Abstract
Study Objective:
Positional therapy (PT) is an effective therapy in positional obstructive sleep apnea syndrome (POSAS) when used, but the compliance of PT is low. The objective of this study was to investigate whether a new kind of PT is effective and can improve compliance.
Methods:
29 patients were treated with the sleep position trainer (SPT), 26 patients with the tennis ball technique (TBT). At baseline and 1 month polysomnography, Epworth Sleepiness Scale (ESS) and the Quebec Sleep Questionnaire (QSQ) were taken. Daily compliance was objectively measured in both groups.
Results:
Both therapies prevent supine sleep position to a median of 0% (min-max: SPT 0.0% to 67%, TBT 0.0% to 38.9%), resulting in a treatment success (AHI < 5) in 68.0% of the SPT and 42.9% of the TBT patients. The ESS at baseline was < 10 in both groups. Sleep quality parameters, such as wake after sleep onset (WASO; p = 0.001) and awakenings (p = 0.006), improved more in the SPT group. Total QSQ scores (0.4 ± 0.2, p = 0.03), the QSQ domains nocturnal symptoms (0.7 ± 0.2, p = 0.01), and social interactions (0.8 ± 0.3, p = 0.02) changed in favor of the SPT group. Effective compliance (≥ 4 h/night + ≥ 5 days/week) was 75.9% for the SPT and 42.3% for the TBT users (p = 0.01).
Conclusion:
In mild POSAS with normal EES the new SPT device and the standard TBT are equally effective in reducing respiratory indices. However, compared to the TBT, sleep quality, quality of life, and compliance improved significantly more in the SPT group.
Citation:
Eijsvogel MM, Ubbink R, Dekker J, Oppersma E, de Jongh FH, van der Palen J, Brusse-Keizer MG. Sleep position trainer versus tennis ball technique in positional obstructive sleep apnea syndrome. J Clin Sleep Med 2015;11(2):139–147.
Keywords: positional therapy, compliance, obstructive sleep apnea
Obstructive sleep apnea syndrome (OSAS) is a common disorder affecting at least 2% to 4% of the middle-aged population.1 It is characterized by recurrent episodes of complete or partial obstruction in the upper airway during sleep with consequences such as daytime somnolence, increased cardiovascular mortality, and traffic accidents.2–4 As measured by polysomnography (PSG), the severity of OSAS is expressed by the apnea-hypopnea index (AHI); AHI of 5–15/h indicates mild OSAS, 15–30/h is moderate, and an AHI ≥ 30 is severe. A supine sleeping position can influence OSAS severity and can be the only precipitating factor for OSAS. This so-called positional dependent OSAS (POSAS) is defined as a two-fold increase in AHI in supine compared to non-supine position and mostly with an AHI < 5 in non-supine position .5–7 Using this definition, Mador et al. observed that POSAS is very common in patients with OSAS and inversely related to the severity of OSAS: 49.5% in mild OSAS, 19.4% in moderate OSAS, and 6.5 % in severe OSAS.6 In all patients, but especially in patients with mild OSAS, conservative measures such as weight reduction, alcohol and smoking abstinence, and optimization of nasal patency can diminish OSAS but seldom cure it. In patients with mild OSAS, continuous positive airway pressure (CPAP) compliance is poo r.8,9 Mandibular reposition device (MRD) application is a highly effective therapy in mild and moderate OSAS or in CPAP failure, but health insurance reimbursement in most countries is still a problem; also 25% of the patients have a contraindication for MRD therapy because of dental and periodontal abnormalities.10 Due to difficulties in treating mild OSAS and the frequent presence of POSAS in these patients, treatment of POSAS is gaining new interest.11 Positional therapy (PT) can be defined as preventing patients from sleeping in the supine sleeping position. Until recently positional therapy consisted of the so-called tennis ball technique (TBT). It consists of a bulky mass (tennis ball, bulge of hard foam, or inflated airbags) attached between the shoulder blades to prevent the patient from adopting a supine position during sleep.5,12–16 In mild POSAS patients, the normalization of the AHI with TBT is equivalent to CPAP during the overnight observation.5 Unfortunately, reported compliance with the TBT in the long term (≥ 6 months) is poor, varying from 6% to 29%, mainly due to discomfort and no improvement in sleep quality or daytime alertness.13,16,17 Therefore, there is a need for an effective positional therapy with better compliance. Recently, a new generation of PTs has been introduced: small, mainly indirect working devices, which give off a vibrating stimulus when sensing supine position. As the devices are more comfortable, it is predicted that compliance will be better.18–20 A recent study with the new sleep position trainer device (SPT) but without a control group showed indeed promising results.18 So the objectives of this randomized controlled study were to assess the efficacy and objectively measured daily compliance in patients with mild to moderate POSAS treated with the SPT or the TBT.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Standard positional therapy, with the tennis ball technique, in patients with positional obstructive sleep apnea prevents supine position and is an effective therapy (as long as it is used) but the compliance is low. New small easy to wear, in supine position vibrating devices, can perhaps be as effective and improve compliance; our study is the first randomized controlled trial comparing such a new device with standard therapy.
Study Impact: The main results of this study are twofold: both therapies are highly and equally effective in patients with mild positional obstructive sleep apnea who still use their therapy at one month, but a substantially better, objectively measured compliance, was observed in favor of the sleep position trainer. Long-term compliance studies are now needed.
METHODS
Patients
Patients were eligible for this study if referred to the sleep medicine department in a large teaching hospital, based in the Netherlands because of suspected OSAS between January and August 2011. A thorough sleep history was taken, physical examination performed, and patients were screened with a portable home sleep-monitoring unit (PM). Patients were diagnosed with OSAS If they met the following criteria as defined by the American Academy of Sleep Medicine (AASM): complaints of excessive daytime sleepiness (naps during day/evening) or ≥ 2 of the following that were not better explained by other factors: choking or gasping during sleep, recurrent awakenings from sleep, refreshing sleep, daytime fatigue, and/or impaired concentration, in combination with an AHI ≥ 5.21 POSAS was defined as ≥ 2-fold AHI supine versus AHI non-supine, AHI < 10 in non-supine position, and sleeping in supine position between 10% and 90% of time. Patients in this study had to fulfill the POSAS and OSAS criteria twice (during screening and at baseline PSG). The maximum AHI was restricted to 30. Exclusion criteria were as follows: central sleep apnea syndrome, nasal obstruction or major facial or pharyngeal anatomic abnormalities likely to require surgery, night or rotating shift work, severe chronic heart failure, known history of a known cause of daytime sleepiness and severe sleep disruption (e.g. insomnia, periodic limb movement disorder, narcolepsy), seizure disorder, mental retardation, psychiatric disease, memory disorders. Consecutive POSAS patients with an AHI 5–30 received spoken and written study information and were invited for study participation by telephone call after at least one week.
