Abstract
Objectives:
In this study, we examine the association of asthma (asthma symptoms, asthma control, lung function) and sleep problems in a group of urban children. The role of allergic rhinitis (AR), a comorbid condition of asthma, on children's sleep problems is also examined. Finally, we investigate whether sleep hygiene moderates the association between asthma and sleep problems, and whether there are differences in these associations based on ethnic background.
Methods:
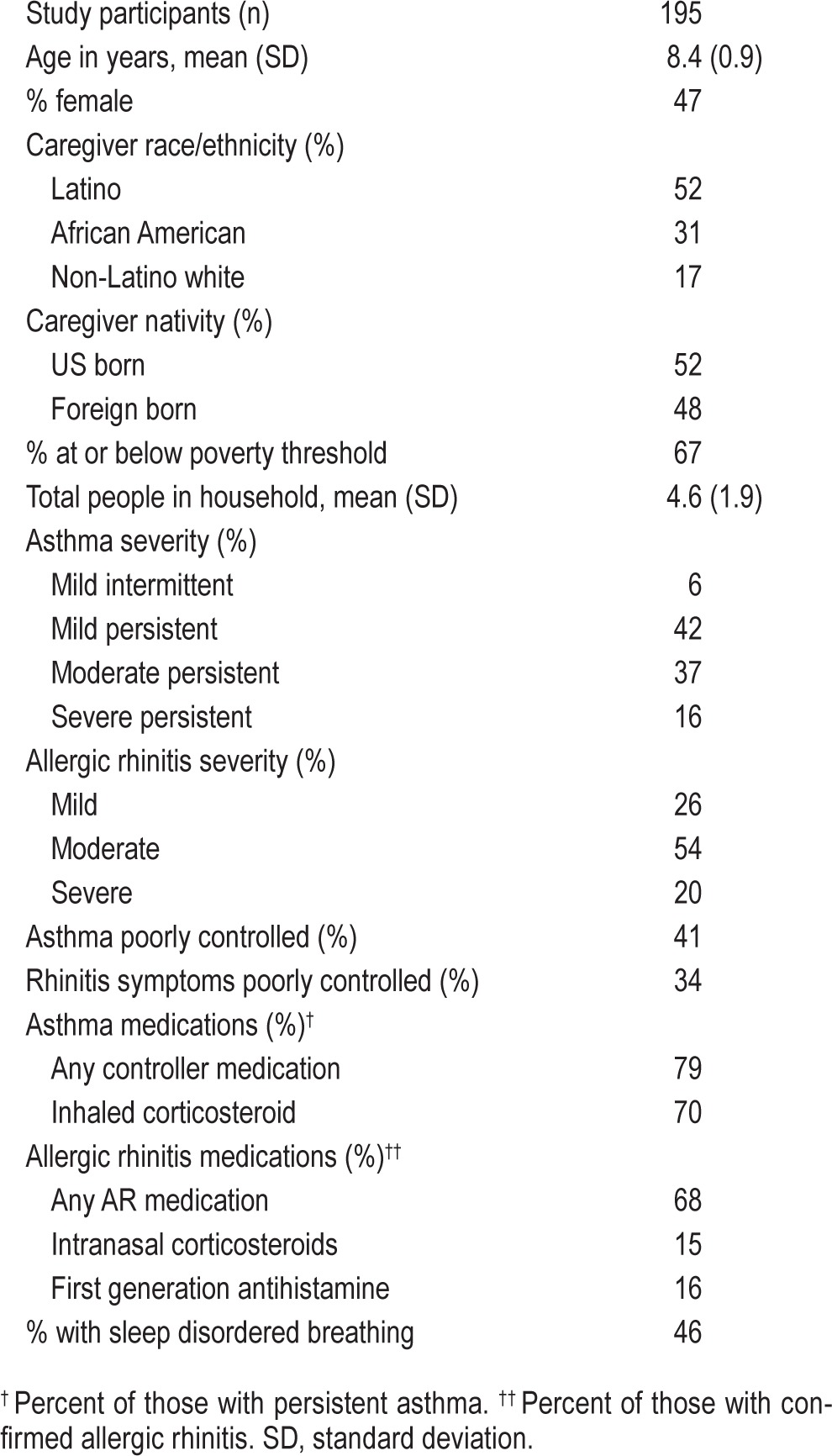
Non-Latino White, Latino, and African American urban children with asthma (n = 195) ages 7–9 (47% female) and their primary caregivers participated in a baseline visit involving interview-based questionnaires on demographics, asthma and rhinitis control, and caregiver report of children's sleep problems and sleep hygiene. Children and their caregivers participated in a clinical evaluation of asthma and AR, followed by a month monitoring period of children's asthma using objective and subjective methods.
Results:
Total sleep problem scores were higher in children of the sample who were from African American and Latino backgrounds, compared to non-Latino white children. Poor asthma control was predictive of higher levels of sleep problems in the entire sample. Poorer AR control also was related to more sleep problems, over and above children's asthma in the sample. This association was more robust in non-Latino white children. Poor sleep hygiene heightened the association between poor asthma control and sleep problems in the entire sample and in African American children.
Conclusions:
Multidisciplinary interventions integrating the co-management of asthma, AR, and the effects of both illnesses on children's sleep, need to be developed and tailored to children and their families' ethnic background.
Citation:
Koinis-Mitchell D, Kopel SJ, Boergers J, Ramos K, LeBourgeois M, McQuaid EL, Esteban CA, Seifer R, Fritz GK, Klein RB. Asthma, allergic rhinitis, and sleep problems in urban children. J Clin Sleep Med 2015;11(2):101–110.
Keywords: urban children, sleep behaviors, asthma
Urban minority children with asthma are at a disadvantage with respect to asthma burden.1 In urban cities in the US, rates of asthma are found to be 20% and higher among children.2 African American (AA) and Latino children, particularly Puerto Rican (PR) and Dominicans (DR), have greater disease prevalence and morbidity compared to non-Latino white (NLW) children.3 Urban minority children are exposed to higher levels of poverty, environmental triggers, acculturative stress, discrimination, and language and medication barriers, which affect asthma management behaviors and morbidity.4–6
Approximately 80% of all children with asthma have coexisting allergic rhinitis (AR).7 Managing asthma and AR may be challenging for urban children and families, given the complexity of each illness' treatment regimen,8,9 and the potential for urban stressors (e.g., environmental triggers, neighborhood stress), which can challenge the control of each illness.
Asthma and Sleep
Urban children with asthma miss more sleep when their asthma is in poor control.10 Nocturnal asthma symptoms disrupt the continuity of sleep by frequent awakenings, and affect daytime sleepiness, school performance, and increase school absences.11–13 Non-adherence to controller asthma medications, as well as mechanistic links between asthma and sleep (e.g., increase in inflammation at night which can increase symptoms) make children with asthma more vulnerable to night wakings.14,15 Home allergens/irritants, pollen counts, and sleep posture, can facilitate mucous production, nocturnal symptoms, and awakenings.15 Moreover, co-occurring sleep conditions (sleep disordered breathing), found twice as likely in minority children,16 can affect sleep duration and daytime sleepiness.17 Thus, urban and minority children with asthma are at an increased risk for sleep disruptions due to asthma symptoms, which can affect their overall quality of sleep.15 Co-occurring nighttime AR symptoms (e.g., nasal congestion) may further contribute to sleep disruptions, daytime fatigue, and somnolence.18
BRIEF SUMMARY
Current Knowledge/Study Rationale: Urban minority children are disproportionately affected by both asthma and allergic rhinitis, which in turn may increase sleep problems and negatively affect sleep behaviors and patterns. Study results highlight the role of asthma and AR control in potentially improving children's sleep and how specific groups may be more at risk for poor sleep problems.
