Abstract
Study Objectives:
Obstructive sleep apnea (OSA) has been associated with psychiatric pathology. Psychiatric comorbidity in OSA may affect patient quality of life and adherence to CPAP. A focused evaluation of OSA in highly selected groups of primarily psychiatric patients may provide further insights into the factors contributing to comorbidity of OSA and psychopathology. The goal of this study is to examine the prevalence and treatment of OSA in psychiatric populations.
Methods:
A systematic review following the PRISMA guidelines was conducted to determine the prevalence of OSA in schizophrenia and other psychotic disorders, mood disorders, and anxiety disorders, and to examine potential interventions. The PubMed, EMBASE, and PsycINFO databases were searched (last search April 26, 2014) using keywords based on the ICD-9-CM coding for OSA and the DSM-IV-TR diagnostic groups.
Results:
The search retrieved 48 records concerning studies of OSA in the selected disorders. The prevalence studies indicate that there may be an increased prevalence of OSA in individuals with major depressive disorder (MDD) and posttraumatic stress disorder (PTSD), despite considerable heterogeneity and a high risk of bias. There was insufficient evidence to support increased OSA in schizophrenia and psychotic disorders, bipolar and related disorders, and anxiety disorders other than PTSD. Studies of treatment of OSA indicate an improvement in both OSA and psychiatric symptoms. CPAP adherence was reduced in veterans with PTSD.
Conclusions:
OSA prevalence may be increased in MDD and PTSD. In individuals with OSA and psychiatric illness, treatment of both disorders should be considered for optimal treatment outcomes.
Citation:
Gupta MA, Simpson FC. Obstructive sleep apnea and psychiatric disorders: a systematic review. J Clin Sleep Med 2015;11(2):165–175.
Keywords: obstructive sleep apnea, psychiatry, PTSD, depression, comorbidity
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder characterized by repeated episodes of upper airway obstruction during sleep.1 According to a major US study of OSA diagnosed by polysomnography (PSG), the prevalence of OSA, as defined by an apnea-hypopnea index (AHI) ≥ 5 and without inclusion of a daytime sleepiness criterion, was reported as 24% for men and 9% for women under the age of 65 years; addition of a daytime sleepiness criterion reduced these estimates to 4% for men and 2% for women.1,2 OSA is commonly associated with metabolic syndrome including comorbid obesity, hypertension, and diabetes.1 Upper airway obstruction may present as apneas, hypopneas, or respiratory effort-related arousals (RERAs), resulting in oxygen desaturation, repeated arousals and sleep fragmentation.1 Recently, there has been an increase in reports of comorbidity of OSA with psychological/psychiatric symptoms. Psychiatric comorbidity in OSA has been reported to adversely affect the quality of life of OSA patients and adherence to CPAP therapy.3–5
Psychological symptoms such as depression and anxiety are commonly reported in adults with OSA; however, the relationship between OSA and full psychiatric syndromes is less clear. Global prevalence studies and reviews have suggested that there are elevated rates of psychological symptoms in individuals with OSA.6–16 These studies are limited in their ability to confirm psychiatric diagnoses, as psychiatric symptoms are commonly evaluated using self- or clinician rated psychiatric severity scales, not a diagnostic evaluation by an experienced clinician. Sleep symptoms may also artificially elevate patient scores on psychiatric scales. Popular scales, such as the Beck Depression Inventory (BDI), Profile of Mood States (POMS), and Minnesota Multiphasic Personality Inventory (MMPI), have questions relating to sleep symptoms such as insomnia and fatigue that are common to both OSA and psychiatric conditions.17–19 Studies that have evaluated the prevalence of OSA in the highly selected groups of psychiatric populations may provide additional insight into the factors contributing to the comorbidity of OSA and psychopathology.
Our objectives were (1) to perform a comprehensive evaluation of the prevalence of OSA in the major psychiatric disorders including schizophrenia and other psychotic disorders, mood disorders, and anxiety disorders; and (2) to perform a narrative evaluation of interventions for the treatment of OSA in individuals with schizophrenia and other psychotic disorders, mood disorders, and anxiety disorders.
METHODS
The methodology for the systematic review was carried out according to the PRISMA guidelines.20
Eligibility Criteria
The inclusion criteria are: subjects with clinically diagnosed schizophrenia and other psychotic disorders, mood disorders, and anxiety disorders and an OSA diagnosis conducted using PSG. A clinical diagnosis for a psychiatric disorder must have been established by a clinician using an interview or clinician-rated scale. Psychiatric disorders were classified based on the system used in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM-IV-TR), although studies using prior editions were also acceptable.21 This diagnosis could be determined prospectively from the patient, through a chart review, or through the use of insurance coding using the International Classification of Diseases, Ninth Revision (ICD-9) or 10th Revision (ICD-10).22,23 The criteria for OSA required a PSG (single or split-night) meeting the International Classification of Sleep Disorders, 2nd edition (ICSD-2) criteria for OSA with AHI ≥ 5 events/h or the respiratory disturbance index (RDI) equivalent.1 The exclusion criteria were: non-English language articles, review articles, and animal studies.
Information and Sources
The search was conducted in PubMed (including MEDLINE), EMBASE (from 1974) via OVID, and PsycINFO (from 1806) via OVID up to April 26, 2014. The search terms were based on the ICD-9 codes for schizophrenia and other psychotic disorders, mood disorders, and anxiety disorders. These diagnostic groups coincided with the respective DSM-IV-TR categories for these disorders.21,22 The full search strategy for PubMed can be viewed in Table S1 (Appendix 1). Additional articles were identified by hand search of the reference sections of relevant papers.
Study Selection
Full text articles were evaluated for inclusion by 2 independent reviewers (FS and KK), and disagreements were resolved by discussion to reach a consensus. Study populations discussed in multiple articles were grouped under a single study identifier by the final report (i.e., preliminary conference abstracts would be found under the identifier for the final published journal article) (Appendix 2).
Data Extraction
Data extraction was conducted independently by FS using a standard form for prevalence and intervention studies. The data collected for prevalence studies included the study identifier, sample size for any groups, gender, characteristics of the study population, the mean age, mean BMI, psychiatric medications used, psychiatric diagnostic criteria, and OSA diagnostic criteria. The data collected for intervention studies included the study identifier, sample size for any groups, gender, characteristics of the study population, psychiatric medications used, psychiatric diagnostic criteria, OSA diagnostic criteria, type of intervention, duration of intervention, study outcome measures, and results of the outcome measures. The authors of studies with missing or incomplete data were contacted via email to request additional data. In several large epidemiological studies of concurrent diagnoses of OSA and psychiatric disorders, the scores of the prevalence of psychiatric disorders in OSA were converted into scores for the prevalence of OSA in psychiatric disorders where the data permitted conversion.24–26
Risk of Bias
The quality of the included studies was evaluated by 2 independent reviewers (FS and KK) using the Hoy tool for assessing risk of bias (RoB) in prevalence studies and the Cochrane Risk of Bias tool for interventions.27,28 Case reports were not assessed for RoB. The inter-rater reliability between the reviewers' RoB assessments was assessed using Cohen's κ in IBM SPSS Statistics 20 (Armonk, NY).29
RESULTS
Included Studies
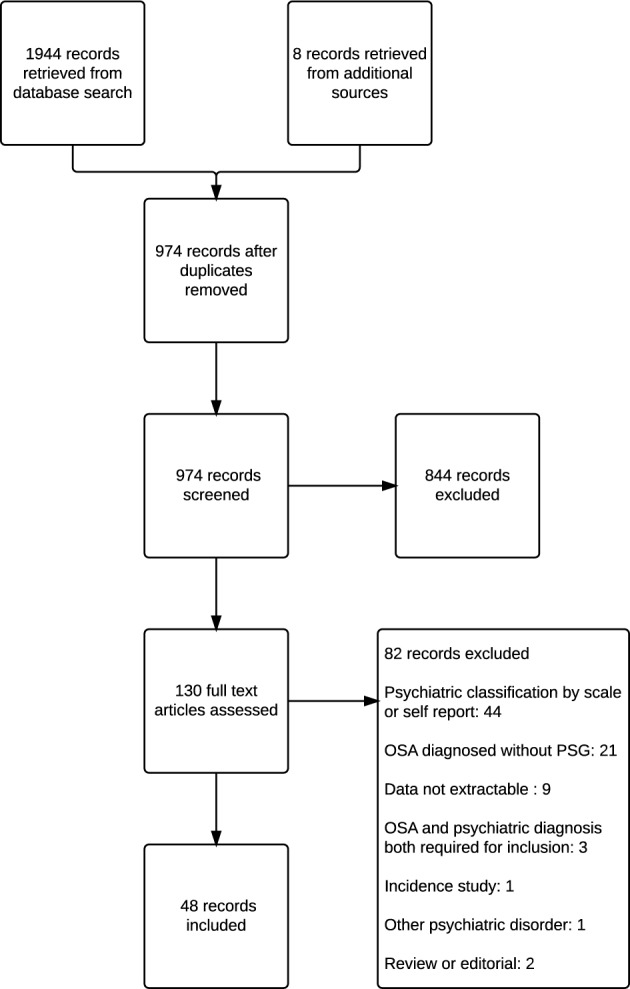
Our search identified 974 individual manuscripts relating to psychiatric disorders in subjects diagnosed with OSA. Of these, 48 records containing 44 studies concerned subjects with clinically diagnosed schizophrenia and other psychotic disorders, mood disorders, and anxiety disorders, who were evaluated by polysomnography for OSA (Figure 1; Table S2, Appendix 1).
