Abstract
Neurotrophins, such as brain-derived neurotrophic factor (BDNF), are important in modulating neuroplasticity and promoting recovery after spinal cord injury. Intrathecal delivery of BDNF enhances functional recovery following unilateral spinal cord hemisection (SH) at C2, a well-established model of incomplete cervical spinal cord injury. We hypothesized that localized delivery of BDNF-expressing mesenchymal stem cells (BDNF-MSCs) would promote functional recovery of rhythmic diaphragm activity after SH. In adult rats, bilateral diaphragm electromyographic (EMG) activity was chronically monitored to determine evidence of complete SH at 3 days post-injury, and recovery of rhythmic ipsilateral diaphragm EMG activity over time post-SH. Wild-type, bone marrow-derived MSCs (WT-MSCs) or BDNF-MSCs (2×105 cells) were injected intraspinally at C2 at the time of injury. At 14 days post-SH, green fluorescent protein (GFP) immunoreactivity confirmed MSCs presence in the cervical spinal cord. Functional recovery in SH animals injected with WT-MSCs was not different from untreated SH controls (n=10; overall, 20% at 7 days and 30% at 14 days). In contrast, functional recovery was observed in 29% and 100% of SH animals injected with BDNF-MSCs at 7 days and 14 days post-SH, respectively (n=7). In BDNF-MSCs treated SH animals at 14 days, root-mean-squared EMG amplitude was 63±16% of the pre-SH value compared with 12±9% in the control/WT-MSCs group. We conclude that localized delivery of BDNF-expressing MSCs enhances functional recovery of diaphragm muscle activity following cervical spinal cord injury. MSCs can be used to facilitate localized delivery of trophic factors such as BDNF in order to promote neuroplasticity following spinal cord injury.
Key words: : diaphragm muscle, neuroplasticity, neurotrophin, respiration, spinal hemisection
Introduction
Neurotrophins, such as brain-derived neurotrophic factor (BDNF), are important in modulating neuroplasticity and promoting recovery after spinal cord injury.1 A commonly used model of incomplete cervical spinal cord injury is the unilateral spinal cord hemisection (SH) at C2 model. Phrenic motoneurons innervating the diaphragm muscle are located in cervical spinal cord segments C3–C6 in rats,2–4 and receive predominantly ipsilateral descending excitatory bulbospinal inputs.5,6 SH involves a unilateral transection of anterolateral funiculi at C2, which removes premotor drive to phrenic motoneurons, paralyzing the ipsilateral diaphragm muscle.7–14 Following SH, there is gradual recovery of rhythmic diaphragm activity ipsilateral to injury,15–21 suggesting neuroplasticity and strengthening of spared contralateral synaptic inputs to phrenic motoneurons. Importantly, recovery of ipsilateral diaphragm activity after SH can be enhanced by intrathecal delivery of BDNF at the level of the phrenic motor nucleus.14 Unfortunately, nonlocalized administration of BDNF by systemic,22 or even intrathecal, treatment23,24 in human studies is linked to adverse effects, mainly related to altered sensory processing and pain,25–27 muscle spasticity,28,29 and other behavioral effects22 that prevent its therapeutic use.
Mesenchymal stem cells (MSCs) can be used to facilitate localized delivery of trophic factors such as BDNF, and minimize side effects associated with systemic delivery. Advantages of using MSCs include their endogenous release of neurotrophic factors,30–35 and that they can be engineered to release higher levels of trophic factors such as BDNF.31,36–39 We hypothesized that localized delivery of BDNF-expressing MSCs (BDNF-MSCs) will promote functional recovery of rhythmic diaphragm activity after SH. Localized delivery was attained using intraspinal injections of adult rat MSCs engineered to produce releasable BDNF by retroviral treatment.36,40,41 Our results demonstrate that localized delivery of BDNF-MSCs promotes functional recovery of rhythmic diaphragm activity after SH, and that this effect depends upon released BDNF, because wild-type MSCs (WT-MSCs) did not enhance functional recovery in this model of incomplete spinal cord injury.
Methods
Experimental animals
Adult male Sprague–Dawley rats (colony 236, Harlan, Indianapolis, IN; initial body weight ∼300 g) underwent unilateral SH. The Mayo Clinic Institutional Animal Care and Use Committee approved all experimental procedures. All surgical procedures and experimental measurements were performed under appropriate anesthesia induced by intramuscular ketamine (90 mg/kg) and xylazine (10 mg/kg) and maintained by intermittent repeat dosing as needed.
SH
The surgical procedure for SH has been previously published.8,9,11,12,14,21 Briefly, the cervical spinal cord was exposed with a bilateral dorsal laminectomy at C2, and the right anterolateral cord was transected at C2 (i.e., involving the lateral and ventral funiculi, and preserving the dorsal funiculus). All animals were observed daily after surgery, and intramuscular buprenorphine and oral acetaminophen were administered for the first 3 days.
Chronic diaphragm electromyographic (EMG) recordings
A pair of electrode wires (0.28 mm diameter - model AS631, Cooner Wire Inc., Chatsworth, CA) were stripped ∼3 mm at the tip, and implanted into both the right and left diaphragm midcostal regions at 3 days prior to the SH surgery, as previously described.14,21,42–46 The uninsulated portion was embedded within the diaphragm. The wires were tunneled subcutaneously to the dorsum of the animal and externalized. Diaphragm EMG activity was recorded in anesthetized rats during SH surgery and at 3 days post-SH (SH 3D) to verify absence of ipsilateral diaphragm activity and completeness of SH. Diaphragm EMG activity was also recorded during eupnea in anesthetized rats at 7 days post-SH (SH 7D) and 14 days post-SH (SH 14D). The EMG signal was differentially amplified (2000×), band-pass filtered (20–1000 Hz), and analog-to-digitally converted at 2 kHz using a LabView data acquisition board and software (National Instruments, Austin, TX). Signals were digitally stored until blinded analyses could be performed on the entire dataset.
MSCs
BDNF-green fluorescent protein (GFP) expressing MSCs (BDNF-MSCs; obtained from G.E. Rooney at the Regenerative Medicine Institute, National University of Ireland, Galway, Ireland),36,40,41 were derived from the bone marrow of adult transgenic rats expressing GFP and transduced with murine leukemia virus-encoding BDNF. WT-MSCs were derived from the bone marrow of adult WT rats. Both WT-MSCs and BDNF-MSCs were grown to ∼70% confluence in rat MSCs media (αMEM-F12, 10% fetal bovine serum [FBS], 1% penicillin-streptomycin) and incubated at 37°C in 5% CO2 and 90% humidity. Media was changed every 3–4 days. MSCs were characterized by consistent cell surface expression of CD105 and lack of cell surface expression of CD45, indicating an undifferentiated MSCs phenotype. The amount of BDNF produced and released in vitro by BDNF-MSCs (n=5; passage 7–15, ∼1.2×105 cells/mL) and WT-MSCs (n=4; passage 11–12, ∼1.4×105 cells/mL) was measured by enzyme-linked immunosorbent assay (ELISA) (Human BDNF Quantikine kit, R&D Systems, Minneapolis, MN) following the manufacturer's protocol. Duplicate assays showed that BDNF-MSCs released significantly greater levels of BDNF compared to WT-MSCs (37.9±7.9 compared to 0.2±0.01 ng/mL, respectively; p=0.004).