Study Impact: By understanding associations between asthma, AR, sleep hygiene, and overall sleep problems in specific groups at risk for poor asthma morbidity and poor sleep, interventions can be better tailored to address asthma and sleep in a multifaceted way. Focused questions targeted on children's missed sleep may prioritize where to enhance educational and treatment strategies with urban families who have children with asthma and AR.
Parent-Reported Sleep Problems in Children with Asthma
Sleep problems are common among healthy school-aged children, but may be even more common among urban minority children and children with chronic illnesses.19–21 Several studies of children with chronic illnesses and healthy children have measured sleep problems using the Children's Sleep Habits Questionnaire,19 a well-validated, reliable, and widely used parent-report instrument. These studies have found more sleep problems in children who have chronic illnesses (e.g., juvenile rheumatoid arthritis [JRA], sickle cell disease [SCD], and chronic pain) than in children without chronic illnesses.22–24 In this paper, we focus on “sleep problems” as measured by the total score on the CSHQ. Sleep problems, according to this instrument, are defined as sleep behaviors and patterns which can disturb the overall quality of children's sleep. Subscales of the CSHQ include a focus on bedtime resistance, sleep-onset delay, sleep duration, sleep anxiety, night wakings, parasomnias, sleep disordered breathing, and daytime sleepiness. Higher total scores on this measure are indicative of higher levels of sleep problems.
To date, only one study has used the CSHQ in a sample of low-income and ethnically diverse children with asthma symptoms.12 In this study, the average CSHQ total score of the sample (51) was above the clinical cutoff (41), indicating a high level of sleep problems. Further, children with more frequent nocturnal asthma symptoms (reported by parents in the past week) had a higher level of sleep problems than children with less frequent symptoms. Gaps in the literature include measurement of asthma by self-report only, lack of consideration for common comorbidities of asthma (e.g., AR), and factors related to urban context and the sleep environment (e.g., sleep hygiene).
Sleep Hygiene, Asthma, and Sleep Problems
Risks of urban poverty (e.g., family stress affecting bedtime routines, crowded housing) can affect children's sleep environment (noise, sleep disruptions in the child's bedroom) and sleep behaviors (e.g., inconsistent bedtimes and wake up times, and inconsistent sleep locations).25 Sleep hygiene, or the extent to which children and families practice consistent sleep behaviors and habits (e.g., consistent bedtime and wake time, removing disruptions from the child's bedroom such as electronics, eliminating caffeine before bedtime) has been found to influence sleep problems and insufficient sleep in youth.26 Interventions to enhance sleep hygiene have improved health outcomes and daytime sleepiness in children.27 Urban children with asthma may be at an increased risk for poor sleep hygiene compared to their healthy counterparts, as urban poverty may increase children's exposure to risk factors that may challenge both asthma control (e.g., allergens and irritants in the child's bedroom),28 and families' abilities to practice optimal sleep hygiene. To date, no studies have examined how sleep hygiene may affect the association between asthma and sleep problems in urban children.
The Current Study
Our study sought to build on the results from previous research, to examine the association between asthma, allergic rhinitis control, and sleep problems in a sample of urban children with asthma. In this study, we included several indicators and measurements of asthma status. Children's asthma symptoms were measured through the use of caregiver and child symptom reports via a daily diary and the Asthma Control Test,29 and through lung function measurement (home spirometry to assess FEV1 percent predicted) over a 4-week period. Children's sleep problems were assessed by the total score on the CSHQ.
Were first sought to investigate the association between asthma and children's sleep problems (measured by the total sleep score), and whether this association differed by care-giver ethnicity, to identify groups of children who may be at increased risk for sleep problems. We expected that poorer asthma control would be associated with more sleep problems in the entire sample. We also expected this association would be more pronounced in children from Latino and African American backgrounds, given previous research described above.
Second, we were interested in examining the extent to which AR control was associated with children's sleep disturbances, over and above asthma. Given previous work showing that AR can exacerbate asthma,9 we expected that poorer AR control would be associated with more sleep problems, even when asthma was taken into account. We also examined these associations by ethnic group and expected them to be more pronounced in ethnic minority children. Finally, we sought to examine moderators of asthma and children's sleep behaviors that may be relevant to urban children, such as children's sleep hygiene. We expected that the link between asthma activity and sleep problems would be more robust in the context of poor sleep hygiene, and ethnic minority children would be at further risk.
METHODS
Data for this study were collected from a larger study, Project NAPS (Nocturnal Asthma and Performance in School), that assesses the co-occurrence of asthma and AR symptoms, sleep quality, and academic functioning in urban children (7–9 years of age) with persistent asthma (R01 HD057220, Koinis-Mitchell, PI) across one academic year. The current study includes data from asthma participants who completed the first 4 years of the study.
Recruitment of participants for Project NAPS occurred in the 4 largest and adjacent urban school districts in an urban Northeast city, in hospital-based, ambulatory pediatric clinics, and in a hospital-based asthma educational program. Consent-to contact forms (forms signed by caregivers providing permission for a study research assistant to call the family) were distributed across recruitment settings. Research assistants called interested caregivers and screened them to determine eligibility.
Eligibility criteria for the study required that the: child's age be between 7 and 9 years old, child's legal guardian was willing to participate, caregiver ethnicity was self-identified as Latino (Dominican or Puerto Rican), non-Latino white (NLW), or black/African American (AA), child attended public school in 1 of 4 of the largest urban school districts in the greater Providence area, and that the child had physician-diagnosed asthma or breathing problems in the last 12 months. In addition, at screening each child met persistent asthma status either by current prescription of an asthma controller medication, and/ or recurrent daytime symptoms, nighttime symptoms, activity limitation, rescue medication use, or ≥ 2 oral steroid bursts the previous 12 months.30 Exclusionary criteria were moderate-to-severe cognitive impairment as determined by school placement, use of stimulant medication for ADHD, another pulmonary or chronic health condition, or a diagnosed sleep disorder (e.g., restless leg syndrome, chronic insomnia) that would confound the primary hypotheses of the larger study.
Data included in the current study were collected during the fall and early winter period of each study year (August 15–December 31). Demographic information and information regarding asthma and AR medication use was collected at the initial study visit (“baseline”). The second session occurred at our hospital-based clinic ≥ 2 weeks after the baseline session. During this time, asthma and AR diagnosis and severity, and allergy status were evaluated by the study clinicians, and asthma and AR medication use was confirmed. Immediately following this visit, children and their caregiver participated in a 4-week home-monitoring period in which they recorded information on the child's asthma symptoms twice each day in a daily diary, and during which the child used a portable device twice daily to assess lung function (see below). Information on AR control and sleep behaviors was collected. Standardized training procedures on the use of these devices were provided during the baseline and clinic visits (see below).
