Abstract
Study Objectives:
The prevalence of sleep disturbances and heart failure increases with age. We aimed to evaluate the associations of incident heart failure and cardiac dysfunction with changes in sleep quality.
Methods:
This prospective population-based study was conducted in the Rotterdam Study. Of the 3,445 eligible persons (mean age 72.0 ± 7.1 years) available for cross-sectional analyses, 8.9% (n = 307) had prevalent clinical heart failure. In longitudinal analyses, 1,989 eligible persons (mean age 70.0 ± 5.8 years) were followed for an average of 6.5 ± 0.4 years, of which 4.6% (n = 91) had prevalent or incident clinical heart failure. Heart failure was assessed according to European Society of Cardiology criteria. To estimate cardiac function, we measured left ventricular fractional shortening, left ventricular systolic function, and E/A ratio by echocardiography. Heart failure and cardiac dysfunction were studied with linear regression in relation to sleep quality, assessed by the Pittsburgh Sleep Quality Index.
Results:
No associations between clinical heart failure and sleep quality were observed in cross-sectional analyses. Clinical heart failure predicted a reduction of sleep quality (B = 1.00 points on the Pittsburgh Sleep Quality Index; 95% CI 0.40, 1.60) in longitudinal assessment. This association was driven by the sleep onset latency and sleep quality components of the Pittsburgh Sleep Quality Index. Cardiac dysfunction was not related to sleep quality in cross-sectional or longitudinal analyses.
Conclusions:
Clinical heart failure, but not cardiac dysfunction measured by echocardiography, increases the risk of poor sleep quality in the general population over time. These findings suggest that clinical manifestations of heart failure negatively affect sleep.
Citation:
Zuurbier LA, Luik AI, Leening MJ, Hofman A, Freak-Poli R, Franco OH, Stricker BH, Tiemeier H. Associations of heart failure with sleep quality: the Rotterdam Study. J Clin Sleep Med 2015;11(2):117–121.
Keywords: sleep quality, heart failure, echocardiography, population-based, epidemiology
Sleep disturbances have been reported in around 60% of patients with heart failure (HF).1,2 Despite improved treatment and an overall decline in mortality after a cardiovascular event, the number of HF patients is increasing among the elderly.3 This is probably due to an aging population and to the improved chances of survival for HF patients.4 Nevertheless, only 25% to 35% of patients with clinical HF survive up to 5 years after diagnosis, and HF poses a great burden in terms of treatment, hospitalization and quality of life.5–7 HF is related to multiple physical and mental problems, including shortness of breath, depressive symptoms, cognitive impairment, and sleep problems.8,9 Little is known about the changes in sleep occurring in these patients over time.
Previous studies investigating HF and sleep have been mostly cross-sectional and cannot establish a temporal relation. Prospective sleep studies have demonstrated that short and long sleep durations increase the risk of cardiovascular disease.10–13 Furthermore, difficulty maintaining and initiating sleep are associated with incident HF.14 However, there is evidence for a bi-directional relation between sleep and heart disease. For example, poor sleep quality and difficulty maintaining and initiating sleep are consequences of cardiovascular disease.15 How sleep quality changes over time in patients with cardiac dysfunction, prevalent HF or new-onset HF is unclear.
BRIEF SUMMARY
Current Knowledge/Study Rationale: With increasing age, the prevalence of heart failure and sleep disturbances increases. How sleep quality changes over time in patients with cardiac dysfunction, prevalent or new-onset heart failure is unclear.
Study Impact: Clinical heart failure, but not echocardiographic indicators of cardiac dysfunction, increases the risk of poor sleep quality in the general population. In this population-based study no cross-sectional associations were observed. These findings suggest that clinical manifestations of heart failure negatively affect sleep.
We examined whether prevalent and incident HF and echocardiographic indicators of cardiac dysfunction are associated with sleep quality in a community-dwelling population using both cross-sectional and longitudinal designs. Also, the associations between HF and the separate component scores of the Pittsburgh Sleep Quality Index (PSQI) were analyzed.
