Abstract
Our previous studies have established that (-)-epigallocatechin-3-gallate (EGCG) has both neuroprotective and -regenerative capacity after sciatic nerve injury. Moreover, this improvement was evident on the behavioral level. The aim of this study was to investigate the central effects of ECGC on spinal cord motor neurons after sciatic nerve injury. Our study showed that administering 50 mg/kg intraperitoneally i.p. of EGCG to sciatic nerve-injured rats improved their performance on different motor functions and mechanical hyperesthesia neurobehavioral tests. Histological analysis of spinal cords of EGCG-treated sciatic nerve-injured (CRUSH+ECGC) animals showed an increase in the number of neurons in the anterior horn, when compared to the naïve, sham, and saline-treated sciatic nerve-injured (CRUSH) control groups. Additionally, immunohistochemical study of spinal cord sections revealed that EGCG reduced the expression of glial fibrillary acidic protein and increased the expression of growth-associated protein 43, a marker of regenerating axons. Finally, EGCG reduced the ratio of B-cell lymphoma 2 (Bcl-2)-associated X protein/Bcl-2 and increased the expression of survivin gene. This study may shed some light on the future clinical use of EGCG and its constituents in the treatment of peripheral nerve injury.
Key words: : apoptosis, green tea, neuroprotection, neuroregeneration, surviving
Introduction
Nerves are delicate and can be easily injured by pressure, stretching, or cutting. Injury to a peripheral nerve can stop sensory and motor signals to and from the brain and spinal cord, causing paralysis and/or weakness, and a loss of sensations in the area innervated by the injured nerve. Injury to the peripheral nerves can occur through a variety of trauma, such as laceration, focal contusion (gunshot wounds), stretch/traction injury, compression, drug injection injury, and electrical injury. Further, traumatic neuronal damage resulting from road traffic accidents represents a major burden on health care systems globally. According to the recent global status report,1 road traffic accidents (RTAs) are the eighth leading cause of death globally. Additionally, a report from the World Health Organization states that, every year, an estimated number between 20 and 50 million sustain nonfatal injuries resulting from RTAs.1 These injuries include injuries to the motor neurons caused by fractures or other types of injuries.
Many studies have shown the effect of green tea extracts on different neurological disorders as promising therapeutic and preventive agents. Green tea is a widely consumed beverage worldwide. Polyphenols, also known as catechins, are the major compounds found in green tea; of these polyphenols, (-)-epigallocatechin-3-gallate (EGCG) is the most abundant, accounting for 50–80% of total phenols found in green tea. 2 EGCG has been shown to display many beneficial actions on different tissues, such as anti-oxidant, -inflammatory, and -cholesterolemic effects, as well as various modulating effects on apoptosis.3–5 Recently, we have demonstrated that EGCG treatment enhanced the functional recovery in rats after a crush nerve injury.6 Moreover, upon histological examination, we were able to prove that EGCG treatment improved axonal and myelin regeneration in this group of rats.
Studies have shown that EGCG displays neuroprotective effects as a result of its antiapoptotic and -oxidative properties. EGCG protected effectively cultured retinal ganglion cells (RGCs) against H2O2 oxidative-stress injury by markedly reducing the number of apoptotic cells and attenuating intracellular reactive oxygen species (ROS) generation.7 Lately, EGCG produced a higher neurofilament L (NF-L) protein expression and higher density of RGC after optic nerve crush.8 In addition, administration of EGCG before axotomy promoted RGC survival through mediating antiapoptotic and cell-survival–signaling pathways.9 Some studies have reported that the production of ROS can affect the expression of some of the B-cell lymphoma 2 (Bcl-2) family members, both antiapoptotic and proapoptotic effectors, and consequently trigger the induction of the apoptosis. Therefore, to gain insight into mechanisms controlling apoptosis, we looked at the antiapoptotic effect of EGCG on the expression of Bcl-2-associated X protein (Bax), a proapoptotic protein, Bcl-2, an antiapoptotic protein, and survivin, an inhibitor of apoptosis. Studies have also shown that Bax/Bcl-2 ratio increases during apoptosis.10 It is also known that p53 could regulate the expression of the above genes in response to stress signals, thus contributing to the decision making of apoptosis induction or repression.
The aims of this study were to test whether EGCG can produce a neurobehavioral improvement in rats with sciatic nerve injury and investigate the mechanism by which EGCG can improve the survival of motor neurons in the spinal cord in sciatic nerve crush injuries through the modulation of the expression of apoptosis regulating genes, particularly Bax and Bcl-2.
Methods
Animals
Forty male Wistar rats, each weighing between 250 and 300 g, were obtained from the animal facility of the Health Science Center, Kuwait University (Safat, Kuwait). Animals were kept under conditions of constant temperature (23±2°C) and humidity with a 12-h light/dark cycle. Moreover, rats were housed in pairs with free access to food and water ad libitum. An ethical approval was obtained for all procedures from the animal ethics committee at the Health Sciences Center, Kuwait University, in accord with the guidelines of laboratory animal welfare and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications no. 8023, revised 1978). All efforts were made to minimize animal suffering and reduce the number of animals used in this study.
Surgical procedure
Animals were randomly assigned to one of the three treatment groups (n=10): group 1: sham-operated rats; group 2: saline-treated crushed sciatic nerve rats (CRUSH); or group 3: 50-mg/kg EGCG-treated crushed sciatic nerve (CRUSH+EGCG) rats. In addition, a group of naïve control rats were also maintained (group 4). Animals were anesthetized with an intraperitoneal (i.p.) injection of a mixture of Ketallar® (62.5 mg/kg, Tekam® 50 [ketamine HCl]; Hikma Pharmaceuticals, Amman, Jordan) and Rompun® (3.2 mg/kg, 2% xylazine HCl; Bayer AG, Leverkusen, Germany). Surgical sites were shaved and cleaned with 70% alcohol. An incision was made in the right gluteal region, and the sciatic nerve was exposed by splitting the gluteal muscle with the aid of a surgical microscope. The sciatic nerve was crushed at a 10-mm distance from the sciatic notch for 60 sec using a micro mosquito forceps (12.5 cm [S] [500451] [09F S/N]; World Precision Instruments, Inc., Sarasota, FL). The nerve was then examined to ensure that the epineurial sheath was intact, but translucent (axotomy), and returned back under the muscle.11,12 The skin was sutured with fine silk sutures after repositioning the muscles. The recommended dose of EGCG, in many studies, was 50 mg/kg i.p.4,13–17 Therefore, we injected 50 mg/kg (i.p.) of EGCG daily for 3 days, starting 1 h after sciatic nerve crush injury in the treated group.
We chose the sciatic nerve crush technique to induce acute blunt sciatic nerve injury because it allows us to perform a standard direct trauma in each rat, and it also results in a lesion similar to those observed in patients with peripheral nerve injury. Factors such as duration and severity of the injury, as well as dose selection, can alter results. Moreover, uniform distribution of rats among different groups and random allocation provide some control of confounding factors. In order to minimize animal-animal variation in producing the injury in the accepted model for testing pharmacological substances on neuronal regeneration and recovery after an injury, we took the following approved parameters by many investigators using this well-established model.12,18–20 The same investigator conducted all surgeries while keeping all parameters identical. Nerve injury may depend on the length of time of crush insult. During our earlier trials, we tried different time frames, ranging from 30 to 120 sec. The 60-sec compression injury in the sciatic nerve showed all the motor and sensory symptoms of a typical crushed nerve reported previously by many investigators.21–23 The crush-lesioned rats were randomly distributed among groups 2 and 3 to minimize group variations as much as possible.
Assessment of functional recovery
The different animal groups were evaluated for walking-track analysis preoperatively and once a week after surgery. All the tests were repeated three times (with 30-min interval), and the average of the three readings was used for data analysis. Investigators were blinded to all treatments in all experiments. Several tests of reflexive sciatic nerve function (foot position, toe spread, extensor postural thrust [EPT], and hopping tests) were conducted as described in our previous studies.6,24 Briefly, the time course of functional recovery was measured using EPT after crush injury and EGCG treatment. EPT was measured by calculating the functional deficit in this feature; thus, a higher value indicates a poor outcome. The hopping test was done to test several integrated functions. Each leg of the rat was suspended above a horizontal surface and was slowly moved laterally, with one foot touching the table surface. The rat was scored (1 or 0) based on whether it hopped on the foot or not.
Neurobehavioral sensory tests were performed as follows
Tactile allodynia
The von Frey methodology was used to assess the sensitivity of the skin to tactile stimulation.24,25 von Frey filaments were calibrated to have a characteristic bending force when pressure is applied. Mechanical allodynia was tested in different animal groups by the application of von Frey filaments on different rat groups and then measuring the withdrawal reflex threshold (mean±standard error of the mean [SEM]).
Mechanical hyperalgesia
Nociceptive mechanical thresholds expressed in grams were measured with an analgesic meter (model 21025; UGO Basile, Monvalle Varese, Italy), which has been previously used to measure hyperalgesia.24–26 Briefly, nociceptive mechanical threshold was assessed in the different animal groups by applying a steady pressure on the hindlimb and measuring the time needed for the rat to withdraw the limb. The mean of three consecutive values were measured, and the mean±SEM of the latency periods were calculated and plotted.
Hot-plate analgesia
Thermal nociception was measured by a modified hot-plate test. The rat was covered in a surgical towel and held in an upright position with one foot placed on a wooden board and the other on the hot plate (50°C; model 39D Hot Plate Analgesia Meter; Columbus Instruments, Columbus, OH). The withdrawal response latency was characterized as a brief paw flick recorded to the nearest 0.1 sec; a standard cut-off latency of 20 sec was employed to prevent tissue damage.27 Results are presented as mean and SEM. WRL is defined as the time elapsed from the onset of hot-plate contact to withdrawal of the hind paw and measured with a stopwatch. Normal rats withdraw their paws from the hot plate within 4 sec or less. The affected limbs were tested three times, with an interval of 20 min between consecutive tests to prevent for sensitization, and the three latencies were averaged to obtain a final result.
Tail flick
The spinally mediated nociceptive thresholds were determined using an analgesia meter apparatus (model 7360; UGO Basile). The animal was gently restrained by hand, and radiant heat (IR Intensity 20) was directed onto the proximal two-thirds part of its tail. The amount of time taken for the animal to move (flick) its tail away from the heat was recorded. The cut-off time was 20 sec to prevent tissue damage.27
Tissue processing for immunostaining and Nissl staining
Both sham control and sciatic nerve-lesioned animals were deeply anesthetized with a mixture of Ketallar 62.5 mg/kg and Rompun 3.2 mg/kg injection (i.p.) and were perfused transcardially with 50 mL of ice-cold saline, followed by 350 mL of 4% paraformaldehyde (PFA) in phosphate buffer (PB; 0.1 M, pH 7.4). Spinal cords were removed carefully and post-fixed in 4% PFA overnight at 4°C. Spinal cords were cryoprotected in 10%, 20%, and 30% sucrose solution in PB containing 0.06% sodium azide to prevent bacterial and fungal growth before sectioning. Cryostat sections of 30 μm thick were cut transversely and collected serially in PB in a 24-well culture plate. These sections were used for Nissl staining (for cytoarchitecture of spinal cord), neuronal nuclear-specific protein (NeuN), glial fibrillary acidic protein (GFAP), growth-associated protein 43 (GAP-43), Bax, and Bcl-2 immunostaining. Ventral gray and dorsal horn regions of spinal cord were studied. Number of neurons, immunoreactivity for astrocytes, GAP-43, Bax, and Bcl-2 in dorsal and ventral horn regions were quantified.
Nissl staining
For Nissl (cresyl violet) staining, every 15th cross section of the lumbar spinal cord (a total of 10 sections per spinal cord; n=6 in all groups) were processed. Sections were mounted on gelatinized slides and air-dried overnight. Sections were hydrated in graded alcohol and stained with 0.1% aqueous cresyl violet (Sigma-Aldrich, St. Louis, MO). Stained sections were differentiated in 70% ethanol and dehydrated in graded alcohol, cleared in xylene, and cover-slipped with DPX.
