the treatment of hearing loss.
Introduction
Human umbilical cord mesenchymal stromal cells (hUCMSCs) are believed to be multipotent stem cells and have shown promising results in gene delivery and tissue engineering applications (Rachakatla et al., 2007; Wang et al., 2011). hUCMSCs are isolated from Wharton's jelly of umbilical cords and have some properties in common with bone marrow mesenchymal stem cells (BMSCs) (Weiss et al., 2006). Umbilical cords represent an abundant and inexpensive cell source. hUCMSCs can be an excellent source for cell transplantation therapies and regenerative medicine because of the abundance of umbilical cords, as well as their low immune rejection and nontumorigenic properties (Fong et al., 2007). However, to make any stem cell useful in clinical applications, it must be differentiated into a specific cell type. hUCMSCs can differentiate into a number of cell types and offer significant potential in gene delivery techniques (Baksh et al., 2007; Can and Karahuseyinoglu 2007; Qian et al., 2010; Rachakatla et al., 2007; Sarugaser et al., 2005; Wang et al., 2004). Differentiation can be achieved by delivering growth factors and genes in several ways. One approach is to use a viral vector to deliver a specific gene of interest. Although some studies have investigated the transduction of BMSCs (Conget and Minguell 2000; Meyerrose et al., 2008), there have been few reports on the transduction of hUCMSCs. Qian et al. (2010) reported lentivirus-mediated gene delivery in hUCMSCs, and Rachakatla et al. (2007) reported adenoviral transduction of hUCMSCs using a recombinant fiber-modified adenovector.
Hair cells are produced only for limited period of time during the early embryonic development of the cochlea in mammals. After this period, no new hair cells are generated and, unlike birds, the mammalian ear is not capable of regenerating damaged and lost hair cells. In contrast to the existing treatments, hair cell restorations via gene delivery– and stem cell–based therapies hold a potential to cure deafness. A cell source that has the potential to regenerate inner ear hair cells would have tremendous potential in clinical applications. Mesenchymal stem cells from the Wharton's jelly of umbilical cords can differentiate into cell types from all three germ layers (Wang et al., 2011). Studies have documented in vitro differentiation of these hUCMSCs into osteocytes, chondrocytes, hepatocytes, adipocytes, neural cells, and pancreatic cells (Campard et al., 2008; Chao et al., 2008; Karahuseyinoglu et al., 2007; Mitchell et al., 2003). Recent work has shown that inner ear progenitor cells can be generated from bone marrow mesenchymal stem cells by using a combination of growth factors and forcing the expression of the Atoh1 transcription factor (Jeon et al., 2007). Several studies in inner ear gene therapy have reported successful delivery of the Atoh1 gene using adenoviral vectors (Huang et al., 2009; Kawamoto et al., 2003; Praetorius et al., 2009; Staecker et al., 2007; Zheng and Gao 2000). Atoh1 is a protein belonging to the basic helix–loop–helix family of transcription factors. It is expressed in inner ear hair cells and neural cells in the hindbrain, spinal cord, and germinal layer of the cerebellum (Bermingham et al., 1999). Studies have reported that Atoh1-null mice failed to produce inner ear hair cells and overexpression of Atoh1 led to the production of numerous ectopic hair cells in mice (Bermingham et al., 1999; Zheng and Gao 2000). Therefore, the Atoh1 gene plays a key role in the formation of inner ear hair cells.
Gene delivery studies in mesenchymal stem cells have been reported using different viral vectors. However, the transduction efficiencies in mesenchymal stem cells vary with each vector (Allay et al., 1997; Conget and Minguell 2000; Marx et al., 1999; Tsuda et al., 2003). Our preliminary adenoviral transduction studies with Ad5, Ad28, Ad35, and Ad41 resulted in high transduction efficiencies with Ad5 and Ad28 when compared with the other serotypes, and therefore we decided to pursue our further experiments with these two serotypes. Although lentiviral transduction has proven to be more efficient than adenoviral transduction in several cell types, lentiviruses can integrate into the host genome, leading to mutation of genes and activation of oncogenes. A lentiviral transduction system offers long-term gene expression; however, with hair cell regeneration, only short-term expression of the Math1 gene is required. This can be accomplished with the adenoviral transduction system. Adenoviral vectors offer high transfection efficiency and have been extensively investigated in clinical trials for ocular disease and cystic fibrosis (Alton and Kitson 2000; Campochiaro et al., 2006). Furthermore, prior experience with adenovectors in the clinic can make them more desirable for commercial and clinical applications over other viral vectors.
In this study, we evaluated the feasibility of transducing hUCMSCs with two different adenoviral serotype vectors—Ad5 and Ad28—at varying multiplicities of infections (MOI) in the presence of protamine sulfate as a cross-linking agent. Adenoviral binding and entry into susceptible cells is mediated by interactions of fibers projecting from the viral capsid and coxsackievirus–adenovirus receptor (CAR) (Roelvink et al., 1998). hUCMSCs do not express the CAR, so adenoviral transfection of these cells is generally very poor. There are several approaches for improving adenoviral transduction efficiency in human stem cells and overcoming gene transfer issues associated with CAR-dependent entry of the adenovirus. Use of lipofectamine has been reported to improve the transduction efficiency of human hematopoietic stem cells (Byk et al., 1998). Lactoferrin can be used as a bridge to enhance binding between the host cell and the virus to increase transduction efficiency (Adams et al., 2009). Increasing the viral concentration can increase efficiency; however, this can be toxic to the cells and cause an inflammatory response (Gonzalez et al., 1999; Smith et al., 1999). Viral adsorption and uptake can be enhanced using polycations like Polybrene, protamine sulfate, and polylysine (Clark et al., 1999). The positively charged polycationic molecules mask the negative charges on the host cell surface and increase the availability of cell-surface receptors. The viral vectors can be complexed with the polycations and enter the cells via endocytosis (Jacobsen et al., 2006; Lin et al., 2003). Protamine sulfate is a polycation that increases transduction efficiency by enhancing viral uptake via a fiber-independent pathway and is especially effective in cells that lack the CAR receptor. We compared the transduction efficiencies of the different vectors and MOIs in hUCMSCs by fluorescence imaging of green fluorescent protein (GFP). The vector concentration that resulted in the maximum number of GFP-positive cells from this study was used as the concentration for further studies with AdAtoh1 vectors. Next, we investigated the ability of hUCMSCs to respond to the Atoh1 gene using the serotype and MOI that resulted in the highest gene expression. We assessed the ability of hUCMSCs to express Atoh1 driven by two different promoter types—human cytomegalovirus (hCMV) and glial fibrillary acidic protein (GFAP) promoters. We also examined the potential of differentiated hair cell–like cells to repair damaged sensory epithelium by co-culturing with murine macular organs.