At the time of the SH surgery, animals were randomly assigned to the following groups: BDNF-MSCs treated (n=7), WT-MSCs treated (n=4) and untreated (n=6). For in vivo transplantation, MSCs were resuspended at a concentration of ∼5×104 cells/μL in sterile media containing F-12. Around the site of SH injury (C2 level), 2 μL of cell suspension were injected unilaterally ∼1 mm rostral and ∼1 mm caudal to the C2 level using a Hamilton syringe (total injected volume, 4 μL), 2 mm lateral to midline and at a depth of 3.5 mm (corresponding to the anterolateral funiculus). The concentration of cells injected in vivo (200,000 cells total) would be expected to generate ∼24.4 ng BDNF per day in BDNF-MSCs treated SH animals compared with ∼0.2 ng BDNF per day in WT-MSCs treated SH animals. In preliminary studies, survival of transplanted BDNF-MSCs at the site of injection was confirmed at 1 day post-transplantation.
EMG analyses
As in previous studies,14,21,46 two outcome measures defining functional recovery were determined from EMG analyses: 1) the proportion of animals displaying recovery of rhythmic ipsilateral diaphragm EMG activity; and 2) the amplitude of the ipsilateral diaphragm root mean squared (RMS) EMG signal (i.e., extent of functional recovery). The criteria for classification of functional recovery included a diaphragm EMG signal that: 1) was both rhythmic (i.e., in phase with contralateral signal) and periodic (i.e., occurring across multiple eupneic bursts); 2) was composed of more than one motor unit; and 3) displayed RMS EMG amplitude >10% of the pre-SH amplitude. For each recording period (i.e., D0, D3, D7, and D14) and hemidiaphragm, RMS EMG amplitude was calculated using a moving window of 50 ms.21,44,47
Immunohistochemistry
In BDNF-MSCs treated SH animals, cholera toxin subunit B (CTB) (List Biological Laboratories, Campbell, CA) was used to retrogradely label phrenic motoneurons by intrapleural injection at 3 days before the terminal experiment, as previously reported.12,48,49 At SH 14D, rats were anesthetized, euthanized by exsanguination after administration of heparin, and transcardially perfused with 4% paraformaldehyde in phosphate-buffered saline (PBS). The cervical spinal cord was excised and post-fixed in 4% paraformaldehyde overnight, and then transferred to 24% sucrose in PBS for 24–72 h. Longitudinal 50 μm thick sections were cut with a Reichert–Jung Frigocut cryostat (Reichert Microscope Services, Depew, NY). Spinal cord sections were blocked with 10% donkey serum in 0.3% Triton tris-buffered saline (TBS), followed by overnight incubation with CTB antibody (goat polyclonal, #703; List Biological Laboratories) and GFP antibody (rabbit polyclonal, ab6556; Abcam, Cambridge, MA). Secondary antibodies DyLight 649-conjugated anti-goat and Cy3-conjugated anti-rabbit (Jackson Immunoresearch, West Grove, PA) were used. Tissue sections were mounted on slides, dehydrated in graded alcohol concentrations, and cover-slipped with DPX mountant (Fluka, Sigma–Aldrich, St. Louis, MO).
Primary antibody validation
Immunoreactivity with the GFP antibody was previously validated in the rat spinal cord by using intrapleural delivery of AAV7 expressing GFP (2×1011 genome copies/animal), which specifically transduces phrenic motoneurons present in the cervical ventral horn region.46 GFP immunoreactivity was absent in spinal cord from control (untreated) rats. In addition, we confirmed that the GFP antibody recognized a single band (∼27 kDa) in Western blot analyses of rat spinal cord that was treated with AAV7-GFP.
Immunoreactivity with the CTB antibody was validated by lack of staining in the spinal cords of rats not injected with intrapleural CTB. Retrogradely labeled phrenic motoneurons in rats injected with CTB had a staining pattern identical to previous studies.12,46,48,49
Confocal microscopy
Spinal cord sections were imaged using a Nikon C1 laser scanning confocal system (Nikon Instruments, Melville, NY) mounted on an upright Nikon Eclipse 90i microscope with argon (488 nm) and solid state (561 and 640 nm) lasers. Phrenic motoneurons and GFP immunoreactivity were imaged with a 20×oil immersion lens (NA 0.75), as well as GFP autofluoresence, to define the borders of white and gray matter in the spinal cord. The entire width of the cervical spinal cord was imaged from the C2 to C4 segments, with individual images having a 10% overlap to allow for stitching. Nikon Elements (version 4.13, Nikon Instruments) was used to stitch individual images to create the merged three channel image. Images were exported into Adobe Photoshop (version 7.0), and, as needed, were changed to gray scale and inverted, and levels were adjusted linearly. For each primary/secondary antibody pair, additional studies, not including the primary antibody (blank), were conducted. Sections from both experimental groups and blank controls were processed in parallel for all immunohistochemical reactions and animals.
Statistical analyses
All statistical evaluations were performed using JMP statistical software (version 9.0.1, SAS Institute Inc., Cary, NC). The proportion of animals displaying functional recovery was compared across groups using Pearson's χ2 test. Diaphragm RMS EMG amplitude was normalized to the eupneic value before SH for the same animal, and differences between treatment groups were examined using one way analysis of variance (ANOVA). Statistical significance was established at the 0.05 level. All experimental data are presented as mean±SE, unless otherwise specified.
Results
Intraspinal injection
Animals were successfully injected with intraspinal BDNF-MSCs or WT-MSCs. No deaths occurred, and no adverse effects of the injection were noted. All animals displayed normal weight gain and behavior, compared with the untreated SH group. All SH animals displayed gross deficits related to the cervical spinal cord injury such as altered movements of the rib cage and abdomen consistent with hemidiaphragm paralysis.8,9
Histological evidence of transplanted MSCs survival
The presence of MSCs in the cervical spinal cord was determined by GFP immunoreactivity at SH 14D (n=6). Retrograde labeling with CTB allowed for visualization of phrenic motoneurons, as in previous studies,2,12,48–50 in order to localize transplanted MSCs relative to the phrenic motor pool. As expected, expression of GFP by transplanted BDNF-MSCs was localized near the injection sites (i.e., both rostral and caudal to C2), and primarily in the white matter (Fig. 1). Expression of GFP was evident between C2 and C3, with no evidence of GFP immunoreactivity present in the vicinity of phrenic motoneurons. GFP immunoreactivity was not present contralateral to the injection sites (i.e., on the uninjured side). Evidence of MSCs survival 14 days following transplantation was limited to two animals, however; one animal displayed extensive GFP immunoreactivity.
FIG. 1.