Neuronal nuclear-specific protein, glial fibrillary acidic protein, growth-associated protein 43, B-cell lymphoma 2, and B-cell lymphoma 2-associated X protein immunohistochemistry
Sections were processed for NueN, GFAP, GAP43, Bcl-2, and Bax immunohistochemical (IHC) staining, as described previously.28–30 Briefly, every 15th transverse section of lumbar spinal cord (a total of 10 sections per spinal cord; n=6 in all groups) was selected for free floating immunostaining in a 24-well culture plate. In control spinal cord, regions of the spinal cord identical to that in the sciatic nerve-lesioned animal were selected for staining. Sections were washed in phosphate-buffered saline (PBS; pH 7.4) and then treated with 3% H2O2 (Fluka Chemika, Buchs, Switzerland) and 10% methanol, in PBS for 20 min to quench the endogenous peroxidase activity. Sections were then washed three times (5 min each) with PBS and incubated for 30 min with appropriate blocking serum solution (5% normal horse serum [NHS]/normal goat serum [NGS] and 0.01% Triton X-100 in PBS) before applying the primary antibody (Ab). Sections were incubated overnight in primary Abs (mouse anti-NeuN, 1:1000; Millipore, Billerica, MA; rabbit anti-GFAP, 1:50; Zymed Laboratories, San Francisco, CA; rabbit polyclonal anti-GAP-43, 1:1000; Abcam, Cambridge, MA; rabbit polyclonal anti-Bax, 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA; and rabbit polyclonal anti-Bcl2, 1:1000; Santa Cruz Biotechnology). Sections were washed in PBS and treated with biotinylated secondary Abs (horse antimouse IgG [1:200] or goat-anti-rabbit IgG [1:200]; Vector Laboratories, Burlingame, CA) and 1% NHS or NGS for 1 h at room temperature. Sections were then washed three times in PBS and treated with avidin biotin complex (Vector Laboratories) for 1 h at room temperature. Sections were color developed with 3-diaminobenzidine (DAB) as a chromogen (DAB kit; Vector Laboratories). Finally, sections were washed in distilled water, dehydrated in graded ethanol, cleared in xylene, and mounted with DPX (Fluka Chemika). Sections were observed under a light microscope.
Neuronal count
Neuronal count in the dorsal horn and ventral horn regions of the spinal cord were done using the NIS-Elements D image analysis system. From each animal, every 15th section stained well with NeuN was selected for this analysis (a total of 10 sections per spinal cord; n=6 in all groups). The region of the spinal cord under analysis was focused at 40× magnification, and an image was transferred to a computer monitor with a high-resolution digital Olympus camera (DP-72; Olympus, Tokyo, Japan) attached to an Olympus microscope. The total number of neurons in the entire dorsal and ventral horn regions was counted. Slides were coded to avoid observer's bias. Mean number of neurons per section was calculated for statistical analysis.
Quantitation of glial fibrillary acidic protein, growth-associated protein 43, B-cell lymphoma 2-associated X protein, and B-cell lymphoma 2 immunoreactivity
The areas occupied by GFAP and GAP-43 immunoreactive elements in the spinal cord were quantitatively determined by Scion Image for Windows, which is based on the National Institutes of Health ImageJ.28 Every 15th section from each animal was used for this quantification in all groups (a total of 10 sections per spinal cord; n=6 in all groups). The areas occupied by the GFAP-, GAP-43-, Bax-, and Bcl-2-positive immunoreactive structures per unit area of tissue were determined. Briefly, a digital microscopic image was taken from dorsal and ventral horn regions of the spinal cord (using a 40× objective lens) in an Olympus microscope attached with a high-resolution digital Olympus camera (DP-72). Sections were well focused before taking the images. The same intensity of light in the microscope, magnification, and the same parameters in the digital camera were used to digitize all the images in grayscale from different animals. Images were saved as BMP files for subsequent measurements using Scion Image. Each image was taken with a scale bar on it for calibration purposes. The area occupied by the GFAP-positive structures in each image was measured by selecting the “Analyze Particles” command of the Scion Image program. The summed area of all particles was stored for further calculations and statistical analyses.
RNA extraction and reverse transcription
Total RNA was extracted from the lumbar region of the spinal cord tissue from the three animal groups (n=4) using TRIzol (Invitrogen, Carlsbad, CA), according to the manufacturer's Instruction. RNA samples were subjected to DNase I digestion (Qiagen, Inc., Valencia, CA) to avoid DNA contamination. Reverse transcription (RT) was performed using the SuperScriptTM First Strand Synthesis System for RT polymerase chain reaction (RT-PCR; Invitrogen). Complementary DNA was synthesized from 2 μg of total RNA by extension with Oligo (dT)18 in the presence of 200 U of M-MLV reverse transcriptase (Invitrogen) for 1 h at 55°C in a final volume of 20 μL. RNA concentration was measured by reading the absorbance at 260 nm, and the purity and integrity were verified by the 260/280-nm ratio >1.8 and by denaturing agarose gel.6,25
Real-time polymerase chain reaction
Gene expression levels of Bax, Bcl-2, survivin, and p53 were measured by real-time PCR based on TaqMan technology using the ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA), as described previously.6,25 Gene-specific assay sets (predesigned and validated primers and TaqMan probes) were purchased from Applied Biosystems. PCR amplification reactions were run in triplicates in a final volume of 20 μL of TaqMan master mix. Real-time PCR conditions used were as follows: 2 min at 50°C, 10 min at 95°C for enzyme activation followed by 40 cycles of 15 sec at 95°C for denaturation, and 1 min at 60°C for annealing and extension. Relative gene expression was calculated by the comparative ΔΔCT method.31
Statistical analysis
All data were analyzed by two-way repeated-measures analysis of variance (ANOVA) followed by Bonferroni's F- and Fisher's least significant difference post-hoc comparison test or Student's t-test, as appropriate, using the SPSS® statistical program (v17; SPSS, Inc., Chicago, IL). Results were considered significant when p<0.05. All biochemical RT-PCR data were analyzed by one-way repeated-measures ANOVA followed by the Neuman-Keuls' test, using the SPSS statistical program (v17; SPSS, Inc.). Results were considered significant when p<0.05. Data are represented as mean±SEM. Graphs were generated in Microsoft® Excel and Publisher (Microsoft Corp., Redmond, WA).
Results
Assessment of functional recovery
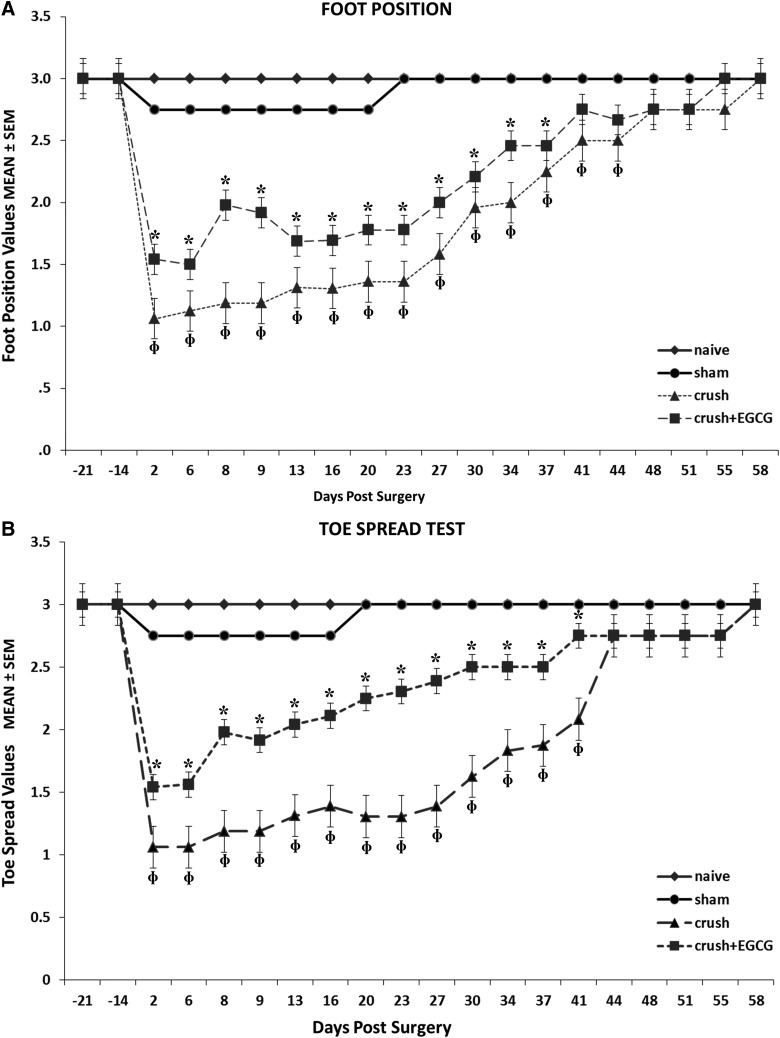
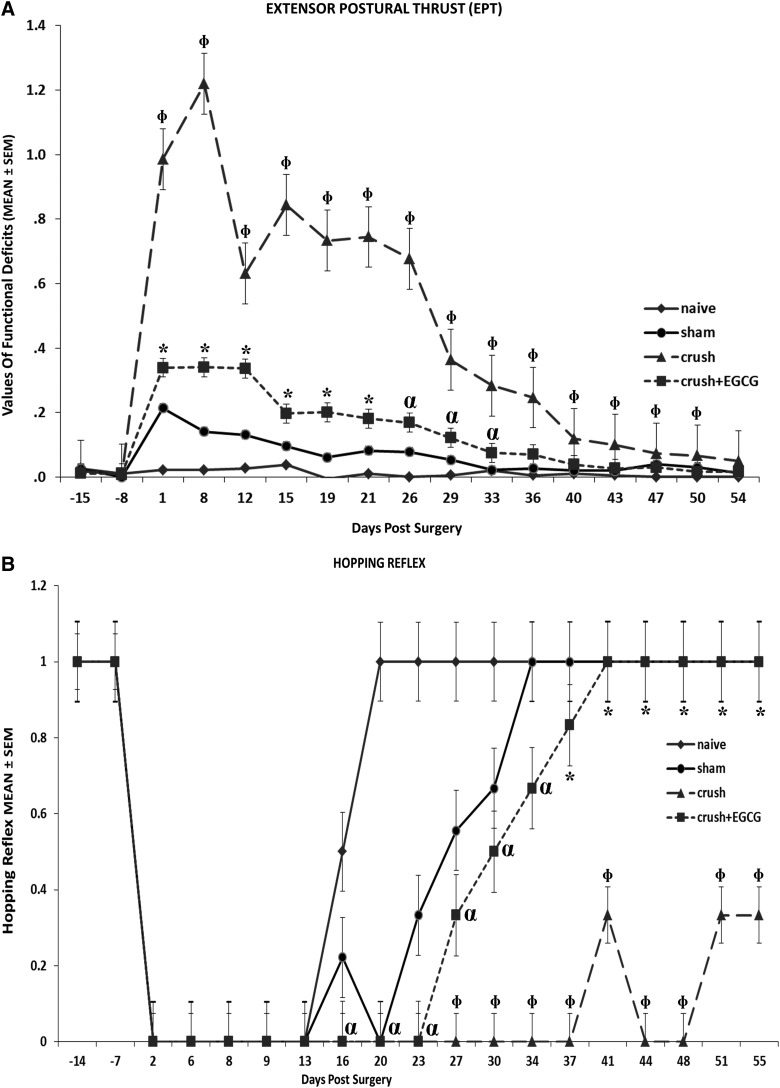
Analysis of foot position for the four groups of rats showed a significant and steady improvement recovery in the CRUSH+EGCG group, when compared to the CRUSH group (p<0.001). On the other hand, the sham group showed a slight displacement in foot position, but recovered completely on day 23 (Fig. 1A). Similarly, CRUSH+EGCG animals showed a consistent and faster improvement, in terms of the toe spread test, when compared to the CRUSH group (p<0.001); again, the sham group showed minimal change in toe spread displacement (Fig. 1B). The CRUSH rats started off at a lower value, but both CRUSH and CRUSH+EGCG groups returned to the sham level by the end of testing. The CRUSH group started off significantly at a lower value on each time point the animals were evaluated. The EPT test was performed to reveal the functional deficit in hindlimb motor performance between different groups, taking the naïve group as a reference (Fig. 2A). The EPT showed only a 40% deficit in motor function of the CRUSH+EGCG group, in comparison to 120% in the CRUSH group, during the first week postsurgery (Fig. 2A). Moreover, the CRUSH+EGCG group showed a significant decrease in functional motor deficit (p<0.001), and this decrease was significantly sustained throughout the period of the study until full recovery was achieved at day 40 postsurgery. In contrast, CRUSH rats showed signs of nearly full recovery at 54 days postsurgery.
