Abstract
Fulminant hepatic failure (FHF) is a clinical syndrome characterized by sudden and severe impairment of liver function. Mesenchymal stem cells (MSCs) have been proposed as a promising therapeutic approach for FHF. In this study we used Propionibacterium acnes (P. acnes)-primed, lipopolysaccharide (LPS)-induced liver injury in mice as an animal model of human FHF. We demonstrated that administration of MSCs significantly ameliorated liver injury and improved the survival rates of mice subjected to P. acnes plus LPS-induced FHF. Allogeneic MSCs showed similar treatment efficacy as autologous MSCs did in FHF. Treatment efficacy of MSCs could be attributed to decreased infiltration and activation of CD4+ T cells in the liver, inhibition of T helper 1 cells, and induction of regulatory T cells (Tregs). Moreover, decreased DNA copies of P. acnes were detected in the liver of MSC-treated mice. Intriguingly, a distinct liver population of CD11c+MHCIIhiCD80loCD86lo regulatory dendritic cells (DCs) was induced by MSCs. Moreover, these DCs induced Treg differentiation through transforming growth factor-β production. Further mechanistic studies demonstrated that MSC-derived prostaglandin E2 and one of its receptors, EP4, played essential roles in the differentiation of CD11c+B220− DC precursors into regulatory DCs in a phosphoinositide 3-kinase-dependent manner. Conclusion: MSCs induce regulatory DCs from CD11c+B220− DC precursors. This study elucidates an immunoregulatory mechanism of MSCs and lays a foundation for application of MSCs in FHF therapy. (Hepatology 2014;59:671–682)
Fulminant hepatic failure (FHF), which develops secondary to infection, toxin, or immune-mediated attack, is a potentially fatal clinical syndrome characterized by rapid development of hepatocellular dysfunction, especially coagulopathy with diffuse intrahepatic infiltration of inflammatory cells and massive multilobular necrosis.1,2 The mechanisms leading to such profound hepatic damage are not fully understood. Inflammatory responses are involved in the pathophysiology of hepatic cell death and liver injury and are associated with failure of hepatic regeneration.3 Mortality without supportive management or liver transplantation is high. Orthotopic liver transplantation is the current gold standard of care, but its use is limited because of organ donor shortage, financial considerations, and the requirement for lifelong immunosuppression.4
Mesenchymal stem cells (MSCs) of multiple origins can differentiate into osteocytes, chondrocytes, adipocytes, neuron-like cells, and hepatocyte-like cells.5 In addition to their wide use in regeneration therapy, the unique immunologic characteristics of MSCs, such as low immunogenicity and immunoregulatory properties, have attracted increased attention.5 MSCs mediate a systemic immunosuppression and have been tested in the treatment of immune disorders such as experimental autoimmune encephalomyelitis, rheumatoid arthritis, and diabetes in several preclinical and clinical studies.5–7 Various mediators are proposed to be responsible for the immunosuppressive capacity of MSCs, such as transforming growth factor (TGF)-β, indoleamine 2,3-dioxygenase, inducible nitric oxide synthase (iNOS), and prostaglandin E2 (PGE2).8 As such, these properties have suggested great therapeutic potential for MSCs in the context of cell-based therapy. Recently, several studies reported that MSCs could relieve liver fibrosis and promote liver regeneration.9 Whether MSCs are effective in the treatment of FHF and the underlying immunoregulatory mechanisms remain elusive.
Dendritic cells (DCs) are the most potent, professional antigen-presenting cells derived from CD34+ hematopoietic progenitor cells (HPCs). DCs are key mediators for the initiation and regulation of both innate and adaptive immune responses. In contrast to the well-known capacity of conventional DCs to prime T cells effectively, regulatory DCs serve to induce immune tolerance and have been used successfully to prevent the onset of delayed-type hypersensitivity, colitis, and collagen-induced arthritis.10 Recently, several groups reported that MSCs may drive the differentiation of regulatory DCs from HPCs.11,12 However, the underlying mechanisms are still poorly understood and the induction of regulatory DCs in vivo remains controversial.
Priming of mice with heat-killed Propionibacterium acnes (P. acnes) followed by a low dose of lipopolysaccharide (LPS) results in acute liver injury in mice, and is one of the commonly used animal models mimicking FHF in humans. In this model, liver injury is pathophysiologically classified into two phases.1,13–16 In the priming phase, as we previously reported, CD11c+B220− DC precursors are mobilized rapidly into the circulation in mice injected with P. acnes.14,15 This is an initial event and a prerequisite for liver injury in this model. Then, DC precursors are recruited into inflammatory liver tissue and differentiate into mature DCs, activating P. acnes-specific CD4+ T cells in the hepatic lymph nodes, which are then recruited to the liver.15 The accumulation of these mononuclear cells (MNCs) in the liver leads to granuloma formation.1,16 In the eliciting phase, LPS injection further increases liver inflammation, leading to massive hepatic cell death and rapid liver failure.16 Therefore, inhibition of inflammatory infiltration and immune responses evoked by DCs is beneficial for treatment of the disease.
In this study, using P. acnes-primed, LPS-induced FHF in mice, we found that MSC treatment effectively attenuated the severity of liver injury in mice and reduced mortality of FHF through inhibiting T helper (Th) 1 cells and induction of regulatory T cells (Tregs). More important, we demonstrate that MSCs ameliorated clinical symptoms and disease progression by secretion of PGE2, which induced the differentiation of liver regulatory DCs from CD11c+B220− DC precursors by activating EP4 and the associated phosphoinositide 3-kinase (PI3K)/extracellular signal-regulated kinase (ERK) 1/2 signaling pathway.
Materials and Methods
Induction of Liver Injury
C57BL/6 mice were primed intravenously with 1 mg of heat-killed P. acnes. For survival analysis, mice were injected intravenously with 1 μg of LPS on day 7 after P. acnes priming. For the indicated experiments, a total of 1 × 106 MSCs or vehicle was injected intravenously on days 0, 2, and 4 (a prophylactic protocol), or on days 3, 5, and 7 (a therapeutic protocol for granulomatous hepatitis). In some MSC-treated mice, NS398 (500 μg/mouse), NG-monomethyl-L-arginine acetate salt (L-NMMA; 500 μg/mouse) (both from Sigma-Aldrich, St. Louis, MO), or vehicle was administered intraperitoneally daily from day 0 until day 6.
Induction of DC Maturation
Mature DCs were generated from CD11c+B220− DC precursors as previously described with some modifications.14 In brief, purified CD11c+B220− cells were cultured in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF; 10 ng/mL) and interleukin (IL)-4 (5 ng/mL) (both from PeproTech, Rocky Hill, NJ) for 5 days to induce immature DCs (immature DC induction phase). Cells were further incubated with GM-CSF and tumor necrosis factor (TNF)-α (50 ng/mL; PeproTech) on type I collagen-coated plates for 3 more days to induce mature DCs (mature DC induction phase).