Materials and methods
Isolation and expansion of hUCMSCs
hUCMSCs were isolated from human umbilical cords according to protocols approved by the University of Kansas Human Subjects Committee (KU-Lawrence IRB approval #15402, KU Medical Center IRB approval # 10951). The cords were obtained from the hospital and harvested within 24 h after delivery. The cords were rinsed in phosphate-buffered saline (PBS) and cut into small pieces of 2–3 cm. The vascular tissue was then removed, and the cords were minced and incubated in 0.2% type II collagenase (298 U/mg; Worthington Biochemical, Lakewood, NJ, USA) in low-glucose Dulbecco's modified Eagle medium (DMEM) for 4 h at 37°C on a shaker. The digested homogeneous solution was diluted in sterile PBS at a 1:4 ratio and centrifuged for 5 min at 1100 rpm. The supernatant was discarded, and the cells were frozen for future use. Frozen hUCMSCs were thawed, plated at a density of 7000 cells/cm2, and cultured in low-glucose DMEM with 10% fetal bovine serum (FBS-MSC quantified) and 1% penicillin/streptomycin (Invitrogen Life Technologies, Carlsbad, CA, USA). Cells were fed every 2 days and maintained in a cell culture incubator at 37°C. At 80–90% confluency, the cells were trypsinized and passaged. hUCMSCs were isolated from four different human umbilical cords (n=4) and expanded to passage 4 (P4) for the experiments. All experiments were performed in quadruplicate for each cord.
Adenoviral vectors and vector production
The backbone of the Ad5 (Adf11D, AdAtoh1.11D, and Ad5.GFAP.Atoh1) vectors had the E1, E3, and E4 regions deleted. The AdAtoh1.11D and Ad5.GFAP.Atoh1 vectors had deleted regions replaced with an expression cassette of Atoh1 driven by the hCMV and human GFAP promoters, respectively. Adf11D had the deleted regions replaced with an expression cassette of GFP driven by the hCMV promoter. The production system for the vector provided robust replication of the adenovector and purified stocks at 5×1011 and 2×1012 total particle units/mL (pu/mL), with a total particle-to-particle ratio ranging from 3 to 10 pu/fluorescent focus units. The backbone of the Ad28 (Ad28t.eGFP and Ad28.GFAP.Atoh1) vectors had the E1 region deleted with the transgene expression cassette inserted at the E1 region. Ad28t.eGFP had the GFP gene inserted and was driven by the hCMV promoter, and Ad28.GFAP.Atoh1 had the Atoh1 gene inserted and was driven by the GFAP promoter. All vectors were produced using 293-ORF cells. The production system for the vector provided robust replication of the adenovector and purified stock at 3×1012 fluorescent focus units/mL. The total particle units were determined by a spectrophotometric assay that was standardized and qualified to reliably and robustly quantify the total particles within a single lot of adenovector. The adenovector lots were purified, aliquoted, and stored at −80°C. Individual aliquots were used for each experiment to prevent loss of activity associated with freeze–thaw cycles. The expression cassette in all vectors contained an open reading frame and an SV40 polyadenylation site and transcriptional stop site at the 3′ end of the open reading frame (GenVec Inc., Gaithersburg, Md., USA) (Pfannenstiel et al., 2009; Schlecker et al., 2011; Staecker et al., 2007).
Adenoviral transduction of hUCMSCs
To investigate the feasibility of transducing hUCMSCs, these cells were trypsinized at P4 and the cells were plated in 48 wells at 1000 cells/well (n=4), and 500 μL of hUCMSC medium (low-glucose DMEM, 10% FBS, 1% penicillin/streptomycin) was added per well. Cells were allowed to attach for 24 h before the virus was added directly to the medium at 10, 50, and 100 MOI in the presence of 8 μg/mL of protamine sulfate (MP Biomedicals, Irvine, CA, USA) (Cornetta and Anderson 1989; Qian et al., 2010). For all transductions using vector containing the Atoh1 gene, hUCMSCs at P4 were plated in eight-well Millicell EZ Slides (Millipore, Billerica, MA) at 1000 cells/well (n=4) with 500 μL of hUCMSCs medium. Cells were allowed to attach for 24 h before the virus was added directly to the medium at an MOI of 100 in the presence of 8 μg/mL of protamine sulfate (Cornetta and Anderson 1989; Qian et al., 2010). Controls did not have any vector added. The plate or chamber slide was gently rocked after adding the vector and incubated at 37°C. The medium was changed after 24 h and every 48 h for a culture period of 10 days.
Fluorescent imaging of transduced cells
Transient gene expression of GFP was imaged and measured at days 3 and 5. At day 10, the cells were fixed for 10 min in 4% paraformaldehyde in PBS, followed by washing the cells three times in PBS. The cells were then stained using ProLong Gold antifade with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen Life Technologies, Carlsbad, CA, USA) and imaged. Microscopic images of enhanced (e) GFP-positive cells were acquired using an inverted fluorescent microscope (Nikon Eclipse E2000-U, Melville, NY, USA), QImaging Retiga 2000R camera, and QCapture software. Transduction efficiencies at day 10 were quantified by averaging the number of GFP-positive cells at four random microscopic fields.
Immunocytochemistry
Cells were fixed for 10 min with 4% paraformaldehyde in PBS. Immunostaining was initiated by blocking the slides for 1 h with 5% FBS followed by four 0.2% Tween washes. The cells were then permeabilized with 0.2% Triton X-100 for 10 min. Atoh1 gene expression was characterized by immunostaining with myosin VIIa, and hair cell differentiation in culture was characterized by immunostaining with the hair cell–specific marker myosin VIIa, the glial cell marker GFAP, and the neuronal marker neurofilament. Double-antibody staining was performed by adding two primary antibodies together—rabbit polyclonal to myosin VIIa (1:100) (Proteus Biosciences, Ramona, CA, USA) and chicken polyclonal to GFAP (1:100) (Abcam, Cambridge, MA, USA). Mouse monoclonal to anti-neurofilament (PAN; Invitrogen, Carlsbad, CA, USA) was stained separately at a 1:100 dilution. The cells were incubated overnight at 4°C. This was followed by secondary antibody incubation for 30 min at room temperature. The secondary antibodies with Alexa visualizing fluorescent signals (1:400) used were Alexa-555-conjugated goat anti-rabbit IgG, Alexa-488-conjugated goat anti-chicken IgG, and Alexa-555-conjugated goat anti-mouse IgG (all from Molecular Probes, Eugene, OR, USA). The slides were rinsed in 0.2% Tween and mounted in ProLong Gold antifade with DAPI and cover slipped. Microscopic images of cells counterstained with DAPI were acquired using an upright fluorescent microscope (Nikon Eclipse E800 and Q Image Aqua camera) and QCapture software. Images were photomerged and analyzed with Adobe Photoshop CS5 extended (Adobe Systems, San Jose, CA).