Study Design
This was a prospective randomized study with 2 parallel groups of patients with POSAS, but otherwise non-selected successive patients. One group of patients received the SPT, the other the TBT. Randomized allocation of treatment took place after baseline PSG. The investigators seeing the patients and the patients themselves could not be blinded; however, randomization, PSG assessment, and data analyses were blinded. The study was approved by the Medical Ethical Committee of Medisch Spectrum Twente hospital (Enschede, Netherlands), Trial number: NL34934.044.10.
Patients participating in the study underwent a PSG and questionnaires at baseline and at the end of the study after 1 month (with PT). To objectively measure daily compliance in both study arms a SPT device in a non-vibrating but sensing mode was also built in the TBT equipment (see Appendix). The SPT accelerometer and thermometer data give absolute information whether and how long the therapy is used.18 Patients were instructed after randomization how to use their allocated PT modality and told to use the PT as soon and often as possible. Information that compliance was measured was not provided to the patients. Patients were seen at the outpatient clinic 2 weeks after starting therapy to check for problems and replace the batteries if needed.
Positional Treatment
The SPT (NightBalance, Delft, Netherlands) is a small lightweight device, placed in a pocket of a neoprene strap, attached around the patient's chest.18 In this SPT a thermometer, accelerometer, microcomputer, battery and vibrating equipment and USB-connection are built-in. Data storage for ≥ 90 days is possible. After a sleep-in period of 30 minutes, when a patient remains in a supine position, a vibration will occur in a progressive manner from day 3 on. The idea is to gradually train patients in avoiding supine sleep position. During our study in 2011, we used the first generation SPT. The commercially online available Rematee band was used as control therapy. This is a TBT, the classical form of PT, whereby 3 inflated airbags are positioned on the back with an elastic band around the chest preventing directly supine position (see Appendix).
Measurements
Sleep Studies
At baseline and after one month a PSG was performed at home with the following in-hospital sensor placements: an electroencephalogram with F4-M1, C4-M1 derivations, nasal airflow (cannula), thoracic and abdominal respiratory movements with inductive plethysmography, chin EMG, vertical and horizontal eye movements, heart rate, and oximetry on the index finger. Sensor choice, settings, and scoring were performed according to the AASM 2007 rules.22 The alternative hypopnea 2007 definition (≥ 50% nasal flow amplitude drop with ≥ 3% O2 desaturation or arousal) was applied.22 Snoring was derived from the nasal flow sensor and expressed in the number of snores per total sleep time (TST) hour (snore index), periods of > 5 repetitive snores in TST (periodic snoring), and percentage of all snores occurring in supine position (snoring supine position percentage). Scoring was done by one technologist unaware of the type of PT the patient had worn. The morning after baseline home PSG, using a preliminary automatic PSG report, the data were checked for data loss and to recheck the OSAS and POSAS inclusion criteria. Home PSG was performed with the Alice PDx (Philips-Respironics) and analyzed with BrainRT (OSG, Rumst, Belgium) software.
Questionnaires and Scales
The Epworth Sleepiness Scale (ESS) is an 8-point self-completed questionnaire assessment of the tendency to fall asleep during 8 various daytimes situations, with a score of 0 to 3 for each question.23 The Quebec Sleep Questionnaire (QSQ) is a validated OSAS-specific quality of life (QoL) questionnaire; 32 items are divided over 5 domains: sleepiness, diurnal symptoms, nocturnal symptoms, emotions and social interactions; each item is scored on a 7-point scale. The minimal clinical important difference (MCID) perceived by the patient before and during (CPAP) treatment is earlier described.24 A higher score means improvement. The QSQ was translated into Dutch by a forward- and back-translation process. Visual analogue scales ([VAS], range −50 to +50) were used to address change in snoring intensity, partner-perceived breathing stops, nightly restlessness and movements, alertness and tiredness during daytime, and for perceived treatment effectiveness (last VAS with range 0–100). Preference of therapy had to be filled in to answer the question whether or not they would want to continue the therapy after the trial period. The ESS and QSQ were done twice; the VAS scores and the preference question only had to be filled in at the end of the study.
Compliance
All objectively measured daily compliance data were measured with the SPT device used in the SPT group but also in the TBT group (see study design) and expressed as: hours use per night, percentage of used days, everyday use, effective compliance, and adjusted compliance. Every day use was defined as use of PT for more than zero hours per day. Effective compliance was defined as > 4 h/day and > 5 of 7 days of the week use of PT.25 Two home PSGs, one without (baseline) and one with used PT (at end study) allowed us to calculate the individual TST. Adjusted compliance (%) was consequently calculated as (median) therapy use in hours per night use divided by the individual TST.26 In all except the adjusted compliance, dropouts were included in the analysis.
Treatment Effectiveness
Treatment effectiveness is presented in several ways: as treatment response defined as AHI reduction ≥ 50%, treatment success defined as AHI < 5, supine position reduction, and as supine-AHI reduction. Therapeutic efficacy can be defined as percentage improvement of the AHI between baseline and therapy.26 Mean disease alleviation (MDA) is than given by the product of therapeutic efficacy and adjusted compliance and expressed as percentage.26 This new measure makes different therapies in OSAS each with its own difference in compliance and effectiveness more comparable.27
Statistics
Sample size calculation: positional therapy using the SPT was seen as equivalent to the TBT if the 95% confidence interval of the difference in percentage of total supine time was within ± 5%. With an assumed reduction to 12%, standard deviation of 6%, α of 0.025, and power of 80%, we needed to include 22 patients within each group. Due to the high probability of skewed data that necessitates nonparametric testing, we aimed at 30 patients per group. Baseline characteristics are displayed as mean with standard deviation or median with range for continuous variables or as number with percentage for categorical variables. Differences between SPT and TBT in continuous variables were tested with a T-test or Mann-Whitney U test depending on the distribution. Differences in categorical variables were tested with χ2 or Fisher exact tests as appropriate. Statistical analysis was performed using SPSS (version 15: SPSS Inc.; Chicago).