Histology and immunofluorescence, flow cytometric analysis, DC precursor-MSC cocultures, quantitative real-time polymerase chain reaction (PCR), western blot, and all other materials and methods are described in the Supporting Information.
Results
MSCs Ameliorate the Severity of Bacteria-Induced Liver Injury and Reduce the Mortality of FHF
To determine the efficacy of MSCs in FHF, MSCs from C57BL/6 mice or vehicle was injected intravenously after P. acnes priming. For the vehicle-treated group, all C57BL/6 mice died within 18 hours post-LPS injection. By contrast, MSC treatment with either a prophylactic protocol or a therapeutic protocol for granulomatous hepatitis effectively improved the survival rate of FHF, and all mice survived more than 7 days post-LPS injection (Fig. 1A; Supporting Fig. S1A). These were consistent with a dramatic decrease in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in the serum of MSC-treated mice (Fig. 1B; Supporting Fig. S1B). Histology showed that large nodules, severe infiltration of lymphocytes, and granuloma formation were observed in liver tissues on day 7 post-P. acnes priming, liver weight increased considerably (Fig. 1C; Supporting Figs. S1C, S2A,B). Moreover, Fas ligand expression was also elevated (Fig. 1D). By contrast, livers isolated from mice treated with MSCs displayed normal morphology without nodules, much less infiltration of lymphocytes, markedly reduced granulomas, normal weight, and remarkably reduced Fas ligand expression (Fig. 1C,D; Supporting Figs. S1C, S2A,B). Importantly, MSCs from BALB/c mice also ameliorated FHF in C57BL/6 mice (Supporting Fig. S3A,B). Taken together, these data demonstrate that MSC treatment effectively attenuated the severity of bacteria-induced liver injury and improved the survival rate of FHF. Interestingly, MSCs were efficacious in amelioration of concanavalin A (ConA)-induced acute liver injury as evidenced by significantly decreased serum levels of ALT and AST, reduced areas of focal necrosis, and less lymphocyte infiltration around the central veins in the liver compared to those of controls (Supporting Fig. S4A,B). Additionally, we also investigated the in vivo tumorigenesis of MSCs and no tumor was detected in mice inoculated with MSCs during a period of 1 month observation (Supporting Fig. S5).
Figure 1.
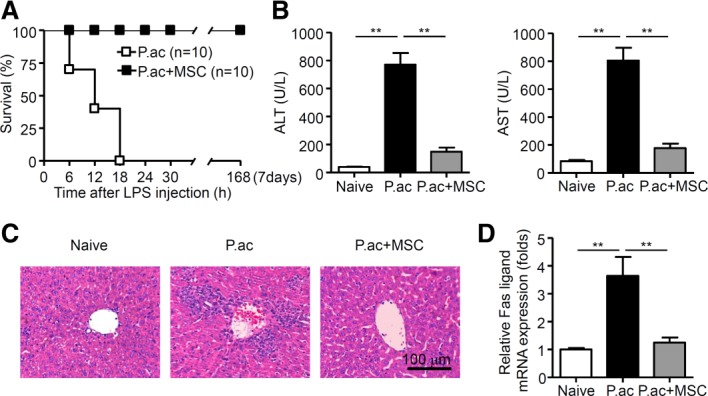
MSCs ameliorate the severity of bacteria-induced liver injury. Mice were injected with P. acnes (P.ac) suspended in 100 μL of phosphate-buffered saline (PBS). PBS or MSCs were administered intravenously on days 0, 2, and 4, and 1 μg of LPS in 100 μL of PBS was injected on day 7 to induce FHF. (A) Cumulative survival rates of mice were analyzed. Data from two independent experiments are combined (n = 10 mice per group). (B-D) Serum and liver tissues from naive, PBS, or MSC-treated mice were sampled on day 7 after P. acnes priming. Serum levels of ALT and AST (B; n = 8 mice per group), and mRNA level of Fas ligand in livers (D; n = 6 mice per group) were measured. Results are mean ± SEM from three independent experiments. (C) Liver tissues were sectioned for histological examination. Scale bar = 100 μm. Representative images from one experiment out of three are shown. **P < 0.01.
MSCs Reduce Migration and Activation of CD4+ T Cells in the Liver
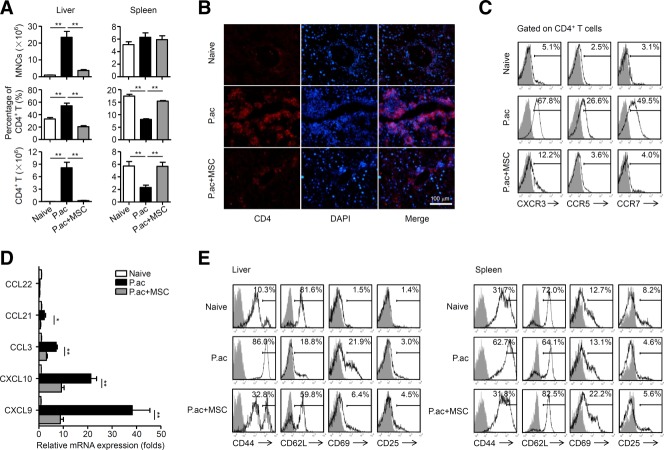
It is known that T-cell-mediated inflammation plays an important role in P. acnes-induced liver injury.15 MNCs accumulated significantly in the liver of P. acnes-primed control mice, and both the percentage and absolute number of CD4+ T cells increased dramatically in the liver (Fig. 2A,B). However, fewer CD4+ T cells were detected in the spleen (Fig. 2A). Intriguingly, infiltration of MNCs and CD4+ T cells decreased significantly in the liver of MSC-treated mice, while CD4+ T cells in the spleen remained at levels similar to those of naive mice (Fig. 2A,B). Accordingly, expression of CXCR3, CCR5, and CCR7 on CD4+ T cells and their respective chemokines CXCL9, CXCL10, CCL3, and CCL21 in the liver of MSC-treated mice were reduced considerably (Fig. 2C,D). These results indicated that MSC treatment suppressed the chemotaxis of pathogenic CD4+ T cells into the liver. In addition, MSC-treated mice showed decreased expression of CD44 and CD69 and increased expression of CD62L (Fig. 2E), suggesting that MSCs suppressed CD4+ T-cell activation in the mice.
Figure 2.