FIG. 1.
(A) Foot position analysis for the four groups: naïve; sham; CRUSH; and CRUSH+EGCG. The figure shows a faster and a steady improvement of foot position in the CRUSH+EGCG animals. This improvement was statistically significant (*p<0.01) from the CRUSH, sham, and naïve groups; Φp<0.0001, CRUSH animals compared to naïve and sham rats. (B) Toe spread analysis figure. Again, CRUSH+EGCG rats showed both clinically and statistically significant recovery in toe spread after sciatic nerve injury. *p<0.02, CRUSH+EGCG animals compared to CRUSH, sham, and naïve groups; Φp<0.001, CRUSH animals compared to sham and naïve groups. EGCG, (-)-epigallocatechin-3-gallate; SEM, standard error of the mean.
FIG. 2.
(A) Time course of functional recovery as measured using the extensor postural thrust (EPT) after crush injury and EGCG treatment. EPT was measured by calculating the functional deficit in this feature; thus, a higher value indicates a poor outcome. Note that CRUSH+EGCG rats displayed 50% improvement in motor recovery when compared to the CRUSH group. *p<0.01 and αp<0.05, CRUSH+EGCG rats compared to sham and naïve groups; Φp<0.001, CRUSH animals compared to CRUSH+EGCG, sham, and naïve groups. (B) The hopping test was done to test several integrated functions. The CRUSH+EGCG group displayed an early hopping response (as calculated by the percent of recovered animals) in the beginning of the third week postsurgery. *p<0.05, CRUSH+EGCG rats compared to CRUSH group; αp<0.05, CRUSH+EGCG rats compared to the naïve group; Φp<0.01, CRUSH animals compared to sham and naïve groups. EGCG, (-)-epigallocatechin-3-gallate; SEM, standard error of the mean.
The hopping test was done to test several integrated functions (Fig 2B). The CRUSH+EGCG group displayed an early hopping response (as calculated by the percent of recovered animals) at the beginning of the third week postsurgery (p<0.05). This positive hopping response was significantly sustained until the end of the experiment and was not different from sham rats (Fig. 2B). In contrast, the CRUSH group maintained significant (p<0.01) absence of the hopping response. Unpredictably, our results show that naïve animals displayed loss of the hopping reflex task on days 2–16 in spite of the fact that all safeguard measures were taken to perform all the behavioral tests in a secluded and quiet laboratory destined for these experiments. Further, all the behavioral tests were performed by two well-experienced and trained individuals. We speculate that some disturbance in the experimental environment that we could not identify occurred during days 2–16 of the testing period. Subsequent testing of naïve animals showed a normal hopping task throughout the testing period (data not shown).
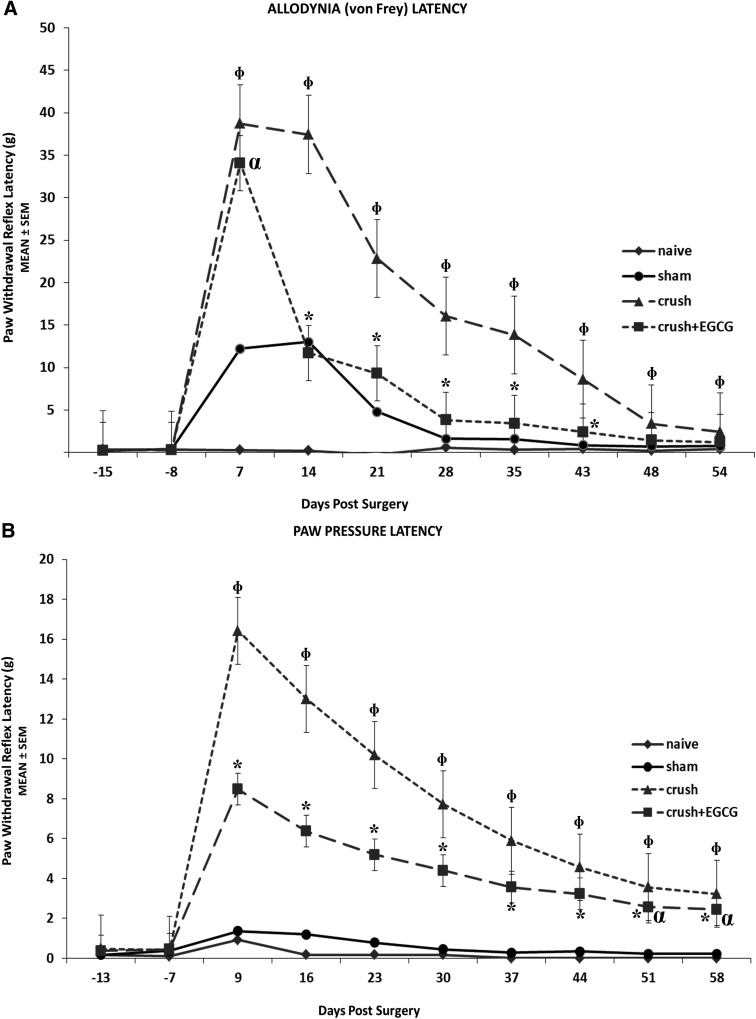
Allodynia latency and paw pressure latency tests were performed to measure the withdrawal reflex time in response to a nociceptive stimulus. Both tests showed similar results in terms of faster and steady decrease in latency periods of the CRUSH+EGCG animals, when compared to the CRUSH group (p<0.001; Fig. 3A,B). Moreover, CRUSH+EGCG animals' performance during the allodynia latency test was comparable to the sham group from day 14 until the end of the experiment (Fig. 3A). However, the paw pressure latency test performance in CRUSH+EGCG animals was significantly (p<0.001) different, compared to sham and naïve groups, throughout the experiment (Fig. 3B).
FIG. 3.
(A) Assessment of allodynia time latency. The CRUSH+EGCG group showed a significant reduction in withdrawal reflex latency time, compared to the CRUSH group. Moreover, it was noticed that the latency times of the CRUSH+EGCG animals followed that of sham group. *p<0.001, CRUSH+EGCG rats compared to CRUSH, sham, and naïve groups; αp<0.001, CRUSH+EGCG rats compared to naïve group; Φp<0.001, CRUSH animals compared to CRUSH+EGCG, sham, and naïve groups. (B) Assessment of paw pressure latency (mechanical hyperalgesia). CRUSH+EGCG rats showed a significant decrease in paw withdrawal reflex latency periods, compared to the CRUSH group. *p<0.001, CRUSH+EGCG rats compared to CRUSH, sham, and naïve groups; αp<0.03, CRUSH+EGCG rats compared to CRUSH group; Φp<0.001, CRUSH animals compared to CRUSH+EGCG, sham, and naïve groups. EGCG, (-)-epigallocatechin-3-gallate; SEM, standard error of the mean.
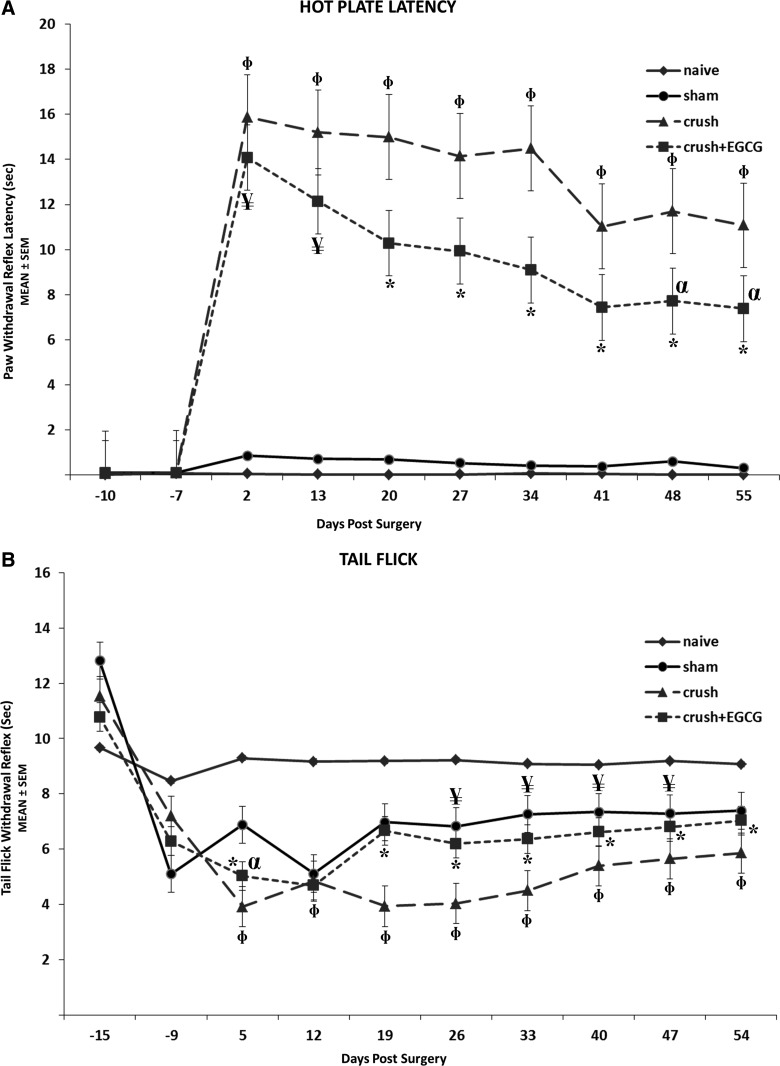
Further, nociception recovery was measured using the hot-plate latency. During this test, the time elapsed between the onsets of hot-plate contact to withdrawal of the hind paw and measured with a stopwatch. The shorter the response time, or the latency period, the better was the result. CRUSH+EGCG and CRUSH groups showed similar latency periods at day 2 postsurgery; however, the former showed decreased latency periods throughout the experiment time, and this decrease was significant (p<0.001; Fig. 4A). Interestingly, the EGCG-treated animals sustained significant (p<0.001) heat nociception latency throughout the experimental period. Spinally mediated nociception was assessed by measuring the time taken by the rat to move its tail after its exposure to radiant heat. The CRUSH+EGCG group showed a similar recovery of sham-operated animals, and this recovery was much faster and sustainable than CRUSH rats (p<0.001; Fig. 4B).
FIG. 4.
(A) Withdrawal reflex latency (WRL) performed weekly 2 weeks before surgery and postsurgery using a hot-plate analgesia meter. Nerve crush injury produced a severe nociception deficit in the CRUSH group. In contrast, the CRUSH+EGCG group showed a significant nociceptive recovery. *p<0.001, CRUSH+EGCG rats compared to CRUSH, sham, and naïve groups; αp<0.01, CRUSH+EGCG rats compared to CRUSH group; ¥p<0.001, CRUSH+EGCG rats compared to sham and naïve groups; Φp<0.001, CRUSH animals compared to CRUSH+EGCG, sham, and naïve groups. (B) Effect of EGCG on the tail-flick withdrawal latency (mean±SEM) in crush-lesioned rats. The CRUSH+EGCG group showed significant nociceptive recovery, compared to CRUSH saline-treated rats by the end of the second week postsurgery. *p<0.001, CRUSH+EGCG rats compared to crush and naïve groups; αp<0.003, CRUSH+EGCG rats compared to sham group; ¥p<0.03, CRUSH+EGCG rats compared to sham group; Φp<0.001, CRUSH animals compared to CRUSH+EGCG, sham, and naïve groups. EGCG, (-)-epigallocatechin-3-gallate; SEM, standard error of the mean.