All assessments were administered verbally in English or Spanish by research staff fluent in both languages, and according to participants' preference. Standardized procedures were used for the translation of instruments.31 Monetary compensation was provided. Approval for the larger study was obtained from the Institutional Review Board of Rhode Island Hospital, Providence, Rhode Island.
Measures
Demographic and Descriptive Information
Primary caregivers provided key demographic information (see Table 1). Poverty status was determined by dividing the family's annual income by the Federal per capita poverty threshold for a family of that size.32 Primary caregivers reported on children's risk for sleep disordered breathing using a well-validated and reliable questionnaire.33
Table 1.
Participant characteristics.
Physician Query
Child participants' primary care providers as well as asthma/ allergy specialists (when applicable) completed a checklist detailing the date of child's last office visit, asthma and/or AR diagnosis, suspected allergy triggers, significant past medical history, and current asthma and rhinitis treatment. This query was used by the study clinician as background information to evaluate the child's asthma and allergy status (see below).
Asthma Diagnosis and Classification of Asthma Severity
The clinic study visit consisted of a medical history and physical examination, allergy skin prick testing, and pulmonary function testing. Confirmation of asthma and classification of severity were made by a study clinician using standard NHLBI EPR-3 guidelines.30 Current medication use was also confirmed. Lung function (FEV1, FEV1/FVC) was measured using the Koko incentive spirometer (nSpireHealth, Longmont, CO) before and after short acting β-agonist administration.34
AR Diagnosis and Classification of AR Severity
Previous diagnosis of allergic rhinitis was not a requirement for study entry; however, AR was evaluated by (1) evidence on physical examination, (2) type and frequency of parent report of AR symptoms in the past month (AR Symptom Summary),8,35 and (3) allergy skin prick testing (Greer Laboratories, NC) to perennial and seasonal allergens common to the Northeastern US. If participants were found to have AR, severity was classified according to clinical practice guidelines (from the Allergic Rhinitis and its Impact on Asthma - ARIA guidelines)8 as Intermittent or Persistent (persistent status defined as AR symptoms > 4 days a week for every week of the month) and Mild, Moderate, or Severe.36
Daily Diary: Asthma Symptoms
Families were asked to complete a daily diary twice each day to indicate whether the child had breathing problems and/ or coughing during the night or day. Research assistants oriented primary caregivers and children to the diary at each research session, reviewed the diary at each visit, and queried any missing information. Diary data were validated by examining associations between daily diary reports and objective lung function data (FEV1) collected daily during the same period. Analyses nested by case indicate an association between these 2 measures of asthma (F = 2.1, p < 0.001). Diary-reported symptoms were summarized across the 1-month monitoring period by computing a proportion of monitored days during which any breathing problems were noted.
Lung Function: FEV1 Percent Predicted
Children's lung function was measured twice daily (in the am and pm during the 4-week monitoring period) by a hand-held computerized spirometer (Jaeger AM2; VIASYS Health-care; Yorba Lina, CA). The best of 3 FEV1 % predicted values per trial were retained. A series of data cleaning and reduction steps were employed, which are available upon request.37 Data were downloaded from the device by RAs at the home during the mid-point and end of each 4-week monitoring period. At the beginning of each session, RAs oriented both the parent and child to the proper use of the device as well as how to conduct spirometry using standard procedures from our previous research.37 Participants were instructed to complete 3 “blows” prior to any asthma or allergy medications in the am and pm.
Asthma Control
At the end of the monitoring period, parents and children completed the Asthma Control Test (ACT),29 a well-validated questionnaire of asthma-related impairment commonly used in the classification of asthma severity. Using standardized scoring procedures,38 we dichotomized scoring using a total cutoff score of 19; those below were considered to have poor asthma control, and those above to have well-controlled asthma. Continuous asthma control scores (higher scores = better control) were maintained for selected analyses, an approach used in previous research with children who have asthma.39
Allergic Rhinitis Control
At the end of the monitoring period, parents completed the Rhinitis Control Assessment Test (RCAT),40 a well-validated questionnaire which assesses rhinitis disease control in patients with allergic rhinitis. The rating scales of 6 items with 5 response choices (frequency of nasal congestion, sneezing, and watery eyes; sleep interference; activity avoidance; and self-assessed control) are added for a continuous total score (6–30). Higher scores indicate better AR control. A dichotomous variable was also computed for descriptive purposes: well versus not well controlled based on a cutoff value of 21.40
Children's Sleep Problems
The Children's Sleep Habits Questionnaire (CSHQ) is a widely used 45-item parent questionnaire that examines children's sleep problems or sleep disturbances, sleep behavior, and sleep patterns. The instrument assesses bedtime resistance, sleep-onset delay, sleep duration, sleep anxiety, night wakings, parasomnias, sleep disordered breathing, and daytime sleepiness. Parents are queried about the child's sleep behaviors over a “typical” recent week and indicate the frequency of each behavior on a 3-point scale (“usually” for 5 to 7 times per week; “sometimes” for 2 to 4 times per week; and “rarely” for 0 to 1 time per week). Higher scores on the total score and all sub-scales indicate a higher frequency of sleep problems. A cutoff value applied to the total score allows for classification of children with a clinical level of sleep disturbance.19 The CSHQ has valid psychometric properties in U.S. children, with adequate internal consistency in both community and clinical samples19,26 and within our own sample (Cronbach α = 0.77).
Children's Sleep Hygiene
The Children's Sleep Hygiene Scale (CSHS) is a 22-item measure examining parent report of sleep hygiene.41 Caretakers were asked about various behaviors relating to hygiene, behaviors that affect sleep initiation and maintenance, with responses ranging across a 6-point scale, from never to always, with higher scores indicating better sleep hygiene. The scale has acceptable internal consistency when used with school-aged children (Cronbach α = 0.76)41 and within our own sample (Cronbach α = 0.70).
Data Analysis Plan
Associations among demographic variables, asthma indicators (FEV1, diary symptoms, asthma control), AR control, and the sleep problems total score were examined via Pearson correlations when both variables were continuous, or analyses of variance (ANOVA) when examining continuous variables across discrete groups. Chi-square analyses tested relationships between categorical variables. Preliminary analyses identified demographic or clinical variables (e.g., asthma or AR severity) related both to the independent variable (e.g., asthma control) and to the sleep problems total score, to be accounted for in subsequent analyses.
In our first set of analyses we examined the association between each asthma indicator (the independent variable) and the total sleep problems score (the dependent variable) in separate linear regression analyses. The next set of analyses assessed the extent to which AR control emerged as a unique predictor of sleep problems, over and above asthma. Separate linear regression models were tested for each asthma indicator, entered in the first step, followed by AR severity (covariate), and then AR control. Following a strategy used in recent studies,39 we maintained Asthma Control and Rhinitis Control scores as continuous variables versus dichotomizing scores into “well controlled” and “not well controlled” in order to maintain the predictive information of continuous scores that can be lost when dichotomizing variables.42
In our final set of analyses, hierarchical regression analyses tested the moderational role of sleep hygiene in the association of asthma control and sleep problems, and then AR control and sleep problems. Covariates were entered in the first step when indicated; in the next two steps the two variables representing potential main effects, sleep hygiene and either asthma or AR control, respectively, were then entered. This was followed by the interaction term of the asthma or AR indicator and sleep hygiene in the final step. Prior to analysis, a mean centered interaction term was computed for each analysis; raw IVs and a centered interaction term were then included in each model. Finally, we conducted post hoc probing to clarify interaction terms that were significant or represented statistical trends.