METHODS
Participants
This study is part of the Rotterdam Study, a prospective population-based cohort of the general population in Rotterdam, The Netherlands.16 The study conforms to the principles outlined in the Declaration of Helsinki, and was approved by the Medical Ethics Committee according to the Wet Bevolkingsonderzoek ERGO (Population Study Act Rotterdam Study), executed by the Ministry of Health, Welfare and Sports of The Netherlands. All participants provided written informed consent.
Between 2002 and 2005, 3,711 participants aged ≥ 55 years completed the PSQI and underwent echocardiography. Of these, 266 participants were excluded because of (1) diagnosis of HF that did not fulfill the criteria of the European Society of Cardiology; (2) poor cognitive function (Mini-Mental State Examination score ≤ 2317); or (3) < 6 valid PSQI component scores. This left 3,445 participants for cross-sectional analyses. Five hundred forty-one participants died during follow-up. Between 2009 and 2012, 2,105 participants completed the PSQI again. Of these, 116 participants were excluded because of (1) poor cognitive function at follow-up or (2) < 6 valid PSQI component scores at follow-up, leaving 1,989 participants for the longitudinal analyses.
Clinical Heart Failure
Assessment of HF in the Rotterdam Study has been described previously.18 In brief, HF was determined in accordance with the guidelines of the European Society of Cardiology, requiring objective evidence of cardiac dysfunction, together with typical symptoms of heart failure such as breathlessness, ankle swelling, pulmonary crepitation, or use of cardiovascular medication for HF.19 In this study, HF was defined as prevalent if the date of diagnosis was before the date of the PSQI baseline measure. Incident HF was diagnosed if it occurred between the baseline and repeated PSQI assessment. Information on incident HF cases was obtained by digital linkage with medical records of general practitioners, which allowed continuous monitoring. Only definite and probable HF diagnoses were included in the analyses.20
Echocardiography
A resting echocardiogram was obtained from each participant at baseline to assess left ventricular systolic function (fractional shortening and visual assessment [normal, fair, moderate, or poor]) and diastolic function (E/A ratio [ < 0.75, 0.75–1.50, > 1.50]) according to a standardized protocol.21 Fractional shortening was computed as (left ventricular end diastolic dimension – left ventricular end systolic dimension) divided by left ventricular end diastolic dimension*100%. E/A ratio was calculated as Doppler peak E filling velocity divided by Doppler peak A filling velocity. The echocardiograms were made with one of two commercially available systems. In the analyses, moderate and poor left ventricular systolic function were combined because of the low number of participants with poor function.
Sleep Quality
Sleep quality was measured with the PSQI, a self-rated 19-item questionnaire.22 The global PSQI score comprises of sleep onset latency, sleep efficiency, sleep quality, sleep disturbances, sleep duration, daytime dysfunction, and use of sleeping medication in the past month (range 0–21). Higher scores represent a poorer sleep quality. Participants with < 6 valid PSQI component scores were excluded. The global PSQI score was calculated as the sum of the component scores. If a participant had only 6 valid PSQI component scores, we calculated the global PSQI score, but weighted the summed score by multiplying with 7/6 (7*the sum of the 6 valid component scores)/6). The PSQI has a good test-retest reliability and validity.22
Statistical Analyses
Clinical HF, fractional shortening, left ventricular systolic function, and E/A ratio were studied as determinants of sleep quality. Clinical HF was continuously monitored, the echocardiographic indicators of cardiac function and confounders were measured at baseline, and sleep quality was measured at baseline and at follow-up. We performed cross-sectional and longitudinal analyses to examine whether clinical HF and echocardiographic indicators of cardiac dysfunction are associated with sleep quality. To assess sleep quality changes in the longitudinal analyses, we adjusted for baseline sleep quality. Incident HF was also studied separately from prevalent HF. Additionally, the associations between HF and the separate component scores of the PSQI were analyzed longitudinally.