Immunohistochemistry
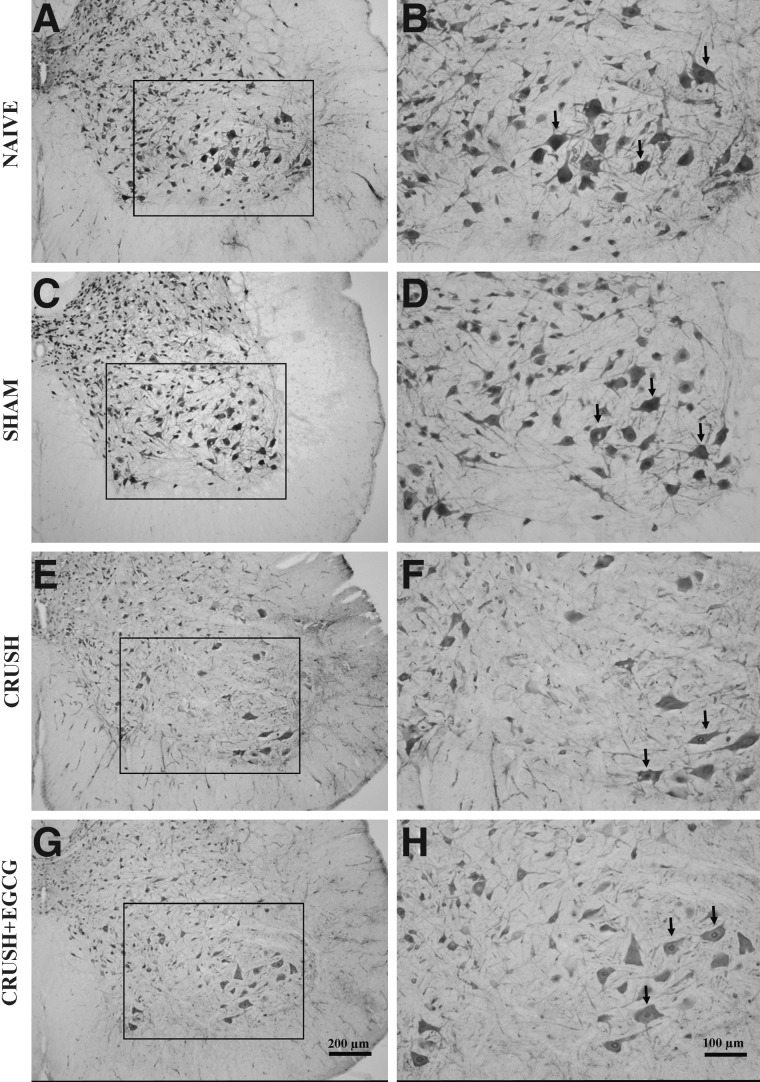
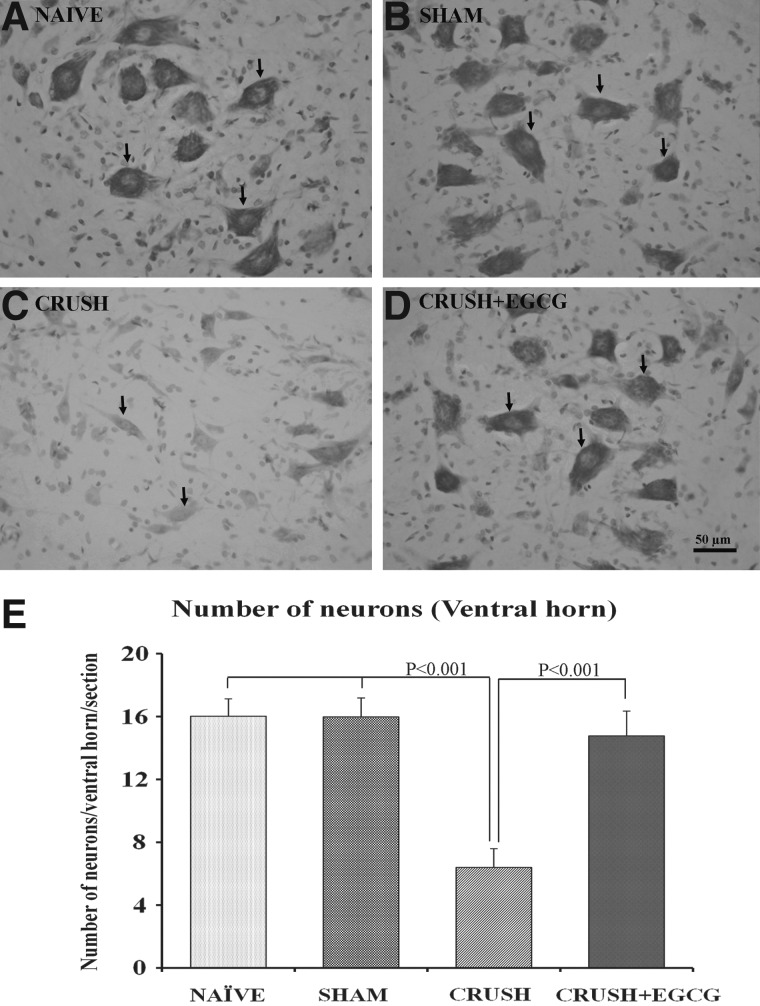
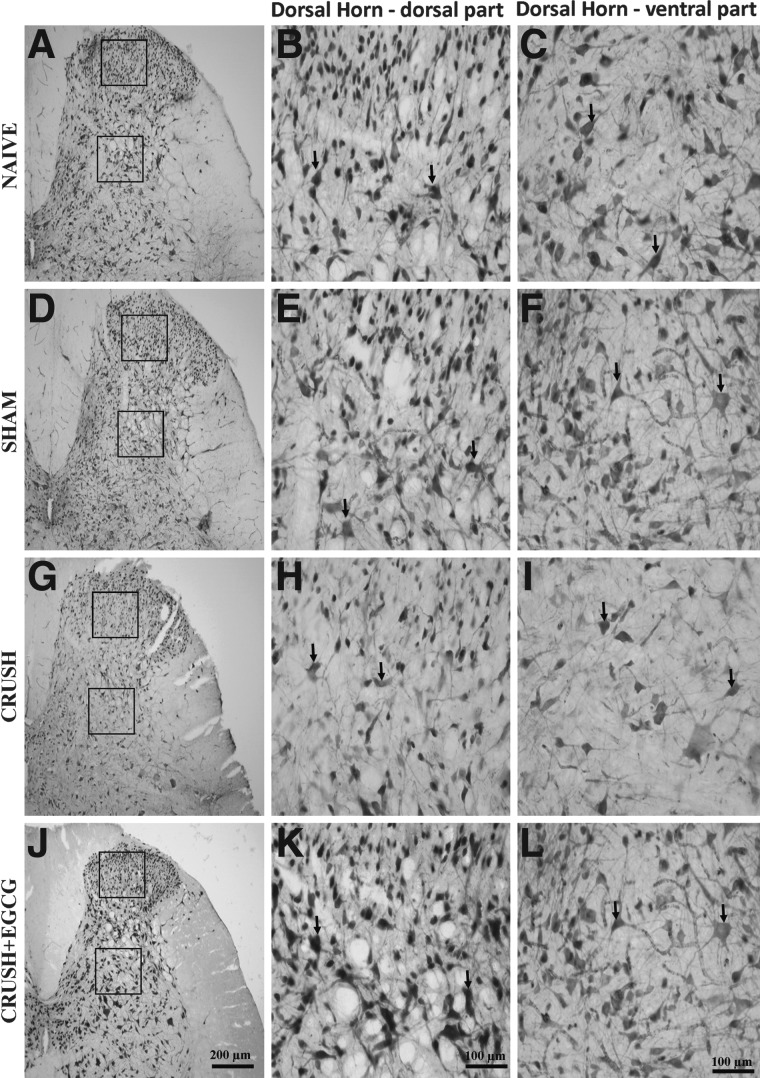
Histological examination of spinal cord cross-sections taken from the lumbosacral region of different animal groups 1 week postprocedure revealed an increase in the number neurons in the ventral horn of spinal cord in CRUSH+EGCG rats, when compared to CRUSH rats (p<0.001; Figs. 5 and 6). Moreover, on cresyl-violet–stained sections, it was noted that the CRUSH group showed Nissl body devoid of neurons, chromatolysed neurons, indicating that these cells were affected by the crush injury in contrast to the neurons obtained from other groups, including the CRUSH+EGCG group (Fig. 6A–D). Moreover, examination of the dorsal horns of spinal cords of different groups 1 week after injury revealed an increase in the number of neurons in both the ventral and dorsal parts of the ventral horn in the CRUSH+EGCG group, when compared to the CRUSH group (Fig. 7). This increase was statistically significant (p<0.001) and preserved throughout different laminae of the dorsal horn of the spinal cord (Fig. 8).
FIG. 5.
Photomicrographs of ventral horns of spinal cords of naïve (NAÏVE: A, B), sham-operated (SHAM: C, D), sciatic nerve crushed and treated with saline (CRUSH: E, F), and sciatic nerve crushed and treated with EGCG (CRUSH+EGCG: G, H) rats at 1 week postsurgery stained with neuronal nuclear antibody. (A), (C), (E), and (G) are low-magnification photomicrographs, whereas (B), (D), (F), and (H) are magnified views of the boxed area in (A), (C), (E), and (G). Arrows point to the stained neurons. Note the significant decrease in the number of neurons in the CRUSH group, compared to the naïve and sham groups. Moreover, the ventral horn of the spinal cord of the CRUSH+EGCG group showed an increase in the number of motor neurons, when compared to the CRUSH group. Scale bar: in (G)=200 μm, applicable to (A), (C), and (E); in (H)=100 μm, applicable to (B), (D), and (F). EGCG, (-)-epigallocatechin-3-gallate.
FIG. 6.
(A–D) Photomicrographs of ventral horns of spinal cords (cresyl violet stained) from naïve (NAÏVE: A), sham-operated (SHAM: B), sciatic nerve crushed and treated with saline (CRUSH: C), and sciatic nerve crushed and treated with EGCG (CRUSH+EGCG: D) rats at 1 week postsurgery. Arrows indicate neurons with prominent Nissl substance in (A), (B), and (D) and chromatolyzed (Nissl body devoid) neurons in (C). Note the significant decrease in the number of neurons in the CRUSH group, compared to the naïve and sham groups. Moreover, the ventral horn of the spinal cord of the CRUSH+EGCG group showed an increase in the number of motor neurons, when compared to the CRUSH group. Scale bar=50 μm, applicable to (A–C). (E) Bar chart indicating the number of neurons in the ventral horn of the spinal cords in different groups at 1 week postsurgery. Note the significant increase in the number of neurons in the CRUSH+EGCG group, when compared to CRUSH (p value less than 0.001). Data represents mean±SEM (n=6 in all groups; ANOVA followed by Bonferroni's post-test). EGCG, (-)-epigallocatechin-3-gallate.
FIG. 7.
Photomicrographs of dorsal horns of spinal cords (NeuN stained) from naïve (NAÏVE: A, B, C), sham-operated (SHAM: D, E, F), sciatic nerve crushed and treated with saline (CRUSH: G, H, I), and sciatic nerve crushed and treated with EGCG (CRUSH+EGCG: J, K, L) rats at 1 week postsurgery. Left panel (A, D, G, and J) are low-magnification photomicrographs; middle panel (B, E, H, and K) and right panel (C, F, I, and L) are magnified views of the dorsal and ventral parts of the dorsal horn (boxed area in A, D, G, and J). Arrows indicate the neurons. Note the significant decrease in the number of neurons in the CRUSH group, compared to naïve and sham groups. Moreover, the ventral and dorsal parts of the dorsal horn of the spinal cord of the CRUSH+EGCG group showed a significant increase in the number of neurons, when compared to the CRUSH group. Scale bar: in (J)=200 μm, applicable to (A), (D), and (G); in (K)=100 μm, applicable to (B), (E), and (H); in (L)=100 μm, applicable to (C), (F), and (I). EGCG, (-)-epigallocatechin-3-gallate.