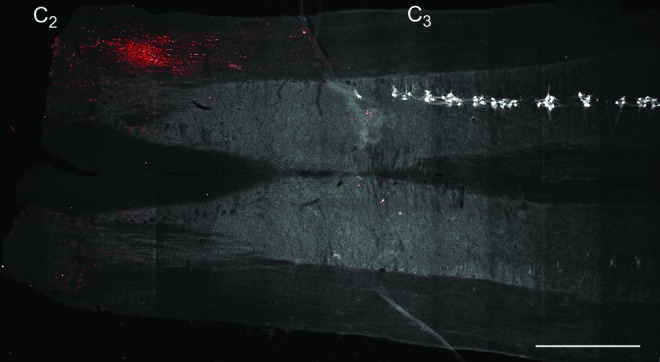
Delivery of mesenchymal stem cells engineered to produce releasable brain-derived neurotrophic factor (BDNF)-green fluorescent protein (GFP) (BDNF-expressing mesenchymal stem cells [BDNF-MSCs]) to the spinal cord was confirmed by GFP expression. Representative stitched image composed of 91 fields of view of a longitudinal spinal cord section at ∼C2–C4. The left side of the spinal cord is on the bottom and the right side is on the top of the image. Autofluorescence at 488 nm was used to create the background gray scale image. Note that white matter appears darker than the gray matter. Cholera toxin subunit B-labeled phrenic motoneurons were converted to gray scale and inverted to facilitate visualization (white). Phrenic motoneurons were present on the right side of the spinal cord starting near C3, and were not located on the left side of the spinal cord in this section. Intraspinal injection of BDNF-MSCs (into right side of spinal cord, rostral and caudal to C2; n=6) resulted in GFP immunoreactivity (red) between C2 and C3, and primarily in the white matter. Some background fluorescence was visible in this section contralateral to the injections (left side), but overall GFP expression was not evident in the contralateral side. Bar, 1 mm. Color image is available online at www.liebertpub.com/neu
Proportion of animals displaying functional recovery after SH
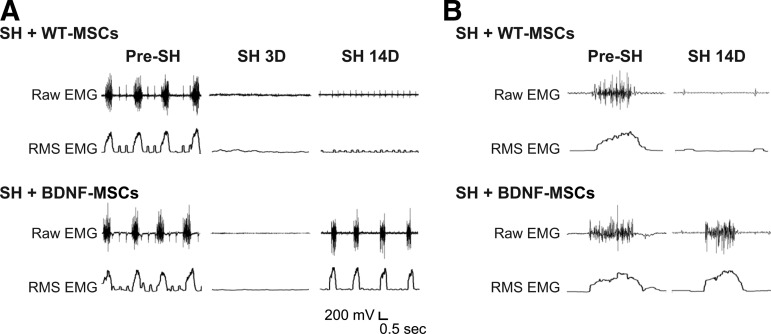
Diaphragm EMG electrodes were successfully implanted in all SH animals at 3 days before surgery (n=17). Diaphragm EMG was recorded during the surgery to verify the completeness of SH surgery. Both ipsilateral and contralateral diaphragm EMG activity in anesthetized animals occurred in bursts, and was rhythmic. As in previous studies,14,21,46 absence of ipsilateral diaphragm EMG activity 3D post-SH was also used to assess complete functional hemisection. None of the animals displayed rhythmic ipsilateral EMG activity during eupnea at SH 3D, confirming complete interruption of descending drive to phrenic motoneurons ipsilateral to SH. Diaphragm EMG was also recorded during eupnea at SH 7D and SH 14D. Representative diaphragm EMG recordings and RMS EMG tracings are shown in Figure 2.
FIG. 2.
Representative raw diaphragm electromyographic (EMG) recordings and root mean squared (RMS) EMG tracings after C2 hemisection (SH) and injection with wild-type mesenchymal stem cells (WT-MSCs) or brain-derived neurotrophic factor (BDNF)-green fluorescent protein (GFP) expressing MSCs (BDNF-MSCs). Intraspinal WT-MSCs treated SH (SH+WT-MSCs; n=4) and intraspinal BDNF-MSCs treated SH (SH+BDNF-MSCs; n=7) animals were monitored under anesthesia using EMG recordings obtained during eupnea via chronically implanted diaphragm electrodes. (A) EMG recordings obtained prior to SH (pre-SH), 3 days after SH (SH 3D) and 14 days after SH (SH 14D) for one animal from each group are shown. Diaphragm EMG activity occurred in rhythmic bursts. Electrocardiographic activity is visible in some recordings as narrow spikes. In this example, there is a lack of diaphragm EMG activity in the SH+WT-MSCs group. Untreated SH animals (n=6) displayed qualitatively similar results to the WT-MSCs treated SH group and are not shown. (B) Expanded EMG recordings for SH+WT-MSCs and SH+BDNF-MSCs groups, obtained pre-SH and at SH 14D. Each tracing is the first burst of the corresponding EMG recording in A. Notice multiple active motor units (with varying spike morphology) in the diaphragm EMG signal at SH 14D with intraspinal BDNF-MSCs treatment.
The proportion of animals displaying functional recovery after SH (i.e., rhythmic ipsilateral diaphragm EMG activity) was evaluated up to 14 days post-injury. Over time, a subset of SH animals displayed spontaneous recovery of ipsilateral diaphragm EMG activity during eupnea. At SH 7D, two out of six untreated SH animals (33%) displayed functional recovery of ipsilateral hemidiaphragm EMG activity. By SH 14D, three untreated SH animals (50%) displayed functional recovery. These recovery proportions are similar to what has been previously published for the SH model.12,14,46 A subset of animals were treated with WT-MSCs (n=4). No animals in the WT-MSCs group displayed functional recovery of ipsilateral EMG activity at SH 7D. At SH 14D, one out of four WT-MSCs treated SH animals displayed minimal recovery (5% of the pre-SH RMS EMG amplitude), which is less than the established criterion of 10% of pre-SH RMS EMG amplitude to be classified as functional recovery. The proportion of WT-MSCs treated SH animals displaying functional recovery was not different than that for untreated SH animals (p=0.20 at SH 7D and p=0.09 at SH 14D); therefore, these groups were combined into one group called “Control/MSCs treated SH” (Fig. 3). Overall (n=10), two Control/MSCs treated SH rats (20%) displayed functional recovery at SH 7D and three rats (30%) displayed functional recovery at SH 14D.
FIG. 3.
Proportion of animals displaying functional recovery of ipsilateral hemidiaphragm eupneic electromyographic (EMG) activity after C2 hemisection (SH). Chronic diaphragm EMG recordings were used to establish the proportion of animals displaying functional recovery after SH. The criteria for classification of functional recovery are detailed in the Methods section. A subset of animals were treated with wild-type mesenchymal stem cells (WT-MSCs) (n=4) and were not different than untreated SH animals (n=6); therefore, these groups were combined into one group (SH+Control/MSCs). Diaphragm EMG activity was absent in all animals at 3 days after SH (SH 3D), confirming complete interruption of descending ipsilateral drive to phrenic motoneurons and resulting diaphragm muscle paralysis. At 7 days after SH (SH 7D), 2 out of 7 animals with intraspinal injection of brain-derived neurotrophic factor (BDNF)-MSCs (SH+BDNF-MSCs) displayed functional recovery, compared with 2 out of 10 SH+Control/MSCs treated animals (p=0.68). By SH 14D, all of the BDNF-MSCs treated SH animals displayed functional recovery, compared with 3 out of 10 SH+Control/MSCs treated animals (*p=0.004).