All analyses described above were conducted first across the entire sample, and then stratified by ethnicity. An α level of 0.05 was used for all statistical tests. Effect sizes for analyses of variance were expressed as partial omega squared (ω2p), which are interpreted as small (0.01), medium (0.06), or large (0.14).43 R2-adjusted are presented for multiple regression results.
RESULTS
Participant characteristics appear in Table 1. One hundred ninety-five children with asthma completed the protocol through the fall monitoring period (children's mean age = 8.4 years). Forty-eight percent of children's primary caregivers were born outside of the US (68% of Latinos, 35% of African Americans, and 9% of NLWs were foreign born).
Asthma Clinical Characteristics
Fifty-three percent of children had moderate-severe persistent asthma, 42% were Mild Persistent (Table 1). Although persistent asthma status was a study-entry criterion, 10 participants (6%) whose asthma appeared to be persistent at screening were classified as intermittent at the clinic visit. These cases were nevertheless permitted to continue participation in the study, as their overall clinical presentation was found to be on the borderline of mild persistent and intermittent asthma, yet they did not qualify for persistent asthma based on clinical guidelines. This group of participants with intermittent asthma did not have significantly different sleep disturbance scores (mean = 50.1, SD = 8.2) compared to those with persistent asthma (mean = 49.0, SD = 7.9). Seventy-nine percent of children classified with persistent asthma (n = 166) reported using a physician-prescribed asthma controller medication (any controller), and 70% reported using an inhaled corticosteroid. Forty-three percent of the sample had mean FEV1 % predicted < 80% during the monitoring period, indicating compromised lung function; this did not differ by ethnic group. Forty-one percent of children were classified as having poorly controlled asthma, as measured by the Asthma Control Test. A significantly larger proportion of Latinos (67%) had poorly controlled asthma, relative to NLWs (58%) and African Americans (45%), χ2 = 6.6, p < 0.05.
AR Clinical Characteristics
After participating in the study's clinic evaluation, 72% met the criteria for AR. Seventy-four percent had moderate/severe persistent AR, which according to clinical guidelines,8 necessitates the use of an intranasal steroid (Table 1). Of the child participants who met criteria for moderate/severe persistent AR, 15% were reported to be taking intranasal steroids. Only 34% percent of those diagnosed with AR were using the appropriate medications according to their severity level and clinical guidelines.8 Sixteen percent were reportedly taking oral first generation antihistamines, which have sedating properties.
Thirty-four percent of children with AR were classified according to the RCAT as poorly controlled; and there were ethnic differences in AR control, F2,51 = 3.4, p < 0.05, with NLW participants having higher scores on the RCAT or better AR control (mean = 27, SD = 3.1) relative to Latinos (mean = 23, SD = 5.0) and African Americans (mean = 24.8, SD = 4.2).
Table 2 contains the total CSHQ score and sleep hygiene scores for the entire sample and by caregiver ethnicity. Across the sample, 86% of children had sleep problem scores that were above the clinical cutoff, indicating significant sleep disturbances. Latinos and African Americans had marginally higher Sleep Problems scores relative to NLWs, F2,171 = 3.0, p = 0.05. Non-
Table 2.
Sleep problems and sleep hygiene.
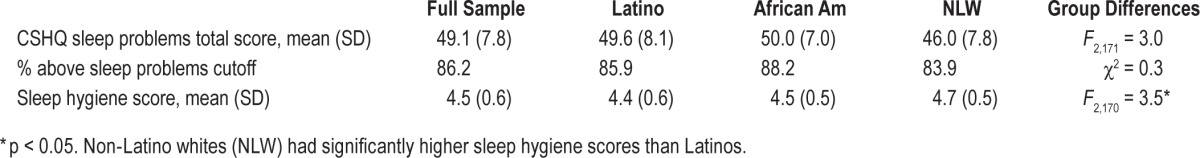
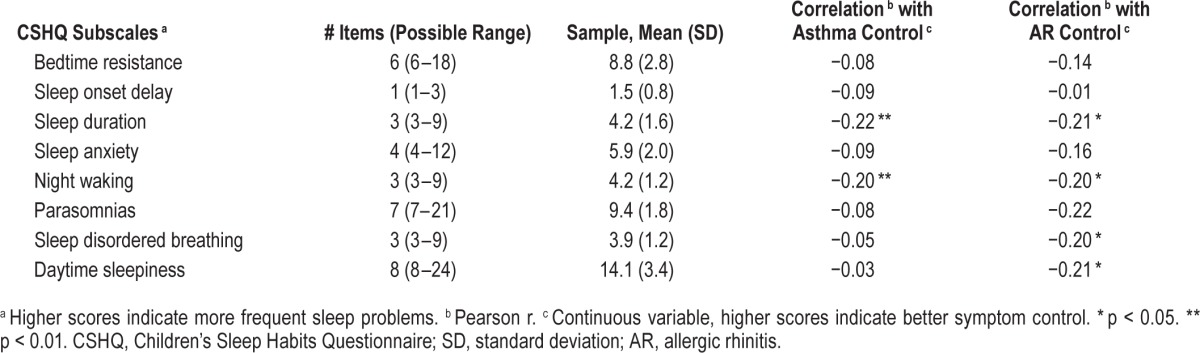
Latino whites had significantly higher hygiene scores than Latinos (African Americans were not different than the other groups), F2,170 = 3.5, p < 0.05 (Table 2). For descriptive purposes, CSHQ subscale scores are listed in Table 3, along with their correlations to Asthma and AR control. Means of the subscales in our sample are quite similar to those of a study of nocturnal asthma symptoms and poor sleep quality in urban school children that found significant associations between these subscales and nocturnal asthma symptoms.44 Sleep duration problems were associated with worse asthma control (r = −0.22, p < 0.01) and worse AR control (r = −0.21, p < 0.05). Similarly a higher frequency of night wakings were related to worse asthma (r = −20, p < 0.01) and AR (r = −0.20, p < 0.05) control. Frequency of sleep disordered breathing and daytime sleepiness was also associated with worse AR control (r's = −0.20 and −0.21, respectively, p's < 0.05).
Table 3.
Children's Sleep Habits Questionnaire subscale scores for the full sample.