RESULTS
Patients and Baseline Data
Seventy-seven POSAS patients gave informed consent (Figure 1). Eight patients were directly excluded due to the study inclusion and exclusion criteria. Twenty-one patients declined participation in the study because as holiday plans, finding 2 extra PSGs too cumbersome, therapy preference, or no reason at all. Eighteen patients did not meet the POSAS criteria again with repeat baseline PSG, and 4 patients did not show up at the baseline PSG appointment. Of those 18 patients one patient, one day after randomization, when the preliminary PSG data were re-analyzed had to be excluded due to not meeting the POSAS AHI inclusion criteria at baseline; the patient was not started with his PT (TBT). With permission of the METC this patient was excluded. Hence, 55 patients were randomized. Twenty-six patients were assigned to using the TBT, and 29 patients to using the SPT. Due to 5 and 2 dropouts (see compliance and dropouts), 21 and 27 patients in, respectively, the TBT and SPT groups did have a PSG after one month of therapy. Comparing baseline data between the 2 groups, 3 parameters where significantly different: sleep efficiency (p = 0.047), number of awakenings (p = 0.03), and a lower score for the QSQ-domain social interactions (p = 0.04). So the SPT group started with a lower sleep quality and a bit lower QoL.
Figure 1. Flow chart of inclusion, exclusion and randomization of patients.
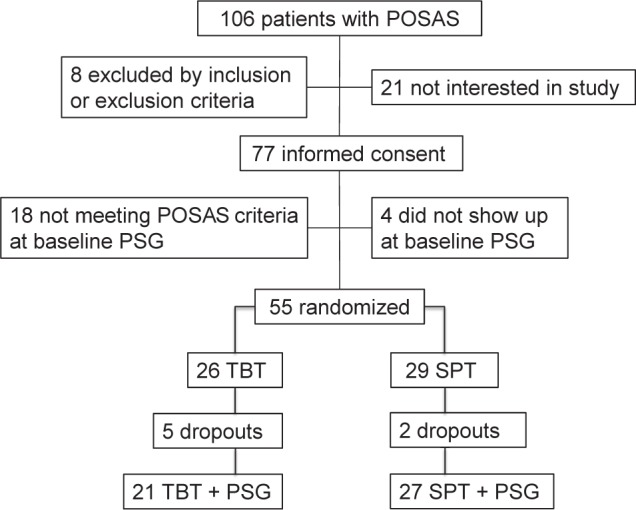
POSAS, positional obstructive sleep apnea syndrome; PSG, polysomnography; TBT, tennis ball technique; SPT, sleep position trainer.
Respiratory Data and Treatment Results
All patients used their therapies during the final home PSG night. Both therapies were equally effective in reducing supine position in TST, supine AHI, and snoring in supine position to a median of zero (Table 1); these 3 parameters when compared to baseline, showed a highly significant improvement without significant difference between the TBT and SPT groups (Table 1). A reflection of this is the 100% median supine position and supine AHI reduction (Table 2). A median of zero means that at least half the patients had at least a zero value, as illustrated in the AHI-supine during PT (Figure 2). Median AHI was significantly (both p = 0.02) reduced to 5.8 (0.2–23.1) and 3.9 (0.4–30.8), for, respectively, the TBT and SPT groups, without significant difference between the TBT and SPT groups (Table 1). The values for the snore index and periodic snoring indicated persistent snoring in the non-supine positions during therapy. Treatment response (AHI < 50%) was not different between the 2 groups. Treatment success, defined as AHI < 5, for the TBT and SPT was, respectively, 43% (9/21) and 68% (17/25); this numeric difference between TBT and SPT treatment response was, however, not statistically different (Table 2).
Table 1.
Characteristics, questionnaires, and polysomnography at baseline and 1 month.