Figure 1. Flow diagram of study selection.
Study Design
The included studies for prevalence were categorized based on whether they were population-based samples, clinical or inpatient samples. Overall, most prevalence studies were based on sleep clinic referrals or inpatient psychiatric populations. Due to the heterogeneous nature of the study populations, no pooled estimates of population prevalence were possible (Appendix 3, Section A). The inter-rater reliability score for RoB for prevalence studies was 0.871.
The search for intervention studies resulted in a combination of case reports and prospective and retrospective studies. These studies also presented a wide variety of interventions for OSA in subjects with psychiatric disorders, including CPAP, armodafinil, and uvulopalatopharyngoplasty (UPPP). RoB was only assessed for prospective and retrospective studies meeting the inclusion criteria, due to the obvious sample size bias of a report on a single individual (Appendix 3, Section B). The inter-rater reliability score for RoB for intervention studies was 0.828. Intervention data, categorized by psychiatric diagnosis, are subsequently presented.
Subjects
Excluded Studies
There were 82 excluded studies based on the full text review (Figure 1; Table S3, Appendix 1). The reasons for exclusion are presented in Table S4 (Appendix 1). The most common reason for exclusion was the lack of a clinical psychiatric diagnosis, followed by lack of diagnostic PSG.
Prevalence
SCHIZOPHRENIA AND PSYCHOTIC DISORDERS
Schizophrenia: There have been 2 clinic-based studies conducted on the prevalence of OSA in schizophrenia (Table 1; Table S5, Appendix 2).24,30 The clinic-based studies reported a prevalence range of 0.73% to 48.0%, and both studies had high risk of selection bias. The discrepancy between the reported prevalence is likely due to Levine reporting prevalence in consecutive psychiatric inpatients, while Winkelman reported prevalence in psychiatric patients with sleep clinic referrals, which is a substantial bias towards a higher prevalence of OSA.24,30
Table 1.
Summary of findings: prevalence.
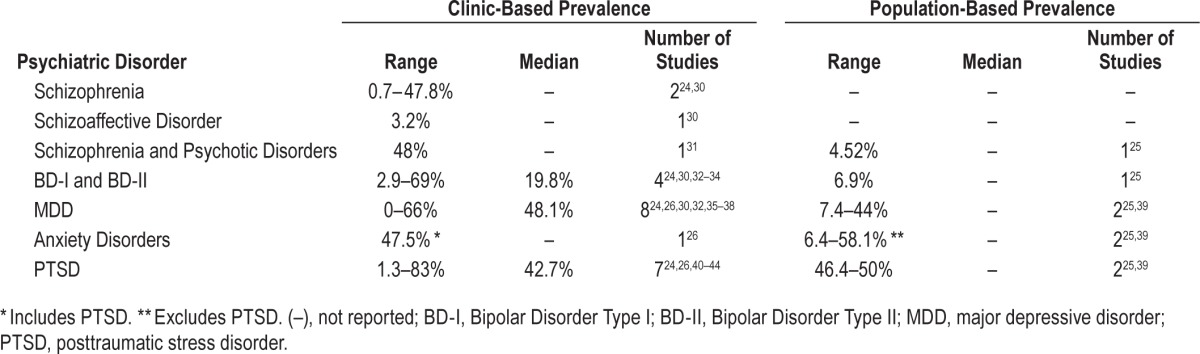
Schizoaffective Disorder: A single clinic-based prevalence study has been conducted on the prevalence of OSA in schizoaffective disorder (Table 1; Table S5, Appendix 2).30 Levine found that the prevalence of OSA was 3.2% in 93 subjects with schizoaffective disorder.
Pooled Schizophrenia and Psychotic Disorders: There has been one clinic-based and one population-based study on pooled schizophrenia and other psychotic disorders.25,31 The clinic-based population showed a 48% prevalence of OSA in mixed schizophrenia and schizoaffective disorder participants.31 The population-based study conducted using the United States Veterans Health Administration (VHA) records reported an OSA prevalence in pooled schizophrenia and psychotic disorders of 4.52%.25
MOOD DISORDERS
Bipolar I and II Disorder: There were 5 studies that reported the prevalence of OSA in bipolar I and II disorders (BD); 4 clinical studies and a single population-based study (Table 1; Table S6, Appendix 2).24,25,30,32–34 In the clinic-based studies, the prevalence of OSA ranged from 2.9% to 69%. All 4 studies were at a high risk of selection bias. The range of prevalence may be related to the clinical population studied. Hattori reported the highest prevalence of 69% in a population of individuals with BD requiring a depression score on the HAMD ≥ 10 and clinical signs of OSA.32 This study has the greatest selection bias for OSA due to the requirement for active depressive symptoms and clinical signs of OSA. The lowest prevalence reported was 2.9% by Levine in a study of consecutive psychiatric patients at a state hospital where no pre-existing sleep symptoms were required for inclusion.30 The populations of psychiatric inpatient sleep clinic referrals and consecutive BD patients showed moderate prevalence of 18.5% and 21%, respectively.24,33 In Winkelman, there is a selection bias for psychiatric inpatients with sleep disturbance that is not specific to OSA and a reduced diagnostic threshold of an RDI > 10.24 In Kelly, the study participants are consecutive outpatients, but the criteria for OSA are more stringent; requiring presenting EDS for diagnosis if the AHI is > 5 and < 15 events per hour or an AHI ≥ 15.33,34 In the population-based study, the reported rate of OSA in BD was 6.94% in the VHA database.25
Major Depressive Disorder: Ten studies on the prevalence of OSA in individuals with major depressive disorder (MDD) were identified—8 clinic-based studies and 2 population-based studies (Table 1; Table S7, Appendix 2).24–26,30,32,35–39 The clinic-based studies range in prevalence from 0% to 66%. Seven studies were at high risk of selection bias, and one study was at moderate risk of selection bias. The lowest prevalence of 0% was found by Levine in a sample of consecutive psychiatric inpatients without sleep symptoms.30 The highest prevalence of 66% was found by Carney in subjects with comorbid coronary heart disease who have an increased risk for both depression secondary to CHD and for OSA due to pathophysiologic factors underlying CHD including obesity.36 High selection bias for pre-existing sleep symptoms was present for sleep disturbance in Winkelman (12%) and insomnia in Ong (39%).24,37 A specific depression severity was required for inclusion for the studies of Deldin (53%), Hattori (53%), and Summers (46.7%).32,35,38 The final study, Mysliwiec, was conducted in a military population where routine PSG is a post-combat requirement, which may increase the reporting bias for OSA.26 Overall, 6 of the 8 clinic-based samples reported an elevated rate of OSA in MDD. In population based studies, Sharafkhaneh reported a prevalence of 7.4% in the VHA vs. 44% for a community population sample in Hrubos-Strom.25,39 This difference may be affected by sample size, as the final sample of MDD patients in Hrubos-Strom was 36 subjects, compared to 358,817 in Sharafkhaneh.