FIG. 8.
(A) Bar chart illustrating the total number of neuronal nuclear-specific protein–positive neurons in the dorsal horn per section in different animal groups. (B) Bar chart illustrating the total number of neurons in laminae 1–4 of dorsal horn in different experimental groups at 1 week postsurgery. Note the significant decrease in the number of neurons in CRUSH group, compared to naïve and sham groups, in both the dorsal horn of the spinal cord and different laminae. Data represent mean±SEM (n=6 in all groups; ANOVA followed by Bonferroni's post-test). EGCG, (-)-epigallocatechin-3-gallate; GFAP, glial fibrillary acidic protein; DH, dorsal horn.
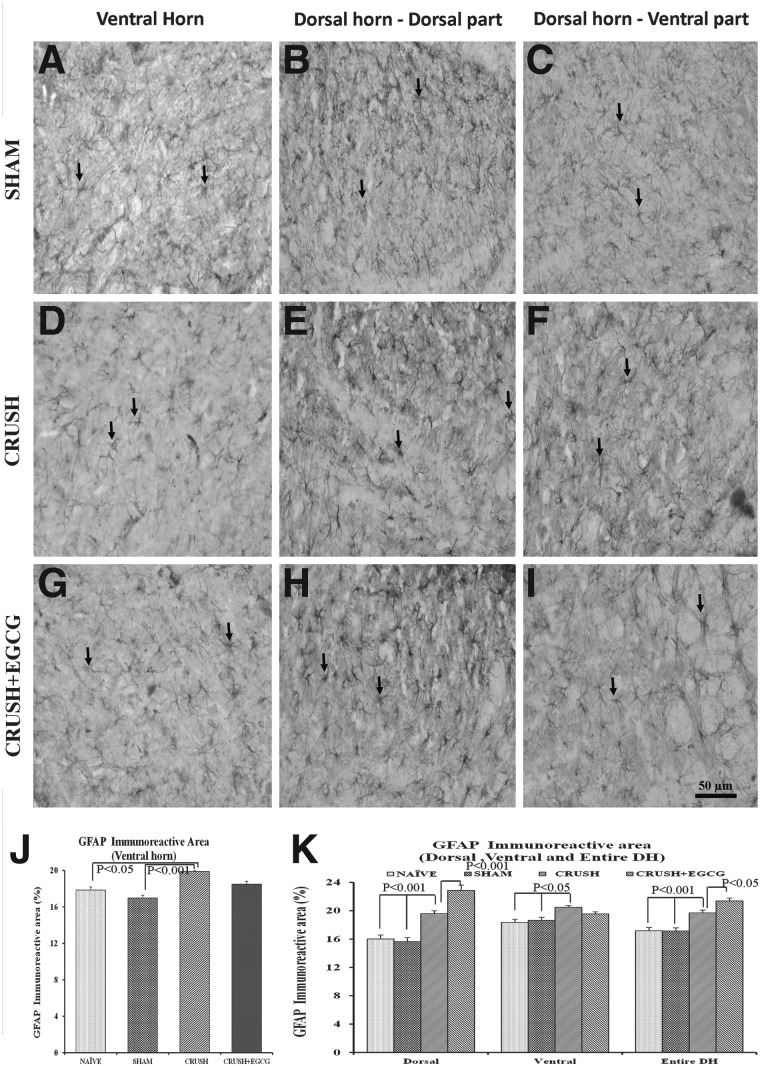
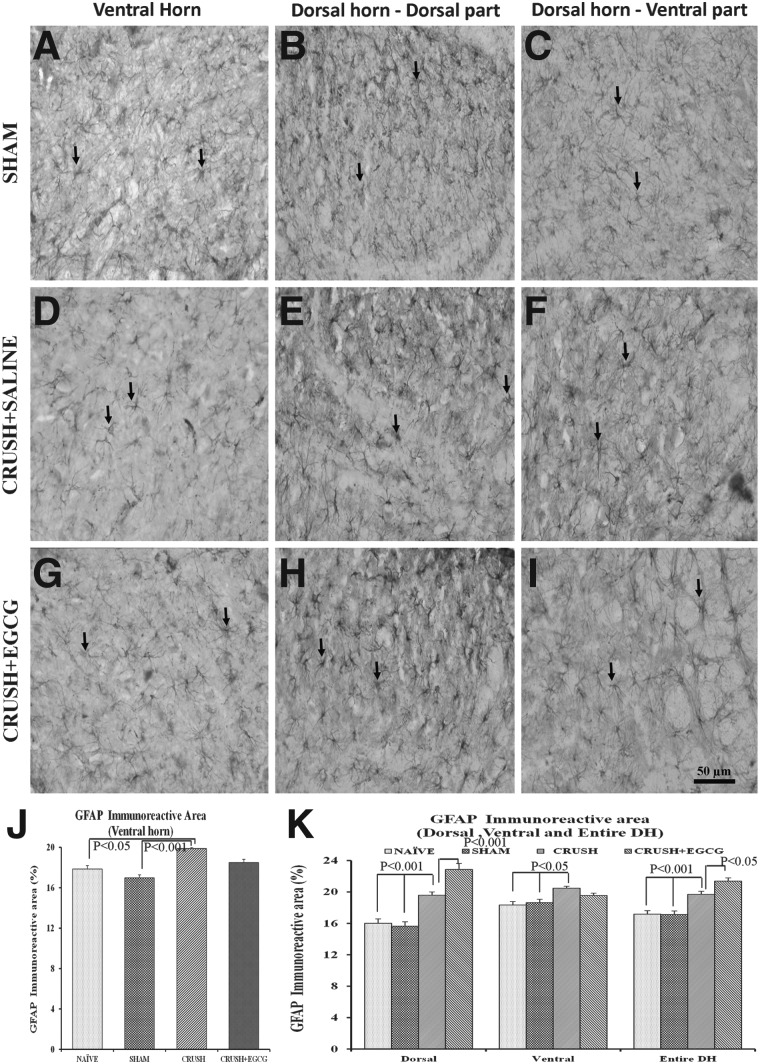
Staining spinal cord sections with GFAP IHC (Fig. 9) revealed a statistically significant (p<0.001 and p<0.05) increase in the GFAP immunoreactive area in the ventral horn of spinal cord sections of the CRUSH group, when compared to the sham and naïve groups, respectively. However, this increase was not significant, when compared to CRUSH+EGCG animals. On the other hand, upon examining the GFAP immunoreactive area on the different laminae of the dorsal horn of the spinal cord, mixed results were obtained from the dorsal horn, depending on the laminae.
FIG. 9.
(A–I) GFAP-stained photomicrographs of ventral horn (left panel: A, D, G), dorsal part of dorsal horn (middle panel: B, E, H), and ventral part of dorsal horn (right panel: C, F, I) from sham, CRUSH, and CRUSH+EGCG groups of rats at 1 week postsurgery. Arrows indicate the GFAP-positive astrocytes. Scale bar=50 μm, applicable to all photomicrographs. (J and K) GFAP immunoreactive area (%) in the ventral horn (J) and dorsal horn (K), as measured by Scion Image analysis software (National Institutes of Health), in different groups. Data represent mean±SEM (n=6 in all groups; ANOVA followed by Bonferroni's post-test). EGCG, (-)-epigallocatechin-3-gallate; GFAP, glial fibrillary acidic protein; DH, dorsal horn.
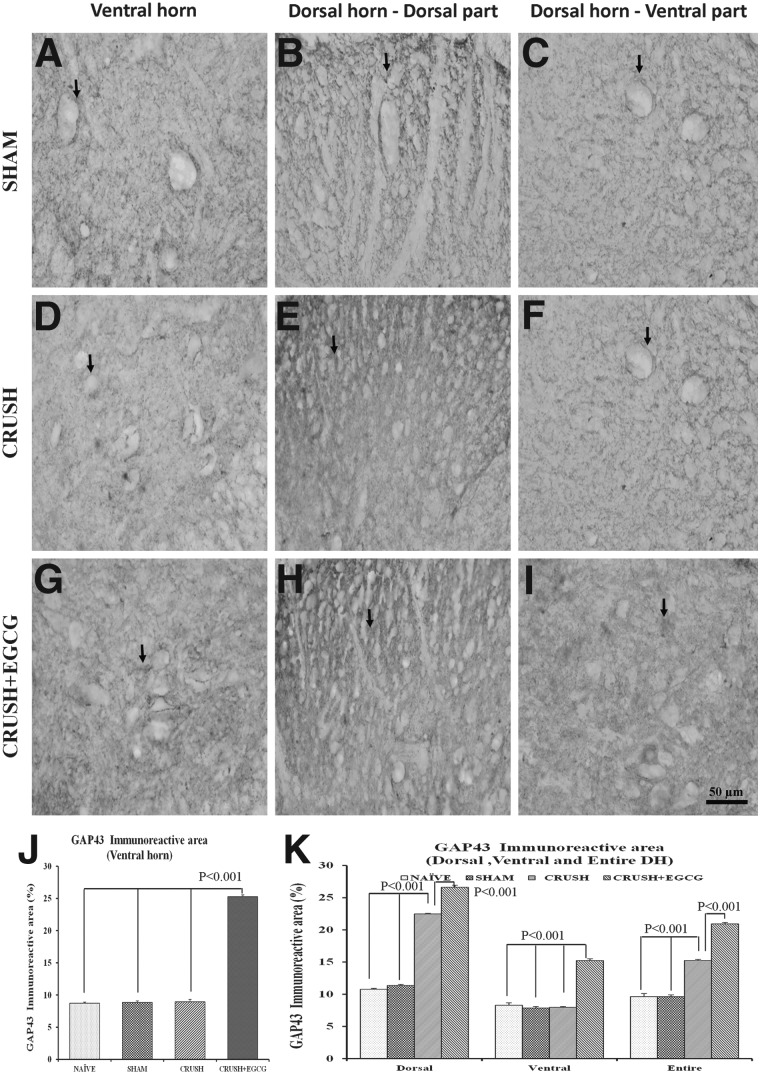
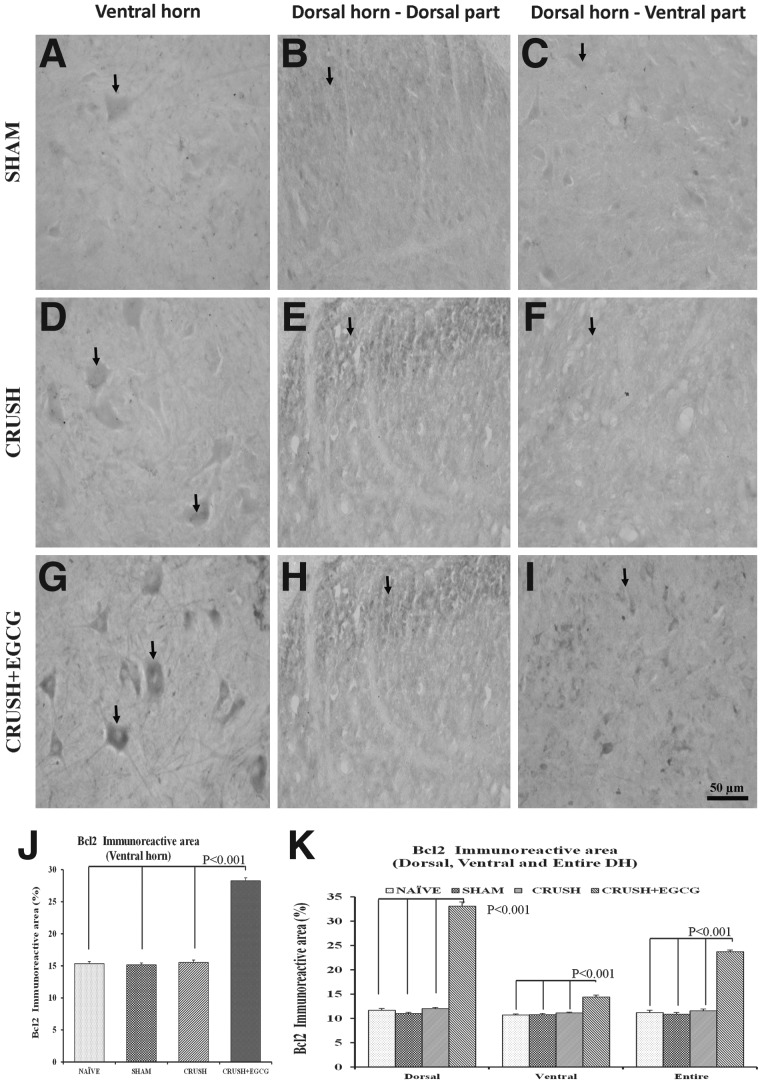
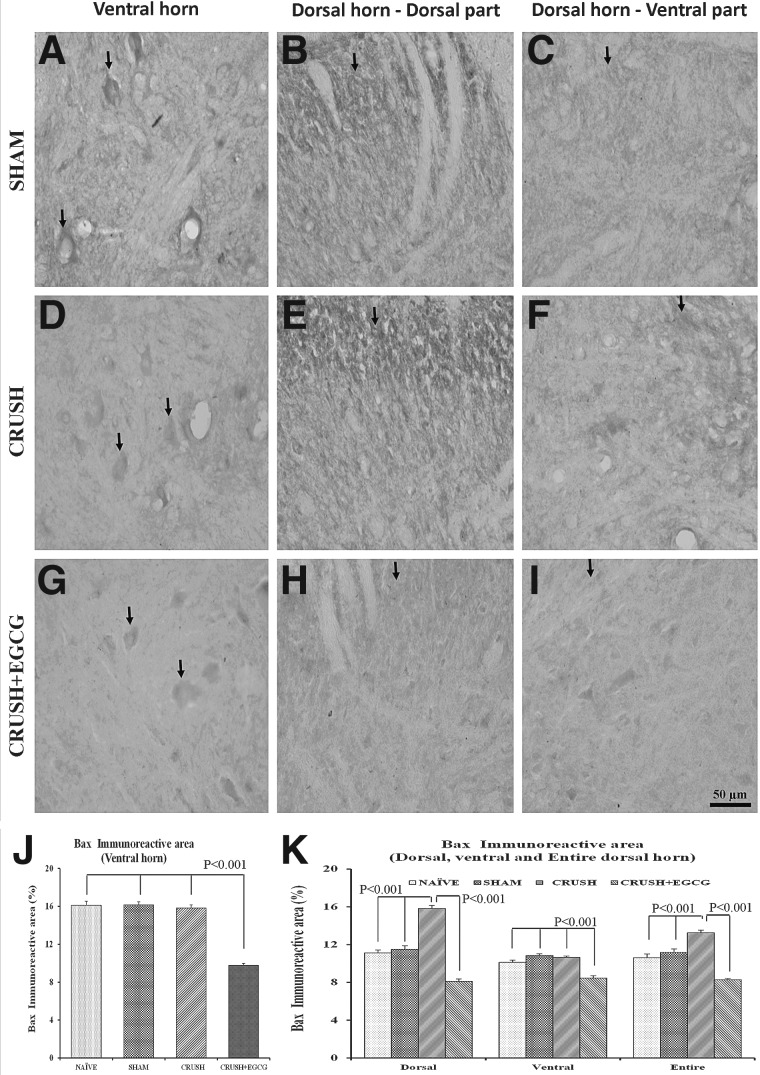
In addition, the neurons in both the ventral and dorsal horns of the spinal cord sections of the CRUSH+EGCG group showed increased expression of GAP-43, when compared to control groups (Fig. 10A–I), and this increase was highly significant (p<0.001; Fig. 10J,K). Similar results were obtained from spinal cord sections stained with Bcl-2 1 week postsurgery. CRUSH+EGCG rats showed increased Bcl-2 immunoreactivity, compared to CRUSH rats (Fig. 11A–I), and the Bcl-2 immunoreactive area percentage in both the ventral and dorsal horns of CRUSH+EGCG rats was significantly increased (p<0.001), compared to control groups. On the other hand, the CRUSH+EGCG group showed low affinity to Bax stain in both the ventral and dorsal horns of the spinal cord, compared to control groups (Fig. 12A–I). Analysis of the Bax immunoreactive area of the ventral and dorsal horn of spinal cord showed a significant decrease (p<0.001) in the immunoreactive area in the CRUSH+EGCG group, compared to control groups. However, the CRUSH group showed a significant increase (p<0.001) in the Bax immunoreactive area only in the dorsal horn laminae of the spinal cord (Fig. 12J,K).
FIG. 10.
(A–I) GAP-43-stained photomicrographs of the ventral horn (left panel: A, D, G), dorsal part of the dorsal horn (middle panel: B, E, H), and ventral part of the dorsal horn (right panel: C, F, I) from SHAM, CRUSH, and CRUSH+EGCG groups of rats at 1 week postsurgery. Arrows indicate the GAP-43 staining. Note the significant increase in GAP-43 immunoreactivity in the CRUSH+EGCG group in the dorsal and ventral horns. Scale bar=50 μm, applicable to all photomicrographs. (J and K) Bar chart illustrating GAP-43 immunoreactive area (%) in the ventral horn (J) and dorsal horn (K), as measured by Scion Image analysis software (National Institutes of Health) in different groups. Data represent mean±SEM (n=6 in all groups; ANOVA followed by Bonferroni's post-test). EGCG, (-)-epigallocatechin-3-gallate; GAP-43, growth-associated protein 43; DH, dorsal horn.