FM1-43 staining and fixation
FM1-43 FX (Invitrogen, Carlsbad, CA, USA) is a lipophilic dye that has been shown to enter hair cells through transduction channels and is used to detect active transducer channels in the differentiated hair cell–like cells (Gale et al., 2001; Taura et al., 2010). Then 5 μg/mL of FM1-43 FX staining solution was prepared in ice-cold Hanks' balanced salt solution (HBSS) (Sigma-Aldrich, St. Louis, MO, USA). The cells were quickly stained for 1 min in the staining solution and then fixed in ice-cold 4% paraformaldehyde in HBSS for 10 min on ice. The slides were rinsed in HBSS and mounted in ProLong Gold antifade with DAPI (Invitrogen Life Technologies, Carlsbad, CA) and cover slipped. Images of cells counterstained with DAPI were acquired using an upright fluorescent microscope (Nikon Eclipse E800 and Q Image Aqua camera) and QCapture software. Images were photomerged and analyzed with Adobe Photoshop CS5 extended (Adobe Systems, San Jose, CA).
LIVE/DEAD assay for cell viability
Cell viability at day 3 posttransduction with the AdAtoh1.11D vector was assessed using a LIVE/DEAD assay kit for cell viability (Molecular Probes, Eugene, OR, USA). hUCMSCs at P4 from each of the four cords were plated in a 24-well plate at 5000 cells/well (n=3). Cells were incubated with the LIVE/DEAD reagent (dye concentration 2 mM calcein AM and 4 mM ethidium bromide) for 10 min at 37°C in the dark. Cells were imaged using an inverted fluorescent microscope (Nikon, Melville, NY, USA) and QCapture software. For quantitative analysis, the percentage of live cells in four random microscopic fields in three samples was counted and averaged.
Co-culture of AdAtoh1.11D-transduced hUCMSCs with murine macular organs
Macular organs from mice were harvested as previously described (Staecker et al., 2007). Adult C57B16 mice (12 months old, male and female) were sacrificed with intraperitoneal euthanasia and decapitated under approved Institutional Animal Care and Use Committee (IACUC) protocol (# 2008-1746). The otic capsule was exposed, and the macular organs were identified by finding the otolithic membranes. The saccule and utricle were removed using no. 5 watchmaker forceps. The organs were cultured on a Millicell membrane (Millipore Corp., Bedford, MA, USA) suspended in 2000 μL of DMEM supplemented with N1 (Sigma, St. Louis, MO, USA)+100 U/mL penicillin and 5.5 μL/mL of 30% glucose.
After 24 h in vitro (37°C, 5% carbon dioxide), cultures were treated with 10−3 mol/L neomycin for 48 h. Experimental cultures (n=4 per experimental group) were treated with previously transduced hUCMSCs. Atoh1 gene-expressing cells and GFP-expressing cells were obtained from AdAtoh1.11D- and Ad28.eGFP-transduced hUCMSCs cultures, respectively. The cells were trypsinized at day 10 posttransduction and suspended in organ culture medium at 50,000 cells/mL. A total of 250 μL of this suspension was added to each macular organ (Coleman et al., 2007). Negative control cultures were placed in a medium not treated with neomycin, and no cells were added. The positive myosin VIIa labeling in these cultures indicated the presence of vestibular hair cells in the macular organs. A second negative control culture was placed in complete medium without neomycin, and no cells were added. These cultures failed to show any positive labeling for myosin VIIa and indicated the ototoxic effect of neomycin on the vestibular hair cells. Positive control cultures were treated with GFP-expressing hUCMSCs. Organ cultures were incubated in a CO2 incubator at 37°C for 10 days with medium changes every 3 days.
Characterization of hair cells in stem cell and macular organ co-culture by immunostaining
At day 10, explants were washed in PBS and fixed in 4% paraformaldehyde for 10 min. Immunostaining was initiated by blocking and permeabilizing explants in 10% FBS and 0.3% Triton X-100 for 30 min. This was followed by incubation with primary antibody (1:500) at 4°C overnight. The primary antibody used was rabbit polyclonal to myosin VIIa (Proteus Biosciences, Ramona, CA). The explants were rinsed with PBS and incubated in secondary antibody (1:400) for 30 min. The secondary antibody with Alexa-visualizing fluorescent signals used was Alexa-555-conjugated goat anti rabbit immunoglobulin G (IgG; all from Molecular Probes, Eugene, OR, USA). The explants were rinsed and mounted on Superfrost/Plus microscope slides (Fisher Scientific, Pittsburgh, PA, USA) using ProLong Gold antifade with DAPI (Invitrogen Life Technologies, Carlsbad, CA, USA) and cover slipped. Microscopic images of explants were acquired using anupright fluorescent microscope (Nikon Eclipse E800 and Q Image Aqua camera) and QCapture software. Images were analyzed with Adobe Photoshop CS5 extended (Adobe Systems, San Jose, CA, USA).
Results
Effects of transducing hUCMSCs at different MOIs with Ad5 and Ad28 serotypes
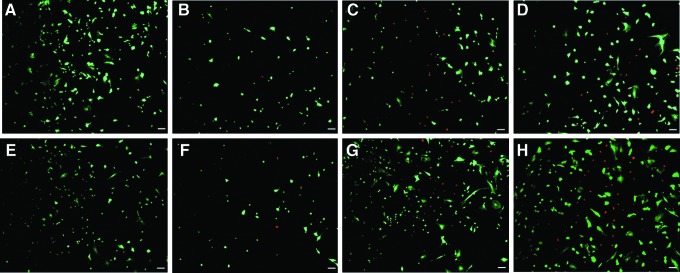
By visual examination, it was observed that the intensity of fluorescence and the number of cells transduced with both vectors gradually increased from MOI 10 to MOI 100. No fluorescence was observed in the controls. To investigate the transduction efficiencies and the transient GFP gene expression between Ad5 and Ad28 in hUCMSCs, the transgene gene expression was imaged at days 3, 5, and 10 (Figs. 1 and 2). With both vectors, the maximum number of GFP-expressing cells was observed at a MOI of 100. The cells had a maximum fluorescence intensity and transduction efficiency at day 5, with gene expression fading off by day 10. However, when the gene expression between both vectors was compared, Ad28 had a higher number of GFP-expressing cells and higher fluorescence intensity at all MOIs on all days when compared to Ad5. The transduction efficiencies at day 10 were quantified. The efficiencies for MOI 10, 50, and 100 for the Ad28 vector were 3.7%, 4.7%, and 2.5%, respectively. For cells transduced with Ad5 vector, the transduction efficiencies at day 10 were less than 0.5%.
FIG. 1.
Adenovirus-mediated expression of GFP in hUCMSCs transduced with Ad28.eGFP vector at 3, 5, and 10 days posttransduction. Vertical panels (left to right) represent transient gene expression at days 3, 5, and 10, respectively. Horizontal panels (top to bottom) represent the effect of MOI on the expression level of adenovirally introduced GFP. Note that maximum gene expression was at day 5 and at MOI 100. The figures represent a random microscopy field for one cord. The results were consistent among cords. Scale bar, 100 μm. Color images available online at www.liebertpub.com/cell
FIG. 2.