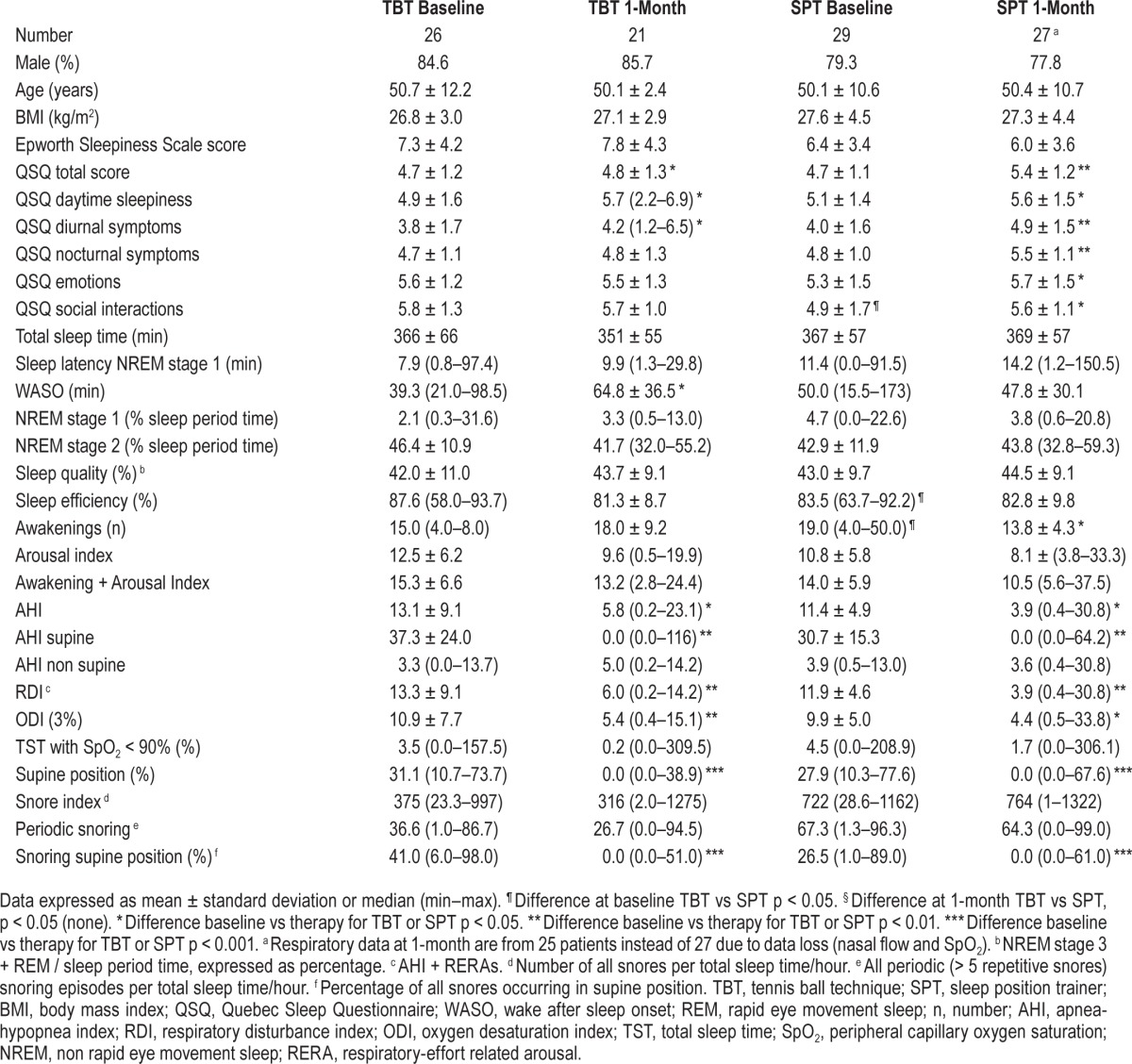
Table 2.
Respiratory data: comparing baseline to treatment.
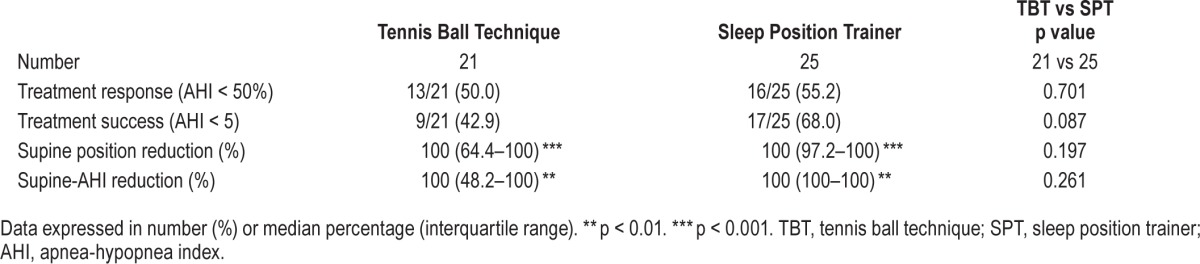
Figure 2. AHI supine.
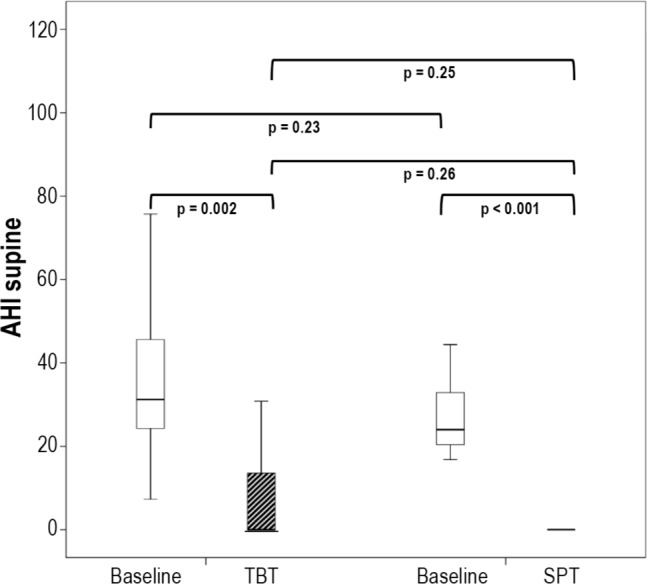
AHI supine at baseline (white boxes) and at 1-month with TBT or SPT (shaded boxes). Medians at 1-month are zero: for the TBT the median is at the bottom of the interquartile range box (25%–75%); the SPT interquartile range box (25%–75%) and median are identical. AHI, apnea-hypopnea index; TBT, tennis ball technique; SPT, sleep position trainer.
Sleep Quality and Treatment Results
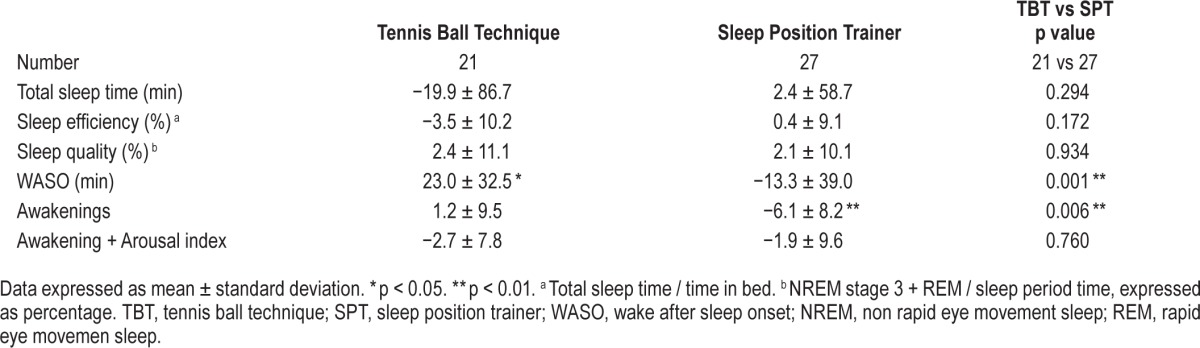
No differences between the TBT and SPT were noted in sleep parameters after one-month of therapy. However a tendency for less disturbed sleep was noted in the SPT treatment arm (Table 1). Compared to baseline awakenings decreased −6.1 ± 8.2 (p < 0.001) only in the SPT group. WASO increased 23.0 ± 32.5 (p = 0.04) minutes in the TBT group and decreased 13.3 ± 39.0 (p = 0.09) minutes in the SPT group (p < 0.001) during therapy, indicating improved sleep quality in the SPT patients (Table 3).