FIG. 11.
(A–I) Bcl-2-stained photomicrographs of the ventral horn (left panel: A, D, G), dorsal part of the dorsal horn (middle panel: B, E, H), and ventral part of the dorsal horn (right panel: C, F, I) from SHAM, CRUSH, and CRUSH+EGCG groups of rats at 1 week postsurgery. Arrows indicate the Bcl-2 immunoreactivity. Note the significant increase in Bcl2 immunoreactivity in the CRUSH+EGCG group, both in the dorsal and ventral horns. Scale bar=50 μm, applicable to all photomicrographs. (J and K) Bcl-2-immunoreactive area (%) in the ventral horn (J) and dorsal horn (K), as measured by Scion Image analysis software (National Institutes of Health) in different groups. Data represent mean±SEM (n=6 in all groups; ANOVA followed by Bonferroni's post-test). EGCG, (-)-epigallocatechin-3-gallate; Bcl2, B-cell lymphoma 2; DH, dorsal horn.
FIG. 12.
(A–I) Bax-stained photomicrographs of the ventral horn (left panel: A, D, G), dorsal part of the dorsal horn (middle panel: B, E, H), and ventral part of the dorsal horn (right panel: C, F, I) from SHAM, CRUSH, and CRUSH+EGCG groups of rats at 1 week postsurgery. Arrows indicate the Bax immunoreactivity. Note the significant decrease in Bax immunoreactivity in the CRUSH+EGCG group. Scale bar=50 μm, applicable to all photomicrographs. (J and K) Bax immunoreactive area (%) in the ventral horn (J) and dorsal horn (K), as measured by Scion Image analysis software (National Institutes of Health) in different groups. Data represent mean±SEM (n=6 in all groups; ANOVA followed by Bonferroni's post-test). EGCG, (-)-epigallocatechin-3-gallate; Bax, B-cell lymphoma 2-associated X protein; DH, dorsal horn.
Expression of apoptosis genes
Genetic expression of apoptotic genes was measured using real-time PCR (as described in the Methods section) in spinal cord sections taken from different animal groups (Table 1). It was noted that Bax/Bcl-2 ratio was significantly increased 3 days after sciatic nerve injury in CRUSH animals, compared to the sham control group (0.71+0.05 vs. 0.31+0.03; p<0.01). On the other hand, CRUSH+EGCG animals showed a Bax/Bcl-2 ratio comparable to the sham control group 3 days after injury (0.30+0.05). In spite of the significant increase in Bcl-2 messenger RNA (mRNA) level (0.64+0.01; p<0.05) that was observed at day 7 postsurgery in CRUSH animals, the Bax/Bcl-2 ratio in those animals was significantly (p>0.05) different from the control sham group (0.22+0.05 vs. 0.33+0.04; respectively). Moreover, survivin mRNA level significantly decreased 3 days postinjury in CRUSH rats, compared to the sham group; however, survivin levels were equal to the control group at days 7 and 14 postsurgery. In contrast, EGCG treatment led to the normalization of survivin levels, when compared to the sham group. p53 expression level was not affected by sciatic nerve injury or EGCG treatment throughout the investigation period.
Table 1.
Spinal Cord Gene Expression Analysis of Apoptosis Genes
| Gene | Sham (3 days) | CRUSH (3 days) | CRUSH+EGCG (3 days) | Sham (7 days) | CRUSH (7 days) | CRUSH+EGCG (7 days) | Sham (14 days) | CRUSH (14 days) | CRUSH+EGCG (14 days) |
|---|---|---|---|---|---|---|---|---|---|
| Bax | 0.18±0.04 | 0.39±0.02* | 0.17±0.05 | 0.2±0.03 | 0.18±0.04 | 0.17±0.03 | 0.2±0.05 | 0.23±0.04 | 0.21±0.06 |
| Bcl2 | 0.56±0.01 | 0.55±0.07 | 0.57±0.05 | 0.59±0.02 | 0.79±0.02* | 0.64±0.01 | 0.58±0.03 | 0.54±0.02 | 0.64±0.04 |
| Survivin | 0.87±0.05 | 0.48±0.02* | 0.85±0.04 | 0.82±0.01 | 0.85±0.01 | 0.83±0.03 | 0.86±0.01 | 0.87±0.03 | 0.85±0.04 |
| p53 | 0.79±0.05 | 0.82±0.07 | 0.81±0.05 | 0.8±0.03 | 0.82±0.04 | 0.78±0.02 | 0.8±0.05 | 0.77±0.05 | 0.79±0.02 |
p<0.05 versus the corresponding sham, as analyzed by ANOVA followed by the Neuman-Keuls' test.
Bax, B-cell lymphoma 2-associated X protein; Bcl2, B-cell lymphoma 2.