Dysthymia: A single study was identified that reported the prevalence of OSA in dysthymia. Hrubos-Strom 2012 reported that 3 of 5 (60%) individuals with dysthymia had a clinical diagnosis of OSA; however, the sample size and moderate RoB limit the generalizability of this data.39
ANXIETY DISORDERS
Three studies reported the prevalence of pooled anxiety diagnoses (Table 1; Table S8, Appendix 2).25,26,39 The clinic-based sample in Mysliwiec reported a prevalence of anxiety disorders excluding PTSD as 47.5% for their time interval.26 This study was at a moderate RoB, as it included all diagnostic PSG performed on postcombats veterans as a routine measure. Hrubos-Strom reported a rate in current anxiety disorders including PTSD as 58.1% in a moderate RoB community survey-based sample.39 This study is also the only study to report prevalence for individual anxiety disorders other than PTSD (Table 1; Table S8, Appendix 2). The prevalence of OSA was 58.8% in panic disorder (n = 17), 100% in agoraphobia without panic disorder (n = 2), 53.8% in social phobia (n = 13), 40% in obsessive compulsive disorder (n = 5), and 57.1% in generalized anxiety disorder (n = 14), respectively. However, the small sample sizes limit the generalizability of these results. The population-based study Sharafkhaneh reported an OSA prevalence of 6.4% in anxiety disorders including PTSD in the VHA.25 The anxiety disorders represented the most heterogeneous group, as some studies pooled all anxiety disorders under one grouping while others either excluded PTSD or reported on individual diagnoses.
Posttraumatic Stress Disorder (PTSD): There were 9 studies that reported the prevalence of OSA in PTSD; 7 studies were clinic-based and 2 studies were population-based (Table 1; Table S9, Appendix 2).24–26,39–44 The clinic-based studies reported a prevalence range of 0.7% to 83%.24,26,40–44 Six studies were considered to have high risk of selection bias, and one had a moderate risk of selection bias due to population sample, sleep symptom requirements, and/or PTSD severity. Kinoshita (83%) and Yesavage (69%) required participants to be male, aged ≥ 55 with a CAPS score ≥ 40, each of which acts as a selection bias towards increased OSA prevalence.41,42 Two studies by Mysliwiec26,43 examined postcombat PSG in military personnel, and the second of these43 required a PTSD Checklist Military Version score ≥ 50, both of which act as selection biases. The van Liempt study also included male veterans with a CAPS score > 50.44 The 2 civilian clinic samples reported the lowest prevalence of OSA in PTSD. Winkelman included consecutive psychiatric inpatients with sleep disturbances as selection bias. Krakow recruited crime victims with nightmares and insomnia with a PTSD severity requirement of Posttraumatic Stress Diagnostic Scale (PSDS) score ≥ 11.40 The population-based studies reported a prevalence range of 46.4% to 50%.25,39 These studies were at a low and moderate RoB, respectively. Six of the 9 studies were conducted in past and present military populations, which reported a range of 42.7% to 50% for both classes of study.25,26,41–44 Civilian populations had the greatest discrepancy in prevalence rates from 0.7% to 50%.24,39,40
Interventions
SCHIZOPHRENIA AND PSYCHOTIC DISORDERS
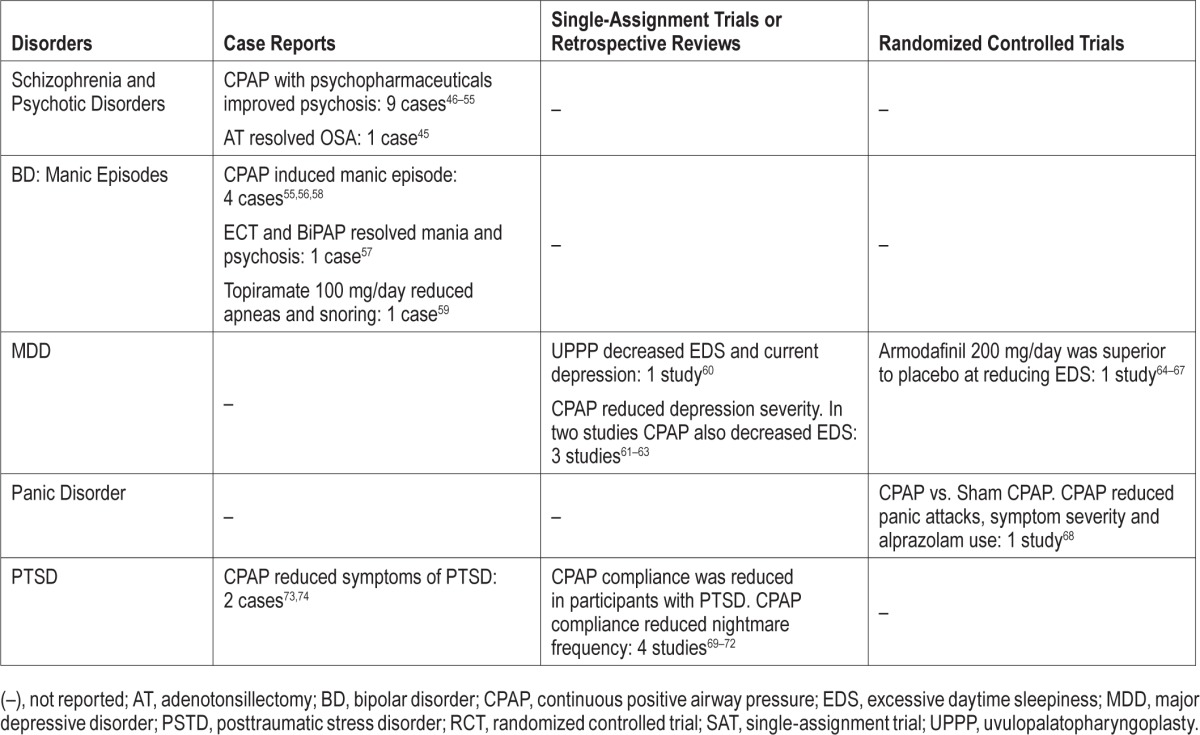
Ten case studies were identified concerning the treatment of a spectrum of schizophrenia and psychotic disorders in individuals with undiagnosed OSA (Table 2; Table S10, Appendix 2).45–54 Case reports concerned men in 83.3% of included studies. The most common intervention for OSA was CPAP in combination with existing psychiatric medications. In all but one case, CPAP resulted in improvement in excessive daytime sleepiness and negative psychotic symptoms. Chiner was the sole report of an acute psychotic episode induced by CPAP therapy.47 Lee was the sole case report on adenotonsillectomy resolving a subject's psychosis and OSA; this patient also had temporal lobe epilepsy, which could have been the basis for the psychotic symptoms.45 There are no available clinical trials evaluating the impact of the treatment of OSA on the presentation of symptoms of schizophrenia.
Table 2.
MOOD DISORDERS
Bipolar Disorder: Four case reports on the relationship between CPAP and manic episodes have been reported for individuals with BD (Table 2; Table S11, Appendix 2).55–58 In 4 cases, male subjects were admitted for depressive episodes where OSA was diagnosed after observation and PSG.55,56,58 The subjects developed mania after 2–4 weeks of CPAP use. These patients required mood stabilizers or atypical antipsychotics to stabilize the manic episode, and it is clear that only 2 subjects continued with long-term CPAP use. The fifth case report concerns a female subject with a mixed manic and psychotic episode, who was treated with 12 sessions of ECT followed by CPAP and psychiatric drugs.57
An additional case study by Weber examined the effect of adding 100 mg/day of topiramate to the existing drug regimen of a 50-year-old man with BD.59 The topiramate resulted in a reduction of snoring and a decrease in apneas from 20.0/h to 6.6/h. There was no weight change observed during the course of treatment.
The systematic review did not identify any clinical trials which evaluated the impact of treating OSA on symptoms of BD. The 4 case studies on emergent mania during treatment with CPAP may not be indicative of the overall effect of treating OSA in individuals with BD, as there is a tendency to publish case reports on exceptional circumstances, not successful routine treatment. The successful use of topiramate, which falls outside the parameters of routine treatment for OSA, further illustrates the high likelihood that publication bias is present for OSA and BD. Randomized controlled trials (RCTs) are required to determine the impact of treating OSA on symptoms of BD.