MSCs reduce migration and activation of CD4+ T cell in the liver. Mice were injected with P. acnes (P.ac). PBS or MSCs were administered intravenously on days 0, 2, and 4 after P. acnes injection. Livers or spleens were isolated from naive, PBS, or MSC-treated mice on day 7. (A) Absolute numbers of total MNCs, percentages and absolute numbers of CD4+ T cells in these tissues were determined by flow cytometry. (B) Immunofluorescence staining of CD4+ T cells in liver tissues. Scale bar = 100 μm. Representative images from one experiment out of three are shown. (C) Levels of CXCR3, CCR5, and CCR7 on CD4+ T cells derived from livers were analyzed by flow cytometry. (D) mRNA levels of chemokines in livers were measured by quantitative real-time PCR. (E) Levels of CD44, CD62L, CD69, and CD25 on CD4+ T cells of livers and spleens were analyzed by flow cytometry. (A,D) Results are mean ± SEM from three independent experiments (n = 6 mice per group). (C,E) Gray shading and solid line indicate immunofluorescence intensity of cells for the control and test antibodies, respectively. Results are representative of three independent experiments. *P < 0.05; **P < 0.01.
MSCs Suppress Th1 Cells but Promote Tregs in the Liver
We previously identified Th1 cells as central players in the pathogenesis of P. acnes-induced liver injury.1 Thus, serum levels of Th1 cytokines TNF-α and interferon (IFN)-γ, Th2 cytokines IL-4 and IL-5, and another proinflammatory cytokine IL-17, were determined. As shown in Fig. 3A, MSCs reduced levels of TNF-α and IFN-γ significantly, but had no effect on IL-4, IL-5, or IL-17 production. Intracellular staining of TNF-α and IFN-γ further confirmed the reduction of TNF-α- and IFN-γ-positive cells within CD4+ T cells (Fig. 3B). By contrast, administration of MSCs significantly increased CD4+CD25+Foxp3+ Tregs as compared with the vehicle treatment (Fig. 3C). These data demonstrate that MSCs suppressed Th1 cells but promoted Tregs in the liver. Interestingly, MSC treatment accelerated elimination of the bacteria, since DNA copies of P. acnes 16S rDNA in the liver of MSC-treated mice were considerably lower from day 1 post-P. acnes priming onwards as compared to those of controls (Supporting Fig. S6A). In addition, MSC-treated mice showed significantly reduced lymphocyte infiltration in the liver, and marked decrease in serum levels of AST, ALT, TNF-α, and IFN-γ on day 28 post-P. acnes priming (Supporting Fig. S6B-D). Taken together, the data suggest that transfer of MSCs down-regulated excessive Th1 response but retained the T-cell response controlling the bacteria in vivo.
Figure 3.
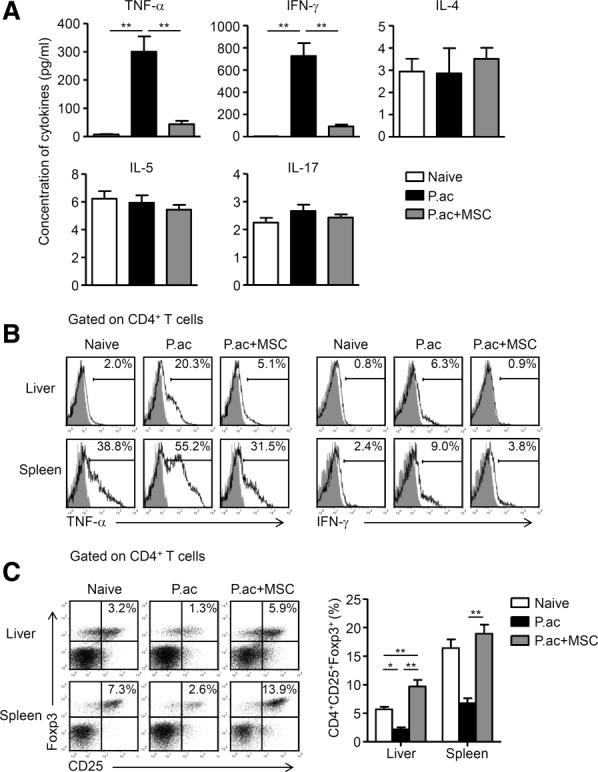
MSCs suppress Th1 cells but promote Tregs in the liver. Mice were injected with P. acnes (P.ac). PBS or MSCs were administered intravenously on days 0, 2, and 4 after P. acnes injection. Peripheral blood, livers, or spleens were isolated from naive, PBS, or MSC-treated mice on day 7. (A) Levels of serum IFN-γ, TNF-α, IL-4, IL-5, and IL-17 were measured by cytometric bead array or enzyme-linked immunosorbent assay. Data are mean ± SEM from three independent experiments (n > 6 mice per group). (B) Inflammatory cytokine production in CD4+ T cells were assessed by intracellular staining and flow cytometry. Gray shading and solid line indicate immunofluorescence intensity of cells for the control and test antibodies, respectively. Data are representative of three independent experiments. (C) MNCs isolated from livers and spleens were stained for surface markers and intracellular expression of Foxp3 and analyzed by flow cytometry. Representative dot plots are shown on the left and summarized results are shown as mean ± SEM from three independent experiments (n = 6 mice per group) on the right. *P < 0.05; **P < 0.01.