Adenovirus-mediated expression of GFP in hUCMSCs transduced with Adf11D.eGFP vector at 3, 5, and 10 days posttransduction. Vertical panels (left to right) represent transient gene expression at days 3, 5, and 10, respectively. Horizontal panels (top to bottom) represent the effect of MOI on the expression level of adenovirally introduced GFP. Note that maximum gene expression was at day 5 and at MOI 100. The figures represent a random microscopy field for one cord. The results were consistent among cords. Scale bar, 100 μm. Color images available online at www.liebertpub.com/cell
Myosin-positive cells from hUCMSCs
The potential of hUCMSCs to respond to Atoh1 was evaluated by transducing hUCMSCs with the AdAtoh1.11D vector driven by the hCMV promoter and the Ad5GFAP.Atoh1 and the Ad28.GFAP.Atoh1 vectors driven by the GFAP promoter at a MOI of 100. The expression of the hair cell–specific marker myosin VIIa in hUCMSCs transduced with all three vectors was detected by immunostaining. Only a small percentage of cells expressed myosin VIIa. However, the AdAtoh11D vector driven by the hCMV promoter had a relatively higher number of myosin VIIa–positive cells when compared to Ad5.GFAP.Atoh1 and Ad28.GFAP.Atoh1 vectors that were driven by the GFAP promoter. The positive cells in AdAtoh11D-transduced cells also demonstrated a degree of anatomical resemblance to hair cells (Figs. 3–5).
FIG. 3.
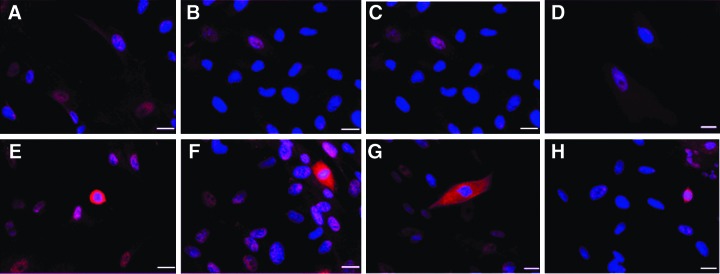
Characterization of Atoh1 gene expression by immunostaining with myosin VIIa in hUCMSCs transduced with the AdAtoh1.11D vector. (A–D) Controls at day 10. (E–H) Myosin VIIap–positive cells at day 10. Vertical panels represent a randomly selected microscopy field for each cord. Cells were infected at MOI 100. The position of nuclei is represented by DAPI staining (blue). Immunostaining indicated myosin VIIa–positive cells (red) and hair cell-like cells. Scale bar, 20 μm. Color images available online at www.liebertpub.com/cell
FIG. 5.

Characterization of Atoh1 gene expression by immunostaining with myosin VIIa in hUCMSCs transduced with the Ad5.GFAP.Atoh1 vector. (A–D) Controls at day 10. (E–H) Myosin VIIa–positive cells at day 10. Vertical panels represent a randomly selected microscopy field for each cord. Cells were infected at MOI 10. The position of nuclei is represented by DAPI staining (blue). Immunostaining demonstrated low levels of myosin VIIa expression (red). Scale bar, 20 μm. Color images available online at www.liebertpub.com/cell
FIG. 4.
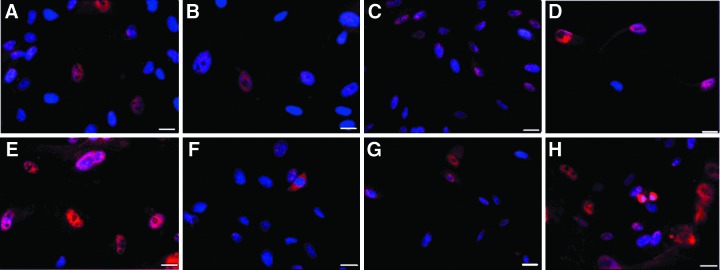
Characterization of Atoh1 gene expression by immunostaining with myosin VIIa in hUCMSCs transduced with the Ad28.GFAP.Atoh1 vector. (A–D) Controls at day 10. (E–H) Myosin VIIa–positive cells at day 10. Vertical panels represent a randomly selected microscopy field for each cord. Cells were infected at MOI 100. The position of nuclei is represented by DAPI staining (blue). Immunostaining demonstrated low levels of myosin VIIa expression (red). Scale bar, 20 μm. Color images available online at www.liebertpub.com/cell
Characterization of hair cell–like differentiation of hUCMSCs in vitro
Differentiated cells (n=4) from hUCMSC cultures were characterized on the basis of morphology and immunocytochemistry. Spindle-shaped fibroblast-like cells (characteristic morphology of hUCMSCs) were observed in the negative controls; however, the transduced cell populations showed a small percentage of pear-shaped cells with extended protrusions resembling the ciliary bundles in hair cells. These cells had centrally located nuclei. The ciliary protrusions and cytoplasm of the pear-shaped cells were positive for myosin VIIa and GFAP antibodies (Fig. 6). In addition, FM1-43 fluorescent labeling was observed in a small percentage of transduced cells (Fig. 7). The transduced cells did not stain positively for the neurofilament antibody (Fig. 8).
FIG. 6.
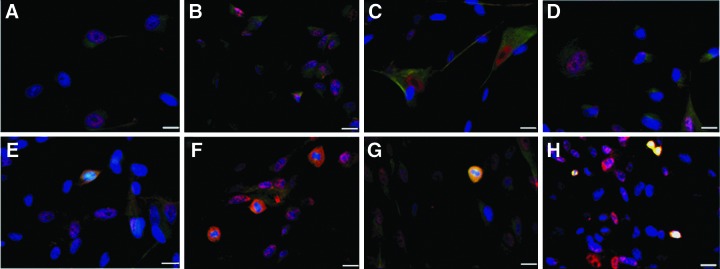
Characterization of hair cell–like cells in hUCMSC culture transduced with AdAtoh1.11D vector using specific hair cell and glial cell markers. (A–D) controls at day 10. (E–H) Myosin VIIa– and GFAP-positive cells at day 10. Vertical panels represent a randomly selected microscopy field for each cord. Cells were infected at MOI. The positions of nuclei are represented by DAPI staining (blue). Immunostaining demonstrated myosin VIIa– and GFAP-positive pear-shaped cells with an extended protrusion at one end, similar in appearance to inner ear hair cells. Yellow color indicates expression of myosin VIIa and GFAP in differentiated cells. Scale bar, 20 μm. Color images available online at www.liebertpub.com/cell
FIG. 7.