Intraspinal injection of BDNF-MSCs (n=7) increased the proportion of animals displaying ipsilateral hemidiaphragm EMG activity during eupnea after SH. At SH 7D, two animals (29%) displayed functional recovery (p=0.68 compared to Control/MSCs treated SH; Fig. 3). By SH 14D, all of the BDNF-MSCs treated SH animals displayed functional recovery (p=0.004 compared with Control/MSCs treated SH).
Extent of eupneic diaphragm EMG activity after SH
Diaphragm RMS EMG amplitude was assessed during eupnea to determine the extent of recovery of rhythmic diaphragm EMG activity after SH. Diaphragm RMS EMG amplitude at SH 7D and SH 14D was normalized to the eupneic value before SH for the same animal. Quantitative measurements were not possible in one BDNF-MSCs treated SH rat at SH 14D even though this animal displayed evidence of recovery of ipsilateral diaphragm EMG activity. There was minimal variability in diaphragm RMS EMG amplitude within each recording session. Localized delivery of BDNF-MSCs significantly increased the extent of recovery after SH. Figure 4 shows a box plot of diaphragm RMS EMG amplitude and the individual animal values at SH 7D and 14D. At SH 14D, RMS EMG amplitude was 12±9% of pre-SH eupneic RMS EMG amplitude in the Control/MSCs treated SH animals (including both those that displayed recovery and those that did not; n=10). In Control/MSCs treated SH animals that displayed recovery at SH 14D, the RMS EMG amplitude was 40±23% of the pre-SH value (n=3). In contrast, in BDNF-MSCs treated SH animals, RMS EMG amplitude was 25±8% of the pre-SH value at SH 7D (n=2 rats that displayed recovery) and 63±16% of the pre-SH value at SH 14D (n=6; p=0.008 compared with Control/MSCs treated SH animals).
FIG. 4.
Extent of functional recovery of ipsilateral rhythmic phrenic activity at 7 days (7D) and 14 days (14D) after C2 hemisection (SH). Individual animal values are depicted by different symbols within each group; filled symbols denote animals that displayed recovery at any point beyond 3 days after SH. Diaphragm root mean squared (RMS) electromyographic (EMG) amplitude was measured during eupnea at 3, 7, and 14 days after SH, and normalized to the pre-SH RMS EMG amplitude for the same animal. In all animals, RMS EMG was 0 at 3 days after SH. Data are shown as box plots (25th to 75th percentile), with the thicker line in the box representing the median value. In the Control/wild-type mesenchymal stem cells (WT-MSCs) treated SH animals (SH+Control/MSCs; n=10), RMS EMG amplitude was reduced at SH 14D compared with pre-SH. Intraspinal injection of BDNF-MSCs (SH+BDNF-MSCs; n=6) increased the extent of diaphragm activity at SH 14D compared with SH+Control/MSCs (p=0.008).
Contralateral diaphragm RMS EMG amplitude at SH 14D increased similarly in both Control/MSCs treated SH (37±17% increase; n=10) and BDNF-MSCs treated SH animals (47±39% increase; n=6) compared with pre-SH EMG activity (repeated measures ANOVA; p=0.04 for time; p=0.79 for across-group comparison; there was no time vs. group interaction).
Respiratory rate was measured from contralateral hemidiaphragm eupneic EMG recordings. Respiratory rate was unaffected by Control/MSCs and/or BDNF-MSCs treatment. In Control/MSCs treated SH animals (n=10), the respiratory rate was 81±5 min−1 before SH and 90±6 min−1 at SH 14D (repeated measures ANOVA; p=0.06). In BDNF-MSCs treated SH animals (n=6), the respiratory rate was 72±4 min−1 before SH and 81±5 min−1 at SH 14D (repeated measures ANOVA; p=0.06). There were no differences in respiratory rate across treatment groups at SH 14D (p=0.13).
Discussion
This study used engineered bone marrow-derived MSCs to localize delivery of BDNF to injured regions of the spinal cord in a well-established model of incomplete cervical spinal cord injury (SH). Localized delivery of BDNF-MSCs that release 120-fold greater levels of BDNF by retrovirus treatment promotes functional recovery of diaphragm muscle activity following SH. In contrast, delivery of WT-MSCs did not promote recovery. Importantly, these results support the importance of motoneuron BDNF/TrkB signaling to promote recovery of respiratory function following incomplete cervical spinal cord injury.14,46 Localized delivery of BDNF may minimize off-target effects associated with systemic or intrathecal delivery of BDNF that prevent its therapeutic utility.22–24,27,28
Beneficial effects of MSCs
MSCs are multipotent stem cells that are attractive for regenerative therapy and specifically for neuroprotective and neurorestorative therapy after spinal cord injury.30,51–55 Some of the beneficial effects of MSCs are mediated by their release of neurotrophic factors.34,35 This allows neurotrophins to be delivered locally to the spinal cord, overcoming issues related to neurotrophin penetration and bioavailability. MSCs may also secrete neurotrophins in response to local cues such as hypoxia, apoptosis, or inflammation.56 As such, MSCs have the potential to function as “intelligent” drug delivery vehicles. Notably, WT-MSCs did not promote recovery following SH, suggesting that local delivery of BDNF was insufficient in the absence of retroviral treatment. The beneficial effects of MSCs on long-term functional recovery from spinal cord injury can be seen up to 6 months57 or 1 year after administration.58 However, there is evidence that MSCs survival is low at 2 weeks after injection,36,59,60 in agreement with the present study showing evidence of transplanted MSCs survival in two out of six animals at 2 weeks. Immunosuppression may enhance MSCs survival,61 and could be used in future experiments examining longer-term effects of BDNF-MSCs transplantation.
Role of BDNF on functional recovery after spinal cord injury
Spinal cord injury itself increases expression of neurotrophins.14,62–65 Specifically, BDNF expression is elevated in the local environment containing phrenic motoneurons at SH 14D, and likely contributes to the spontaneous recovery of ipsilateral hemidiaphragm EMG activity that occurs over time after SH.14 It is known that BDNF plays an important role in neuroplasticity following spinal cord injury,1 and previous studies have shown that BDNF treatment after spinal cord injury increases survival of neurons, axonal sprouting, and/or recovery of function when delivered by intrathecal infusion,66–69 intraspinal viral treatment,28,29,70 or stem cell transplantation.37,71 Unfortunately, nonlocalized administration of BDNF is associated with adverse effects in human studies. Thirteen of 20 amyotrophic lateral sclerosis (ALS) patients receiving intrathecal BDNF reported sensory symptoms, and 9 reported behavioral effects.23 Animal studies have additionally shown induction of muscle spasticity at 4 weeks after AAV-mediated delivery of BDNF.28,29 The potential adverse effects of BDNF were not an outcome of the current study design; therefore, allodynia or spasticity was not systematically assessed. We did not observe behavioral manifestations suggestive of allodynia or spasticity following 14 days of MSCs-based BDNF delivery, in agreement with the lack of adverse effects of intrathecal BDNF treatment at C4.14 However, it is possible that BDNF side effects are delayed, and not observed during this limited survival time of 14 days.