We adjusted the linear regressions for age and gender. Additional adjustments were made for education (low, intermediate, and high), possible sleep apnea, depressive symptoms (Center for Epidemiologic Studies-Depression scale23), diabetes mellitus, cognitive function (Mini-Mental State Examination score), diuretic use, and the echocardiographic system used, as these variables changed effect estimates (> 5%) or were a priori confounders. Possible sleep apnea was based on 2 questions of the PSQI. Apnea was considered possible when participants reported that they snored loudly ≥ 2 nights per week and if they reported occasional respiratory pauses, or if they reported respiratory pauses ≥ 1–2 nights per week.24 Diuretic use (ATC code C03) was assessed with pharmacy records. Participants without a diuretic prescription were considered non-users, participants with a prescription up to the defined daily dose were considered low users, and participants with a prescription higher than the defined daily dose were considered high users. Systolic and diastolic blood pressure, smoking, alcohol intake, and body mass index were not entered as covariates, as they did not change effect estimates.
Missing values were handled by multiple imputation using 5 imputations. The maximum amount of missing data was for fractional shortening (4.9 %). A p value < 0.05 was considered statistically significant. Analyses were performed in SPSS version 20 (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY).
A validation of PSQI measures was performed within 2 months in a random subsample of 173 participants with actigraphy and sleep diary measures conducted over the course of one week. The global PSQI score was correlated to sleep quality measured with three questions of the sleep diary (r = −0.41, p < 0.001), sleep duration correlated well with actigraphic assessment of average sleep duration (r = 0.33, p < 0.001).