Distinct Regulatory DC Population Is Induced by MSC Treatment
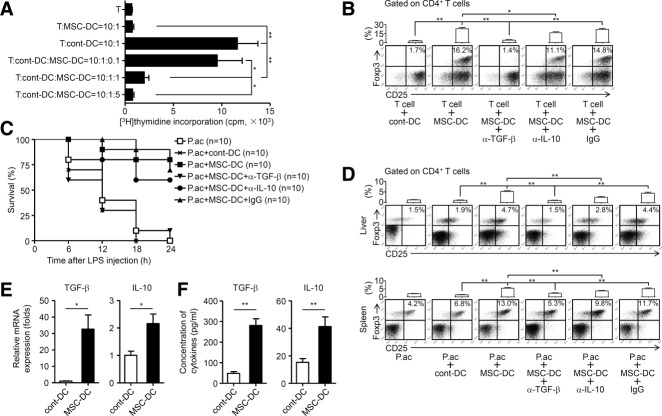
In the P. acnes-induced liver injury model, antigen-presenting cells, such as Kupffer cells and DCs, elicit inflammatory responses by presenting antigens to CD4+ T cells. The DC-T cell interactions are then amplified by continuous stimulation of recruited DCs to liver-infiltrating CD4+ T cells, resulting in subsequent liver injury.13 In this regard, we investigated whether MSC treatment could influence activation and function of antigen-presenting cells in the liver. MSC treatment did not affect the expression of MHC class II (MHCII) and costimulatory molecules (e.g., CD80 and CD86) in F4/80+ Kupffer cells, but dramatically reduced CD80 and CD86 expression in CD11c+ DCs and increased their MHCII expression level (Fig. 4A). In addition, these DCs displayed the same phenotype after further stimulation with GM-CSF and TNF-α in vitro (Fig. 4B). They were defined as MSC-DCs thereafter. In a functional study, these MSC-DCs showed a much lower capability to evoke an allogeneic mixed lymphocyte reaction (MLR) as compared with DCs isolated from control mice (cont-DCs) (Fig. 4C), although these DCs could uptake more FITC-dextran than cont-DCs (Fig. 4D). In addition, MSC-DCs produced lower levels of proinflammatory cytokines including TNF-α, IL-1β, and IL-12 than cont-DCs (Fig. 4E). These data suggested that these MSC-DCs were distinct from either immature or mature conventional DCs. Furthermore, MSC-DCs significantly suppressed allogeneic CD4+ T cell proliferation elicited by cont-DCs in a dose-dependent manner (Fig. 5A). Interestingly, MSC-DCs, but not cont-DCs, were able to induce Tregs efficiently when cocultured with naive T cells (Fig. 5B). Notably, MSCs from BALB/c mice were also able to induce MSC-DCs in C57BL/6 mice (Supporting Fig. S3C-E). In vivo studies were then performed to validate the immunoregulatory functions of MSC-DCs. MSC-DC treatment effectively improved mice survival rate of FHF, as compared with cont-DCs, by inducing the generation of Tregs (Fig. 5C,D). Besides, transfer of MSC-DCs significantly decreased serum levels of ALT and AST, and reduced lymphocyte infiltration in the liver compared to those of cont-DCs (Supporting Fig. S7A,B). Altogether, these data demonstrated that these CD11c+MHCIIhiCD80loCD86lo MSC-DCs resemble the regulatory DC population, but are distinct from conventional stimulatory DCs.
Figure 4.
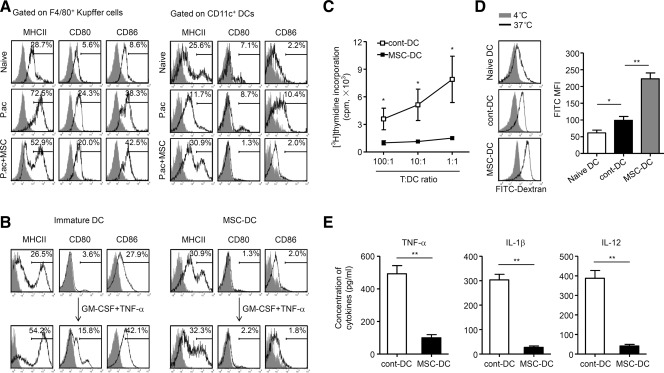
MSCs induce a distinct population of regulatory DCs. Mice were injected with P. acnes (P.ac). PBS or MSCs were administered intravenously on days 0, 2, and 4 after P. acnes injection. Livers were isolated from naive, PBS, or MSC-treated mice on day 7. (A) Levels of MHCII, CD80, and CD86 on F4/80+ Kupffer cells or CD11c+ DCs of livers were analyzed by flow cytometry. (B) Immature DCs induced from CD11c+B220− DC precursors of PBS-treated mice or MSC-DCs magnetically purified from liver MNCs of MSC-treated mice were stimulated with GM-CSF (10 ng/mL) and TNF-α (50 ng/mL) for 3 days in vitro. The phenotypes of both types of cells before and after stimulation were analyzed by flow cytometry. (C) Cont-DCs or MSC-DCs were magnetically purified from liver MNCs of C57BL/6 mice. After lethally irradiated (30 Gy), they were cultured in graded doses with magnetically purified CD4+ T cells (3 × 105 cells/well) from the spleen of naive BALB/c mice. Five days later, T-cell proliferation was measured by incorporation of [3H]thymidine. Data are mean ± SEM (n = 3). (D) Cont-DCs or MSC-DCs were incubated with FITC-dextran (0.5 mg/mL) at 4°C or 37°C for 120 minutes. The capacity of antigen uptake was detected by measuring the mean fluorescence intensities (MFI) of the fraction of FITC-positive cells using flow cytometry. Representative histograms are shown on the left and summarized results are shown as mean ± SEM (n = 6). (E) Collected cont-DCs or MSC-DCs were cultured with GM-CSF (10 ng/mL) in vitro. After 3 days, levels of TNF-α, IL-1β, and IL-12 in supernatants were measured by enzyme-linked immunosorbent assay. Results are mean ± SEM (n = 6). (A,B) Gray shading and solid line indicate immunofluorescence intensity of cells for the control and test antibodies, respectively. Data are representative of three independent experiments. *P < 0.05; **P < 0.01.
Figure 5.
Immunoregulatory function of liver MSC-DCs. Mice were injected with P. acnes (P.ac). PBS or MSCs were administered intravenously on days 0, 2, and 4 after P. acnes injection. Livers or spleens were isolated from PBS or MSC-treated mice on day 7. (A) CD4+ T cells (3 × 105 cells/well) from the spleen of naive BALB/c mice were incubated with or without lethally irradiated (30 Gy) cont-DCs (3 × 104 cells/well) from C57BL/6 mice. In the indicated experiments, MSC-DCs from C57BL/6 mice were added to the coculture system in graded doses. Five days later, T-cell proliferation in response to the indicated treatments was measured by incorporation of [3H]thymidine. Data are mean ± SEM from three independent experiments (n = 3). (B) CD4+CD25− T cells from C57BL/6 mice were cultured with syngeneic cont-DCs or MSC-DCs in the presence of plate-coated anti-CD3 antibody (5 μg/mL) and soluble anti-CD28 antibody (2 μg/mL). In the indicated experiments, anti-TGF-β, anti-IL-10 neutralizing antibody (α-TGF-β or α-IL-10), or control IgG was added into the culture system, respectively. Five days later, cells were stained for surface markers and intracellular expression of Foxp3, and analyzed by flow cytometry. (C,D) C57BL/6 mice were infused intravenously with 5 × 105 cont-DCs or MSC-DCs on days 2 and 4 after P. acnes priming. In some experiments, mice infused with MSC-DCs were also treated with anti-TGF-β, anti-IL-10 neutralizing antibody, or control IgG (7 mg/kg). LPS was injected on day 7, and cumulative survival rates were analyzed. Data from two independent experiments are combined (C; n = 10 mice per group). (D) MNCs isolated from livers or spleens were stained for surface markers and intracellular expression of Foxp3, and analyzed by flow cytometry. (E) mRNA levels of TGF-β and IL-10 in cont-DCs and MSC-DCs were measured by quantitative real-time PCR. Data are normalized to expression levels of cont-DCs. (F) Collected cont-DCs or MSC-DCs were cultured with GM-CSF (10 ng/mL) in vitro. After 3 days, levels of TGF-β and IL-10 in supernatants were measured by enzyme-linked immunosorbent assay. (B,D) Representative dot plots are shown on the bottom and summarized results are mean ± SEM from three independent experiments (n = 6 mice per group) on the top. (E,F) Results are mean ± SEM (n = 6). *P < 0.05; **P < 0.01.