Major Depressive Disorder: Five intervention studies were included concerning the treatment of subjects with depressive disorders and OSA (Table 2; Table S12, Appendix 2).60–67 A single study examined the effect UPPP on patients with a current major depressive episode (MDE).60 At 6-month follow-up, the rate of current MDE had decreased to 10% from 34%, and hypersomnia decreased from 98% to 6%. Three studies examined the effect of CPAP in OSA in individuals with MDD. In all studies, CPAP reduced the severity of depression measured by the BDI and the Hamilton Rating Scale for Depression (HAM-D). Habukawa demonstrated that the decrease in BDI and HAM-D correlated with decreases in the Epworth Sleepiness Scale (ESS) score.62 El-Sherbini reported resolution of MDD for 6 of 11 subjects with Structured Clinical Interview for DSM-IV Disorders diagnosed MDD.63 The final study examined the effect of armodafinil in individuals with MDD or dysthymic disorder on stable antidepressant regimens and a stable CPAP regimen.64–67 The study showed that armodafinil resulted in minimal Clinical Global Impression of Change improvement over placebo and a significant decrease in ESS scores.
There is significant publication and clinical trial design bias present in the studies conducted in individuals with MDD and OSA. UPPP has only been evaluated in a single-open label trial which limits the generalizability of the conclusions of this study. The 3 trials on CPAP were also single-assignment, open-label trials that each had different inclusion criteria for OSA. In addition, Habukawa was the sole study that required concurrent antidepressant treatment. For all 4 single arm trials, the lack of comparison to a sham-control group or alternate active therapy makes it difficult to determine if the depressive symptoms respond to the specific treatment or to placebo effect. The fifth trial, Krystal, fulfills the criteria for a gold standard RCT; however, armodafinil is intended to treat symptoms of EDS secondary to OSA. This therapy may be of benefit to individuals who present with EDS and OSA, but it is not indicative of the effect of treating the primary OSA on symptoms of MDD. RCTs are required to determine the impact of treating OSA on symptoms of MDD.
ANXIETY DISORDERS
Panic Disorder: A randomized, crossover, sham-controlled study of CPAP was conducted in individuals with panic disorder (Table 2).68 In Takaesu, participants were randomized to 4 weeks CPAP, 4 weeks off, and 4 weeks of sham CPAP, or the same regimen with CPAP and sham CPAP reversed. At follow-up, individuals who underwent CPAP therapy showed reduction in panic attacks, symptom severity, and alprazolam use when compared to the sham treated subjects. The presence of a single study evaluating the impact of treating OSA on symptoms of panic disorder implies a high risk of publication bias; further RCTs are required to determine the impact of treating OSA in individuals with panic disorder.
Posttraumatic Stress Disorder: Four studies were identified concerning the treatment of OSA in subjects with PTSD (Table 2; Table S13, Appendix 2).69–72 All 4 studies that had a high RoB were retrospective analyses of subjects undergoing CPAP treatment. CPAP compliance was the primary outcome for all studies, which was lower in individuals with PTSD than controls. In El-Sohl, lack of CPAP adherence was associated with increased baseline nightmare severity and less baseline EDS.70 Krakow, Collen, and Gharaibeh found that reduction in nightmare frequency was associated with CPAP compliance.69,71,72
Two case reports concerning the treatment of OSA in PTSD were also identified (Table 2).73,74 Youakim reported a case of a 42-year-old male veteran with PTSD and severe OSA who was treated with CPAP, resulting in control of OSA symptoms, nightmare reduction, and improvement in PSTD symptoms.74 Yarlagadda reported a case study of a 35-year-old male with DSM-IV PTSD and chronic pain treated with fluoxetine 20 mg/ day and lorazepam 0.5 mg as needed, resulting in OSA, weight gain, and hypertension. CPAP treatment was initiated in combination with lorazepam and antihypertensives.73
The studies of the treatment of OSA in PTSD were all retrospective reviews. The primary outcome for 3 of 4 studies was CPAP compliance. While these studies had the advantage of being able to identify factors that determine CPAP compliance, they are less effective than randomized, prospective studies at examining the treatment impact on PSTD severity. The control groups of these studies were more clearly oriented around CPAP compliance than PTSD symptom reduction. Gharaibeh used non-compliant participants as controls, whereas El-Sohl and Collen employed a control group of individuals with OSA without PTSD. The Krakow, El-Sohl, and Gharaibeh studies also employed comparison to CPAP non-compliant participants who were unaware of their compliance scores to examine baseline and follow-up symptom severity, which helped control for the placebo effect to an extent. Prospective, controlled RCTs with PTSD severity scales as outcome measures are required to determine the impact of treating OSA on PTSD symptoms.
DISCUSSION
Summary of Evidence
The first goal of this systematic review was to determine if there are elevated levels of OSA in individuals with psychiatric disorders. The prevalence of OSA in the general population is 24% for men and 9% for women, using a definition of AHI ≥ 5 without EDS.1,2 Overall, there were insufficient reports on the prevalence of OSA in schizophrenia and other psychotic disorders, mood disorders, and anxiety disorders to draw conclusions about the prevalence of OSA in psychiatric patients. There were two disorders, MDD and PTSD, in which the quantity of reports was higher, although the RoB for these studies was still moderate-high for all clinical populations. Despite the selection bias identified for the studied populations with MDD and PTSD, it appears that there is an elevated prevalence of OSA in these disorders. The median prevalence of OSA in MDD in eight clinical populations was 48.1% (range: 0% to 66%), which is substantially higher than the general population. In the population-based samples, the range was 7.4% to 44%; however, the 7.4% prevalence for OSA in MDD is higher than the 3.3% prevalence of OSA in the total study population. The median prevalence of OSA in PTSD was 42.7% in seven clinic-based populations (range: 1.3% to 83%). Both population-based studies also identified an elevated prevalence of OSA in PSTD (range: 46.4% to 50%). In contrast, four clinical reports on the prevalence of OSA in BD have a median prevalence of 19.8%, which is within the range of the general population, despite a similarly wide range of prevalence of 2.9% to 66%. Due to the high RoB for these studies, future prevalence studies will be required to confirm the findings in MDD and PTSD and to begin to evaluate the prevalence of OSA in other psychiatric diagnoses.
The second goal of this review was to identify and evaluate interventions for OSA in individuals with psychiatric disorders. The systematic review found reports on the use of CPAP, UPPP, adenotonsillectomy, and armodafinil alone and in conjunction with psychopharmaceuticals in psychiatric populations. These studies were predominantly case studies, single assignment trials, and retrospective chart reviews with high RoB. The only RCT included was for the use of armodafinil in subjects with MDD. CPAP therapy had positive outcomes in all populations tested, except for BD. A series of case studies suggests that CPAP may be linked to the development of manic episodes in patients with BD, so these patients should be observed carefully in the first months of treatment. In MDD, single assignment trials of CPAP were associated with improved symptom severity and decreased EDS. In subjects with PTSD, CPAP reduced nightmare frequency and PTSD symptoms; however, PTSD was also a predictive factor for CPAP non-compliance. UPPP conducted in unmedicated subjects with MDD decreased hypersomnia by 92% and reduced current depression to 10% from 34%. The use of armodafinil in participants with a current major depressive episode in conjunction with an antidepressant resulted in improvement in subjective, but not objective symptoms of EDS. However, many of these results were obtained in studies without placebo or sham controls, so these conclusions are preliminary and indicate a need for further study. Due to the scarcity of studies evaluating the treatment of OSA in individuals and the high RoB for the included studies, RCTs are required to assess the efficacy of OSA treatment in individuals with psychiatric disorders.
Limitations
Overall, the systematic review reveals that the relationship between OSA and psychiatric disorders is an area requiring substantial further study. There are few studies examining OSA in individuals with clinically diagnosed psychiatric disorders. The available data are largely concentrated in patient populations with MDD or PTSD. This may be related to (1) the clinical observation that patients with OSA show increased depressive symptoms and (2) the interest of the military in the effective treatment of PTSD from a multisystem perspective.