Discussion
The present study indicates that EGCG treatment of sciatic nerve-injured rats enhanced the survival of spinal cord neurons through modulating the expression of neuronal growth genes along with pro- and antiapoptotic genes and, accordingly, conserving the structures of the cell bodies located in the spinal cord. Moreover, the functional recovery of the affected limb was measured in the study through performing EPT, foot position, and allodynia and paw pressure latencies, along with other tests. The findings of this study were consistent with our previous studies6,32 and other investigations that examined the neuroprotective properties of EGCG on peripheral neuropathies,33 degenerative diseases, such as Alzehiemer's disease and Parkinson's disease, and traumatic brain injury.34–36
Parallel to other studies, our study demonstrates that administering EGCG (50 mg/kg) to sciatic nerve-injured rats improved neurological recovery in these rats and increased the survival of neurons in both the dorsal and ventral horns of spinal cords of EGCG-treated rats on histological examination. Neurological recovery was measured as a function of the EPT, allodynia latency, paw pressure latency, hot-plate latency, and tail flick. The results of this study were comparable to other studies conducted on the effects of EGCG on peripheral neuropathy.25,33,37 Lee and colleagues33 demonstrated that administering 60 mg/kg of EGCG i.p. improved the sensory and thermal thresholds in oxaliplatin-induced peripheral neuropathy animals; these thresholds were tested using von Frey filaments and tail flick test, respectively. However, Lee and colleagues failed to demonstrate any electrophysiological changes or significant difference in the number of apoptotic cells in the dorsal root ganglia (DRG) between EGCG-treated and control animals. This may be a result of the use of terminal deoxynucleotidyl transferase dUTP nick end labeling stain in the detection of apoptotic cells, instead of quantifying the number of neurons in DRG. Likewise, Baluchnejadmojarad and Roghani showed similar behavioral effects in a streptozotocin-induced diabetic neuropathic rat model.37 However, they only looked at malondialdehyde, nitrite content, and erythrocyte superoxide dismutase activity as serum markers of oxidative stress. Further, other studies looked the effect of EGCG on the central nervous system (CNS) after spinal cord injury (SCI). Tian and colleagues demonstrated that an intrathecal administration of 10 mg/kg of EGCG after SCI enhanced locomotor activity.38 Similar results were obtained from our recent work on the effects of intravenous ///i.v. infusion of EGCG 20 mg/kg/h after an SCI.32 Neuroprotective properties of EGCG are believed to be a result of its antioxidant properties, along with its ability to modulate genetic expression and transduction pathways. The antioxidant effects of green tea catechins are well established in the literature, both in vivo and in vitro, and are beyond the scope of this study.35,39,40 Our study explores the effect of EGCG on several genes and their expression. GAP-43 is a membrane protein involved in neuronal development and plasticity. It is considered also an essential factor for proper neuronal regeneration.41 It has been established that GAP-43 expression increases (both centrally and peripherally) after peripheral neuronal injury and then it is transported peripherally, where it contributes to neuronal regeneration.41,42 IHC staining revealed a significant increase in GAP-43 expression in CRUSH+EGCG animals, when compared to sham and CRUSH groups. This finding is consistent with our previous work32 and other studies that proved the GAP-43 expression increases with EGCG administration, whether in vivo or in vitro.43,44 On the other hand, GFAP is the main intermediary filament for astrocytes and is a marker for reactive gliosis, which is related to neuronal damage and aging.45 Our study showed a significant reduction in the expression of GFAP in the ventral horn of the spinal cord of EGCG-treated rats, when compared to the saline-treated group, indicating a neuroprotective effect of i.p. administration of EGCG after sciatic nerve injury. his neuroprotection is achieved by hindering the process of gliosis and neuronal damage, therefore, promoting cellular regeneration. Rrapo and colleagues reported a decrease in GFAP expression, and therefore a reduction in neuronal loss, in the brains of human immunodeficiency virus type 1 (HIV-1) transgenic (Tg) rats after supplying them with EGCG in the drinking water with a concentration of 6 mg/mL. It has to be noted that this neuroprotective effect was achieved by the oral route of administration, which is different from our study.46
However, the association between EGCG administration after sciatic nerve injury and GFAP expression was less clear in the dorsal horn of the spinal cord, and the correlation was inconstant between different laminae. More-detailed work needs to be performed on the DRG and dorsal horn laminae in order to understand the role of GFAP in these anatomical areas.
The effects of EGCG in modulating genetic expression of apoptotic genes were measured by Bax/Bcl-2 ratio using IHC staining and real-time PCR. It is known that Bcl2 is an antiapoptotic gene, whereas Bax is a proapoptotic gene. Bcl-2 acts by inhibiting the release of cytochrome c from the mitochondria, preventing apoptosis.47 The mere value of either Bcl2 or Bax expression does not reflect the survival potential of the neuron; however, Bax/Bcl-2 ratio does determine the fate of the cell.48 From our study, it is evident that EGCG down-regulated the expression of Bax and up-regulated the expression of Bcl-2 in spinal cord neurons, both dorsal and ventral neurons, after peripheral nerve crush injury. This finding is consistent with previous studies that demonstrated the antiapoptotic effect of EGCG on the CNS and peripheral nerves after different injuries.6,32,35,38,49 These findings are to further confirm the proposed mechanism for the antioxidant effect of EGCG that underlies neurodegeneration. Both Hall and colleagues and Smith and colleagues showed that it was lipid peroxidation (LPO) that was responsible for inducing apoptotic neural death in rats.50,51 However, a future study is planned to fully investigate this relation.
Apparent inconsistent results were observed between the IHC and RT-PCR results for Bcl-2 in CRUSH+EGCG rats, with an increase noted on immunostaining that was not observed at any time point measured in PCR. Although Bcl-2 mRNA level was greater at day 7 postsurgery in CRUSH animals, the Bax/Bcl-2 ratios in those animals was significantly lower from the control sham. IHC experiments showed an increase in the expressed Bcl-2 protein observed in both the ventral and dorsal horns of the CRUSH+EGCG group, compared to CRUSH rats. On the other hand, the CRUSH+EGCG group showed low affinity to Bax stain in both the ventral and dorsal horns of the spinal cord, compared to control groups. The increased levels of Bcl-2 after EGCG treatment may be as compensated balance resulting from overactivation of Bax in crush injury. Further, these findings may implicate that activation of the apoptotic marker, Bax, is the primary pathway of apoptotic stimulation by crush injury, rather than through its inhibitory effect on antiapoptotic molecules such as Bcl-2. In this respect, the ratio of antiapoptotic molecules to proapoptotic molecules might be more important than absolute amounts of each of them. In the present study, EGCG may produce its neuroprotective effects on spinal cord neurons through the modulation of the Bax/Bcl-2 ratio of the apoptotic pathway.
Survivin is an inhibitor of apoptosis genes that is required for neuronal survival and proliferation; lack of this gene is found in cases of neuronal damage and ischemia.52,53 Survivin mRNA expression was decreased in saline-treated rats, reflecting a possible mechanism for inducing apoptosis in this group. On the other hand, EGCG increased survivin mRNA expression to a level comparable to that in the sham group. The previous findings were in line with our earlier studies.6,32,54 p53 expression was not affected either by sciatic nerve injury or different treatments. However, more-elaborate experiments need to be performed in the presence of the small interfering RNA against Bax, Bcl2, and survivin in order to support our working hypotheses suggesting a role of these proteins in the antiapoptotic effect of EGCG.
Through its antioxidant and -apoptotic effects, EGCG was found to be a neuroprotective agent. Administration of EGCG (25 and 50 mg/kg, i.p.) to rats with cerebral ischemia-reperfusion injury significantly improved the functional neurological outcome parameters and significantly reduced the brain infarction size volume, in comparison to control untreated rats.16 Many studies have also demonstrated a neuroprotective effect of EGCG at a dose of 50 mg/kg, administered i.p. against neuronal damage, brain edema, and LPO in in vivo and in vitro experiments on gerbil brain ischemia.13–15 ECGC produced a higher NF-L protein expression and higher density of retinal ganglionic cells after optic nerve crush.8 In addition, EGCG dramatically reduced astrogliosis, as demonstrated by GFAP expression and neuronal loss in an HIV-1 Tat Tg mouse model. This decrease was accompanied by mild reduction in activated microglia and a significant decrease in neuronal loss.46 In an organotypic culture of a rat spinal cord model, EGCG has recently been shown to protect motor neurons by blocking the glutamate excitotoxicity caused by threohydroxyaspartate, an inhibitor of glutamate transporter.55 Nonetheless, more experiments should be performed to clarify the mechanism of action of EGCG. The signal-transduction pathways should be examined in order to determine whether the EGCG effect on the number of the neurons depends on the interaction with a specific receptor or strictly relates to its antioxidant and -apoptotic properties.
In conclusion, this study provides evidence for the neuroprotective effects of the administration of EGCG on the anterior horn motor neurons after peripheral nerve injury. We believe that this effect is the result of the combination of the antioxidant and -apoptotic effect of EGCG. This study demonstrates that the administration of EGCG reduced the Bax/Bcl-2 ratio and therefore exhibits an antiapoptotic effect. Further, EGCG increased the expression of survivin, which is an inhibitor of apoptosis. In accord with histological findings, EGCG improved the functional capacity of the sciatic nerve-injured rats on both motor and sensory levels.
Acknowledgments
The authors thank Mrs. Preethi George and Mrs. Shabeeba Pattillath for their excellent technical assistance. Also, the authors acknowledge the support of the Animal Resource Center, Health Science Center, Kuwait University.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.World Health Organization. (2013). Global status report on road safety: time for action. Geneva:WHO [Google Scholar]
- 2.Sang S., Lambert J.D., Ho C.T., and Yang C.S. (2011). The chemistry and biotransformation of tea constituents. Pharmacol. Res. 64, 87–99 [DOI] [PubMed] [Google Scholar]
- 3.Feng B., Fang Y., and Wei S.M. (2013). Effect and mechanism of epigallocatechin-3-gallate (EGCG) against the hydrogen peroxide-induced oxidative damage in human dermal fibroblasts. J. Cosmet. Sci. 64, 35–44 [PubMed] [Google Scholar]
- 4.Sutherland B.A., Rahman R.M., and Appleton I. (2006). Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. J. Nutr. Biochem. 17, 291–306 [DOI] [PubMed] [Google Scholar]
- 5.van Praag H., Lucero M.J., Yeo G.W., Stecker K., Heivand N., Zhao C., Yip E., Afanador M., Schroeter H., Hammerstone J., and Gage F.H. (2007). Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice. J. Neurosci. 27, 5869–5878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renno W.M., Al-Maghrebi M., Alshammari A., and George P. (2013). (-)-Epigallocatechin-3-gallate (EGCG) attenuates peripheral nerve degeneration in rat sciatic nerve crush injury. Neurochem. Int. 62, 221–231 [DOI] [PubMed] [Google Scholar]
- 7.Fan B., Li G.Y., Li Y.P., and Cui J.Z. (2008). Neuroprotective effect of epigallocatechin gallate on oxidative-stress-induced retinal cells. Zhonghua Yi Xue Za Zhi 88, 1711–1714 [PubMed] [Google Scholar]
- 8.Xie J., Jiang L., Zhang T., Jin Y., Yang D., and Chen F. (2010). Neuroprotective effects of epigallocatechin-3-gallate (EGCG) in optic nerve crush model in rats. Neurosci. Lett. 479, 26–30 [DOI] [PubMed] [Google Scholar]