MSC-DCs Suppress T-Cell Immune Responses and Induce Treg Differentiation Through Production of TGF-β
Besides the expression of low levels of costimulatory molecules, MSC-DCs may execute their suppressive functions through expression of a group of inhibitory molecules such as CD200R3, Aldh1a (a gene involved in retinoid acid metabolism), IL-10, and TGF-β (Supporting Table 1).17,18 Among them, we found that messenger RNA (mRNA) levels of TGF-β and IL-10 were remarkably elevated in MSC-DCs as compared with those of cont-DCs (Fig. 5E; Supporting Fig. S8). Accordingly, MSC-DCs secreted more TGF-β and IL-10 than cont-DCs (Fig. 5F). We then evaluated the function of TGF-β and IL-10 in MSC-DC-mediated Treg differentiation. As shown in Fig. 5B, anti-TGF-β neutralizing antibody significantly reversed Treg induction by MSCs; anti-IL-10 neutralizing antibody also blocked Treg differentiation but to a much less extent. Moreover, in vivo experiments also provided evidence demonstrating that MSC-DCs could induce Treg generation, an effect significantly blocked by infusion of anti-TGF-β neutralizing antibody and partially blocked by anti-IL-10 neutralizing antibody (Fig. 5C,D). These data demonstrate that TGF-β played an important role in MSC-DC-mediated Treg differentiation.
MSCs Induce Differentiation of CD11c+B220− DC Precursors Into Regulatory DCs
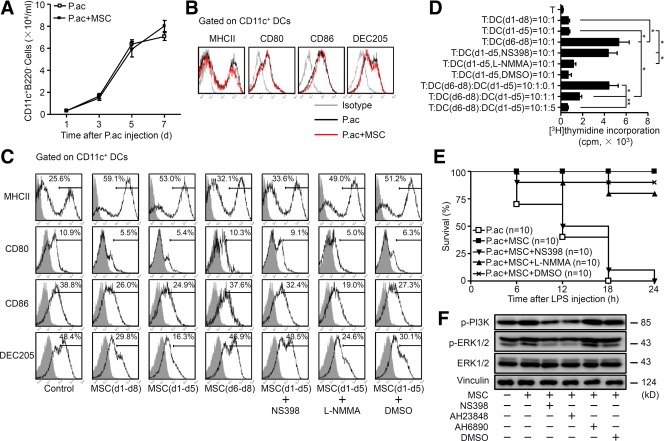
Our previous studies showed that after P. acnes priming, circulating CD11c+B220− DC precursors could be mobilized to the liver and gradually differentiate into functional, mature DCs to elicit immune responses.14 Thus, we set out to determine whether MSCs altered the characteristics of DC precursors. The number of DC precursors in periphery blood increased markedly and peaked on day 7 post-P. acnes priming to a similar level in vehicle and MSC-treated groups (Fig. 6A). In addition, DC precursors from both groups displayed a similar capability to differentiate into conventional, mature DCs (Fig. 6B), suggesting that MSC treatment did not influence recruitment of DC precursors into the periphery blood or their potential to differentiate into mature DCs.
Figure 6.
PGE2 secreted by MSCs is essential for the generation of regulatory DCs. Mice were injected with P. acnes (P.ac). PBS or MSCs were administered intravenously three times on days 0, 2, and 4 after P. acnes injection. (A) Periphery blood of PBS or MSC-treated mice was collected on days 1, 3, 5, or 7. CD11c+B220− DC precursors were analyzed by flow cytometry. Data are shown as mean ± SEM from three independent experiments (n = 6). (B) Purified DC precursors from PBS or MSC-treated mice blood on day 7 were sorted out and incubated with GM-CSF (10 ng/mL) plus IL-4 (5 ng/mL) for 5 days and GM-CSF plus TNF-α (50 ng/mL) for 3 more days. Cells collected on day 8 were stained for MHCII, CD80, CD86, and DEC205 and analyzed by flow cytometry. (C) Induction of mature DCs from DC precursors was performed as described in (B). In the indicated experiments, MSCs were included in the culture during the immature DC induction phase (d1-d5), mature DC induction phase (d6-d8), or both phases (d1-d8). In some experiments with MSC treatment, NS398 (5 μM), L-NMMA (1 mM), or DMSO was added to the culture. Cells were stained for MHCII, CD80, CD86, and DEC205, and analyzed by flow cytometry. (D) Induction of DCs from DC precursors was performed as described in (B,C). DCs induced from DC precursors in the presence of MSCs during various phases were indicated as DC(d1-d5), DC(d6-d8), or DC(d1-d8), respectively. For those cocultured with MSCs during d1-d5, NS398, L-NMMA, or DMSO was also included in the culture. Then, DCs were sorted out and cultured with CD4+ T cells derived from BALB/c mice as indicated. Five days later, T-cell proliferation was measured by incorporation of [3H]thymidine. Data are mean ± SEM (n = 3). (E) P. acnes-primed mice were intravenously injected with PBS or MSCs. Those treated with MSCs were subsequently injected with nothing, NS398 (500 μg/mouse), L-NMMA (500 μg/mouse), or DMSO. All animals were then injected with LPS and survival rates were followed for 24 hours. Data from two independent experiments are combined (n = 10 mice per group). (F) Purified DC precursors were incubated with GM-CSF and IL-4, with or without MSCs. In some experiments, NS398, AH23848, AH6809, or DMSO was added to the culture system. After 48 hours, levels of p-PI3K, p-ERK1/2, ERK1/2, and vinculin from cultured cells were determined by western blot analysis. (B,C,F) Results are representative of three independent experiments. *P < 0.05; **P < 0.01.