RESULTS
Sample Characteristics
The cross-sectional sample consisted of 3,445 eligible participants (mean age 72.0 ± 7.1 years; 43.2% male), of which 307 (8.9%) participants had prevalent HF at baseline (baseline characteristics can be found in Table S1, supplemental material). The sample available for longitudinal analyses consisted of 1,989 participants (mean age 70.0 ± 5.8 years; 41.9% male), of which 40 (2.0%) participants had prevalent HF at baseline and 51 (2.6%) had incident HF during follow-up (mean follow-up 6.5 ± 0.4 years). Therefore a total of 91 participants with HF were studied (baseline characteristics for the 1,989 participants in the longitudinal analyses can be found in Table 1).
Table 1.
Baseline characteristics (n = 1,989).
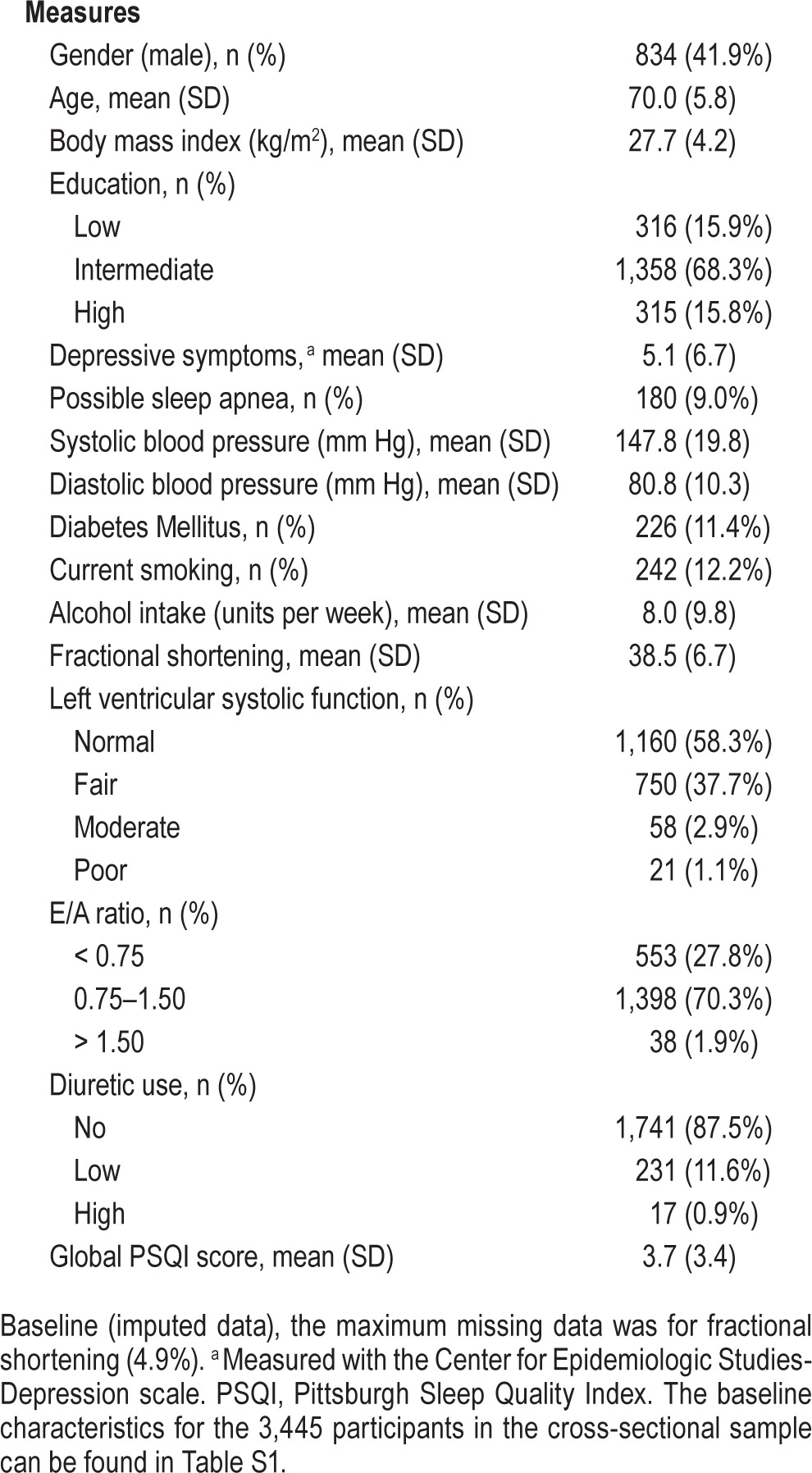
Linear Regression
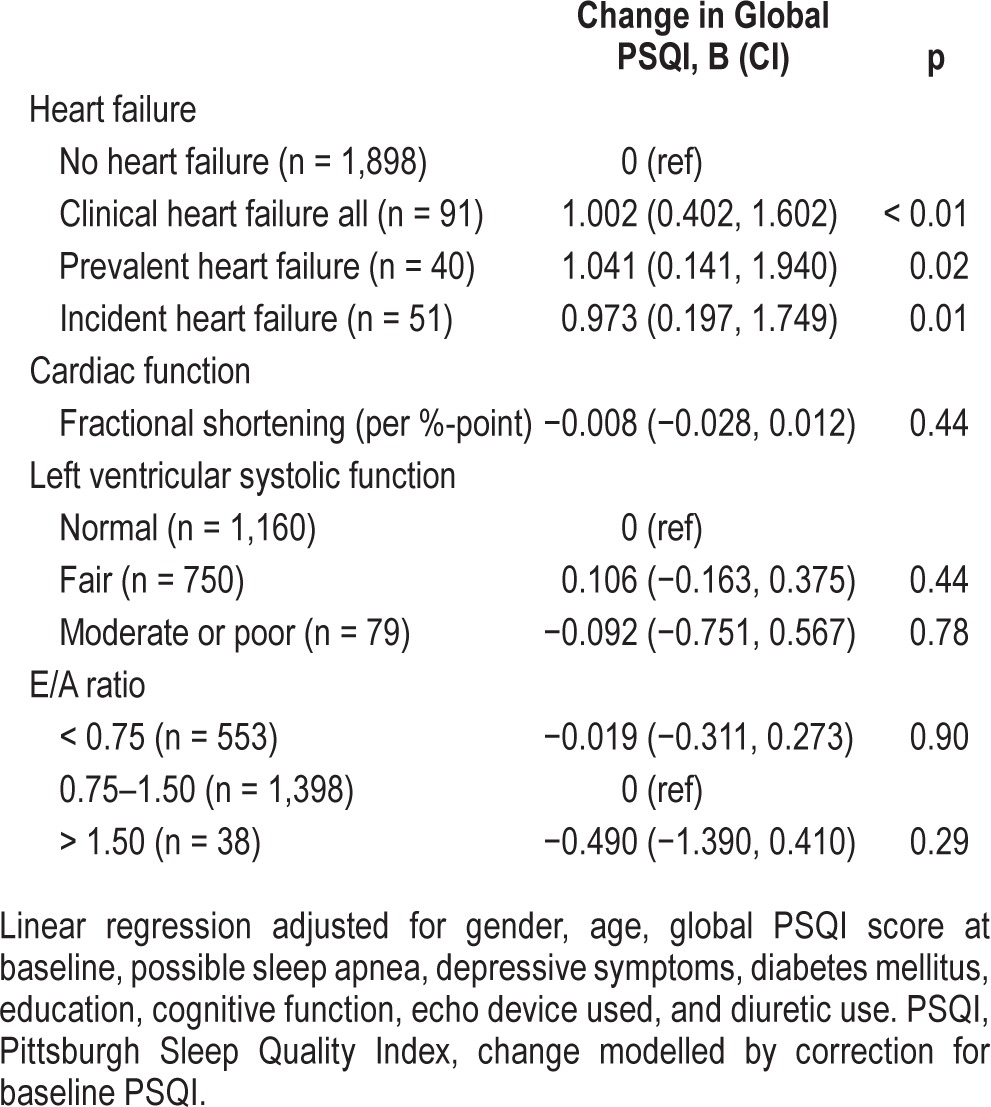
No associations between clinical HF or cardiac dysfunction and sleep quality were found cross-sectionally (Table 2). The cross-sectional analyses only including the 1,989 participants from the longitudinal analyses showed similar results (data available upon request). In Table 3, we only present the fully adjusted model, as the age- and gender-adjusted model had similar results. In longitudinal analyses, HF was related to a poorer sleep quality, i.e., an increase in global PSQI score between baseline and follow-up (B = 1.00 points on the PSQI; 95% CI 0.40, 1.60; p < 0.01; Table 3). This association was found in both prevalent (B = 1.04 points on the PSQI; 95% CI 0.14, 1.94; p = 0.02) and incident (B = 0.97 points on the PSQI; 95% CI 0.20, 1.75; p = 0.01) HF cases. None of the echocardiographic cardiac parameters were associated with changes in sleep quality.