Associations among Demographic Characteristics, Asthma, AR, and Sleep Problems Scores
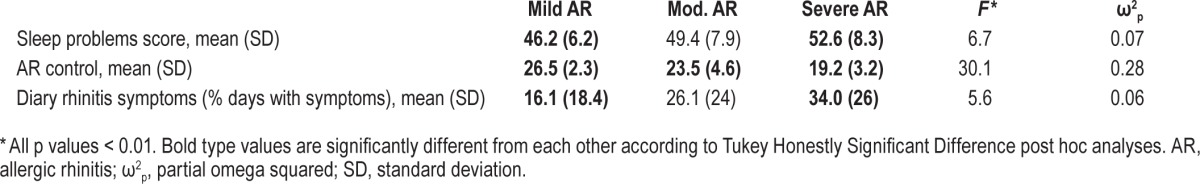
Preliminary analyses were conducted to examine associations among demographic and clinical variables (child age and gender, caregiver ethnicity and, number of people in the household, family's poverty status, asthma and AR severity), asthma indicators (FEV1 % predicted, asthma control, and diary-reported breathing problems), AR control, and the sleep problems total score. Demographic and clinical characteristics shown to be significantly correlated both with the predictor (asthma or AR control) and the outcome variable (sleep problems) were then held constant in subsequent regression models including those specific indicators. Total sleep problems scores differed by AR severity level (F2,161 = 6.7, p < 0.01; ω2p = 0.07). Post hoc analyses indicated sleep problems were higher in children with severe AR (mean = 52.6, SD = 8.3) than those with mild AR (mean = 46.2, SD = 6.2). AR control (F 2,145 = 30.1, p < 0.01; ω2p = 0.28) also differed by AR severity level (Table 4). Based on these associations, AR severity was controlled in regressions including AR control and sleep problems. No other demographic or clinical characteristics were identified as covariates.
Table 4.
Differences in sleep problems, AR control and rhinitis symptoms by AR severity in children who have asthma in the full sample.
Associations between Asthma Indicators and Sleep Problems
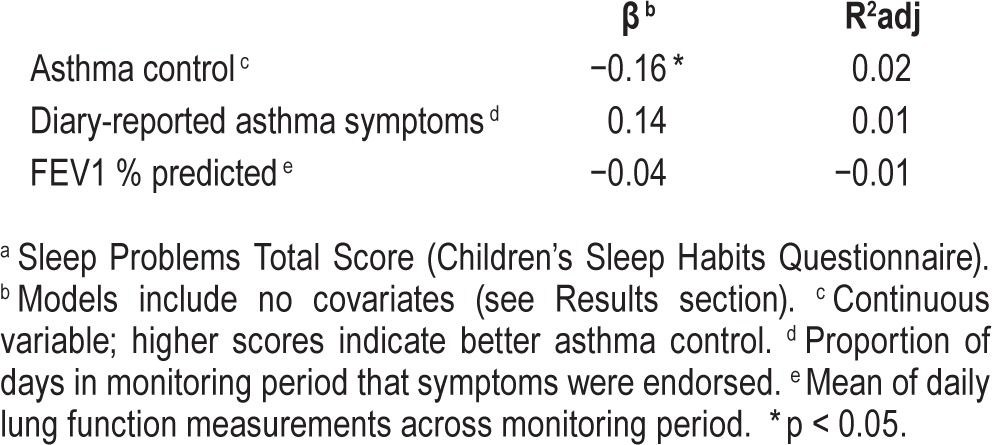
We first sought to examine the extent to which objectively measured lung function, diary-reported asthma symptoms, and asthma control were predictive of parent-reported sleep problems in children (Table 5). Across the entire sample, a significant negative association was found between asthma control and sleep problems (β = −0.16, p = 0.04; R2 adjusted = 0.02). Asthma control did not emerge as a significant predictor of sleep problems in analyses stratified by ethnicity. No statistically significant associations emerged between lung function or diary-reported days with asthma symptoms and sleep problems across the sample or in the ethnic subgroup analyses.
Table 5.
Associations between asthma indicators and sleep problems a (full sample).
Associations between AR Control and Sleep Problems in the Context of Asthma Symptoms
Next, a series of multiple regression analyses were conducted to assess the association between AR control and sleep problems, above and beyond each asthma indicator, and controlling for AR severity (Table 6).
Table 6.
Association between allergic rhinitis control and sleep problems a in the context of asthma.
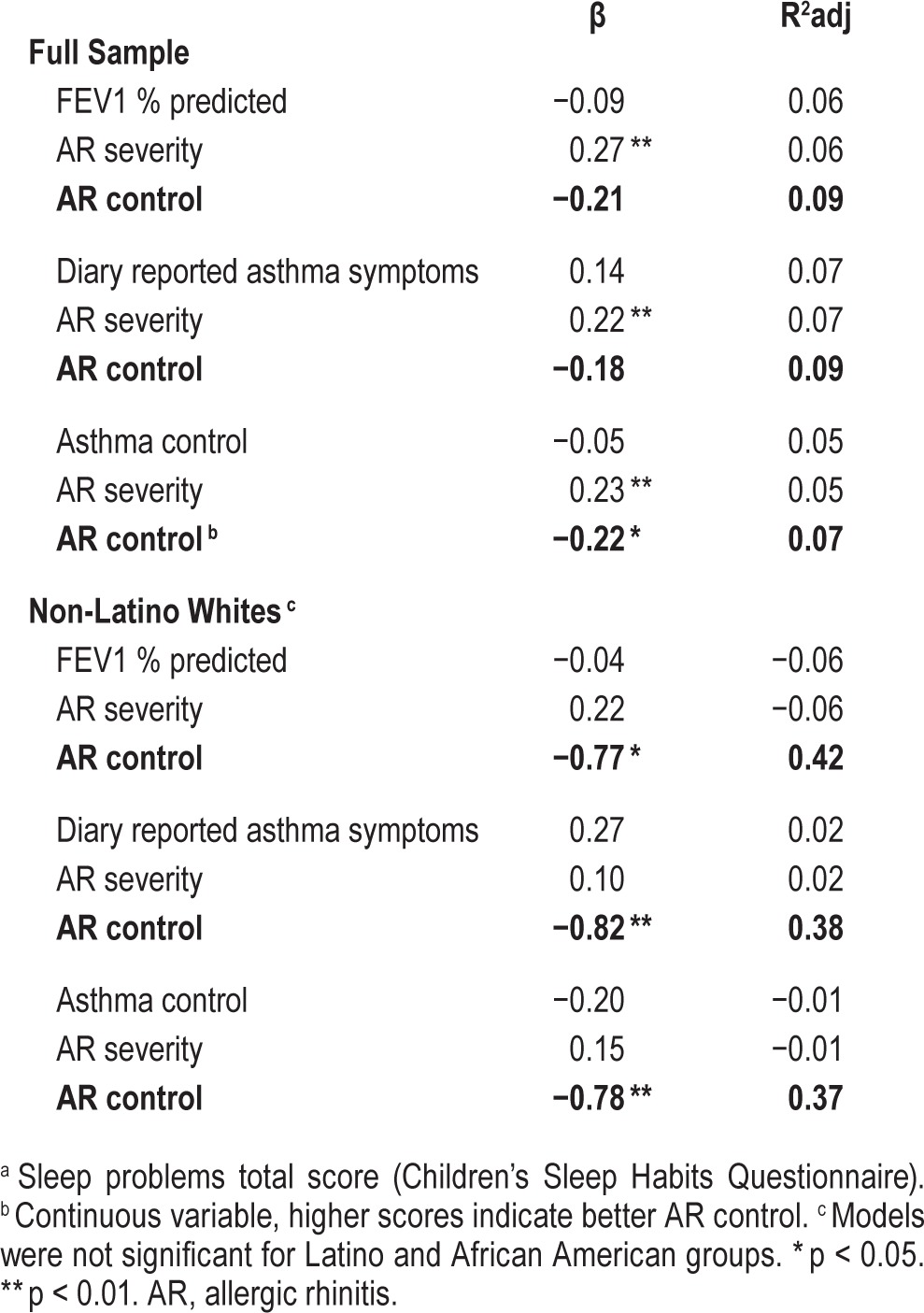
Across the entire sample, in the model including asthma control and controlling for AR severity, AR control was predictive of sleep problems (β = −0.22, p = 0.04; R2 adusted = 0.07). In models stratified by caregiver ethnicity and including FEV1 (controlling for AR severity), AR control was predictive of sleep problems in NLW children (β = −0.77, p < 0.01; R2 adjusted = 0.42). Similarly in NLWs, AR control was a significant predictor of sleep problems in models including diary reported asthma symptoms (β = −0.82, p < 0.01; R2 adjusted = 0.38) and asthma control (β = −0.78, p < 0.01; R2 adjusted = 0.37).