Table 3.
Sleep data, comparing baseline to treatment.
Questionnaires, VAS, and Therapy Preference
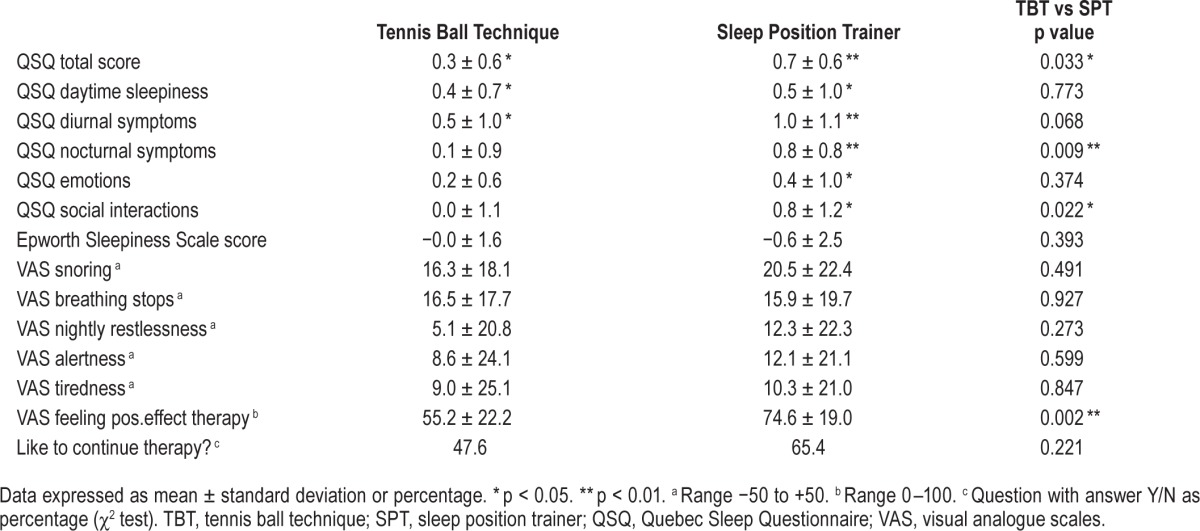
After one month, no statistical differences in ESS, VAS, and in the 5 domains or total QSQ score were found between the 2 groups (Table 1). Compared to baseline, significant improvements were noted in all 5 QSQ-domains and total QSQ score in the SPT and in the domains daytime sleepiness, diurnal symptoms, and total QSQ score for the TBT group (Table 4). Comparing change from baseline in the TBT and SPT group a significantly higher improvement in QSQ total score (p = 0.03), domain nocturnal symptoms (p = 0.01), and the domain social interactions (p = 0.02) for the SPT patients was observed (Table 4). However the changes did not reach the “minimal clinically important difference” (MCID) as defined for CPAP therapy.24 All VAS scores improved, but without significant difference between the therapy groups. The question “Do you like to continue your therapy” was answered positive respectively in 10/21 (47.6 %) and 17/26 (65.4%) of the patients in the TBT and SPT group (Table 4). Patients using the SPT preferred their therapy more than patients using the TBT (p = 0.002).
Table 4.
Questionnaires and visual analogue scales, comparing baseline to treatment.
Compliance and Dropouts
Effective compliance defined in line with the CPAP compliance definition (≥ 4 h/day + ≥ 5 days/week) was significant (p = 0.01) different between the 2 groups: 42.3% (11/26) for the TBT and 75.9% (22/29) for the SPT group of patients (Table 5). Highly significant different in favor of the SPT was every day and the percentage of days usage (both p = 0.005). The median hours use per night and the number of dropouts were statistically not different between the 2 therapy groups. The intention to treat analysis included dropouts in all compliance data. Five dropouts in TBT and 2 in SPT were noted (p = 0.24). Rea -sons for dropouts were shoulder/back pain (TBT), inability to turn on the back and (TBT/SPT) or vibrating noise (SPT). We measured daily compliance for both treatment arms. A negative trend for the compliance (≥ 4 h/night) is observed during one-month of use (Figure 3). At day 1, neither group started at 100% compliance for several reasons: not all patients started right away with their therapy, and therapy use ≥ 4 h is required.
Table 5.
Compliance.
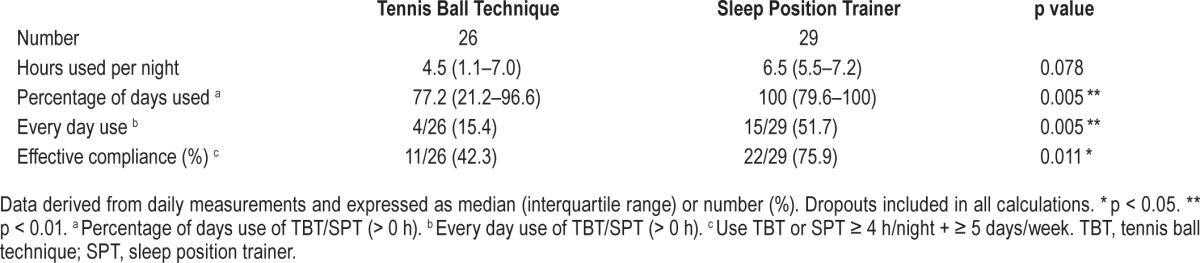
Figure 3. Position device: compliance (≥ 4 h/night).