Table 2.
The cross-sectional association of heart failure and echocardiographic parameters with sleep quality (n = 3,445).
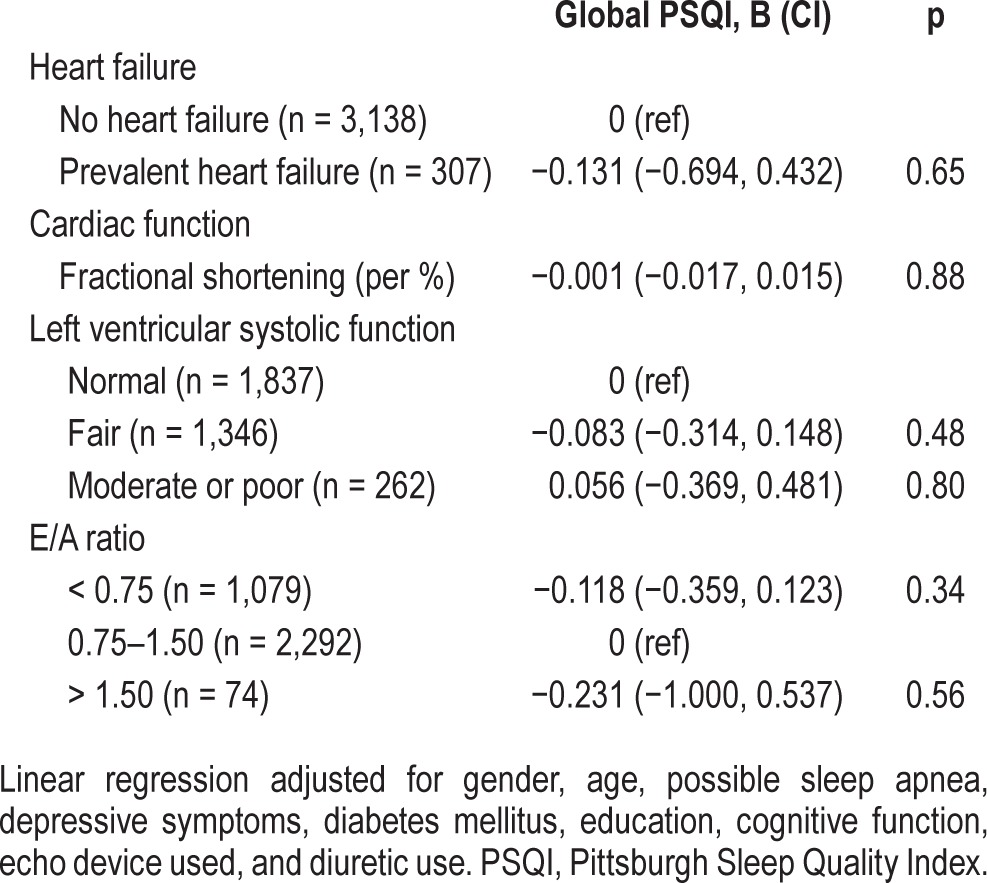
Table 3.
The longitudinal associations of heart failure and echocardiographic parameters with sleep quality (n = 1,989).
We analyzed the components of the global PSQI score separately with logistic regression. This enabled us to test how clinical HF affects the change of these aspects of sleep. HF was associated with changes in sleep quality (OR = 2.63; 95% CI 1.41, 4.93) and sleep onset latency (OR = 2.03; 95% CI 1.11, 3.72; component scores 0, 1 versus 2, 3; fully adjusted), but was not associated with changes in sleep duration, sleep efficiency, sleep disturbances, daytime dysfunction, or sleep medication (Table 4).
Table 4.
The longitudinal association of heart failure with the component scores of the Pittsburgh Sleep Quality Index (n = 1,989).

To disentangle the effect of sleep medication on the association between HF and change in global PSQI score, we omitted the sleep medication component from the global PSQI score (at baseline and at follow-up) and added sleep medication use at baseline as extra covariate in the analysis. The association between HF and global PSQI score was only modestly attenuated (B = 0.86; 95% CI 0.31, 1.40).
DISCUSSION