To further dissect the cellular mechanisms involved in regulatory DC induction by MSCs, peripheral DC precursors, derived from P. acnes-primed mice, were cultured to induce immature DCs, and then fully mature DCs. MSCs were added into the culture either during the immature DC induction phase, mature DC induction phase, or both phases. Compared with the high expression of MHCII, CD80, CD86, and DEC205 in the control group, culture with MSCs for 8 days induced a regulatory DC phenotype; the cells displayed higher expression of MHCII, but decreased expression of CD80, CD86, and DEC205 (Fig. 6C). Interestingly, culture with MSCs during the immature DC induction phase, but not during the mature DC induction phase, resulted in the typical phenotype of regulatory DCs (Fig. 6C). Furthermore, functional studies indicated that, phenotypically, regulatory DCs could not elicit allogeneic CD4+ T-cell proliferation, but indeed significantly suppress allogeneic CD4+ T-cell proliferation elicited by DCs treated with MSCs only during the mature DC induction phase (Fig. 6D). These results indicated that regulatory DCs could be induced from CD11c+B220− DC precursors by MSC treatment in vitro.
MSC-Derived PGE2 Plays an Essential Role in the Induction of Regulatory DCs From DC Precursors by Activating the PI3K/ERK1/2 Pathway
PGE2 and iNOS are important effectors derived from MSCs. Thus, we evaluated whether PGE2 or iNOS participated in the induction of regulatory DCs. The PGE2-specific inhibitor NS398, but not the iNOS inhibitor L-NMMA, significantly blocked the differentiation of regulatory DCs from DC precursors as well as the suppressive function of regulatory DCs (Fig. 6C,D). Furthermore, an in vivo study showed that inhibition of PGE2 significantly reversed the treatment effect of MSCs on P. acnes plus LPS-induced FHF (Fig. 6E). Altogether, these results indicated that MSC-derived PGE2 was essential for the differentiation of CD11c+B220− DC precursors into regulatory DCs.
To further explore the molecular mechanisms involved in differentiation of regulatory DCs, we assayed phosphorylation of PI3K and ERK1/2 as activation indicator in MSC-induced regulatory DCs. PI3K and ERK1/2 are known signaling downstream kinases following binding of PGE2 and its respective receptors, EP2 and EP4.19,20 Markedly elevated phosphorylation of PI3K and ERK1/2 was detected in CD11c+B220− cells after coculture with MSCs. Intriguingly, NS398 as well as the EP4 antagonist AH23848 markedly inhibited phosphorylation of PI3K and ERK1/2, whereas AH6809, an EP2 antagonist, did not affect the activation of these proteins (Fig. 6F). Thus, MSC-derived PGE2 plays an essential role in the induction of regulatory DCs from DC precursors by activating EP4 and associated PI3K/ERK1/2 signaling pathway.
Discussion
Our findings demonstrate that MSCs effectively attenuate the severity of bacteria-induced liver injury and increase survival rate of mice subjected to P. acnes plus LPS-induced FHF. Mechanistically, MSCs induce differentiation of a distinct, functional CD11c+MHCIIhiCD80loCD86lo regulatory DC population from CD11c+B220− DC precursors through production of PGE2 in a PI3K-dependent manner. These regulatory DCs inhibit Th1 cells and induce Tregs, resulting in amelioration of FHF.
MSCs can differentiate in vitro along the hepatogenic lineage, and have been extensively investigated as potential sources for liver regeneration.21 In the current study, we focused on dissecting the underlying immunoregulatory mechanisms for application of MSCs in FHF treatment. We provide clear evidence that delivery of MSCs dramatically decreases mortality and alleviates liver injury. Detailed analyses demonstrated that MSCs induced a distinct DC population characterized by a CD11c+MHCIIhiCD80loCD86lo phenotype with potent regulatory function. These DCs were able to phagocytose antigens efficiently but could not present the antigens properly due to their insufficient expression of CD80 and CD86. In addition, MSC-DCs produced low levels of proinflammatory cytokines including TNF-α, IL-1β, and IL-12, and were defective in stimulating proliferation of allogeneic T cells in MLR cultures, but were able to suppress T-cell proliferation and induce Tregs. These findings are reminiscent of a recent study, which showed that a population of regulatory DCs expressed the phenotype of MHCIIhiCD80loCD86lo and possessed potent ability to repress inflammatory T-cell responses.22 Conventional mature DCs express high levels of antigen-presenting molecules (e.g., MHCII) and costimulatory molecules, produce large amounts of proinflammatory cytokines (e.g., TNF-α, IL-1β, and IL-12), have great ability to stimulate proliferation and expansion of allogeneic T cells in a MLR culture. However, the immature DCs are characterized by low T-cell activation potential accompanied by low expression of MHCII and costimulatory molecules. Upon inflammatory stimulation, immature DCs are able to acquire both phenotypic and functional properties of mature DCs.23,24 Notably, despite in vitro stimulation with GM-CSF plus TNF-α for 3 days, which has been shown to effectively induce maturation of conventional immature DCs,14 MSC-DCs fail to up-regulate the expression of CD80 and CD86. Taken together, these observations suggest that MSC-DCs resemble to regulatory DCs, but are distinct from either immature or mature conventional DCs which can elicit primary T-cell responses. Thus, it is likely the active phagocytosis may represent a unique property of MSC-DCs rather than maturity conditions. In fact, various regulatory DC populations have been identified in distinct settings during the last years,22,23,25–27 which support the concept of significant heterogeneity of regulatory DC populations.
Regulatory DCs comprise a heterogeneous population located in various tissues, where they use distinct mechanisms to induce and maintain central and peripheral tolerance. In the current study, we found MSC-DCs highly express TGF-β and IL-10 as compared with cont-DCs. Moreover, these MSC-DCs induce Treg differentiation in a TGF-β-dependent manner. IL-10 also contributed to Treg generation, but to a lesser extent. Our data are consistent with a recent study, which showed that CD103+ regulatory DCs migrated from the lamina propria to the mesenteric lymph nodes to present locally administered antigen to naive CD4+ T cells and drive the differentiation of Tregs in a TGF-β-mediated manner.28 Nevertheless, other groups also reported DCs promote Tregs through activation of retinoid acid and production of indoleamine 2, 3-dioxygenase.18,29 All these data demonstrate the important regulatory properties of regulatory DCs.
Several studies demonstrated previously that MSCs might interfere with the differentiation of CD34+ HPC into mature DCs.11,12 Recently, Li et al.30 showed that human bone marrow-derived MSCs may induce HPCs to generate regulatory DCs through activation of the Notch pathway. Liu et al.11 reported that embryonic fibroblast-derived MSCs induced generation of mouse regulatory DCs from HPCs by activation of SOCS3 in a IL-10-dependent manner. In contrast to previous studies, we demonstrated that MSCs induce differentiation of circulating CD11c+B220− DC precursors into regulatory DCs. Additionally, we identified that it was the differentiation of DC precursors to immature DCs, rather than differentiation of immature DCs to mature DCs, that was required for generation of regulatory DCs. This is important for understanding the ontogeny of regulatory DCs and for generating large numbers of regulatory DCs for immunotherapy.