Moderation Analyses: Asthma, Sleep Hygiene, and Sleep Problems
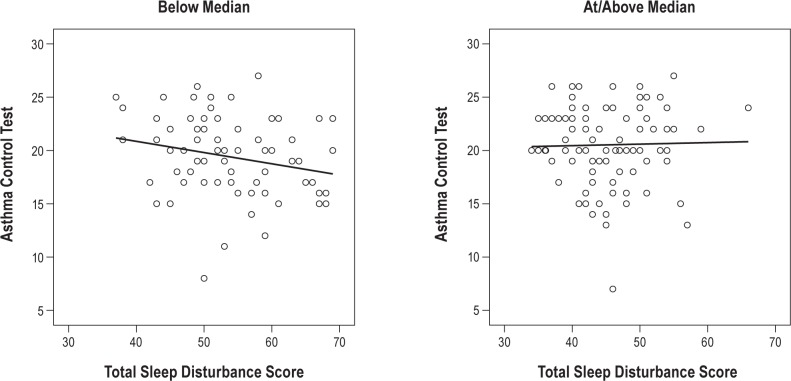
Results of regression analyses examining whether sleep hygiene moderated the association between asthma control and sleep problems indicated a significant main effect for sleep hygiene (β = −0.53, p < 0.001; R2 adjusted = 0.29) but not for asthma control (β = −0.13, p = 0.05). The inter -action term (asthma control × sleep hygiene) was significant (β = 0.16, p < 0.05; R2 adjusted = 0.31), indicating the association between asthma control and sleep problems depends on the child's level of sleep hygiene. To further explore the interaction, sleep hygiene was dichotomized by median split, and follow-up regression analyses were conducted examining asthma control and sleep problems at high and low levels of sleep hygiene (Figure 1). For participants in the lower sleep hygiene group, poorer asthma control was marginally related to higher levels of sleep problems (β = −0.23, p = 0.05; R2 adjusted = 0.04), whereas no relationship emerged in the group with higher sleep hygiene scores (β = 0.02, R2 adjusted = −0.01).
Figure 1. Asthma control and sleep disturbance scores by sleep hygiene (median split).
In models stratified by caregiver ethnicity, sleep hygiene significantly moderated the association between asthma control and sleep problems for children of African American caregivers. There was a significant main effect for sleep hygiene (β = −0.43, p < 0.01) but not for asthma control (β = −0.17), model R2 adjusted = 0.19. The interaction term was also significant (β = 0.38, p < 0.01, model R2 adjusted = 0.32), suggesting that within this subsample, the relationship between asthma control and sleep problems may depend, in part, to the level of sleep hygiene. For children of African American caregivers, simple regressions to probe the moderation did not yield statistically significant results for those below the sleep hygiene median (β = −0.18, R2 adjusted = 0.01), nor for those above (β = 0.22, R2 adjusted = 0.01), likely due to the small size of these subsamples.
Moderation Analyses: AR, Sleep Hygiene, and Sleep Problems
Analyses were repeated testing whether sleep hygiene moderated the association between AR control and sleep problems, controlling for AR severity. Significant main effects emerged both for AR control (β = −0.21, p < 0.05) and for sleep hygiene (β = −0.51, p < 0.01), model R2 adjusted = 0.33; however, the interaction term was not significant (β = 0.08), model R2 adjusted = 0.33. Analyses examining these associations by care-giver ethnicity yielded a similar pattern of results.
DISCUSSION
In our study of urban children with asthma, we found both a substantial burden of asthma and sleep problems among our sample. Nearly half of our sample (43%) had a mean FEV1 below 80% predicted across the monitoring period, and 41% were classified as having poorly controlled asthma for this same time period. Further, a high proportion of the children in our study (86%) scored above the clinical cutoff of 41 on the CSHQ, indicating pervasive sleep problems, according to caregiver report. The average total score for sleep problems in our sample was 49, which is consistent with the mean total CSHQ score (51) of the only other published report including urban children with asthma.12 While this previous study found increased asthma severity to be associated with an increase in total sleep disturbances, in our study, the average total sleep problems score remained consistent and above the clinical cutoff regardless of children's classification of asthma severity. Further, we found differences in total sleep problems scores by ethnic group, with Latinos and African American children in the sample having higher levels of sleep problems than their non-Latino white counterparts.
Our study is unique in that it focused on differences in the association between asthma and sleep problems by ethnic group, which can inform tailored sleep and asthma treatment approaches for specific groups of children at high risk for poor asthma and/or AR. We used a range of subjective (asthma control, asthma symptom diaries) and objective asthma assessments (FEV1 percent predicted by home spirometry) assessed twice daily, over a month-long monitoring period. The role of AR in potentially complicating sleep problems in urban children with asthma was also assessed. Interventions to address undertreated and undiagnosed AR may have relevance for sleep in urban children with persistent asthma, as uncontrolled AR may exacerbate asthma and contribute to increased night wakings.45 Asthma and AR diagnosis and severity was also assessed through state of the art methods (e.g., clinical evaluation, symptom reports, and spirometry).30 Finally, the role of sleep hygiene in potentially affecting the association between asthma or AR and sleep problems was also assessed. Sleep hygiene may be an important, modifiable target for intervention in this high-risk group.
Results from our study showed that poor asthma control may increase the risk for caregiver-reported sleep problems in our entire sample of urban children. The direction of the association between poor asthma control and more sleep problems was consistent with what we expected for the ethnic minority groups of children; however, the associations were not significant. The small sample sizes of these subgroups may have affected these results. It is important to note that poverty threshold was also not related to children's sleep disturbances. Further research is needed with larger samples of urban and ethnic minority children to understand which specific risk (e.g., urban and/or family level risks such as noise and family stress-ors) and protective processes (e.g., family level factors such as social support from extended kin, family connectedness) may moderate the association between asthma management and sleep problems. It is also possible that specific barriers to sleep quality, such as disruptions to sleep (e.g., electronics), may be more likely present in households with more resources. This bears further investigation in future research.