The primary limitation of this systematic review is the overall quality of evidence. The RoB assessment was high for 78.9% of the included prevalence studies. In addition, there is substantial heterogeneity in study design for both prevalence and intervention studies with a lack of global agreement on the OSA and psychiatric diagnostic criteria. The retrospective studies for both prevalence and intervention are also further complicated by the use of lifetime psychiatric disorder diagnoses (as opposed to current episodes), which confounds the relationship between the severity of the psychiatric disorder and the presence and severity of OSA.
It is also apparent that many studies are conducted in samples of convenience, such as sleep clinic patient referrals with psychiatric diagnoses, psychiatric inpatients, or members of the military. While these studies present important preliminary findings, they are highly subject to publication bias. Populations selected from sleep clinic referrals for PSG due to reported symptoms of OSA are also subject to a selection bias. A sample that presents with clinical signs of OSA is significantly more predisposed to a high rate of OSA than a random sample of individuals with psychiatric disorders. Psychiatric inpatients have a selection bias due to their clinical status. Inpatients may have more severe psychiatric disorders than community dwelling individuals, which may increase the levels of obesity due to more expansive drug regimens that may contribute to metabolic syndrome. In addition, inpatients are likely to have a lower level of activity than community dwelling subjects due to the restrictions placed on their movements, which in turn could contribute to obesity and increased risk of developing OSA. The military population is primarily, if not exclusively, composed of male combat veterans and is the subject of the largest population-based study included in this review. This population may also have a different presentation of psychiatric disorders than the general population, as psychological testing a is a routine part of joining the armed forces and military personnel are exposed to different acute stressors than the general population.
The intervention studies are also subject to an overall high RoB. The scarcity of data is problematic, as the major interventions for OSA such as CPAP, adenotonsillectomy, UPPP, and wake-promoting drugs have not been studied in many psychiatric disorders. It is also problematic that many of these studies are single-assignment, open-label studies or retrospective chart reviews. In the absence of adequate sham or active controls, participant and personnel blinding, and randomization, it is difficult to determine if the improvement in psychiatric symptom severity is an effect of the treatment or the result of the placebo effect. Prospective, randomized controlled trials will be required to improve the quality of evidence to support the conclusions of these studies across all diagnoses.
CONCLUSIONS
The results of this systematic review point to limited evidence that OSA may be elevated in MDD and PTSD, with insufficient evidence to draw conclusions on any other DSMIV-TR diagnoses for schizophrenia and other psychotic disorders, mood disorders, and anxiety disorders. This does not necessarily indicate the absence of elevated levels of OSA in these populations, but points to the necessity of further study.
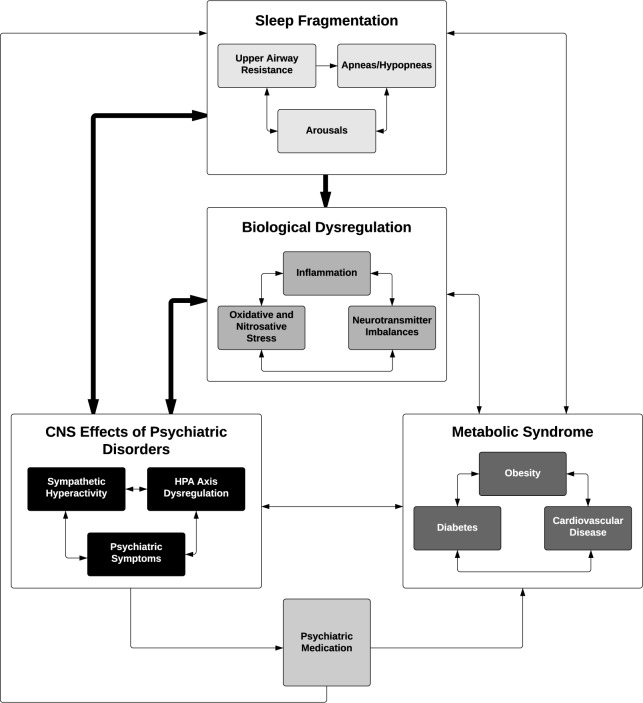
A number of factors may play a role in the association between psychiatric disorders such as MDD and PTSD with OSA (Figure 2). Oxidative and nitrosative stress, inflammation, and neurotransmitter imbalances play a role in all of the included psychiatric disorders.75 This underlying molecular dysregulation manifests as psychiatric symptoms, but it also alters the neurobiological and endocrine function of these individuals, leading to the association of psychiatric disorders with obesity, diabetes, and cardiovascular disease. Psychiatric disorders have independent associations with obesity, metabolic syndrome, cardiovascular disease, and smoking, which are all independent predictors of OSA.75–82 Central nervous system (CNS) alterations in psychiatric disorders may also lead to an increased risk of OSA, as sympathetic hyperactivity and hyperarousal states and resultant sleep fragmentation may lead to upper airway instability, which may contribute to subsequent OSA.83,84 In chronic psychiatric disorders such as MDD and PTSD, it can be hypothesized that the severity of the dysfunction in each of these areas leads to slow and incremental increases in CNS activation and endocrine dysregulation. Eventually, as all of these changes affect homeostasis, the individual responds with further biological dysregulation, and the feed-forward process continues with a probable end result of metabolic syndrome, severe CNS dysregulation, and OSA.
Figure 2. Proposed feed-forward pathway for the development of symptoms of upper airway instability and OSA from biological, psychiatric, and metabolic dysregulation.
Each symptom cluster is an independent entry point to the cycle. If left untreated, the presence of a risk factor increases the likelihood of the synergistic development of more symptoms from each cluster, resulting in OSA. The bolded arrows denote the most salient associations in the model.
The addition of psychiatric medication to correct the underlying molecular dysfunction and treat psychiatric symptoms may alleviate the issues in the CNS, but contributes to the further development of metabolic syndrome and possibly upper airway resistance as a result of extrapyramidal side effects, which in turn continue to stimulate the proposed feed-forward mechanisms leading to OSA.85–87 The association between obesity and psychiatric medication is well known,79,82,85,87,88 but the direct effects of these drugs on the upper airway and breathing during sleep are important factors. The tranquilizing effects of sleep medications and benzodiazepines may have direct effects on breathing during sleep which results in airway obstruction.89–93 Atypical antipsychotics, a group of medications that are generally associated with fewer extrapyramidal side effects, have been associated with an increased AHI in a cross-section of psychiatric patients, even when compared to patients with similar BMI and neck circumference taking benzodiazepines, opioids, and sleeping agents.86 This result points to an obesity independent effect of antipsychotics on OSA, possibly due to their extrapyramidal side effects.
The feed-forward mechanism proposed for the development of OSA (Figure 2) is a general hypothesis for the co-evolution of OSA in psychiatric disorders, and not all aspects will be present in every patient. Psychiatric disorders are associated with different types of neurobiological dysregulation that manifests as specific secondary effects. For example, HPA axis dysregulation is a prominent feature of PTSD and MDD, both of which appear to be associated with increased OSA. Anxiety disorders are associated with increased activity in the amygdala and insula, but only PTSD is correlated with additional decreased activity in the hippocampus, anterior cingulate cortex, and medial prefrontal cortex, which leads to a reduced ability to regulate the fear response.94 This contributes to sympathetic hyperarousal during both sleep and wakefulness, and the hypervigilant states observed in PTSD and resultant sleep fragmentation, which may lead to instability of upper airways during sleep and upper airway resistance. In MDD, HPA axis dysregulation is a result of increased corticotrophin releasing hormone sensitivity, glucocorticoid resistance, and increased cortisol levels.93,95 The downstream effects of this dysregulation are more likely to manifest as metabolic syndrome. These are both examples of disorder specific HPA axis dysregulation that manifest differently, but which have feed-forward effects into sleep symptoms eventually manifesting as OSA.
The most clinically significant finding of this review is the importance of recognizing and treating OSA when it occurs in an individual with a psychiatric disorder. OSA results in chronic intermittent hypoxia and arousals from sleep leading to sleep fragmentation, which has been shown to cause neurocognitive and mood deterioration in otherwise mentally robust individuals.96,97 In psychiatric populations who already experience dysregulation of mood and possible neurocognitive deficits, it is likely that the same degree of hypoxic insult and sleep fragmentation may result in greater decompensation of the psychiatric disorder. Case study reports and clinical trials both suggest that the treatment of OSA with CPAP can help to reduce the need for psychopharmaceuticals, and to help clarify which symptoms originate from the primary psychiatric illness. Polysomnographic evaluation of treatment-resistant psychiatric patients (for example, MDD and PTSD) can be considered an excellent tool to determine if a sleep disorder is complicating a refractory psychiatric disorder. In cases where OSA is present, a combination of CPAP and pharmaceutical treatment may result in greater therapeutic efficacy than traditional psychopharmacology alone.