We also performed splenectomy to determine whether splenic immune response contributes to MSC-induced protection against liver injury. Surprisingly, MSCs are still efficacious in ameliorating FHF in splenectomized mice. There is no change in DC precursors, regulatory DCs, Tregs in the liver, or serum levels of TNF-α and IFN-γ. Migration and residence of MSCs in the liver are also unaffected as compared to sham controls (data not shown). It is known that the white pulp of the spleen consists of aggregates of lymphoid tissue, which can identify antigens and mount an immune response to antigens within the blood, and thus plays a vital role in immune homeostatics and fighting infections.31,32 However, in the current model it seems that splenic immune response is redundant to MSC-mediated attenuation of bacteria-induced FHF. Our results are concordant with previous study which demonstrated the importance of hepatic local DC-T cell interaction in the liver injury,13 and further strengthen the concept that suppression of immune response in the hepatic microenvironment by MSCs is essential for the treatment effects on FHF in mice.
MSCs have been shown to exert their immunoregulatory effects through secretion of soluble factors such as PGE2 and indoleamine 2,3-dioxygenase.5 Recently, several studies showed that when cultured in the presence of PGE2, DCs have a lower capacity to present antigens to T cells, and they produce lower levels of IL-12 and higher levels of IL-10 or arginase-1.33,34 We demonstrated that MSC-derived PGE2 is responsible for the generation of CD11c+MHCIIhiCD80loCD86lo regulatory DCs from CD11c+B220− DC precursors. Moreover, we showed that this process is mediated by EP4, which is the receptor of PGE2, and subsequent activation of PI3K/ERK1/2 pathway. Thus, our data provide new evidence to demonstrate the importance of PGE2 and related downstream pathways in MSC-mediated immunoregulatory function.
It is of interest to elucidate the role of Th1 response in the pathogenesis of P. acnes-induced liver injury. Although appropriate Th1 cell traffic into the inflamed liver is necessary to eliminate microorganisms,14 excessive influx of Th1 cells may cause more severe inflammation.13 Thus, the final outcome of T-cell-mediated disease is dependent on the extent and duration of effector T-cell recruitment into the target tissue. We found that modulation of Th1 response by MSCs accelerated the elimination of bacteria in the liver, reduced inflammatory infiltration, and improved liver functions in the long run. Thus, MSCs may down-regulate excessive Th1 response, retain a certain extent of Th1 capability, and enable the host to eradicate an invading pathogen with minimum tissue damage. Notably, administration of MSCs led to a marked reduction of DNA copies of P. acnes in the liver 1 day after bacterial priming. Since 24 hours after bacterial injection may not be enough for the generation of an efficient Th1 response, Th1 cells are unlikely to mediate the reduction of P. acnes DNA copies at this stage. MSCs are able to activate macrophages through a direct physical cell-to-cell interaction and secretion of cytokines such as IL-6 and GM-CSF.35,36 Kupffer cells are specialized macrophages that can phagocytose and degrade pathogenic bacteria through formation of phagolysosomes. It is possible that MSCs can augment the capability of Kupffer cells in the liver, leading to an accelerated clearance of P. acnes early after priming.
Our data demonstrated that MSC treatment is therapeutic for granulomatous hepatitis and ConA-induced FHF, and is preventive for the P. acnes plus LPS-induced FHF model. This is consistent with results from a recent study showing that MSC-conditioned medium was able to ameliorate D-galactosamine-induced FHF by inhibiting hepatocellular death and stimulating hepatocyte regeneration.37 More important, MSCs transplantation showed favorable short-term efficacy in patients with liver failure.38 Finally, allogeneic MSCs are able to induce regulatory DCs in the host to exert immunoregulatory functions, which is of significance for clinical application of allogeneic MSCs in treatment of FHF.
In conclusion, the current study shows that MSC-based therapy has profound inhibitory effects on inflammatory responses and that it ultimately improves survival in mice undergoing P. acnes plus LPS-induced FHF. Furthermore, we demonstrate direct induction of CD11c+MHCIIhiCD80loCD86lo regulatory DCs from CD11c+B220− DC precursors both in vivo and in vitro. This work shows that MSCs induce an integrated response to liver disease and further lays a solid foundation for application of MSCs in the treatment of this devastating disorder.
Acknowledgments
The authors thank Dr. Yongping Gu (Soochow University, Suzhou, China) for histopathologic assessment, and Dr. Gary Brewer (University of Medicine and Dentistry of New Jersey) for critical review of the article.
Glossary
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ConA
concanavalin A
- DC
dendritic cell
- ERK
extracellular signal-regulated kinase
- FHF
fulminant hepatic failure
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HPC
hematopoietic progenitor cell
- IFN
interferon
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- L-NMMA
NG-monomethyl-L-arginine acetate salt
- LPS
lipopolysaccharide
- MHCII
MHC class II
- MLR
mixed lymphocyte reaction
- MNC
mononuclear cell
- MSC
mesenchymal stem cell
- PGE2
prostaglandin E2
- PI3K
phosphoinositide 3-kinase
- TGF
transforming growth factor
- Th
T helper
- TNF
tumor necrosis factor
- Treg
regulatory T cell
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Supporting Information
References
- 1.Yoneyama H, Harada A, Imai T, Baba M, Yoshie O, Zhang Y, et al. Pivotal role of TARC, a CC chemokine, in bacteria-induced fulminant hepatic failure in mice. J Clin Invest. 1998;102:1933–1941. doi: 10.1172/JCI4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoofnagle JH, Carithers RL, Jr, Shapiro C, Ascher N. Fulminant hepatic failure: summary of a workshop. Hepatology. 1995;21:240–252. [PubMed] [Google Scholar]
- 3.Moniaux N, Song H, Darnaud M, Garbin K, Gigou M, Mitchell C, et al. Human hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein cures fas-induced acute liver failure in mice by attenuating free-radical damage in injured livers. Hepatology. 2011;53:618–627. doi: 10.1002/hep.24087. [DOI] [PubMed] [Google Scholar]
- 4.Nemes B, Gelley F, Piros L, Zadori G, Gorog D, Fehervari I, et al. The impact of Milan criteria on liver transplantation for hepatocellular carcinoma: first 15 years’ experience of the Hungarian Liver Transplant Program. Transplant Proc. 2011;43:1272–1274. doi: 10.1016/j.transproceed.2011.03.077. [DOI] [PubMed] [Google Scholar]