Clinical HF increased the risk of sleep problems in this sample of middle-aged and elderly persons. In our longitudinal analyses, we observed that sleep quality was reduced in participants with either prevalent or incident HF at follow-up. This relation could not be demonstrated with echocardiographic indicators of cardiac dysfunction. Therefore, findings suggest that it is clinical manifestations of HF that specifically affect sleep negatively.
Several studies have related poor sleep quality to prevalence of cardiovascular disease; however, these studies have not reported repeated sleep assessments.2,11,12,25 Therefore, any effect of cardiovascular disease upon sleep must be inferred from case-control studies, which have been undertaken in a clinical setting. Only one of the studies focused specifically on HF and observed that HF patients had a lower sleep quality than controls.2 In our cross-sectional analyses, we did not observe an association between HF and sleep. In clinical studies HF is probably more severe than in our study, which might explain the absence of the association in our cross-sectional analyses. For our longitudinal analyses we assessed sleep quality repeatedly. Our study suggests that clinical HF might lead to sleep problems. The reason why the association between HF and sleep quality was observed in longitudinal analyses, but not in cross-sectional analyses might be due to two reasons. First, in the longitudinal analyses, new-onset HF cases are included. Patients with new-onset HF probably experience the most substantial decline in sleep quality. Second, in the longitudinal analyses we tested the individual change in global PSQI score, while in cross-sectional analyses, the absolute level of this score was tested. Participants who develop HF might show a decline in global PSQI score compared to participants who do not develop HF, while their baseline global PSQI scores might not differ.