Characterization of hair cell-like cells in hUCMSC culture transduced with AdAtoh1.11D vector using a specific hair cell marker, FM1-43. (A–D) Controls at day 10. (E–H) FM1-43 positive cells (green) at day 10. Vertical panels represent a randomly selected microscopy field for each cord. Cells were infected at MOI 100. The positions of nuclei are represented by DAPI staining (blue). FM1-43 labels stereocilia transduction channels in hair cells, indicating transduction activity in differentiated hair cell-like cells. Scale bar, 20 μm. Color images available online at www.liebertpub.com/cell
FIG. 8.

Characterization of neuronal expression by immunostaining with anti-neurofilament (PAN) in hUCMSCs transduced with AdAtoh1.11D vector. (A) Negative control at day 10. (B) Positive control. This section of a mouse cochlea represents the positive control. Immunostaining with anti-neurofilament (PAN)-stained spiral ganglion cells. (C) Transduced cells that did not show neurofilament-positive cells, indicating an absence of neural differentiation in Atoh- transduced cells. Cells were infected at MOI 100 and immunostained 10 days posttransduction. The positions of nuclei are represented by DAPI staining (blue). The figures represent a random microscopy field for one cord. The results were consistent among cords. Scale bar, 20 μm. Color images available online at www.liebertpub.com/cell
Co-culture of AdAtoh1.11D-transduced cells and macular organ explants
The potential of AdAtoh1.11D-transduced hUCMSCs to repair damaged sensory epithelium in macular organ explants was evaluated by immunostaining. After 10 days of co-culture with macular organs, hair cell–like cells were found in one of the four explants (Fig. 9). Negative controls without neomycin showed the presence of hair cells in the sensory epithelium. Negative controls with neomycin treatment did not show any myosin VIIa–positive hair cells due to the absence of hair cells. In addition, the positive controls failed to show any migration of GFP-expressing hUCMSCs (Fig. 9).
FIG. 9.
Co-culture of transduced hUCMSCs and macular organs. (A and B) Negative controls without and with neomycin treatment, respectively. Both the negative controls were not treated with cells. (C) Positive control treated with neomycin and GFP-expressing hUCMSCs. (D) Experimental group treated with neomycin and differentiated hUCMSCs. Cultures were stained for myosin VIIa at day 10. The results demonstrated myosin VIIa–positive cells (red) in experimental group. Arrows point to myosin VIIa–positive cells. All figures represent one explant from each group. Scale bar, 20 μm. Color images available online at www.liebertpub.com/cell
Transduced cell viability
Cell viability was determined with Calcein AM (green) and Ethidium Bromide (red) fluorescent markers. Green color indicated live cells, whereas the red color indicated dead cells. hUCMSCs transduced with the AdAtoh1.11D vector at a MOI of 100 demonstrated high cell viabilities at 3 days posttransduction, indicating low cytotoxic effects of adenovirus-mediated gene delivery in hUCMSCs (Fig. 10). The percentage of live cells for the transduced group was quantified to be 90.5% when compared to the controls, which were at 85.2%.
FIG. 10.
LIVE/DEAD images for AdAtoh1.11D-transduced hUCMSCs. (A–D) Controls from each cord. (E–H) hUCMSCs from each cord transduced with AdAtoh1.11D vector at MOI 100 at day 3 posttransduction. Live cells are stained green, whereas dead cells are stained red. The results demonstrated high cell viability in transduced cells indicating low cytotoxic effects of AdAtoh1.11D. Scale bar, 100 μm. Color images available online at www.liebertpub.com/cell
Discussion
In the current study, we characterized the feasibility of transducing hUCMSCs using Ad5 and Ad28 vectors and compared the transduction efficiencies between the two serotypes. Overall, Ad28 was observed to transduce hUCMSCs better than Ad5 in terms of both the intensity of fluorescence and the number of cells transduced (Figs. 1 and 2). Both vectors showed transgene expression at all MOIs, with a MOI of 100 giving the best gene expression. The cells were imaged at days 3, 5, and 10 to evaluate the transient gene expression in both vectors. Gene expression reached a maximum level on day 5 and gradually declined by day 10. Although the highest MOI of 100 was the most effective, using even higher MOIs may not be advisable, because MOIs greater than 100 may cause damage to the cells.
The use of protamine sulfate as a cross-linking agent may have enhanced the uptake of adenoviral particles and contributed to improving the transduction efficiency in both the Ad5 and Ad28 vectors (Lanuti et al., 1999; Yang and Hsieh 2001). Lentiviral transduction studies of hUCMSCs have used Polybrene as a cross-linking agent to enhance efficiency (Qian et al., 2010). However, preliminary studies of adenoviral transduction in hUCMSCs with Polybrene indicated cytotoxicity and cell death. Protamine sulfate is another polycation reported to be an excellent alternative to Polybrene (Cornetta and Anderson, 1989). Studies with protamine sulfate have demonstrated essentially the same infection efficiency as Polybrene, with a low cytotoxicity on a range of cell types (Cornetta and Anderson, 1989), and therefore protamine sulfate was selected and used as an alternative to Polybrene.
To further explore the potential of hUCMSCs to respond to the Atoh1 transcription factor, we transduced the cells with Atoh1-expressing vectors driven by two different promoter types— hCMV and GFAP. It was demonstrated that hUCMSCs were capable of expressing the Atoh1 gene by immunostaining for myosin VIIa. The Atoh1 gene is essential for hair cell formation, thus hUCMSCs expressing Atoh1 should stain positively for myosin VIIa (Bermingham et al., 1999). In the current study, AdAtoh1.11D demonstrated a higher number of myosin VIIa–positive cells when compared to the Ad5.GFAP.Atoh1 and Ad28.GFAP.Atoh1 vectors. The controls also expressed a certain level of myosin VIIa–positive cells; however, the expression was minimal when compared to the vector-treated cells, and there were no cells morphologically resembling hair cells. Previous studies using adenoviruses or adeno-associated viruses for inner ear gene delivery in the cochlea using the CMV and GFAP promoters demonstrated that the expression driven by the GFAP promoter was limited only to vestibular supporting cells, demonstrating the specificity of this promoter type (Praetorius et al., 2010; Stone et al., 2005). These previous findings explain the low gene expression in hUCMSCs with the transgene cassette driven by the GFAP promoter.