In the SH model of spinal cord injury used in this study, increasing BDNF availability by intrathecal delivery of BDNF at the level of phrenic motoneurons enhanced functional recovery after SH, with 100% of rats displaying rhythmic phrenic activity at SH 14D,14 compared with ∼40% of untreated SH rats.12,14,46 Intraspinal stem cell transplantation offers the advantage of targeted, contextual release of BDNF that is not possible with intrathecal or systemic infusion and, therefore, may contribute to reduced side effects.
It is of note that the genetically engineered MSCs delivered a lower level of BDNF compared with intrathecal BDNF infusion in the same SH model.14 The concentration of cells injected in this study released ∼24.4 ng BDNF per day, estimated from in vitro BDNF production measurements. In contrast, chronic infusion of BDNF using an intrathecal catheter and implanted mini-osmotic pump delivered ∼180 ng BDNF per day.14 Both doses of BDNF, however, resulted in functional recovery of 100% of rats at SH 14D. In addition, the extent of recovery was similar between the localized, lower dose achieved by BDNF-MSCs and the higher dose administered via intrathecal infusion (63±16% vs. 76±17% of pre-SH RMS EMG, respectively). These findings support targeting of BDNF to phrenic motoneurons being important in the functional response, and suggest a minimal dose requirement for functional improvement. In the present study, we found that functional recovery of WT-MSCs treated SH rats (which deliver ∼0.2 ng BDNF per day) was not different from that of untreated SH controls (overall, 30% at SH 14D).
Mechanism of action
Neurotrophins contribute to recovery after spinal cord injury by a number of mechanisms, including enhancing synaptic input from descending premotor pathways, increasing the strength of pre-existing synaptic connections, increasing sprouting and generation of new synaptic connections, and/or increasing growth or sprouting of descending corticospinal and bulbospinal axons.1,14,31,46,72 Furthermore, BDNF treatment can directly modulate synaptic efficacy in the adult rat.73,74 Some of the beneficial effects of MSCs are mediated by release of neurotrophic factors33,34 and, therefore, may be independent of cellular incorporation and differentiation.32,35 MSCs increase axonal sprouting and motoneuron survival in contusive30 and transection31 models of spinal cord injury, despite limited cell differentiation. Several strategies have been used to enhance MSCs release of neurotrophic factors such as BDNF, including retroviral36,37 or adenoviral treatment of MSCs.31,38,39 Therefore, several studies have engineered MSCs to produce elevated levels of BDNF, and have found that they elicited enhanced neuroprotective effects compared with WT-MSCs.31,37–39 The comparison between the functional impact of WT-MSCs and BDNF-MSCs supports the role of BDNF in enhancing recovery after SH, suggesting that optimal localized delivery may be attained using a cell-based strategy.
MSCs as a vehicle for BDNF production
As seen by GFP immunoreactivity in the cervical spinal cord in rats injected with BDNF-MSCs (which also express GFP), MSCs successfully incorporated into the spinal cord and were capable of expressing GFP. GFP expression was evident in two (out of six) animals at 14 days post-transplantation, and was primarily localized near the injection sites. There was no localization of MSCs in the vicinity of phrenic motoneurons, as identified by retrograde labeling with CTB. Taken together, these results suggest that the transplanted cells migrated minimally during the 14 day experimental period. It is likely that incorporation of MSCs into neural tissue was limited, consistent with previous reports.36,75,76 Other studies have evaluated markers of MSCs differentiation (e.g., CD90, CD105, CD45, or CD34)77 or markers of neural, oligodendrocyte, or astrocytic phenotype36 to determine cell fate following stem cell transplantation. However, such characterization would not enhance this study. Release of BDNF was the main therapeutic effect of MSCs transplantation, with MSCs modifying the environment around the site of spinal cord injury by providing sustained release of BDNF into the injured spinal cord. This is evidenced by the significant increase in both the proportion of animals that recovered and the extent of that recovery after treatment with BDNF-MSCs, compared with WT-MSCs.
This study evaluated EMG activity during eupnea as a measure of spontaneous recovery after SH. The animal-to-animal variability in extent of recovery following SH (i.e., RMS EMG amplitude) was consistent with previous studies using the SH model.14,21,46 It is important to note that ventilation during eupnea can be sustained across a range of species by generation of transdiaphragmatic pressure that is ∼20% of the total force generating capacity of the diaphragm.47,78–80 It is unknown if injection of BDNF-MSCs after SH also increased EMG activity during other ventilatory (hypoxia-hypercapnia) and nonventilatory behaviors (i.e., airway occlusion, sneezing) that would require generation of larger pressures and force by the diaphragm muscle. Future studies could explore treatments to enhance recovery of expulsive behaviors, as maintenance of airway clearance is an important predictor of survival following spinal cord injury.81,82 Based on a recent study using the SH model, it is tempting to speculate that improved results could be obtained by combinatorial therapies such as BDNF-MSCs treatment in the presence of AAV-mediated upregulation of TrkB.FL expression in phrenic motoneurons.46
In future studies, BDNF production using induced pluripotent stem cells (iPSCs) is an attractive alternative. iPSCs are generated by reprogramming mature, fully differentiated cells into a pluripotent state.83 Their advantage is that easily available cells, such as skin from a spinal cord injury patient, could be reprogrammed, differentiated, engineered, and transplanted. Recent studies have transplanted human iPSC-derived neurospheres or monolayer cultures into the spinal cord after injury, and demonstrated extensive incorporation, improved locomotor recovery, increased expression of neurotrophic factors, angiogenesis, axonal outgrowth, and remyelination.84–86 The beneficial effects of iPSCs after spinal cord injury may enhance the extent of recovery during eupnea, and possibly permit greater levels of recovery of more forceful, nonventilatory behaviors promoting functional recovery of rhythmic diaphragm activity after SH beyond levels achievable even with BDNF-MSCs transplantation.