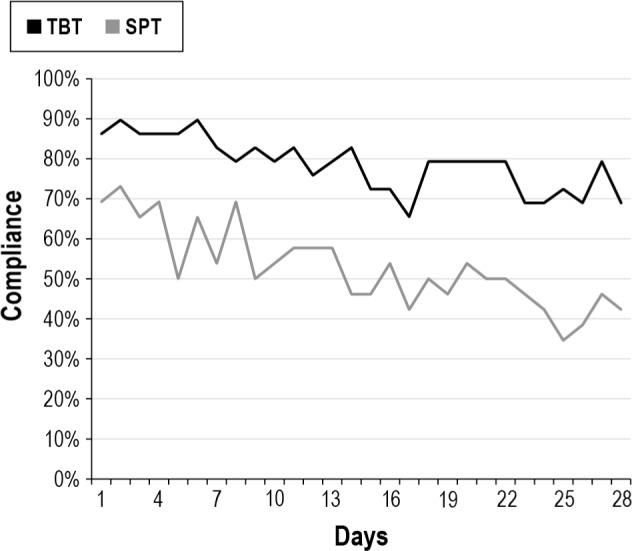
TBT, tennis ball technique; SPT, sleep position trainer.
Mean Disease Alleviation
TBT and SPT reduce the AHI (therapeutic efficacy), respectively, with 61.8% and 68.7%, with an adjusted compliance of 78.7% and 102.7% (p = 0.041). A mean disease alleviation of 48.6% and 70.5% for, respectively, TBT and SPT was observed (p = 0.005; Table 6). Due to calculation of the average TST (baseline PSG + 1-month PSG expressed as mean), adjusted compliance can be higher than 100%.
Table 6.
Mean disease alleviation.
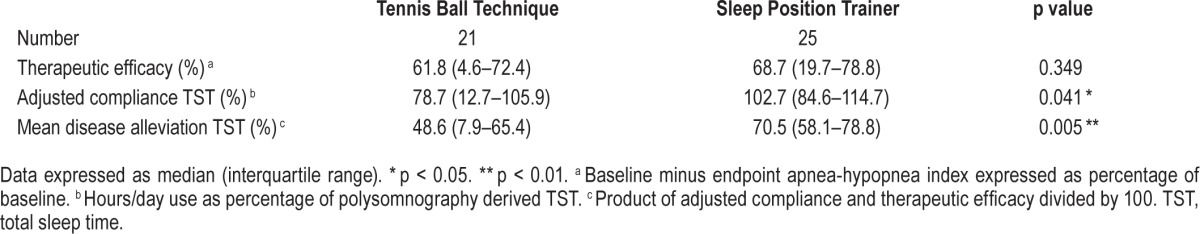
Position Device Data
The reduction in supine sleep position starts on the first day with the TBT (direct prevention of supine position) and for the SPT from day 3 (first 2 days only sensing). This resulted in supine sleep time position drop from 25% to < 5% from day 5 on for the SPT and between 4% and 10% for the TBT during the entire month (Figure 4). The time in bed for those who used the device was identical: 6.58 and 6.56 h, respectively, for TBT and SPT. Only in the SPT group the vibration episodes and reactions (turning to a non-supine position) of the patients where available (Figure 5). The vibration episodes (mean of 5.3) in this first generation SPT were not always followed by a turning reaction (mean 3.6).
Figure 4. Position device: supine position.
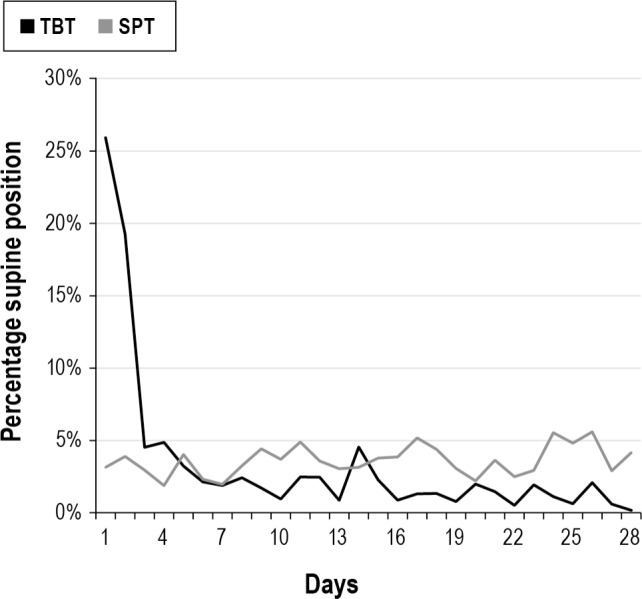
TBT, tennis ball technique; SPT, sleep position trainer.
Figure 5. Position device: vibration activity and reactions.
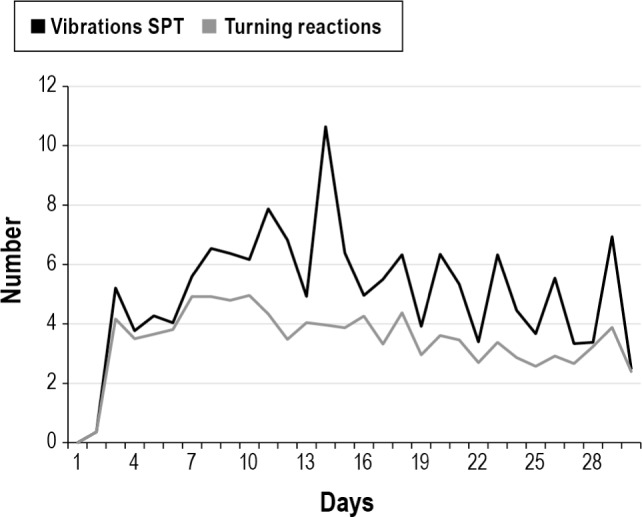
SPT, sleep position trainer.