Previously, the Atoh1 gene has been shown to be expressed in rat and mouse bone marrow mesenchymal stem cells when transfected using Lipofectamine (Edge 2009; Jeon et al., 2007; Qin et al., 2011). However, there have been no reports on viral gene delivery of the Atoh1 gene in mesenchymal stem cells. In the current study, we demonstrated for the first time that hUCMSCs can respond to the Atoh1 transcription factor delivered using a viral vector. The AdAtoh1.11D vector was shown to produce a relatively higher number of myosin VIIa–positive cells when compared to the Ad5.GFAP.Atoh1 and Ad28.GFAP.Atoh1 vectors.
hUCMSCs are believed to be multipotent stem cells and have been reported to differentiate into multiple cell lineages belonging to all three germ layers. hUCMSCs have been shown to give rise to cells from chondrogenic, osteogenic, myogenic, adipogenic, neurogenic, hepatogenic, and pancreatic lineages (Campard et al., 2008; Chao et al., 2008; Kadner et al., 2002; Karahuseyinoglu et al., 2007; Mitchell et al., 2003). In this study, we demonstrated the possibility of extending the range of lineages for hUCMSCs to include inner ear sensory hair cells. Studies of Atoh1 expression in inner ear progenitor cells have indicated that this transcription factor plays an important role in the differentiation of hair cells (Bermingham et al., 1999). Studies with rat bone marrow mesenchymal stem cells have demonstrated their potential to express Atoh1 and differentiate into inner ear progenitor cells in the presence of otic-inducing growth factors (Jeon et al., 2007; Qin et al., 2011).
The current study demonstrated that hUCMSCs treated with an adenovector expressed the Atoh1 transcription factor driven by the hCMV promoter and differentiated into hair cell-like cells. Studies with mesenchymal stem cells have indicated that a stem cell that has differentiated partially or completely into an inner ear progenitor cell can express early otic, hair cell, and neuronal markers (Edge, 2009). Immunocytochemical results on the transduced cell population in our experiments demonstrated differentiated cells that expressed the hair cell–specific marker myosin VIIa and the glial cell marker GFAP and took up the transduction ion channel dye FM1-43. Myosin VIIa is a hair cell–specific marker that stains actin-binding domains in myosin motor proteins present in the transduction channels (Gillespie and Müller, 2009). GFAP is an astrocyte marker expressed in sensory epithelial cells in the inner ear (Rivolta and Holley, 2002). Myosin VIIa– and GFAP-positive cells were pear-shaped and displayed protrusions at one end that resembled ciliary bundles in hair cells. Cell morphology indicated differentiation into hair cell–like cells, and positive expressions of myosin VIIa and GFAP indicated immature features of hair cell precursor cells and neuroepithelial cells (Rivolta and Holley, 2002). FM1-43–positive stains indicated the presence of open mechanotransducer channels in the hair cell–like cells (Meyers et al., 2003). The differentiated cells did not express the neuronal marker neurofilament indicating that the Atoh1 gene did not induce any neuronal phenotypes.
Immunocytochemistry of transduced hUCMSCs and macular organ co-cultures demonstrated the presence of myosin-positive cells, indicating the potential of hUCMSCs to engraft and integrate into the sensory epithelium. The experimental group treated with GFP-expressing hUCMSCs failed to show GFP expression in sensory epithelium owing to the short-term transient gene expression of GFP. Evaluation of the integration of differentiated cells in tissue can be challenging because of the complex structure of the inner ear. More importantly, these results provide the basic information required for further in vitro and cell transplantation studies. Studies have demonstrated the successful co-culture of stem cells with cochlear explants (Coleman et al., 2007; Jeon et al., 2007). Therefore, co-culturing cochlear explants with cells that are genetically manipulated in vitro to express Atoh1 using viral vectors can potentially be used to regenerate inner ear hair cells.
The findings in this study indicated that hUCMSCs may have the potential to differentiate into inner ear sensory cells for use as a source of transplantation in curing SNHL and other hearing disorders. Stem cells in the inner ear lack the capacity to differentiate and replace damaged hair cells (Li et al., 2004). Therefore, a cell source like hUCMSCs with the capacity to express Atoh1 may potentially be used in therapeutic applications. Our findings suggest for the first time that hUCMSCs can differentiate into hair cell–like cells via the adenovirus-mediated gene delivery of Atoh1, thus confirming our hypothesis and providing a significant finding in the field of tissue engineering and a potential strategy to cure sensory neural hearing loss.
Conclusion
Previous studies that attempted to regenerate inner ear hair cells have successfully differentiated embryonic and mesenchymal stem cells using a combination of several otic-inducing growth factors or by expression of an externally delivered Atoh1 gene. This study made the first attempt to use mesenchymal cells from human umbilical cord stroma for regenerating hair cells. The current study examined the potential use of hUCMSCs in stem cell–based and tissue engineering approaches to restore hearing loss.
Hair cell differentiation in hUCMSCs was induced in vitro via adenovirus-mediated gene delivery of the Atoh1 gene. The differentiated cells resembled hair cells in terms of morphology and immunocytochemical markers. Differentiated cells expressed hair cell–specific markers myosin VIIa and the glial cell marker GFAP and took up the FM1-43 dye. The findings indicated the development of hair cell-like cells. However, transduction efficiencies were low and only a small percentage of the cells differentiated into hair cell–like cells, which may suggest partial differentiation of stem cells. The notch signaling pathway, Hes1, Hes5, and Jagged1 genes are known to be potent downregulators of Atoh1 expression. They cause lateral inhibition in which one cell is singled out from a group of cells for a given fate. Notch signaling inhibits Atoh1 gene expression and hair cell differentiation, preventing a cell from committing to the hair cell fate. Future work can investigate the application of notch inhibitors such as γ-secretase inhibitors at varying concentrations to prevent the lateral inhibition caused by notch signaling.
Future studies with the use of hUCMSCs for hair cell differentiation can focus on delivering adenoviral vectors at high MOI and using a combination of growth factors such as insulin-like growth factor-1 (IGF-1), neurotrophin-3 (NT-3), basic fiboblast growth factor (bFGF), epidermal growth factor (EGF), and brain-derived neurotrophic factor (BDNF) to supplement the differentiation process and achieve a higher percentage of differentiated cells. Co-culturing partially differentiated cells with inner ear sensory epithelial cells may also improve differentiation in stem cells due to inductive cell signaling. Additionally, a vector designed to contain an antibiotic selection marker or fluorescence-activated cell sorting (FACS) analysis could be used to isolate the differentiated cells from the transduced population to test their potential to regenerate hair cells in vivo. Detailed analysis of target gene expression can be analyzed by quantitative RT-PCR on an isolated, differentiated cell population.
The success achieved with hUCMSCs in the current study has laid a foundation for exploring numerous ways of inducing hair cell differentiation. We examined the potential of hUCMSCs as a possible source for engineering inner ear hair cells to restore hearing loss. In the future, cell transplantation treatments for hearing loss may include the delivery of a pure, differentiated hUCMSC population to the inner ear, with or without the augmentation of growth factors.
Acknowledgments
We would like to thank Denise Martinek and Dr. Samantha Durland from Lawrence Memorial Hospital and the nursing staff at Stormont-Vail Health Care for providing the umbilical cords used in this study. I would also like to thank Jennifer Brantley for her valuable guidance and help in harvesting macular organs and immunocytochemistry.