Acknowledgments
We acknowledge Jeffrey Bailey, Amy Jorgenson, Laura Restrepo, and Alejandra Arias for their technical assistance in the completion of this project. This work was supported by National Institutes of Health (NIH) grants R01-HL096750 (G.C.S. and C.B.M.), T32-HL105355 (H.M.G.), the Mayo Clinic Center for Regenerative Medicine (H.M.G.), and the Mayo Clinic.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Weishaupt N., Blesch A., and Fouad K. (2012). BDNF: the career of a multifaceted neurotrophin in spinal cord injury. Exp. Neurol. 238, 254–264 [DOI] [PubMed] [Google Scholar]
- 2.Prakash Y.S., Mantilla C.B., Zhan W.Z., Smithson K.G., and Sieck G.C. (2000). Phrenic motoneuron morphology during rapid diaphragm muscle growth. J. Appl. Physiol. 89, 563–572 [DOI] [PubMed] [Google Scholar]
- 3.Song A., Ashwell K.W., and Tracey D.J. (2000). Development of the rat phrenic nucleus and its connections with brainstem respiratory nuclei. Anat. Embryol. (Berl.) 202, 159–177 [DOI] [PubMed] [Google Scholar]
- 4.Goshgarian H.G., and Rafols J.A. (1984). The ultrastructure and synaptic architecture of phrenic motor neurons in the spinal cord of the adult rat. J. Neurocytol. 13, 85–109 [DOI] [PubMed] [Google Scholar]
- 5.Mantilla C.B., Seven Y.B., and Sieck G.C. (2014). Convergence of pattern generator outputs on a common mechanism of diaphragm motor unit recruitment. Prog. Brain Res. 209, 309–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellenberger H.H., Feldman J.L., and Goshgarian H.G. (1990). Ventral respiratory group projections to phrenic motoneurons: electron microscopic evidence for monosynaptic connections. J. Comp. Neurol. 302, 707–714 [DOI] [PubMed] [Google Scholar]
- 7.Zhan W.Z., Miyata H., Prakash Y.S., and Sieck G.C. (1997). Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J. Appl. Physiol. 82, 1145–1153 [DOI] [PubMed] [Google Scholar]
- 8.Miyata H., Zhan W.Z., Prakash Y.S., and Sieck G.C. (1995). Myoneural interactions affect diaphragm muscle adaptations to inactivity. J. Appl. Physiol. 79, 1640–1649 [DOI] [PubMed] [Google Scholar]
- 9.Prakash Y.S., Miyata H., Zhan W.Z., and Sieck G.C. (1999). Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve 22, 307–319 [DOI] [PubMed] [Google Scholar]
- 10.Goshgarian H.G., Ellenberger H.H., and Feldman J.L. (1991). Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei: a possible substrate for the crossed–phrenic phenomenon. Exp. Neurol. 111, 135–139 [DOI] [PubMed] [Google Scholar]
- 11.Mantilla C.B., Rowley K.L., Zhan W.Z., Fahim M.A., and Sieck G.C. (2007). Synaptic vesicle pools at diaphragm neuromuscular junctions vary with motoneuron soma, not axon terminal, inactivity. Neuroscience 146, 178–189 [DOI] [PubMed] [Google Scholar]
- 12.Mantilla C.B., Bailey J.P., Zhan W.Z., and Sieck G.C. (2012). Phrenic motoneuron expression of serotonergic and glutamatergic receptors following upper cervical spinal cord injury. Exp. Neurol. 234, 191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinit S., Gauthier P., Stamegna J.C., and Kastner A. (2006). High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J. Neurotrauma 23, 1137–1146 [DOI] [PubMed] [Google Scholar]
- 14.Mantilla C.B., Gransee H.M., Zhan W.Z., and Sieck G.C. (2013). Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury. Exp. Neurol. 247C, 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golder F.J., and Mitchell G.S. (2005). Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J. Neurosci. 25, 2925–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golder F.J., Fuller D.D., Davenport P.W., Johnson R.D., Reier P.J., and Bolser D.C. (2003). Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J. Neurosci. 23, 2494–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sieck G.C., and Mantilla C.B. (2009). Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Respir. Physiol. Neurobiol. 169, 218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goshgarian H.G. (2003). Plasticity in respiratory motor control: invited review: the crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J. Appl. Physiol. 94, 795–810 [DOI] [PubMed] [Google Scholar]
- 19.Nantwi K.D., El-Bohy A., Schrimsher G.W., Reier P.J., and Goshgarian H.G. (1999). Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehab. Neural Repair 13, 225–234 [Google Scholar]
- 20.Boulenguez P., Gauthier P., and Kastner A. (2007). Respiratory neuron subpopulations and pathways potentially involved in the reactivation of phrenic motoneurons after C2 hemisection. Brain Res. 1148, 96–104 [DOI] [PubMed] [Google Scholar]
- 21.Mantilla C.B., Greising S.M., Zhan W.Z., Seven Y.B., and Sieck G.C. (2013). Prolonged C2 spinal hemisection-induced inactivity reduces diaphragm muscle specific force with modest, selective atrophy of type IIx and/or IIb fibers. J. Appl. Physiol. 114, 380–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.[No authors listed] (1999) A controlled trial of recombinant methionyl human BDNF in ALS: The BDNF Study Group (Phase III). Neurology 52, 1427–1433 [DOI] [PubMed] [Google Scholar]
- 23.Ochs G., Penn R.D., York M., Giess R., Beck M., Tonn J., Haigh J., Malta E., Traub M., Sendtner M., and Toyka K.V. (2000). A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 1, 201–206 [DOI] [PubMed] [Google Scholar]
- 24.Beck M., Flachenecker P., Magnus T., Giess R., Reiners K., Toyka K.V., and Naumann M. (2005). Autonomic dysfunction in ALS: a preliminary study on the effects of intrathecal BDNF. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 6, 100–103 [DOI] [PubMed] [Google Scholar]
- 25.Coull J.A., Beggs S., Boudreau D., Boivin D., Tsuda M., Inoue K., Gravel C., Salter M.W., and De Koninck Y. (2005). BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021 [DOI] [PubMed] [Google Scholar]
- 26.Groth R., and Aanonsen L. (2002). Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB, prevent inflammation-induced hyperalgesia. Pain 100, 171–181 [DOI] [PubMed] [Google Scholar]
- 27.Yajima Y., Narita M., Usui A., Kaneko C., Miyatake M., Yamaguchi T., Tamaki H., Wachi H., Seyama Y., and Suzuki T. (2005). Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J. Neurochem. 93, 584–594 [DOI] [PubMed] [Google Scholar]
- 28.Boyce V.S., Park J., Gage F.H., and Mendell L.M. (2012). Differential effects of brain-derived neurotrophic factor and neurotrophin-3 on hindlimb function in paraplegic rats. Eur. J. Neurosci. 35, 221–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu P., Blesch A., Graham L., Wang Y., Samara R., Banos K., Haringer V., Havton L., Weishaupt N., Bennett D., Fouad K., and Tuszynski M.H. (2012). Motor axonal regeneration after partial and complete spinal cord transection. J. Neurosci. 32, 8208–8218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chopp M., Zhang X.H., Li Y., Wang L., Chen J., Lu D., Lu M., and Rosenblum M. (2000). Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport 11, 3001–3005 [DOI] [PubMed] [Google Scholar]