There are several explanations for the observed association between clinical HF and sleep quality. HF symptoms such as restless legs, orthopnea, and nocturia due to redistribution of extravascular fluid in supine position, can cause disturbances in sleep and changes in the sleep-wake pattern. Another explanation is that HF and sleep problems share common etiological mechanisms. For example, vascular pathologies could independently explain HF and sleep problems.26,27 Furthermore, sleep-disordered breathing is highly prevalent in people with HF.28 We adjusted for possible sleep apnea to take this into account. In our study, possible sleep apnea was assessed by two questions from the PSQI. However, this is not a formal diagnosis of sleep apnea. We cannot rule out that if sleep apnea was measured by polysomnography, results could differ. It would be optimal to replicate results with sleep apnea assessed using polysomnography.
Only 25% to 35% of people with HF survive 5 years after first diagnosis.5,7 In our study, the participants with prevalent HF completed the second PSQI assessment in the longitudinal analyses 10.2 ± 2.8 years after onset of HF. The participants with recent-onset HF completed the PSQI assessment 3.5 ± 1.8 years after onset of HF. Participants with HF in population-based studies are thus more likely to be long-term survivors with less severe HF, and this can lead to an underestimation of the impact of HF on sleep changes. However, in this study, the effect of incident HF upon sleep was only slightly different than the effect of prevalent HF. We could not find any effect of cardiac dysfunction measured with echocardiography, and sleep problems. This suggests that HF symptoms are on the causal pathway between cardiac dysfunction and sleep disturbances.
The major strengths of this study are the prospective data collection and the use of general practitioners' records to assess HF. HF was recorded early in the disease process, often before the patient was in specialist care and, hence, the chance of reverse causality was low. This study also has some limitations. First, healthy participants were more likely to complete the follow-up PSQI, as observed by the reduction in prevalent HF. Consequently, the longitudinal analyses included more relatively healthy older adults and could be less generalizable. Second, the number of participants with HF was relatively small. We had sufficient power to show consistent longitudinal effects, but including more participants with HF might strengthen the findings and would have enabled us to evaluate specific subgroups. Third, information on sleep disorders such as sleep apnea was limited. In our study, possible sleep apnea was assessed with the PSQI. However, this is not a formal diagnosis of sleep apnea. We cannot rule out that formal diagnoses of sleep apnea could change the results.
To conclude, clinical HF, but not cardiac dysfunction as measured by echocardiography, increases the risk of poor sleep quality in the general population over time. Moreover, no cross-sectional associations were observed in this population-based study. These findings suggest that clinical manifestations of HF negatively affect sleep.
DISCLOSURE STATEMENT
This work was supported by grants from the Netherlands Organization for Scientific Research [NWO-VIDI 017.106.370 to Dr.Tiemeier] and the Netherlands Organization for Health Research and Development [ZonMw 80.82500.98.10208 to Dr. Stricker). The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, The Netherlands; Organization for the Health Research and Development (ZonMw); the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam. Dr. Freak-Poli is supported by a NHMRC ECR Fellowship (1053666). The authors have indicated no financial conflicts of interest. This study was conducted at Erasmus Medical Center, Rotterdam, The Netherlands.
ACKNOWLEDGMENTS
The authors thank the participants, the staff of the Rotterdam Study and the participating pharmacists and general practitioners.
SUPPLEMENTAL MATERIAL
Baseline characteristics of the cross-sectional sample (n = 3,445).