- 9.Peng P.H., Chiou L.F., Chao H.M., Lin S., Chen C.F., Liu J.H., and Ko M.L. (2010). Effects of epigallocatechin-3-gallate on rat retinal ganglion cells after optic nerve axotomy. Exp. Eye Res. 90, 528–534 [DOI] [PubMed] [Google Scholar]
- 10.Tsamandas A.C., Thomopoulos K., Zolota V., Kourelis T., Karatzas T., Ravazoula P., Tepetes K., Petsas T., Karavias D., Karatza C., Bonikos D.S., and Gogos C. (2003). Potential role of Bcl-2 and Bax mRNA and protein expression in chronic hepatitis type B and C: a clinicopathologic study. Mol. Pathol. 16, 1273–1288 [DOI] [PubMed] [Google Scholar]
- 11.Mendonca A.C, Barbieri C.H., and Mazzer N. (2003). Directly applied low intensity direct electric current enhances peripheral nerve regeneration in rats. J. Neurosci. Methods 129, 183–190 [DOI] [PubMed] [Google Scholar]
- 12.Varejão AS., Cabrita A.M., Meek M.F., Bulas-Cruz J., Melo-Pinto P., Raimondo S., Geuna S., and Giacobini-Robecchi M.G. (2004). Functional and morphological assessment of a standardized rat sciatic nerve crush injury with a non-serrated clamp. J. Neurotrauma. 21, 1652–1670 [DOI] [PubMed] [Google Scholar]
- 13.Lee S., Suh S., and Kim S. (2000). Protective effects of the green tea polyphenol (-)-epigallocatechin gallate against hippocampal neuronal damage after transient global ischemia in gerbils. Neurosci. Lett. 287, 191–194 [DOI] [PubMed] [Google Scholar]
- 14.Lee S.R., Im K.J., Suh S.I., and Jung J.G. (2003a). Protective effect of green tea polyphenol (-)-epigallocatechin gallate and other antioxidants on lipid peroxidation in gerbil brain homogenates. Phytother. Res. 17, 206–209 [DOI] [PubMed] [Google Scholar]
- 15.Lee S.Y., Kim C.Y., Lee J.J., Jung J.G., and Lee S. (2003b). Effects of delayed administration of (-)-epigallocatechin gallate, a green tea polyphenol on the changes in polyamine levels and neuronal damage after transient forebrain ischemia in gerbils. Brain Res. Bull. 61, 399–406 [DOI] [PubMed] [Google Scholar]
- 16.Choi Y.B., Kim Y.I., Lee K.S., Kim B.S., and Kim D.J. (2004). Protective effect of epigallocatechin gallate on brain damage after transient middle cerebral artery occlusion in rats. Brain Res. 1019, 47–54 [DOI] [PubMed] [Google Scholar]
- 17.Tiwari V., Kuhad A., and Chopra K. (2011). Amelioration of functional, biochemical and molecular deficits by epigallocatechin gallate in experimental model of alcoholic neuropathy. Eur. J. Pain 15, 286–292 [DOI] [PubMed] [Google Scholar]
- 18.Bélanger E., Henry F.P., Vallée R., Randolph M.A., Kochevar I.E., Winograd J.M., Lin C.P., and Côté D. (2011). In vivo evaluation of demyelination and remyelination in a nerve crush injury model. Biomed. Opt. Express 2, 2698–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore A.M., Borschel G.H., Santosa K.A., Flagg E.R., Tong A.Y., Kasukurthi R., Newton P., Yan Y., Hunter D.A., Johnson P.J., and Mackinnon S.E. (2012). A transgenic rat expressing green fluorescent protein (GFP) in peripheral nerves provides a new hindlimb model for the study of nerve injury and regeneration. J. Neurosci. Methods. 204, 19–27 [DOI] [PubMed] [Google Scholar]
- 20.Unal B., Tan H., Orbak Z., Kiki I., Bilici M., Bilici N., Aslan H., and Kaplan S. (2005). Morphological alterations produced by zinc deficiency in rat sciatic nerve: a histological, electron microscopic and stereological study. Brain Res. 1048, 228–234 [DOI] [PubMed] [Google Scholar]
- 21.Hadlock T.A., Koka R., Vacanti J.P., and Cheney M.L. (1999). A comparison of assessments of functional recovery in the rat. J. Peripher. Nerv. Syst. 4, 258–264 [PubMed] [Google Scholar]
- 22.Luís A.L., Amado S., Geuna S., Rodrigues J.M., Simões M.J., Santos J.D., Fregnan F., Raimondo S., Veloso A.P., Ferreira A.J., Armada-da-Silva P.A., Varejão A.S., and Maurício A.C. (2007). Long-term functional and morphological assessment of a standardized rat sciatic nerve crush injury with a non-serrated clamp. J. Neurosci. Methods. 163, 92–104 [DOI] [PubMed] [Google Scholar]
- 23.Senoglu M., Nacitarhan V., Kurutas E.B., Senoglu N., Altun I., Atli Y., and Ozbag D. (2009). Intraperitoneal alpha-lipoic acid to prevent neural damage after crush injury to the rat sciatic nerve. J. Brachial Plex. Peripher. Nerve Inj. 25, 4–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renno W.M., Saleh F., Klepacek I., Al-Khaledi G., Ismael H., and Asfar S. (2006). Green tea pain modulating effect in sciatic nerve chronic constriction injury rat model. Nutr. Neurosci. 9, 41–47 [DOI] [PubMed] [Google Scholar]
- 25.Renno W.M., Al-Maghrebi M., and Al-Banaw A. (2012). (-)-Epigallocatechin-3-gallate (EGCG) attenuates functional deficits and morphological alterations by diminishing apoptotic gene overexpression in skeletal muscles after sciatic nerve crush injury. Naunyn Schmiedebergs Arch Pharmacol. 2385, 807–822 [DOI] [PubMed] [Google Scholar]
- 26.De Vry J., Kuhl E., Franken-Kunkel P., and Eckel G. (2004). Pharmacological characterization of the chronic constriction injury model of neuropathic pain. Eur. J. Pharmacol. 491, 137–148 [DOI] [PubMed] [Google Scholar]
- 27.Kubo K., Nishikawa K., Ishizeki J., Hardy-Yamada M., Yanagawa Y., and Saito S. (2009). Thermal hyperalgesia via supraspinal mechanisms in mice lacking glutamate decarboxylase 65. J. Pharmacol. Exp. Ther. 331, 162–169 [DOI] [PubMed] [Google Scholar]
- 28.Abdel-Rahman A., Rao M.S., and Shetty A.K. (2004). Nestin expression in hippocampal astrocytes after injury depends on the age of the hippocampus. Glia 47, 299–313 [DOI] [PubMed] [Google Scholar]
- 29.Afsari Z.H., Renno W.M., and Abd-El-Basset E. (2008). Alteration of glial fibrillary acidic proteins immunoreactivity in astrocytes of the spinal cord diabetic rats. Anat. Rec. (Hoboken). 291, 390–399 [DOI] [PubMed] [Google Scholar]
- 30.Renno W.M., Alkhalaf M., Afsari Z., Abd-El-Basset E., and Mousa A. (2008). Consumption of green tea alters glial fibriliary acidic protein immunoreactivity in the spinal cord astrocytes of STZ-diabetic rats. Nutr. Neurosci. 11, 32–40 [DOI] [PubMed] [Google Scholar]
- 31.Livak K.J., and Schmittgen T.D. (2011). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 32.Renno W.M., Al-Khaledi G., Mousa A., Karam S.M., Abul H., and Asfar S. (2014). (-)-Epigallocatechin-3-gallate (EGCG) modulates neurological function when intravenously infused in acute and, chronically injured spinal cord of adult rats. Neuropharmacology 77, 100–119 [DOI] [PubMed] [Google Scholar]
- 33.Lee J.S., Kim Y.T., Jeon E.K., Won H.S., Cho Y.S., and Ko Y.H. (2012). Effect of green tea extracts on oxaliplatin-induced peripheral neuropathy in rats. BMC Complement. Altern. Med. 12, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu M., Chen F., Sha L., Wang S., Tao L., Yao L., He M., Yao Z., Liu H., Zhu Z., Zhang Z., Zheng Z., Sha X., and Wei M. (2013). (-)-Epigallocatechin-3-gallate ameliorates learning and memory deficits by adjusting the balance of TrkA/p75NTR signaling in APP/PS1 transgenic mice. Mol. Neurobiol. 49, 1350–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itoh T., Imano M., Nishida S., Tsubaki M., Hashimoto S., Ito A., and Satou T. (2011). (-)-Epigallocatechin-3-gallate protects against neuronal cell death and improves cerebral function after traumatic brain injury in rats. Neuromolecular Med. 13, 300–9 [DOI] [PubMed] [Google Scholar]
- 36.Rezai-Zadeh K., Shytle D., Sun N., Mori T., Hou H., Jeanniton D., Ehrhart J., Townsend K., Zeng J., Morgan D., Hardy J., Town T., and Tan J. (2005). Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J. Neurosci. 25, 8807–8814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baluchnejadmojarad T., and Roghani M. (2012). Chronic oral epigallocatechin-gallate alleviates streptozotocin-induced diabetic neuropathic hyperalgesia in rat: involvement of oxidative stress. Iran J. Pharm. Res. 11, 1243–1253 [PMC free article] [PubMed] [Google Scholar]
- 38.Tian W., Han X.G., Liu Y.J., Tang G.Q., Liu B., Wang Y.Q., Xiao B., and Xu Y.F. (2013). Intrathecal epigallocatechin gallate treatment improves functional recovery after spinal cord injury by upregulating the expression of BDNF and GDNF. Neurochem. Res. 38, 772–779 [DOI] [PubMed] [Google Scholar]
- 39.Nanjo F., Goto K., Seto R., Suzuki M., Sakai M., and Hara Y. (1996). Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic. Biol. Med. 21, 895–902 [DOI] [PubMed] [Google Scholar]
- 40.Park J.W., Jang Y.H., Kim J.M., Lee H., Park W.K., Lim M.B., Chu Y.K., Lo E.H., Lee S.R. (2009). Green tea polyphenol (-)-epigallocatechin gallate reduces neuronal cell damage and up-regulation of MMP-9 activity in hippocampal CA1 and CA2 areas following transient global cerebral ischemia. J. Neurosci. Res. 87, 567–75 [DOI] [PubMed] [Google Scholar]
- 41.Woolf C.J., Reynolds M.L., Molander C., O'Brien C., Lindsay R.M., Benowitz L.I. (1990). The growth-associated protein GAP-43 appears in dorsal root ganglion cells and in the dorsal horn of the rat spinal cord following peripheral nerve injury. Neuroscience 34, 465–478 [DOI] [PubMed] [Google Scholar]
- 42.Sommervaille T., Reynolds M.L., and Woolf C.J. (1991). Time-dependent differences in the increase in GAP-43 expression in dorsal root ganglion cells after peripheral axotomy. Neuroscience 45, 213–220 [DOI] [PubMed] [Google Scholar]
- 43.Avramovich-Tirosh Y., Reznichenko L., Mit T., Zheng H., Fridkin M., Weinreb O., Mandel S., and Youdim M.B. (2007). Neurorescue activity, APP regulation and amyloid-beta peptide reduction by novel multi-functional brain permeable iron-chelating antioxidants, M-30 and green tea polyphenol, EGCG. Curr. Alzheimer Res. 4, 403–411 [DOI] [PubMed] [Google Scholar]
- 44.Gundimeda U., McNeill T.H., Elhiani A.A., Schiffman J.E., Hinton D.R., and Gopalakrishna R. (2012). Green tea polyphenols precondition against cell death induced by oxygen-glucose deprivation via stimulation of laminin receptor, generation of reactive oxygen species, and activation of protein kinase Cɛ. J. Biol. Chem. 287, 34694–34708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Middeldorp J., and Hol E.M. (2011). GFAP in health and disease. Prog. Neurobiol. 93, 421–443 [DOI] [PubMed] [Google Scholar]
- 46.Rrapo E., Zhu Y., Tian J., Hou H., Smith A., Fernandez F., Tan J., and Giunta B. (2009). Green Tea-EGCG reduces GFAP associated neuronal loss in HIV-1 Tat transgenic mice. Am. J. Transl. Res. 1, 72–79 [PMC free article] [PubMed] [Google Scholar]
- 47.Hockenbery D.G., Nuñez G., Milliman C.L., Schreiber R.D., and Korsmeyer S.J. (1990). Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348, 334–336 [DOI] [PubMed] [Google Scholar]
- 48.Zha H., Aimé-Sempé C., Sato T., and Reed J.C. (1996). Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J. Biol. Chem. 271, 7440–7444 [DOI] [PubMed] [Google Scholar]
- 49.Khalatbary A.R., Tiraihi T., Boroujeni M.B., Ahmadvand H., Tavafi M., and Tamjidipoor A. (2010). Effects of epigallocatechin gallate on tissue protection and functional recovery after contusive spinal cord injury in rats. Brain Res. 1306, 168–175 [DOI] [PubMed] [Google Scholar]
- 50.Hall E.D., Smith S.L., and Oostveen J.A. (1996). Inhibition of lipid peroxidation attenuates axotomy-induced apoptotic degeneration of facial motor neurons in neonatal rats. J. Neurosci. Res. 44, 293–299 [DOI] [PubMed] [Google Scholar]
- 51.Smith S.L., Oostveen J.A., and Hall E.D. (1996). Two novel pyrrolopyrimidine lipid peroxidation inhibitors U-101033E and U-104067F protect facial motor neurons following neonatal axotomy. Exp. Neurol. 141, 304–309 [DOI] [PubMed] [Google Scholar]
- 52.Ambrosini G., Adida C., Sirugo G., and Altieri D.C. (1998). Induction of apoptosis and inhibition of cell proliferation by survivin gene targeting. J. Biol. Chem. 273, 11177–11182 [DOI] [PubMed] [Google Scholar]
- 53.Baratchi S., Kanwar R.K., and Kanwar J.R. (2010). Survivin: a target from brain cancer to neurodegenerative disease. Crit. Rev. Biochem. Mol. Biol. 45, 535–554 [DOI] [PubMed] [Google Scholar]
- 54.Al-Ajmi N., Al-Maghrebi M., and Renno W.M. (2013). (-)-Epigallocatechin-3-gallate modulates the differential expression of survivin splice variants and protects spermatogenesis during testicular torsion. Korean J. Physiol. Pharmacol. 17, 259–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu J., Jia Y., Guo Y., Chang G., Duan W., Sun M., Li B., and Li C. (2010). Epigallocatechin-3-gallate protects motor neurons and regulates glutamate level. FEBS Lett. 584, 2921–2925 [DOI] [PubMed] [Google Scholar]