Future studies on prevalence, incidence, and interventions need to be conducted to better ascertain the relationship between psychiatric disorder and OSA. There is a pressing need for additional population-based studies of OSA in community dwelling individuals with psychiatric disorders to allow for better comparison to the general population. Clinicians who wish to initiate trials of interventions for OSA in psychiatric population should consider sham or active comparator RCTs to better understand the impact of specific treatments in psychiatric populations. In light of the heterogeneity in study populations, participants in these studies should be diagnosed with OSA following the newly introduced ICSD-3 criteria, and the use of standard sleep outcomes should be encouraged to ensure that inter-study comparisons are possible in the future. Psychiatric diagnoses should continue to be made using a clinical interview following current DSM criteria, and validated psychiatric severity scales should be included as outcome measures, even where the primary trial goal is ascertaining compliance. This review reveals that there is a substantial opportunity to develop research projects to better understand the relationship between OSA and psychiatric disorders.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. Off-label or Investigational Use: This article reports clinical trial and case study outcomes that may include investigational use of drugs or devices. The authors do not make any recommendations to use drugs or devices off-label.
ACKNOWLEDGMENTS
The authors thank Katie Knapp, MSc, for her assistance in the preparation of this manuscript.
APPENDICIES
Appendix 1: Included and Excluded Studies
PubMed search strategy.
Included studies.
Excluded studies.
Reasons for exclusion.
Appendix 2: Systematic Review Results
Studies of the prevalence of OSA in individuals with schizophrenia and psychotic disorders.24,25,30,31
Studies of the prevalence of OSA in individuals with depressive disorders (MDD).24–26,30,32,35–39
Case studies of interventions for individuals with schizophrenia and psychotic disorders and OSA.45–54
Appendix 3: Risk of Bias
Section A: Prevalence Studies
ANCOLI-ISRAEL 1999
CARNEY 2006
DELDIN 2006
HATTORI 2009
HRUBOS-STROM 2012
KELLY 2013
KINOSHITA 2012
KRAKOW 2006
LEVINE 2001
MYSLIWIEC 2013
MYSLIWIEC 2013B
ONG 2009
SHARAFKHANEH 2005
SUMMERS 2010
VAN LIEMPT 2013
WINKELMAN 2001
YESAVAGE 2012
Section B: Interventions
COLLEN 2012
DAHLOF 2000
EL-SHERBINI 2011
EL-SOHL 2010
GHARAIBEH 2013
HABUKAWA 2010
KRYSTAL 2011
MACKINGER 2004
TAKAESU 2012
REFERENCES
- 1.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2005. The international classification of sleep disorders, 2nd ed.: diagnostic and coding manual. [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Pamidi S, Knutson KL, Ghods F, Mokhlesi B. Depressive symptoms and obesity as predictors of sleepiness and quality of life in patients with REM-related obstructive sleep apnea: cross-sectional analysis of a large clinical population. Sleep Med. 2011;12:827–31. doi: 10.1016/j.sleep.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Sampaio R, Pereira MG, Winck JC. Psychological morbidity, illness representations, and quality of life in female and male patients with obstructive sleep apnea syndrome. Psychol Health Med. 2012;17:136–49. doi: 10.1080/13548506.2011.579986. [DOI] [PubMed] [Google Scholar]
- 5.Diamanti C, Manali E, Ginieri-Coccossis M, Vougas K, et al. Depression, physical activity, energy consumption, and quality of life in OSA patients before and after CPAP treatment. Sleep Breath. 2013;17:1159–68. doi: 10.1007/s11325-013-0815-6. [DOI] [PubMed] [Google Scholar]
- 6.Andrews JG, Oei TP. The roles of depression and anxiety in the understanding and treatment of obstructive sleep apnea syndrome. Clin Psychol Rev. 2004;24:1031–49. doi: 10.1016/j.cpr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Sharafkhaneh A, Richardson P, Hirshkowitz M. Sleep apnea in a high risk population: a study of Veterans Health Administration beneficiaries. Sleep Med. 2004;5:345–50. doi: 10.1016/j.sleep.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Philipsen A, Hornyak M, Riemann D. Sleep and sleep disorders in adults with attention deficit/hyperactivity disorder. Sleep Med Rev. 2006;10:399–405. doi: 10.1016/j.smrv.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Saunamaki T, Jehkonen M. Depression and anxiety in obstructive sleep apnea syndrome: a review. Acta Neurol Scand. 2007;116:277–88. doi: 10.1111/j.1600-0404.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 10.Harris M, Glozier N, Ratnavadivel R, Grunstein RR. Obstructive sleep apnea and depression. Sleep Med Rev. 2009;13:437–44. doi: 10.1016/j.smrv.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Alam A, Chengappa KN, Ghinassi F. Screening for obstructive sleep apnea among individuals with severe mental illness at a primary care clinic. Gen Hosp Psychiatry. 2012;34:660–4. doi: 10.1016/j.genhosppsych.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Anderson KN, Waton T, Armstrong D, Watkinson HM, Mackin P. Sleep disordered breathing in community psychiatric patients. Eur J Psychiatry. 2012;26:86–95. [Google Scholar]
- 13.Gold AR. Functional somatic syndromes, anxiety disorders and the upper airway: a matter of paradigms. Sleep Med Rev. 2011;15:389–401. doi: 10.1016/j.smrv.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Grigg-Damberger M, Ralls F. Cognitive dysfunction and obstructive sleep apnea: from cradle to tomb. Curr Opin Pulm Med. 2012;18:580–7. doi: 10.1097/MCP.0b013e328358be18. [DOI] [PubMed] [Google Scholar]
- 15.Lin WC, Winkelman JW. Obstructive sleep apnea and severe mental illness: evolution and consequences. Curr Psychiatry Rep. 2012;14:503–10. doi: 10.1007/s11920-012-0307-6. [DOI] [PubMed] [Google Scholar]
- 16.Sculthorpe LD, Douglass AB. Sleep pathologies in depression and the clinical utility of polysomnography. Can J Psychiatry. 2010;55:413–21. doi: 10.1177/070674371005500704. [DOI] [PubMed] [Google Scholar]
- 17.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 18.Butcher J, Dahlstrom W, Graham J, Tellegen A, Kaemmer B. Minneapolis, MN: University of Minnesota Press; 1989. The Minnesota Multiphasic Personality Inventory-2 (MMPI-2): manual for administration and scoring. [Google Scholar]
- 19.McNair DM, Lorr M, Droppleman OF. Manual for the Profile of Mood States. San Diego: Educational & Industrial Testing Service; 1971. [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Arlington, VA: American Psychiatric Association; 2005. Text Revision. [Google Scholar]
- 22.International Classification of Diseases, Ninth Revision, Clinical Modification. Los Angeles: Practice Management Information Corporation; 2005. [Google Scholar]
- 23.International Classification of Diseases and Related Health Problems, 10th Revision. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 24.Winkelman JW. Schizophrenia, obesity, and obstructive sleep apnea. J Clin Psychiatry. 2001;62:8–11. doi: 10.4088/jcp.v62n0103. [DOI] [PubMed] [Google Scholar]
- 25.Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28:1405–11. doi: 10.1093/sleep/28.11.1405. [DOI] [PubMed] [Google Scholar]
- 26.Mysliwiec V, McGraw L, Pierce R, Smith P, Trapp B, Roth BJ. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36:167–74. doi: 10.5665/sleep.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoy D, Brooks P, Woolf A, Blyth F, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934–9. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 The Cochrane Collaboration. 2011 [Google Scholar]