Interestingly, despite the range of indicators used in our assessment of asthma (asthma symptom diaries, measurement of lung function, asthma control), asthma control consistently appeared to have a bearing on children's sleep problems. In our future work, we plan to examine the day-to-day correspondence of asthma and sleep quality using objective measurements (home spirometry and actigraphy).
Results also show that AR control appeared to contribute to children's sleep problems in the entire sample, even when children's AR severity and asthma was taken into account. The association between AR control and sleep problems was stronger in children from non-Latino white backgrounds. Of note, NLW children of the sample had higher AR control scores than their Latino and AA counterparts. It may be that although AR control was poorer in the minority groups of this sample, other additional factors (e.g., related to family context) may contribute more so to sleep disturbances, although this needs to be further examined in larger samples in future work. These results point to the important role of untreated or undertreated AR in potentially exacerbating asthma and/or increasing sleep problems in urban children with asthma.
Finally, results suggest that the extent to which asthma may be associated with sleep problems may depend, in part, to children's sleep hygiene. Poorer sleep hygiene seemed to increase the magnitude of the association between poor asthma and a higher level of sleep problems in the entire sample. As we expected, this association was more robust in children from African American backgrounds. This highlights the importance of interventions that address sleep hygiene and sleep quality using a more integrated approach to consider both asthma control and urban family's home settings. However, more research is needed to clarify which components of sleep hygiene and the sleep environment, and which sleep behaviors may increase nocturnal symptoms in children from specific groups.
This study is limited by the small sample size, the disproportionate number of families per ethnic group, and the use of a parent self-report measure to assess sleep problems over one, two-week time period. Our future work will examine sleep efficiency via actigraphy in a larger sample of urban children with asthma. This approach may help shed light on the day-today correspondence between asthma and AR symptoms and sleep. There are also a host of other processes across multiple levels (biological, environmental, family/cultural) that may affect both asthma and sleep behaviors, which were not assessed by this study. Future work should assess factors that can increase risk for nocturnal asthma (e.g., environmental triggers in the child's bedroom, rescue medication use and availability, medication adherence), beliefs about sleep behaviors, and aspects of the sleep environment (sleep disruptions). With respect to family and cultural related factors, given it has been found that immigration-related experiences (e.g., higher levels of acculturative stress, time spent on Mainland US, comfort with navigating the asthma health care system)46 may affect asthma morbidity in subgroups of Latino children (e.g., Puerto Ricans), it is also important to examine whether the associations among asthma, sleep hygiene, and sleep problems are moderated by specific family and cultural-related experiences.
Urban children with asthma are also at higher risk for specific comorbid conditions relevant to sleep quality, such as sleep disordered breathing (SDB). In our sample, 46% of the child participants were at risk for sleep disordered breathing by caregiver report.33 Future research should focus on the extent to which sleep problems are more prevalent in urban minority children with SDB, to clarify pathways of influence in children with asthma and AR. Although many of our children were taking an asthma daily controller medication, it was beyond the scope of this study to assess children's adherence to these medications in an objective manner. We also recognize that some first-generation medications for AR may cause fatigue; 16% of participants in our sample were taking these medications, by caregiver report. Future research needs to track adherence to both daily and as needed asthma and AR medications and their potential side effects, to examine the role of medication adherence on sleep quality in this group.
Although our results suggest that AR symptoms may contribute to sleep disturbances in our sample, it is important to consider the difficulty children and caregivers may have differentiating between upper and lower airway symptoms. As highlighted by the one-airway theory, asthma and AR can be manifestations of an atopic syndrome.7 It is possible that lower ventilatory effort may influence upper airway flow, an effect that may be challenging to account for within this study, despite our best efforts to develop a protocol which teaches and trains children to focus on either their asthma or AR symptoms.
Results from our study suggest that proper assessment of nocturnal asthma and education regarding the consequence of poorly controlled asthma on sleep should be highlighted in discussions that health care providers have with caregivers of children with asthma. Multidisciplinary interventions that integrate education and behavioral strategies about optimal co-management of asthma and AR and basic sleep hygiene need to be developed and tailored for urban families, to enhance both asthma and sleep outcomes.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (R01 HD057220 to D.K.M). The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention, National Center for Health Statistics. United States: Asthma Prevalence, Health Care Use and Mortality; 2007. Asthma Prevalence, Health Care Use and Mortality: United States National Center for Health Statistics. [Google Scholar]
- 2.Clark NM, Brown R, Joseph CL, et al. Issues in identifying asthma and estimating prevalence in an urban school population. J Clin Epidemiol. 2002;55:870–81. doi: 10.1016/s0895-4356(02)00451-1. [DOI] [PubMed] [Google Scholar]
- 3.Lara M, Akinbami L, Flores G, Morgenstern H. Heterogeneity of childhood asthma among Hispanic children: Puerto Rican children bear a disproportionate burden. Pediatrics. 2006;117:43–53. doi: 10.1542/peds.2004-1714. [DOI] [PubMed] [Google Scholar]
- 4.Kattan M, Mitchell H, Eggleston P, et al. Characteristics of inner-city children with asthma: the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997;24:253–62. doi: 10.1002/(sici)1099-0496(199710)24:4<253::aid-ppul4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Koinis-Mitchell D, McQuaid EL, Seifer R, et al. Multiple urban and asthma-related risks and their association with asthma morbidity in children. J Pediatr Psychol. 2007;32:582–95. doi: 10.1093/jpepsy/jsl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McQuaid EL, Everhart RS, Seifer R, et al. Medication adherence among Latino and non-Latino white children with asthma. Pediatrics. 2012;129:e1404–10. doi: 10.1542/peds.2011-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grossman J. One airway, one disease. Chest. 1997;111:11–6. doi: 10.1378/chest.111.2_supplement.11s. [DOI] [PubMed] [Google Scholar]
- 8.Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 9.de Groot EP, Nijkamp A, Duiverman EJ, Brand PL. Allergic rhinitis is associated with poor asthma control in children with asthma. Thorax. 2012;67:582–7. doi: 10.1136/thoraxjnl-2011-201168. [DOI] [PubMed] [Google Scholar]