- 31.Sasaki M., Radtke C., Tan A.M., Zhao P., Hamada H., Houkin K., Honmou O., and Kocsis J.D. (2009). BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J. Neurosci. 29, 14,932–14,941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parr A.M., Tator C.H., and Keating A. (2007). Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant 40, 609–619 [DOI] [PubMed] [Google Scholar]
- 33.Dormady S.P., Bashayan O., Dougherty R., Zhang X.M., and Basch R.S. (2001). Immortalized multipotential mesenchymal cells and the hematopoietic microenvironment. J. Hematother Stem Cell Res. 10, 125–140 [DOI] [PubMed] [Google Scholar]
- 34.Crigler L., Robey R.C., Asawachaicharn A., Gaupp D., and Phinney D.G. (2006). Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp. Neurol. 198, 54–64 [DOI] [PubMed] [Google Scholar]
- 35.Li Y., Chen J., Chen X.G., Wang L., Gautam S.C., Xu Y.X., Katakowski M., Zhang L.J., Lu M., Janakiraman N., and Chopp M. (2002). Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 59, 514–523 [DOI] [PubMed] [Google Scholar]
- 36.Rooney G.E., McMahon S.S., Ritter T., Garcia Y., Moran C., Madigan N.N., Flugel A., Dockery P., O'Brien T., Howard L., Windebank A.J., and Barry F.P. (2009). Neurotrophic factor-expressing mesenchymal stem cells survive transplantation into the contused spinal cord without differentiating into neural cells. Tissue Eng. Part A 15, 3049–3059 [DOI] [PubMed] [Google Scholar]
- 37.Lu P., Jones L.L., and Tuszynski M.H. (2005). BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp. Neurol. 191, 344–360 [DOI] [PubMed] [Google Scholar]
- 38.Kurozumi K., Nakamura K., Tamiya T., Kawano Y., Kobune M., Hirai S., Uchida H., Sasaki K., Ito Y., Kato K., Honmou O., Houkin K., Date I., and Hamada H. (2004). BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol. Ther. 9, 189–197 [DOI] [PubMed] [Google Scholar]
- 39.Nomura T., Honmou O., Harada K., Houkin K., Hamada H., and Kocsis J.D. (2005). I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience 136, 161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMahon S.S., Albermann S., Rooney G.E., Shaw G., Garcia Y., Sweeney E., Hynes J., Dockery P., O'Brien T., Windebank A.J., Allsopp T.E., and Barry F.P. (2010). Engraftment, migration and differentiation of neural stem cells in the rat spinal cord following contusion injury. Cytotherapy 12, 313–325 [DOI] [PubMed] [Google Scholar]
- 41.Moloney T.C., Rooney G.E., Barry F.P., Howard L., and Dowd E. (2010). Potential of rat bone marrow-derived mesenchymal stem cells as vehicles for delivery of neurotrophins to the Parkinsonian rat brain. Brain Res. 1359, 33–43 [DOI] [PubMed] [Google Scholar]
- 42.Dow D.E., Mantilla C.B., Zhan W.Z., and Sieck G.C. (2006). EMG-based detection of inspiration in the rat diaphragm muscle. Conf. Proc. IEEE Eng. Med. Biol. Soc. 1, 1204–1207 [DOI] [PubMed] [Google Scholar]
- 43.Dow D.E., Zhan W.Z., Sieck G.C., and Mantilla C.B. (2009). Correlation of respiratory activity of contralateral diaphragm muscles for evaluation of recovery following hemiparesis. Conf. Proc. IEEE Eng. Med. Biol. Soc. 1, 404–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mantilla C.B., Seven Y.B., Hurtado–Palomino J.N., Zhan W.Z., and Sieck G.C. (2011). Chronic assessment of diaphragm muscle EMG activity across motor behaviors. Respir. Physiol. Neurobiol. 177, 176–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trelease R.B., Sieck G.C., and Harper R.M. (1982). A new technique for acute and chronic recording of crural diaphragm EMG in cats. Electroencephalogr. Clin. Neurophysiol. 53, 459–462 [DOI] [PubMed] [Google Scholar]
- 46.Gransee H.M., Zhan W.Z., Sieck G.C., and Mantilla C.B. (2013). Targeted delivery of TrkB receptor to phrenic motoneurons enhances functional recovery of rhythmic phrenic activity after cervical spinal hemisection. PLoS One 8, e64755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mantilla C.B., Seven Y.B., Zhan W.Z., and Sieck G.C. (2010). Diaphragm motor unit recruitment in rats. Respir. Physiol. Neurobiol. 173, 101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mantilla C.B., Zhan W.Z., and Sieck G.C. (2009). Retrograde labeling of phrenic motoneurons by intrapleural injection. J. Neurosci. Methods 182, 244–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Issa A.N., Zhan W.Z., Sieck G.C., and Mantilla C.B. (2010). Neuregulin-1 at synapses on phrenic motoneurons. J. Comp. Neurol. 518, 4213–4225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinkead R., Zhan W.Z., Prakash Y.S., Bach K.B., Sieck G.C., and Mitchell G.S. (1998). Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J. Neurosci. 18, 8436–8443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ankeny D.P., McTigue D.M., and Jakeman L.B. (2004). Bone marrow transplants provide tissue protection and directional guidance for axons after contusive spinal cord injury in rats. Exp. Neurol. 190, 17–31 [DOI] [PubMed] [Google Scholar]
- 52.Cizkova D., Novotna I., Slovinska L., Vanicky I., Jergova S., Rosocha J., and Radonak J. (2011). Repetitive intrathecal catheter delivery of bone marrow mesenchymal stromal cells improves functional recovery in a rat model of contusive spinal cord injury. J. Neurotrauma 28, 1951–1961 [DOI] [PubMed] [Google Scholar]
- 53.Someya Y., Koda M., Dezawa M., Kadota T., Hashimoto M., Kamada T., Nishio Y., Kadota R., Mannoji C., Miyashita T., Okawa A., Yoshinaga K., and Yamazaki M. (2008). Reduction of cystic cavity, promotion of axonal regeneration and sparing, and functional recovery with transplanted bone marrow stromal cell-derived Schwann cells after contusion injury to the adult rat spinal cord. J. Neurosurg. Spine 9, 600–610 [DOI] [PubMed] [Google Scholar]
- 54.Forostyak S., Jendelova P., and Sykova E. (2013). The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochimie 95, 2257–2270 [DOI] [PubMed] [Google Scholar]
- 55.Osaka M., Honmou O., Murakami T., Nonaka T., Houkin K., Hamada H., and Kocsis J.D. (2010). Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 1343, 226–235 [DOI] [PubMed] [Google Scholar]
- 56.Joyce N., Annett G., Wirthlin L., Olson S., Bauer G., and Nolta J.A. (2010). Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen. Med. 5, 933–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaquero J., Zurita M., Oya S., and Santos M. (2006). Cell therapy using bone marrow stromal cells in chronic paraplegic rats: systemic or local administration? Neurosci. Lett. 398, 129–134 [DOI] [PubMed] [Google Scholar]
- 58.Zurita M., and Vaquero J. (2006). Bone marrow stromal cells can achieve cure of chronic paraplegic rats: functional and morphological outcome one year after transplantation. Neurosci. Lett. 402, 51–56 [DOI] [PubMed] [Google Scholar]