REFERENCES
- 1.Erickson VS, Westlake CA, Dracup KA, Woo MA, Hage A. Sleep disturbance symptoms in patients with heart failure. AACN Clin Issues. 2003;14:477–87. doi: 10.1097/00044067-200311000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Redeker NS, Stein S. Characteristics of sleep in patients with stable heart failure versus a comparison group. Heart Lung. 2006;35:252–61. doi: 10.1016/j.hrtlng.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Stewart S, MacIntyre K, Capewell S, McMurray JJ. Heart failure and the aging population: an increasing burden in the 21st century? Heart. 2003;89:49–53. doi: 10.1136/heart.89.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonneux L, Barendregt JJ, Meeter K, Bonsel GJ, van der Maas PJ. Estimating clinical morbidity due to ischemic heart disease and congestive heart failure: the future rise of heart failure. Am J Public Health. 1994;84:20–8. doi: 10.2105/ajph.84.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleumink GS, Knetsch AM, Sturkenboom MC, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004;25:1614–9. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–72. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 7.Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3:315–22. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 8.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–37. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 9.Vogels RL, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: a systematic review of the literature. Eur J Heart Fail. 2007;9:440–9. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 11.Meisinger C, Heier M, Lowel H, Schneider A, Doring A. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg cohort study. Sleep. 2007;30:1121–7. doi: 10.1093/sleep/30.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki E, Yorifuji T, Ueshima K, et al. Sleep duration, sleep quality and cardiovascular disease mortality among the elderly: a population-based cohort study. Prev Med. 2009;49:135–41. doi: 10.1016/j.ypmed.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Kronholm E, Laatikainen T, Peltonen M, Sippola R, Partonen T. Self-reported sleep duration, all-cause mortality, cardiovascular mortality and morbidity in Finland. Sleep Med. 2011;12:215–21. doi: 10.1016/j.sleep.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Laugsand LE, Strand LB, Platou C, Vatten LJ, Janszky I. Insomnia and the risk of incident heart failure: a population study. Eur Heart J. 2014;35:1382–93. doi: 10.1093/eurheartj/eht019. [DOI] [PubMed] [Google Scholar]
- 15.Jaussent I, Empana JP, Ancelin ML, et al. Insomnia, daytime sleepiness and cardio-cerebrovascular diseases in the elderly: a 6-year prospective study. PLoS One. 2013;8:e56048. doi: 10.1371/journal.pone.0056048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofman A, Darwish Murad S, van Duijn CM, et al. The Rotterdam Study: 2014 objectives and design update. Eur J Epidemiol. 2013;28:889–926. doi: 10.1007/s10654-013-9866-z. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Mosterd A, Hoes AW, de Bruyne MC, et al. Prevalence of heart failure and left ventricular dysfunction in the general population; The Rotterdam Study. Eur Heart J. 1999;20:447–55. [PubMed] [Google Scholar]
- 19.Swedberg K, Cleland J, Dargie H, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): the Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–40. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 20.Leening MJ, Kavousi M, Heeringa J, et al. Methods of data collection and definitions of cardiac outcomes in the Rotterdam Study. Eur J Epidemiol. 2012;27:173–85. doi: 10.1007/s10654-012-9668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kardys I, Deckers JW, Stricker BH, Vletter WB, Hofman A, Witteman JC. Echocardiographic parameters and all-cause mortality: the Rotterdam Study. Int J Cardiol. 2009;133:198–204. doi: 10.1016/j.ijcard.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 22.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 23.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 24.Fogelholm M, Kronholm E, Kukkonen-Harjula K, Partonen T, Partinen M, Harma M. Sleep-related disturbances and physical inactivity are independently associated with obesity in adults. Int J Obes (Lond) 2007;31:1713–21. doi: 10.1038/sj.ijo.0803663. [DOI] [PubMed] [Google Scholar]
- 25.Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, van den Berg JF, Verschuren WM. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34:1487–92. doi: 10.5665/sleep.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gislason T, Almqvist M. Somatic diseases and sleep complaints. An epidemiological study of 3,201 Swedish men. Acta Med Scand. 1987;221:475–81. [PubMed] [Google Scholar]
- 27.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–62. [PubMed] [Google Scholar]
- 28.Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Topfer V. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–7. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics of the cross-sectional sample (n = 3,445).