- 29.SPSS Statistics for Windows, Version 20. Armonk, NY: IBM; 2011. [Google Scholar]
- 30.Levine J, Chengappa KN, Patel A, Vagnucci A, et al. Obesity and medical illnesses in psychiatric patients admitted to a long-term psychiatric facility. J Psychiatr Pract. 2001;7:432–9. doi: 10.1097/00131746-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Ancoli-Israel S, Martin J, Jones DW, Caligiuri M, et al. Sleep-disordered breathing and periodic limb movements in sleep in older patients with schizophrenia. Biol Psychiatry. 1999;45:1426–32. doi: 10.1016/s0006-3223(98)00166-8. [DOI] [PubMed] [Google Scholar]
- 32.Hattori M, Kitajima T, Mekata T, Kanamori A, et al. Risk factors for obstructive sleep apnea syndrome screening in mood disorder patients. Psychiatry Clin Neurosci. 2009;63:385–91. doi: 10.1111/j.1440-1819.2009.01956.x. [DOI] [PubMed] [Google Scholar]
- 33.Kelly T, Douglas L, Denmark L, Brasuell G, Lieberman DZ. The high prevalence of obstructive sleep apnea among patients with bipolar disorders. J Affect Disord. 2013;151:54–8. doi: 10.1016/j.jad.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 34.Kelly TF, Douglas L, Denmark L, Brausell GM, Lieberman DZ. The incidence of sleep apnea in bipolar disorders. Bipolar Disorders. 2011;13:25–26. [Google Scholar]
- 35.Deldin PJ, Phillips LK, Thomas RJ. A preliminary study of sleep-disordered breathing in major depressive disorder. Sleep Med. 2006;7:131–9. doi: 10.1016/j.sleep.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Carney RM, Howells WB, Freedland KE, et al. Depression and obstructive sleep apnea in patients with coronary heart disease. Psychosom Med. 2006;68:443–8. doi: 10.1097/01.psy.0000204632.91178.26. [DOI] [PubMed] [Google Scholar]
- 37.Ong JC, Gress JL, San Pedro-Salcedo MG, Manber R. Frequency and predictors of obstructive sleep apnea among individuals with major depressive disorder and insomnia. J Psychosom Res. 2009;67:135–41. doi: 10.1016/j.jpsychores.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Summers D, Lazowski L, Fitzpatrick M, et al. Prevalence of obstructive sleep apnea in patients with treatment resistant depression. Prevalence of obstructive sleep apnea in patients with treatment resistant depression. Int J Neuropsychopharmacol. 2010:161–2. [Google Scholar]
- 39.Hrubos-Strom H, Einvik G, Nordhus IH, et al. Sleep apnoea, anxiety, depression and somatoform pain: a community-based high-risk sample. Eur Respir J. 2012;40:400–7. doi: 10.1183/09031936.00111411. [DOI] [PubMed] [Google Scholar]
- 40.Krakow B, Melendrez D, Warner TD, et al. Signs and symptoms of sleep-disordered breathing in trauma survivors: a matched comparison with classic sleep apnea patients. J Nerv Ment Dis. 2006;194:433–9. doi: 10.1097/01.nmd.0000221286.65021.e0. [DOI] [PubMed] [Google Scholar]
- 41.Kinoshita LM, Yesavage JA, Noda A, et al. Modeling the effects of obstructive sleep apnea and hypertension in Vietnam veterans with PTSD. Sleep Breath. 2012;16:1201–9. doi: 10.1007/s11325-011-0632-8. [DOI] [PubMed] [Google Scholar]
- 42.Yesavage JA, Kinoshita LM, Kimball T, et al. Sleep-disordered breathing in Vietnam veterans with posttraumatic stress disorder. Am J Geriatr Psychiatry. 2012;20:199–204. doi: 10.1097/JGP.0b013e3181e446ea. [DOI] [PubMed] [Google Scholar]
- 43.Mysliwiec V, Gill J, Lee H, et al. Sleep disorders in US military personnel: a high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144:549–57. doi: 10.1378/chest.13-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Liempt S, Westenberg HG, Arends J, Vermetten E. Obstructive sleep apnea in combat-related posttraumatic stress disorder: a controlled polysomnography study. Eur J Psychotraumatol. 2011:2. doi: 10.3402/ejpt.v2i0.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee S, Chiu HF, Chen CN. Psychosis in sleep apnoea. Aust N Z J Psychiatry. 1989;23:571–3. doi: 10.3109/00048678909062627. [DOI] [PubMed] [Google Scholar]
- 46.Bottlender R, Moller HJ. Negative symptoms due to sleep apnea syndrome in a patient with a delusional disorder. Eur Psychiatry. 1999;14:352. doi: 10.1016/s0924-9338(99)00155-8. [DOI] [PubMed] [Google Scholar]
- 47.Chiner E, Arriero JM, Signes-Costa J, Marco J. Acute psychosis after CPAP treatment in a schizophrenic patient with sleep apnoea-hypopnoea syndrome. Eur Respir J. 2001;17:313–5. doi: 10.1183/09031936.01.17203130. [DOI] [PubMed] [Google Scholar]
- 48.Dennis JL, Crisham KP. Chronic assaultive behavior improved with sleep apnea treatment. J Clin Psychiatry. 2001;62:571–2. doi: 10.4088/jcp.v62n07b13. [DOI] [PubMed] [Google Scholar]
- 49.Wirshing DA, Pierre JM, Wirshing WC. Sleep apnea associated with antipsychotic-induced obesity. J Clin Psychiatry. 2002;63:369–70. doi: 10.4088/jcp.v63n0415f. [DOI] [PubMed] [Google Scholar]
- 50.Boufidis S, Kosmidis MH, Bozikas VP, Daskalopoulou-Vlahoyianni E, Pitsavas S, Karavatos A. Treatment outcome of obstructive sleep apnea syndrome in a patient with schizophrenia: case report. Int J Psychiatry Med. 2003;33:305–10. doi: 10.2190/GGN0-Y09A-QV4X-DBA0. [DOI] [PubMed] [Google Scholar]
- 51.Sugishita K, Yamasue H, Kasai K. Continuous positive airway pressure for obstructive sleep apnea improved negative symptoms in a patient with schizophrenia. Psychiatry Clin Neurosci. 2010;64:665. doi: 10.1111/j.1440-1819.2010.02146.x. [DOI] [PubMed] [Google Scholar]
- 52.Velasco-Rey MC, Sanchez-Munoz M, Gutierrez-Lopez MI, Trujillo-Borrego A, Sanchez-Bonome L. Psychotic depression induced by obstructive sleep apnoea syndrome (OSAS): a case reported. Actas Esp Psiquiatr. 2012;40:43–5. [PubMed] [Google Scholar]
- 53.Troy D, Elcock E, Owen C. Persevering with treatment of co-morbid obstructive sleep apnoea in a psychiatric setting. Australas Psychiatry. 2013;21:180–1. doi: 10.1177/1039856212471408. [DOI] [PubMed] [Google Scholar]
- 54.Seeman MV. Diagnosis and treatment of sleep apnoea in women with schizophrenia. J Ment Health. 2014;23:191–6. doi: 10.3109/09638237.2013.869572. [DOI] [PubMed] [Google Scholar]
- 55.Hilleret H, Jeunet E, Osiek C, Mohr S, Blois R, Bertschy G. Mania resulting from continuous positive airways pressure in a depressed man with sleep apnea syndrome. Neuropsychobiol. 2001;43:221–4. doi: 10.1159/000054893. [DOI] [PubMed] [Google Scholar]
- 56.Berge D, Salgado P, Rodriguez A, Bulbena A. Onset of mania after CPAP in a man with obstructive sleep apnea. Psychosomatics. 2008;49:447–9. doi: 10.1176/appi.psy.49.5.447. [DOI] [PubMed] [Google Scholar]
- 57.Bastiampillai T, Khor LJ, Dhillon R. Complicated management of mania in the setting of undiagnosed obstructive sleep apnea. J ECT. 2011;27:e15–6. doi: 10.1097/YCT.0b013e3181dd7c82. [DOI] [PubMed] [Google Scholar]
- 58.Aggarwal R, Baweja R, Saunders EF, Singareddy R. CPAP-induced mania in bipolar disorder: a case report. Bipolar Disord. 2013;15:803–7. doi: 10.1111/bdi.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber MVK. Topiramate for obstructive sleep apnea and snoring. Am J Psychiatry. 2002;159:872–73. doi: 10.1176/appi.ajp.159.5.872-a. [DOI] [PubMed] [Google Scholar]
- 60.Dahlöf P, Ejnell H, Hällström T, Hedner J. Surgical treatment of the sleep apnea syndrome reduces associated major depression. Int J Behav Med. 2000;7:73–88. [Google Scholar]
- 61.Mackinger HF, Svaldi JJ. Autobiographical memory predicts cognitive but not somatic change in sleep apnea patients vulnerable for affective disorder. J Affect Disord. 2004;81:17–22. doi: 10.1016/S0165-0327(03)00170-8. [DOI] [PubMed] [Google Scholar]