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Adams W.C. Bond E. Havenga M.J.E. Holterman L. Goudsmit J. Karlsson-Hedestam G.B. Koup R.A. Lore K. Adenovirus serotype 5 infects human dendritic cells via a CAR-independent receptor pathway mediated by lactoferrin and DC-SIGN. J. Gen. Virol. 2009;90:1600–1610. doi: 10.1099/vir.0.008342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allay J.A. Dennis J.E. Haynesworth S.E. Majumdar M.K. Clapp D.W. Shultz L.D. Caplan A.I. Gerson S.L. LacZ and interleukin-3 expression in vivo after retroviral transduction of marrow-derived human osteogenic mesenchymal progenitors. Hum. Gene Ther. 1997;8:1417–1427. doi: 10.1089/hum.1997.8.12-1417. [DOI] [PubMed] [Google Scholar]
- Alton E. Kitson C. Gene therapy for cystic fibrosis. Expert Opin. Investig. Drugs. 2000;9:1523. doi: 10.1517/13543784.9.7.1523. [DOI] [PubMed] [Google Scholar]
- Baksh D. Song L. Tuan R. Adult mesenchymal stem cells: Characterization, differentiation, and application in cell and gene therapy. J. Cell. Mol. Med. 2007;8:301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham N. Hassan B. Price S. Vollrath M. Ben-Arie N. Eatock R. Bellen H. Lysakowski A. Zoghbi H. Math1: An essential gene for the generation of inner ear hair cells. Science. 1999;284:1837. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Byk T. Haddada H. Vainchenker W. Louache F. Lipofectamine and related cationic lipids strongly improve adenoviral infection efficiency of primitive human hematopoietic cells. Hum. Gene Ther. 1998;9:2493–2502. doi: 10.1089/hum.1998.9.17-2493. [DOI] [PubMed] [Google Scholar]
- Campard D. Lysy P.A. Najimi M. Sokal E.M. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134:833–848. doi: 10.1053/j.gastro.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Campochiaro P.A. Nguyen Q.D. Shah S.M. Klein M.L. Holz E. Frank R.N. Saperstein D.A. Gupta A. Stout J.T. Macko J. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: Results of a phase I clinical trial. Hum. Gene Ther. 2006;17:167–176. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- Can A. Karahuseyinoglu S. Concise review: Human umbilical cord stroma with regard to the source of fetus derived stem cells. Stem Cells. 2007;25:2886–2895. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- Chao K.C. Chao K.F. Fu Y.S. Liu S.H. Islet-like clusters derived from mesenchymal stem cells in Wharton's jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One. 2008;3:e1451. doi: 10.1371/journal.pone.0001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark P.R. Stopeck A.T. Brailey J.L. Wang Q. McArthur J. Finer M.H. Hersh E.M. Polycations and cationic lipids enhance adenovirus transduction and transgene expression in tumor cells. Cancer Gene Ther. 1999;6:437–446. doi: 10.1038/sj.cgt.7700074. [DOI] [PubMed] [Google Scholar]
- Coleman B. Fallon J. Pettingill L. de Silva M. Shepherd R. Auditory hair cell explant co-cultures promote the differentiation of stem cells into bipolar neurons. Exp. Cell Res. 2007;313:232–243. doi: 10.1016/j.yexcr.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conget P.A. Minguell J.J. Adenoviral-mediated gene transfer into ex vivo expanded human bone marrow mesenchymal progenitor cells. Exp. Hematol. 2000;28:382–390. doi: 10.1016/s0301-472x(00)00134-x. [DOI] [PubMed] [Google Scholar]
- Cornetta K. Anderson W.F. Protamine sulfate as an effective alternative to polybrene in retroviral-mediated gene-transfer: Implications for human gene therapy. J. Virol Methods. 1989;23:187–194. doi: 10.1016/0166-0934(89)90132-8. [DOI] [PubMed] [Google Scholar]
- Edge A.B. Boston, MA: Massachusetts Eye & Ear Infirmary; 2009. Generation of Inner Ear Cells. [Google Scholar]
- Fong C.Y. Richards M. Manasi N. Biswas A. Bongso A. Comparative growth behaviour and characterization of stem cells from human Wharton's jelly. Reprod. Biomed. Online. 2007;15:708–718. doi: 10.1016/s1472-6483(10)60539-1. [DOI] [PubMed] [Google Scholar]
- Gale J. Marcotti W. Kennedy H. Kros C. Richardson G. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J. Neurosci. 2001;21:7013–7025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie P. Müller U. Mechanotransduction by hair cells: Models, molecules, and mechanisms. Cell. 2009;139:33–44. doi: 10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R. Vereecque R. Wickham T.J. Facon T. Hetuin D. Kovesdi I. Bauters F. Fenaux P. Quesnel B. Transduction of bone marrow cells by the AdZ. F (pK7) modified adenovirus demonstrates preferential gene transfer in myeloma cells. Hum. Gene Ther. 1999;10:2709–2717. doi: 10.1089/10430349950016753. [DOI] [PubMed] [Google Scholar]
- Huang Y. Chi F. Han Z. Yang J. Gao W. Li Y. New ectopic vestibular hair cell-like cells induced by Math1 gene transfer in postnatal rats. Brain Res. 2009;1276:31–38. doi: 10.1016/j.brainres.2009.04.036. [DOI] [PubMed] [Google Scholar]
- Jacobsen F. Hirsch T. Mittler D. Schulte M. Lehnhardt M. Druecke D. Homann H. Steinau H. Steinstraesser L. Polybrene improves transfection efficacy of recombinant replication deficient adenovirus in cutaneous cells and burned skin. J. Gene Med. 2006;8:138–146. doi: 10.1002/jgm.843. [DOI] [PubMed] [Google Scholar]
- Jeon S. Oshima K. Heller S. Edge A. Bone marrow mesenchymal stem cells are progenitors in vitro for inner ear hair cells. Mol. Cell. Neurosci. 2007;34:59–68. doi: 10.1016/j.mcn.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner A. Hoerstrup S.P. Tracy J. Breymann C. Maurus C.F. Melnitchouk S. Kadner G. Zund G. Turina M. Human umbilical cord cells: A new cell source for cardiovascular tissue engineering. Ann Thorac Surg. 2002;74:1422–1428. doi: 10.1016/s0003-4975(02)03910-3. [DOI] [PubMed] [Google Scholar]
- Karahuseyinoglu S. Cinar O. Kilic E. Kara F. Akay G.G. Demiralp D. Tukun A. Uckan D. Can A. Biology of stem cells in human umbilical cord stroma: In situ and in vitro surveys. Stem Cells. 2007;25:319–331. doi: 10.1634/stemcells.2006-0286. [DOI] [PubMed] [Google Scholar]
- Kawamoto K. Ishimoto S.I. Minoda R. Brough D.E. Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J. Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanuti M. Kouri C. Force S. Chang M. Amin K. Xu K. Blair I. Kaiser L. Albelda S. Use of protamine to augment adenovirus-mediated cancer gene therapy. Gene Ther. 1999;6:1600–1610. doi: 10.1038/sj.gt.3300987. [DOI] [PubMed] [Google Scholar]