DISCUSSION
The present study demonstrates more than 30% effective compliance difference between the two study arms, in favor of the SPT group. Both therapies equally minimize supine sleep position, supine-AHI, and supine snoring, and reduce AHI to almost normal (AHI < 5) values when PT is used. The SPT was used every day in 51.7% of the patients, whereas the TBT was used every day in 15.4% of the patients. The observed high effective compliance of 75.9% after a month of SPT use in mild (symptomatic, but mean ESS < 10) OSAS patients where therapy adherence due to mild disease severity is known to be poor is striking. Patient dropouts (2 SPT; 5 TBT) due to discomfort of PT in both therapies were included in this analysis, so the compliance in users is higher. Compliance did decline with time, but the gap (Figure 3) between both therapies was wider at the end of the study, suggesting a progressive difference in compliance between both therapies. One can postulate that a cumbersome therapy as the TBT or a vibrating device as the SPT could influence sleep quality. All sleep treatment results pointed to a small improvement in sleep quality in the SPT group and a minor decrease in the TBT group, with significant differences in treatment effect between the two groups in favor of the SPT group for WASO and awakenings.
The QSQ showed significant but modest treatment effects, and the changes did not reach the values for the MCID as described in the original article of the QSQ.24 This questionnaire was developed in OSAS patients with an AHI of 29 and ESS of 14 with response measurement with and without CPAP.24 It can be argued that the MCID described is too restrictive for therapy evaluation in mild OSAS.
Comparing treatment effects after one month of therapy showed significant differences in favor of the SPT for QSQ total score and two QSQ domains. The VAS for “Feeling positive effect of PT therapy” was also in favor of the SPT. As expected during therapy, we observed almost no snoring in supine position. Compared to baseline, overall snoring (snore index and periodic snoring) still remained high, due to persistent snoring in non-supine position. Snoring in non-supine position is probably less loud than snoring in supine position; however, we did not measure decibels.
To really compare different OSAS therapies, MDA can be used. MDA is a proposed new treatment measure; it is the product of the therapeutic efficacy (ability of a treatment to reduce the AHI) and the adjusted compliance (objectively measured compliance over a certain period with individual TST taken into account).26 For MRD and CPAP, MDA of 50% has been published.26–28 We found a high MDA of 70.5% for the SPT and a significantly lower MDA of 48.6% for the TBT. The high MDA of the SPT is the result of a good therapeutic efficacy and very high-adjusted compliance. The reported lower MDA for CPAP is a reflection of lower adjusted compliance.26–28 Looking at the daily SPT performance in the SPT group, we noted that supine position was effectively prevented; remarkably, not all vibration episodes were followed by a movement to a non-supine position. The manufacturer of the SPT commented that after 30 seconds, the vibration was programmed to stop, and that this has been adapted in newer SPT generations.
Limitations of our study are as follows. Firstly, at baseline, three parameters where significantly worse in the SPT group: QSQ-domain social interactions sleep efficiency and number of awakenings (Table 1). So the SPT group started with less QoL for one of the five QSQ domains and less sleep quality in two of the twelve sleep parameters. These baseline differences between the two groups might have occurred by chance. Secondly, as expected, both therapies were effective in preventing supine position and therefore POSAS as long as the PT was used—as was the case during PSG at one month. However, between baseline and one month, the large compliance difference between therapies did not translate into a statistical difference in ESS. One explanation is that our groups were too small to find a statistical difference. Another explanation is perhaps the fact that we had a very mild POSAS group with daily complaints but normal ESS at inclusion; room for improvement is then limited. Still, 75.9% of the SPT group used their therapy more than 4 hours/day and at least 5 days a week during one month; and sleep quality, QSQ, and AHI were also significantly improved. Thirdly the MDA we found is very high; this figure can decline when longer-term compliance results with the SPT are evaluated. One study evaluating the effect of SPT has recently been published; in 36 POSAS patients, the median AHI decreased from 16.4 to 5.2.18 These patients were sleepier than in our study with an ESS of 11. A significant decrease in ESS and Functional Outcome of Sleep Questionnaire and a high compliance (without dropouts) at one month of 92.7% were observed. Finally, the economic aspects of these two therapies were not studied.
In mild OSAS patients the prevalence of POSAS is approximately 50%, suggesting that there is a huge potential for PT.11,29 There is not much doubt anymore that PT can be an effective therapy.5,11,29 New positional devices seem to be capable of improving short-time compliance as has been shown in other studies and in our randomized study.18–20 The main issue is whether the reported poor long-term compliance of the older TBT can be indeed improved with the new PTs using these small ergonomic vibrating devices.13,16,17 Thus, long-term compliance studies are now really needed.
In conclusion, in mild positional depended OSAS the new SPT device and the standard TBT are equally effective. Compared to standard PT with the TBT, compliance, mean disease alleviation, sleep quality, and quality of life are significantly improved with the SPT.
DISCLOSURE STATEMENT
The study was self-funded; the investigators and authors received no financial support in order to avoid interference and pressure from third parties. Sleep studies were performed as usual care (portable monitoring type III selection, PSGs). The TBT (Rematee band) was bought on the commercial market. The SPTs (sleep position trainers) were provided by the producing company NightBalance. NightBalance provided also the sleep position trainers used in a non-vibrating, but sensing mode for daily compliance measurement in the TBT group. In the contract with NightBalance it was explicitly stated that NightBalance had no influence on the publication, no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Based on this agreement the start of the study was approved by the Medical Ethics Committee Twente, The Netherlands. There was no off-label or investigational use. The authors have indicated no financial conflicts of interest. The study was performed at Medisch Spectrum Twente Hospital, Pulmonary and Sleep department, Haaksbergerstraat 55, 7513 ER, Enschede, The Netherlands.
APPENDIX
left: Rematee® (tennis ball technique) with extra pocket for sleep position trainer (in non-vibrating mode) to measure daily compliance. middle: 0–10 cm scale. right: normal sleep position trainer.