- 5.Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20:510–518. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 6.Pileggi A. Mesenchymal stem cells for the treatment of diabetes. Diabetes. 2012;61:1355–1356. doi: 10.2337/db12-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papadopoulou A, Yiangou M, Athanasiou E, Zogas N, Kaloyannidis P, Batsis I, et al. Mesenchymal stem cells are conditionally therapeutic in preclinical models of rheumatoid arthritis. Ann Rheum Dis. 2012;71:1733–1740. doi: 10.1136/annrheumdis-2011-200985. [DOI] [PubMed] [Google Scholar]
- 8.Li W, Ren G, Huang Y, Su J, Han Y, Li J, et al. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 2012;19:1505–1513. doi: 10.1038/cdd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aquino JB, Bolontrade MF, Garcia MG, Podhajcer OL, Mazzolini G. Mesenchymal stem cells as therapeutic tools and gene carriers in liver fibrosis and hepatocellular carcinoma. Gene Ther. 2010;17:692–708. doi: 10.1038/gt.2010.10. [DOI] [PubMed] [Google Scholar]
- 10.Adler HS, Steinbrink K. Tolerogenic dendritic cells in health and disease: friend and foe! Eur J Dermatol. 2007;17:476–491. doi: 10.1684/ejd.2007.0262. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Qu X, Chen Y, Liao L, Cheng K, Shao C, et al. Mesenchymal stem/stromal cells induce the generation of novel IL-10-dependent regulatory dendritic cells by SOCS3 activation. J Immunol. 2012;189:1182–1192. doi: 10.4049/jimmunol.1102996. [DOI] [PubMed] [Google Scholar]
- 12.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 13.Yoneyama H, Narumi S, Zhang Y, Murai M, Baggiolini M, Lanzavecchia A, et al. Pivotal role of dendritic cell-derived CXCL10 in the retention of T helper cell 1 lymphocytes in secondary lymph nodes. J Exp Med. 2002;195:1257–1266. doi: 10.1084/jem.20011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoneyama H, Matsuno K, Zhang Y, Murai M, Itakura M, Ishikawa S, et al. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J Exp Med. 2001;193:35–49. doi: 10.1084/jem.193.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Yoneyama H, Wang Y, Ishikawa S, Hashimoto S, Gao JL, et al. Mobilization of dendritic cell precursors into the circulation by administration of MIP-1alpha in mice. J Natl Cancer Inst. 2004;96:201–209. doi: 10.1093/jnci/djh024. [DOI] [PubMed] [Google Scholar]
- 16.Xiao Y, Xu J, Mao C, Jin M, Wu Q, Zou J, et al. 18Beta-glycyrrhetinic acid ameliorates acute Propionibacterium acnes-induced liver injury through inhibition of macrophage inflammatory protein-1alpha. J Biol Chem. 2010;285:1128–1137. doi: 10.1074/jbc.M109.037705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hattori T, Saban DR, Emami-Naeini P, Chauhan SK, Funaki T, Ueno H, et al. Donor-derived, tolerogenic dendritic cells suppress immune rejection in the indirect allosensitization-dominant setting of corneal transplantation. J Leukoc Biol. 2012;91:621–627. doi: 10.1189/jlb.1011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grangette C. Bifidobacteria and subsets of dendritic cells: friendly players in immune regulation! Gut. 2012;61:331–332. doi: 10.1136/gutjnl-2011-301476. [DOI] [PubMed] [Google Scholar]
- 19.Yen JH, Kocieda VP, Jing H, Ganea D. Prostaglandin E2 induces matrix metalloproteinase 9 expression in dendritic cells through two independent signaling pathways leading to activator protein 1 (AP-1) activation. J Biol Chem. 2011;286:38913–38923. doi: 10.1074/jbc.M111.252932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caparros E, Munoz P, Sierra-Filardi E, Serrano-Gomez D, Puig-Kroger A, Rodriguez-Fernandez JL, et al. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood. 2006;107:3950–3958. doi: 10.1182/blood-2005-03-1252. [DOI] [PubMed] [Google Scholar]
- 21.Chamberlain J, Yamagami T, Colletti E, Theise ND, Desai J, Frias A, et al. Efficient generation of human hepatocytes by the intrahepatic delivery of clonal human mesenchymal stem cells in fetal sheep. Hepatology. 2007;46:1935–1945. doi: 10.1002/hep.21899. [DOI] [PubMed] [Google Scholar]
- 22.Sato K, Yamashita N, Baba M, Matsuyama T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003;18:367–379. doi: 10.1016/s1074-7613(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 23.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 24.Menges M, Rossner S, Voigtlander C, Schindler H, Kukutsch NA, Bogdan C, et al. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 26.Matta BM, Raimondi G, Rosborough BR, Sumpter TL, Thomson AW. IL-27 production and STAT3-dependent upregulation of B7-H1 mediate immune regulatory functions of liver plasmacytoid dendritic cells. J Immunol. 2012;188:5227–5237. doi: 10.4049/jimmunol.1103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popov A, Abdullah Z, Wickenhauser C, Saric T, Driesen J, Hanisch FG, et al. Indoleamine 2,3-dioxygenase-expressing dendritic cells form suppurative granulomas following Listeria monocytogenes infection. J Clin Invest. 2006;116:3160–3170. doi: 10.1172/JCI28996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-βeta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takagi H, Fukaya T, Eizumi K, Sato Y, Sato K, Shibazaki A, et al. Plasmacytoid dendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo. Immunity. 2011;35:958–971. doi: 10.1016/j.immuni.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Li YP, Paczesny S, Lauret E, Poirault S, Bordigoni P, Mekhloufi F, et al. Human mesenchymal stem cells license adult CD34+ hemopoietic progenitor cells to differentiate into regulatory dendritic cells through activation of the Notch pathway. J Immunol. 2008;180:1598–1608. doi: 10.4049/jimmunol.180.3.1598. [DOI] [PubMed] [Google Scholar]
- 31.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol. 2001;1:177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 32.Ochsenbein AF, Pinschewer DD, Odermatt B, Ciurea A, Hengartner H, Zinkernagel RM. Correlation of T cell independence of antibody responses with antigen dose reaching secondary lymphoid organs: implications for splenectomized patients and vaccine design. J Immunol. 2000;164:6296–6302. doi: 10.4049/jimmunol.164.12.6296. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt LM, Belvisi MG, Bode KA, Bauer J, Schmidt C, Suchy MT, et al. Bronchial epithelial cell-derived prostaglandin E2 dampens the reactivity of dendritic cells. J Immunol. 2011;186:2095–2105. doi: 10.4049/jimmunol.1002414. [DOI] [PubMed] [Google Scholar]
- 34.Harizi H, Gualde N. Pivotal role of PGE2 and IL-10 in the cross-regulation of dendritic cell-derived inflammatory mediators. Cell Mol Immunol. 2006;3:271–277. [PubMed] [Google Scholar]
- 35.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, et al. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47:1634–1643. doi: 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- 38.Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820–828. doi: 10.1002/hep.24434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information