- 59.Wu S., Suzuki Y., Ejiri Y., Noda T., Bai H., Kitada M., Kataoka K., Ohta M., Chou H., and Ide C. (2003). Bone marrow stromal cells enhance differentiation of cocultured neurosphere cells and promote regeneration of injured spinal cord. J. Neurosci. Res. 72, 343–351 [DOI] [PubMed] [Google Scholar]
- 60.Urdzikova L.M., Ruzicka J., LaBagnara M., Karova K., Kubinova S., Jirakova K., Murali R., Sykova E., Jhanwar–Uniyal M., and Jendelova P. (2014). Human mesenchymal stem cells modulate inflammatory cytokines after spinal cord injury in rat. Int. J. Mol. Sci. 15, 11,275–11,293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swanger S.A., Neuhuber B., Himes B.T., Bakshi A., and Fischer I. (2005). Analysis of allogeneic and syngeneic bone marrow stromal cell graft survival in the spinal cord. Cell Transplant 14, 775–786 [DOI] [PubMed] [Google Scholar]
- 62.Frisen J., Verge V.M., Cullheim S., Persson H., Fried K., Middlemas D.S., Hunter T., Hokfelt T., and Risling M. (1992). Increased levels of trkB mRNA and trkB protein-like immunoreactivity in the injured rat and cat spinal cord. Proc. Natl. Acad. Sci. USA 89, 11,282–11,286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.King V.R., Bradbury E.J., McMahon S.B., and Priestley J.V. (2000). Changes in truncated trkB and p75 receptor expression in the rat spinal cord following spinal cord hemisection and spinal cord hemisection plus neurotrophin treatment. Exp. Neurol. 165, 327–341 [DOI] [PubMed] [Google Scholar]
- 64.Widenfalk J., Lundstromer K., Jubran M., Brene S., and Olson L. (2001). Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J. Neurosci. 21, 3457–3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dougherty K.D., Dreyfus C.F., and Black I.B. (2000). Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol. Dis 7, 574–585 [DOI] [PubMed] [Google Scholar]
- 66.Bregman B.S., McAtee M., Dai H.N., and Kuhn P.L. (1997). Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Exp. Neurol. 148, 475–494 [DOI] [PubMed] [Google Scholar]
- 67.Novikova L.N., Novikov L.N., and Kellerth J.O. (2000). BDNF abolishes the survival effect of NT-3 in axotomized Clarke neurons of adult rats. J. Comp. Neurol. 428, 671–680 [DOI] [PubMed] [Google Scholar]
- 68.Novikova L.N., Novikov L.N., and Kellerth J.O. (2002). Differential effects of neurotrophins on neuronal survival and axonal regeneration after spinal cord injury in adult rats. J. Comp. Neurol. 452, 255–263 [DOI] [PubMed] [Google Scholar]
- 69.Ye J.H., and Houle J.D. (1997). Treatment of the chronically injured spinal cord with neurotrophic factors can promote axonal regeneration from supraspinal neurons. Exp. Neurol. 143, 70–81 [DOI] [PubMed] [Google Scholar]
- 70.Koda M., Hashimoto M., Murakami M., Yoshinaga K., Ikeda O., Yamazaki M., Koshizuka S., Kamada T., Moriya H., Shirasawa H., Sakao S., and Ino H. (2004). Adenovirus vector-mediated in vivo gene transfer of brain-derived neurotrophic factor (BDNF) promotes rubrospinal axonal regeneration and functional recovery after complete transection of the adult rat spinal cord. J. Neurotrauma 21, 329–337 [DOI] [PubMed] [Google Scholar]
- 71.Lynskey J.V., Sandhu F.A., Dai H.N., McAtee M., Slotkin J.R., MacArthur L., and Bregman B.S. (2006). Delayed intervention with transplants and neurotrophic factors supports recovery of forelimb function after cervical spinal cord injury in adult rats. J. Neurotrauma 23, 617–634 [DOI] [PubMed] [Google Scholar]
- 72.Mitchell G.S., Baker T.L., Nanda S.A., Fuller D.D., Zabka A.G., Hodgeman B.A., Bavis R.W., Mack K.J., and Olson E.B., Jr. (2001). Invited review: Intermittent hypoxia and respiratory plasticity. J. Appl. Physiol. 90, 2466–2475 [DOI] [PubMed] [Google Scholar]
- 73.Kang H., and Schuman E.M. (1995). Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 267, 1658–1662 [DOI] [PubMed] [Google Scholar]
- 74.Mantilla C.B., Zhan W.Z., and Sieck G.C. (2004). Neurotrophins improve neuromuscular transmission in the adult rat diaphragm. Muscle Nerve 29, 381–386 [DOI] [PubMed] [Google Scholar]
- 75.Castro R.F., Jackson K.A., Goodell M.A., Robertson C.S., Liu H., and Shine H.D. (2002). Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science 297, 1299. [DOI] [PubMed] [Google Scholar]
- 76.Koshizuka S., Okada S., Okawa A., Koda M., Murasawa M., Hashimoto M., Kamada T., Yoshinaga K., Murakami M., Moriya H., and Yamazaki M. (2004). Transplanted hematopoietic stem cells from bone marrow differentiate into neural lineage cells and promote functional recovery after spinal cord injury in mice. J. Neuropathol. Exp. Neurol. 63, 64–72 [DOI] [PubMed] [Google Scholar]
- 77.Dominici M., Le Blanc K., Mueller I., Slaper–Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., and Horwitz E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 [DOI] [PubMed] [Google Scholar]
- 78.Fournier M., and Sieck G.C. (1988). Mechanical properties of muscle units in the cat diaphragm. J. Neurophysiol. 59, 1055–1066 [DOI] [PubMed] [Google Scholar]
- 79.Sieck G.C., and Fournier M. (1989). Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J. Appl. Physiol. 66, 2539–2545 [DOI] [PubMed] [Google Scholar]
- 80.Mantilla C.B., and Sieck G.C. (2011). Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors. Respir. Physiol. Neurobiol. 179, 57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brown R., DiMarco A.F., Hoit J.D., and Garshick E. (2006). Respiratory dysfunction and management in spinal cord injury. Respir. Care 51, 853–870 [PMC free article] [PubMed] [Google Scholar]
- 82.Linn W.S., Adkins R.H., Gong H., Jr., and Waters R.L. (2000). Pulmonary function in chronic spinal cord injury: a cross-sectional survey of 222 southern California adult outpatients. Arch. Phys. Med. Rehabil. 81, 757–763 [DOI] [PubMed] [Google Scholar]
- 83.Takahashi K., and Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 [DOI] [PubMed] [Google Scholar]
- 84.Tsuji O., Miura K., Okada Y., Fujiyoshi K., Mukaino M., Nagoshi N., Kitamura K., Kumagai G., Nishino M., Tomisato S., Higashi H., Nagai T., Katoh H., Kohda K., Matsuzaki Y., Yuzaki M., Ikeda E., Toyama Y., Nakamura M., Yamanaka S., and Okano H. (2010). Therapeutic potential of appropriately evaluated safe–induced pluripotent stem cells for spinal cord injury. Proc. Natl. Acad. Sci. USA 107, 12,704–12,709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujimoto Y., Abematsu M., Falk A., Tsujimura K., Sanosaka T., Juliandi B., Semi K., Namihira M., Komiya S., Smith A., and Nakashima K. (2012). Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell-derived long-term self-renewing neuroepithelial-like stem cells. Stem Cells 30, 1163–1173 [DOI] [PubMed] [Google Scholar]
- 86.Nori S., Okada Y., Yasuda A., Tsuji O., Takahashi Y., Kobayashi Y., Fujiyoshi K., Koike M., Uchiyama Y., Ikeda E., Toyama Y., Yamanaka S., Nakamura M., and Okano H. (2011). Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc. Natl. Acad. Sci USA 108, 16,825–16,830 [DOI] [PMC free article] [PubMed] [Google Scholar]