An example of a person with the two studied forms of position therapy in place. The tennis ball technique (Rematee®, with 3 airbags on the subject's back), and sleep position trainer (secured with strap and pocket on the front of the subject) are shown.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–35. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Jennum P. Quality of life, co-morbidity and obstructive sleep apnoea. Clin Respir J. 2010;4:129–30. doi: 10.1111/j.1752-699X.2010.00211.x. [DOI] [PubMed] [Google Scholar]
- 3.Levy P, Bonsignore MR, Eckel J. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J. 2009;34:243–60. doi: 10.1183/09031936.00166808. [DOI] [PubMed] [Google Scholar]
- 4.Sivertsen B, Overland S, Glozier N, Bjorvatn B, Maeland JG, Mykletun A. The effect of OSAS on sick leave and work disability. Eur Respir J. 2008;32:1497–503. doi: 10.1183/09031936.00044908. [DOI] [PubMed] [Google Scholar]
- 5.Permut I, Diaz-Abad M, Chatila W, et al. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med. 2010;6:238–43. [PMC free article] [PubMed] [Google Scholar]
- 6.Mador MJ, Kufel TJ, Magalang UJ, Rajesh SK, Watwe V, Grant BJ. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest. 2005;128:2130–7. doi: 10.1378/chest.128.4.2130. [DOI] [PubMed] [Google Scholar]
- 7.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7:110–4. doi: 10.1093/sleep/7.2.110. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal L, Gerhardstein R, Lumley A, et al. CPAP therapy in patients with mild OSA: implementation and treatment outcome. Sleep Med. 2000;1:215–20. doi: 10.1016/s1389-9457(00)00012-5. [DOI] [PubMed] [Google Scholar]
- 9.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:1108–14. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 10.Hoekema A, Stegenga B, Wijkstra PJ, van der Hoeven JH, Meinesz AF, de Bont LG. Obstructive sleep apnea therapy. J Dent Res. 2008;87:882–7. doi: 10.1177/154405910808700917. [DOI] [PubMed] [Google Scholar]
- 11.Ravesloot MJ, van Maanen JP, Dun L, de Vries N. The undervalued potential of positional therapy in position-dependent snoring and obstructive sleep apnea-a review of the literature. Sleep Breath. 2013;17:39–49. doi: 10.1007/s11325-012-0683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skinner MA, Kingshott RN, Filsell S, Taylor DR. Efficacy of the ‘tennis ball technique’ versus nCPAP in the management of position-dependent obstructive sleep apnoea syndrome. Respirology. 2008;13:708–15. doi: 10.1111/j.1440-1843.2008.01328.x. [DOI] [PubMed] [Google Scholar]
- 13.Oksenberg A, Silverberg D, Offenbach D, Arons E. Positional therapy for obstructive sleep apnea patients: a 6-month follow-up study. Laryngoscope. 2006;116:1995–2000. doi: 10.1097/01.mlg.0000237674.66716.a7. [DOI] [PubMed] [Google Scholar]
- 14.Heinzer RC, Pellaton C, Rey V, et al. Positional therapy for obstructive sleep apnea: an objective measurement of patients' usage and efficacy at home. Sleep Med. 2012;13:425–8. doi: 10.1016/j.sleep.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Cartwright R, Ristanovic R, Diaz F, Caldarelli D, Alder G. A comparative study of treatments for positional sleep apnea. Sleep. 1991;14:546–52. doi: 10.1093/sleep/14.6.546. [DOI] [PubMed] [Google Scholar]
- 16.Bignold JJ, Deans-Costi G, Goldsworthy MR, et al. Poor long-term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. J Clin Sleep Med. 2009;5:428–30. [PMC free article] [PubMed] [Google Scholar]
- 17.Wenzel S, Smith E, Leiacker R, Fischer Y. Efficacy and longterm compliance of the vest preventing the supine position in patients with obstructive sleep apnea. Laryngorhinootologie. 2007;86:579–83. doi: 10.1055/s-2007-966179. [DOI] [PubMed] [Google Scholar]
- 18.van Maanen JP, Meester KA, Dun LN, et al. The sleep position trainer: a new treatment for positional obstructive sleep apnoea. Sleep Breath. 2013;17:771–9. doi: 10.1007/s11325-012-0764-5. [DOI] [PubMed] [Google Scholar]
- 19.van Maanen JP, Richard W, Van Kesteren ER, et al. Evaluation of a new simple treatment for positional sleep apnoea patients. J Sleep Res. 2012;21:322–9. doi: 10.1111/j.1365-2869.2011.00974.x. [DOI] [PubMed] [Google Scholar]
- 20.Bignold JJ, Mercer JD, Antic NA, McEvoy RD, Catcheside PG. Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device. J Clin Sleep Med. 2011;7:376–83. doi: 10.5664/JCSM.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Academy of Sleep Medicine. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 22.American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [PMC free article] [PubMed] [Google Scholar]
- 23.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 24.Lacasse Y, Bureau MP, Series F. A new standardised and self-administered quality of life questionnaire specific to obstructive sleep apnoea. Thorax. 2004;59:494–9. doi: 10.1136/thx.2003.011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 26.Grote L, Hedner J, Grunstein R, Kraiczi H. Therapy with nCPAP: incomplete elimination of Sleep Related Breathing Disorder. Eur Respir J. 2000;16:921–7. doi: 10.1183/09031936.00.16592100. [DOI] [PubMed] [Google Scholar]
- 27.Vanderveken OM, Dieltjens M, Wouters K, De Backer WA, Van de Heyning PH, Braem MJ. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax. 2013;68:91–6. doi: 10.1136/thoraxjnl-2012-201900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravesloot MJ, de Vries N. Reliable calculation of the efficacy of non-surgical and surgical treatment of obstructive sleep apnea revisited. Sleep. 2011;34:105–10. doi: 10.1093/sleep/34.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oksenberg A, Gadoth N. Are we missing a simple treatment for most adults sleep apnea patients? The avoidance of the supine sleep position. J Sleep Res. 2014;23:204–10. doi: 10.1111/jsr.12097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
left: Rematee® (tennis ball technique) with extra pocket for sleep position trainer (in non-vibrating mode) to measure daily compliance. middle: 0–10 cm scale. right: normal sleep position trainer.
An example of a person with the two studied forms of position therapy in place. The tennis ball technique (Rematee®, with 3 airbags on the subject's back), and sleep position trainer (secured with strap and pocket on the front of the subject) are shown.