- 10.Daniel LC, Boergers J, Kopel SJ, Koinis-Mitchell D. Missed sleep and asthma morbidity in urban children. Ann Allergy Asthma Immunol. 2012;109:41–6. doi: 10.1016/j.anai.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stores G, Ellis AJ, Wiggs L, Crawford C, Thomson T. Sleep and psychological disturbance in nocturnal asthma. Arch Dis Child. 1998;78:413–9. doi: 10.1136/adc.78.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fagnano M, Bayer AL, Isensee CA, Hernandez T, Halterman JS. Nocturnal asthma symptoms and poor sleep quality among urban school children with asthma. Acad Pediatr. 2011;11:493–9. doi: 10.1016/j.acap.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calhoun SL, Vgontzas AN, Fernandez-Mendoza J, et al. Prevalence and risk factors of excessive daytime sleepiness in a community sample of young children: the role of obesity, asthma, anxiety/depression, and sleep. Sleep. 2011;34:503–7. doi: 10.1093/sleep/34.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strunk RC, Sternberg AL, Bacharier LB, Szefler SJ. Nocturnal awakening caused by asthma in children with mild to moderate asthma in the childhood asthma management program. J Allergy Clin Immunol. 2002;110:395–403. doi: 10.1067/mai.2002.127433. [DOI] [PubMed] [Google Scholar]
- 15.Koinis-Mitchell D, Craig T, Esteban CA, Klein RB. Sleep and allergic disease: a summary of the literature and future directions for research. J Allergy Clin Immunol. 2012;130:1275–81. doi: 10.1016/j.jaci.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross KR, Storfer-Isser A, Hart MA, et al. Sleep-disordered breathing is associated with asthma severity in children. J Pediatr. 2012;160:736–42. doi: 10.1016/j.jpeds.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramagopal M, Mehta A, Roberts DW, et al. Asthma as a predictor of obstructive sleep apnea in urban African-American children. J Asthma. 2009;46:895–9. doi: 10.3109/02770900903229636. [DOI] [PubMed] [Google Scholar]
- 18.Peroni D, Piacentini G, Alfonsi L, et al. Rhinitis in pre-school children prevalence, association with allergic diseases and risk factors. Clin Exp Allergy. 2003;33:1349–54. doi: 10.1046/j.1365-2222.2003.01766.x. [DOI] [PubMed] [Google Scholar]
- 19.Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–51. [PubMed] [Google Scholar]
- 20.Sheares BJ, Kattan M, Leu CS, Lamm CI, Dorsey KB, Evans D. Sleep problems in urban, minority, early-school-aged children more prevalent than previously recognized. Clin Pediatr (Phila) 2013;52:302–9. doi: 10.1177/0009922813476573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boergers J, Koinis-Mitchell D. Sleep and culture in children with medical conditions. J Pediatr Psychol. 2010;35:915–26. doi: 10.1093/jpepsy/jsq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloom BJ, Owens JA, McGuinn M, Nobile C, Schaeffer L, Alario AJ. Sleep and its relationship to pain, dysfunction, and disease activity in juvenile rheumatoid arthritis. J Rheumatol. 2002;29:169–73. [PubMed] [Google Scholar]
- 23.Daniel LC, Grant M, Kothare SV, Dampier C, Barakat LP. Sleep patterns in pediatric sickle cell disease. Pediatr Blood Cancer. 2010;55:501–7. doi: 10.1002/pbc.22564. [DOI] [PubMed] [Google Scholar]
- 24.Long AC, Krishnamurthy V, Palermo TM. Sleep disturbances in school-age children with chronic pain. J Pediatr Psychol. 2008;33:258–68. doi: 10.1093/jpepsy/jsm129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boergers J, Koinis-Mitchell D. Sleep and culture in children with medical conditions. J Pediatr Psychol. 2010;35:915–26. doi: 10.1093/jpepsy/jsq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owens JA, Jones C, Nash R. Caregivers' knowledge, behavior, and attitudes regarding healthy sleep in young children. J Clin Sleep Med. 2011;15:345–50. doi: 10.5664/JCSM.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan E, Healey D, Gray AR, Galland BC. Sleep hygiene intervention for youth aged 10 to 18 years with problematic sleep: a before-after pilot study. BMC Paediatr. 2012;12:189. doi: 10.1186/1471-2431-12-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kattan M, Gergen PJ, Eggleston P, Visness CM, Mitchell HE. Health effects of indoor nitrogen dioxide and passive smoking on urban asthmatic children. J Allergy Clin Immunol. 2007;120:618–24. doi: 10.1016/j.jaci.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Liu AH, Zeiger R, Sorkness CA, et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007;119:817–25. doi: 10.1016/j.jaci.2006.12.662. [DOI] [PubMed] [Google Scholar]
- 30.National Heart lung and Blood Institute. Guidelines for the Diagnosis and Management of Asthma. National Institutes of Health; 2007. [Google Scholar]
- 31.Canino G, Bravo M. The adaptation and testing of diagnostic and outcome measures for cross-cultural research. Int Rev Psychiatry. 1994;6:281–6. [Google Scholar]
- 32.U.S. Department of Health and Human Services. The 2005 HHS Poverty Guidelines. U.S. Department of Health and Human Services; 2005. [Google Scholar]
- 33.Redline S, Tichler PV, Hans MG, Tosteson TD, Strohl KP. Risk factors for sleep-disordered breathing in children. Am J Respir Crit Care Med. 1997;155:186–92. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 34.American Thoracic Society, European Thoracic Society. ATS/ERS recommendations for standardized procedures for online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 35.Bender B, Milgrom H. Comparison of the effects of fluticasone propionate aqueous nasal spary and loratadine on daytime altertness and performance in children with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2004;92:344–9. doi: 10.1016/S1081-1206(10)61573-6. [DOI] [PubMed] [Google Scholar]
- 36.Van Hoecke H, Vastesaeger N, Dewulf L, De Bacquer D, Van Cauwenberge P. Is the allergic rhinitis and its impact on asthma classification useful in daily primary care practice? J Allergy Clin Immunol. 2006;118:758–9. doi: 10.1016/j.jaci.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Koinis-Mitchell D, McQuaid EL, Kopel S, et al. Symptom perception in children with asthma: cognitive and psychological factors. Health Psychol. 2009;28:226–37. doi: 10.1037/a0013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Okupa AY, Sorkness CA, Mauger DT, Jackson DJ, Lemanske RF. Daily diaries vs retrospective questionnaires to assess asthma control and therapeutic responses in asthma clinical trials. Chest. 2013;143:993–9. doi: 10.1378/chest.12-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schatz M, Meltzer EO, Nathan R, et al. Psychometric validation of the rhinitis control assessment test: a brief patient-completed instrument for evaluating rhinitis symptom control. Ann Allergy Asthma Immunol. 2010;104:118–24. doi: 10.1016/j.anai.2009.11.063. [DOI] [PubMed] [Google Scholar]
- 41.Harsh JR, Easley A, LeBourgeois MK. A measure of children's sleep hygiene. Sleep. 2002:25. [Google Scholar]
- 42.Whisman MA, McClelland GH. Designing, testing, and interpreting interactions and moderator effects in family research. J Fam Psychol. 2005;19:111–20. doi: 10.1037/0893-3200.19.1.111. [DOI] [PubMed] [Google Scholar]
- 43.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 44.Fagnano M, Bayer AL, Isensee CA, Hernandez T, Halterman JS. Nocturnal asthma symptoms and poor sleep quality among urban school children with asthma. Acad Pediatr. 2011;11:493–9. doi: 10.1016/j.acap.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koinis-Mitchell D, Esteban C, Kopel S, Jandasek B, Dansereau K, Klein R. Perceptual accuracy of upper airway compromise in children: clinical relevance and future directions for research. Allergy Rhinol. 2013;4:e54–62. doi: 10.2500/ar.2013.4.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koinis-Mitchell D, Sato A, Kopel S, et al. Immigration and acculturation-related factors and asthma morbidity in Latino children. J Pediatr Psychol. 2011;36:1130–43. doi: 10.1093/jpepsy/jsr041. [DOI] [PMC free article] [PubMed] [Google Scholar]