- Li H. Corrales C. Edge A. Heller S. Stem cells as therapy for hearing loss. Trends Mol. Med. 2004;10:309–315. doi: 10.1016/j.molmed.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Lin T. Gu J. Zhang L. Davis J. Huang X. Cabbini G. Ji L. Fang B. Enhancing adenovirus-mediated gene transfer in vitro and in vivo by addition of protamine and hydrocortisone. J. Gene Med. 2003;5:868–875. doi: 10.1002/jgm.427. [DOI] [PubMed] [Google Scholar]
- Marx J.C. Allay J.A. Persons D.A. Nooner S.A. Hargrove P.W. Kelly P.F. Vanin E.F. Horwitz E.M. High-efficiency transduction and long-term gene expression with a murine stem cell retroviral vector encoding the green fluorescent protein in human marrow stromal cells. Hum. Gene Ther. 1999;10:1163–1173. doi: 10.1089/10430349950018157. [DOI] [PubMed] [Google Scholar]
- Meyerrose T.E. Roberts M. Ohlemiller K.K. Vogler C.A. Wirthlin L. Nolta J.A. Sands M.S. Lentiviral transduced human mesenchymal stem cells persistently express therapeutic levels of enzyme in a xenotransplantation model of human disease. Stem Cells. 2008;26:1713–1722. doi: 10.1634/stemcells.2008-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J.R. MacDonald R.B. Duggan A. Lenzi D. Standaert D.G. Corwin J.T. Corey D.P. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J. Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K. Weiss M. Mitchell B. Martin P. Davis D. Morales L. Helwig B. Beerenstrauch M. Abou Easa K. Hildreth T. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- Pfannenstiel S. Praetorius M. Plinkert P. Brough D. Staecker H. Bcl-2 gene therapy prevents aminoglycoside-induced degeneration of auditory and vestibular hair cells. Audiol. Neurotol. 2009;14:254–266. doi: 10.1159/000192953. [DOI] [PubMed] [Google Scholar]
- Praetorius M. Brough D. Hsu C. Plinkert P. Pfannenstiel S. Staecker H. Adenoviral vectors for improved gene delivery to the inner ear. Hear. Res. 2009;248:31–38. doi: 10.1016/j.heares.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius M. Hsu C. Baker K. Brough D. Plinkert P. Staecker H. Adenovector-mediated hair cell regeneration is affected by promoter type. Acta Oto-laryngol. 2010;130:215–222. doi: 10.3109/00016480903019251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H. Hang X. Xu W. Zhu W. Cao H. Chen Y. Xu X. Wang M. Xie Y. Sun J. Lentivirus-modified human umbilical cord mesenchymal stem cells maintain their pluripotency. Biotechnol. Appl Biochem. 2010;55:53–62. doi: 10.1042/BA20090210. [DOI] [PubMed] [Google Scholar]
- Qin H. Zhao L.D. Sun J.H. Ren L.L. Guo W.W. Liu H.Z. Zhai S.Q. Yang S.M. The differentiation of mesenchymal stem cells into inner ear hair cell-like cells in vitro. Acta Otolaryngol. 2011;131:1136–1141. doi: 10.3109/00016489.2011.603135. [DOI] [PubMed] [Google Scholar]
- Rachakatla R.S. Marini F. Weiss M.L. Tamura M. Troyer D. Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Ther. 2007;14:828–835. doi: 10.1038/sj.cgt.7701077. [DOI] [PubMed] [Google Scholar]
- Rivolta M.N. Holley M.C. Cell lines in inner ear research. J. Neurobiol. 2002;53:306–318. doi: 10.1002/neu.10111. [DOI] [PubMed] [Google Scholar]
- Roelvink P.W. Lizonova A. Lee J.G.M. Li Y. Bergelson J.M. Finberg R.W. Brough D.E. Kovesdi I. Wickham T.J. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarugaser R. Lickorish D. Baksh D. Hosseini M.M. Davies J.E. Human umbilical cord perivascular (HUCPV) cells: A source of mesenchymal progenitors. Stem Cells. 2005;23:220–229. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- Schlecker C. Praetorius M. Brough D. Presler R. Hsu C. Plinkert P. Staecker H. Selective atonal gene delivery improves balance function in a mouse model of vestibular disease. Gene Ther. 2011;18:884–890. doi: 10.1038/gt.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.S. Keller J.R. Lohrey N.C. McCauslin C.S. Ortiz M. Cowan K. Spence S.E. Redirected infection of directly biotinylated recombinant adenovirus vectors through cell surface receptors and antigens. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8855–8860. doi: 10.1073/pnas.96.16.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staecker H. Praetorius M. Baker K. Brough D. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol. Neurotol. 2007;28:223. doi: 10.1097/MAO.0b013e31802b3225. [DOI] [PubMed] [Google Scholar]
- Stone I.M. Lurie D.I. Kelley M.W. Poulsen D.J. Adeno-associated virus-mediated gene transfer to hair cells and support cells of the murine cochlea. Mol. Ther. 2005;11:843–848. doi: 10.1016/j.ymthe.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Taura A. Kikkawa Y.S. Nakagawa T. Ito J. Hydrogen protects vestibular hair cells from free radicals. Acta Otolaryngol. 2010;130:95–100. doi: 10.3109/00016489.2010.486799. [DOI] [PubMed] [Google Scholar]
- Tsuda H. Wada T. Ito Y. Uchida H. Dehari H. Nakamura K. Sasaki K. Kobune M. Yamashita T. Hamada H. Efficient BMP2 gene transfer and bone formation of mesenchymal stem cells by a fiber-mutant adenoviral vector. Mol. Ther. 2003;7:354–365. doi: 10.1016/s1525-0016(02)00062-x. [DOI] [PubMed] [Google Scholar]
- Wang H.S. Hung S.C. Peng S.T. Huang C.C. Wei H.M. Guo Y.J. Fu Y.S. Lai M.C. Chen C.C. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- Wang L. Ott L. Seshareddy K. Weiss M.L. Detamore M.S. Musculoskeletal tissue engineering with human umbilical cord mesenchymal stromal cells. Regen. Med. 2011;6:95–109. doi: 10.2217/rme.10.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M.L. Medicetty S. Bledsoe A.R. Rachakatla R.S. Choi M. Merchav S. Luo Y. Rao M.S. Velagaleti G. Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24:781–792. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- Yang Y. Hsieh Y. Protamine sulfate enhances the transduction efficiency of recombinant adeno-associated virus-mediated gene delivery. Pharm. Res. 2001;18:922–927. doi: 10.1023/a:1010923924844. [DOI] [PubMed] [Google Scholar]
- Zheng J. Gao W. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat. Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]