- 62.Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: contribution of daytime sleepiness to residual depressive symptoms. Sleep Med. 2010;11:552–7. doi: 10.1016/j.sleep.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 63.El-Sherbini AM, Bediwy AS, El-Mitwalli A. Association between obstructive sleep apnea (OSA) and depression and the effect of continuous positive airway pressure (CPAP) treatment. Neuropsychiatr Dis Treat. 2011;7:715–21. doi: 10.2147/NDT.S26341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krystal AD. A double-blind, placebo-controlled study of armodafinil for excessive sleepiness in patients with treated obstructive sleep apnea and comorbid depression: correction. J Clin Psychiatry. 2011;72:1157. doi: 10.4088/JCP.09m05536gry. [DOI] [PubMed] [Google Scholar]
- 65.Krystal AD, Harsh J, Yang R, Rippon GA, Lankford A. Effect of armodafinil on patient functioning and fatigue: a multicenter, randomized, doubleblind, placebo-controlled, parallel-group study in patients with residual excessive sleepiness associated with treated obstructive sleep apnea and a comorbid depressive disorder. Sleep. 2010;33:A7–A8. (Abstract Suppl) [Google Scholar]
- 66.Krystal AD, Harsh JR, Yang R, Rippon GA, Lankford A. Randomized, double-blind, placebo-controlled study of armodafinil in patients with residual excessive sleepiness associated with treated obstructive sleep apnea and comorbid depressive disorders. Chest. 2009;136:70S. doi: 10.4088/JCP.09m05536gry. (4_Meeting abstracts) [DOI] [PubMed] [Google Scholar]
- 67.Krystal AD, Harsh JR, Yang R, Rippon GA, Lankford DA. A double-blind, placebo-controlled study of armodafinil for excessive sleepiness in patients with treated obstructive sleep apnea and comorbid depression. J Clin Psychiatry. 2010;71:32–40. doi: 10.4088/JCP.09m05536gry. [DOI] [PubMed] [Google Scholar]
- 68.Takaesu Y, Inoue Y, Komada Y, Kagimura T, Iimori M. Effects of nasal continuous positive airway pressure on panic disorder comorbid with obstructive sleep apnea syndrome. Sleep Med. 2012;13:156–60. doi: 10.1016/j.sleep.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 69.Krakow B, Lowry C, Germain A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res. 2000;49:291–8. doi: 10.1016/s0022-3999(00)00147-1. [DOI] [PubMed] [Google Scholar]
- 70.El-Solh AA, Ayyar L, Akinnusi M, Relia S, Akinnusi O. Positive airway pressure adherence in veterans with posttraumatic stress disorder. Sleep. 2010;33:1495–500. doi: 10.1093/sleep/33.11.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Collen JF, Lettieri CJ, Hoffman M. The impact of posttraumatic stress disorder on CPAP adherence in patients with obstructive sleep apnea. J Clin Sleep Med. 2012;8:667–72. doi: 10.5664/jcsm.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gharaibeh K, Tamanna S, Ullah M, Geraci SA. Effect of continuous positive airway pressure therapy on nightmares in patients with post-traumatic stress disorder and obstructive sleep apnea. J Invest Med. 2013;61:480–1. [Google Scholar]
- 73.Yarlagadda AR, Brown AB, Clayton AH. Onset of obstructive sleep apnea after initiation of psychotropic agents. Prim Care Companion J Clin Psychiatry. 2007;9:471. doi: 10.4088/pcc.v09n0611h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Youakim JM, Doghramji K, Schutte SL. Posttraumatic stress disorder and obstructive sleep apnea syndrome. Psychosomatics. 1998;39:168–71. doi: 10.1016/S0033-3182(98)71365-9. [DOI] [PubMed] [Google Scholar]
- 75.Lopresti AL, Drummond PD. Obesity and psychiatric disorders: commonalities in dysregulated biological pathways and their implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:92–9. doi: 10.1016/j.pnpbp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 76.Epstein LJ, Kristo D, Strollo PJ, Jr., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 77.Caples SM, Garcia-Touchard A, Somers VK. Sleep-disordered breathing and cardiovascular risk. Sleep. 2007;30:291–303. doi: 10.1093/sleep/30.3.291. [DOI] [PubMed] [Google Scholar]
- 78.Edmondson D, Kronish IM, Shaffer JA, Falzon L, Burg MM. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J. 2013;166:806–14. doi: 10.1016/j.ahj.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luppino FS, Bouvy PF, Giltay EJ, Penninx BW, Zitman FG. The metabolic syndrome and related characteristics in major depression: inpatients and outpatients compared: metabolic differences across treatment settings. Gen Hosp Psychiatry. 2014;36:509–15. doi: 10.1016/j.genhosppsych.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 80.Prados-Torres A, Calderon-Larranaga A, Hancco-Saavedra J, Poblador-Plou B, van den Akker M. Multimorbidity patterns: a systematic review. J Clin Epidemiol. 2014;67:254–66. doi: 10.1016/j.jclinepi.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 81.Carra G, Bartoli F, Carretta D, et al. The prevalence of metabolic syndrome in people with severe mental illness: a mediation analysis. Soc Psychiatry Psychiatr Epidemiol. 2014;49:1739–46. doi: 10.1007/s00127-014-0835-y. [DOI] [PubMed] [Google Scholar]
- 82.Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–9. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 83.Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150:481–5. doi: 10.1164/ajrccm.150.2.8049833. [DOI] [PubMed] [Google Scholar]
- 84.Krakow B, Haynes PL, Warner TD, et al. Nightmares, insomnia, and sleep-disordered breathing in fire evacuees seeking treatment for posttraumatic sleep disturbance. J Trauma Stress. 2004;17:257–68. doi: 10.1023/B:JOTS.0000029269.29098.67. [DOI] [PubMed] [Google Scholar]
- 85.Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One. 2014;9:e94112. doi: 10.1371/journal.pone.0094112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rishi MA, Shetty M, Wolff A, Amoateng-Adjepong Y, Manthous CA. Atypical antipsychotic medications are independently associated with severe obstructive sleep apnea. Clin Neuropharmacol. 2010;33:109–13. doi: 10.1097/WNF.0b013e3181db8040. [DOI] [PubMed] [Google Scholar]
- 87.Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71:1259–72. doi: 10.4088/JCP.09r05346blu. [DOI] [PubMed] [Google Scholar]
- 88.Gibson M, Carek PJ, Sullivan B. Treatment of co-morbid mental illness in primary care: how to minimize weight gain, diabetes, and metabolic syndrome. Int J Psychiatry Med. 2011;41:127–42. doi: 10.2190/PM.41.2.c. [DOI] [PubMed] [Google Scholar]
- 89.Cirignotta F, Mondini S, Zucconi M, Gerardi R, Farolfi A, Lugaresi E. Zolpidempolysomnographic study of the effect of a new hypnotic drug in sleep apnea syndrome. Pharmacol Biochem Behav. 1988;29:807–9. doi: 10.1016/0091-3057(88)90212-2. [DOI] [PubMed] [Google Scholar]
- 90.Berry RB, Kouchi K, Bower J, Prosise G, Light RW. Triazolam in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:450–4. doi: 10.1164/ajrccm.151.2.7842205. [DOI] [PubMed] [Google Scholar]
- 91.Yaddanapudi S, Batra YK, Balagopal A, Nagdeve NG. Sedation in patients above 60 years of age undergoing urological surgery under spinal anesthesia: comparison of propofol and midazolam infusions. J Postgrad Med. 2007;53:171–5. doi: 10.4103/0022-3859.33858. [DOI] [PubMed] [Google Scholar]
- 92.Norton JR, Ward DS, Karan S, et al. Differences between midazolam and propofol sedation on upper airway collapsibility using dynamic negative airway pressure. Anesthesiology. 2006;104:1155–64. doi: 10.1097/00000542-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 93.Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 2013;11:129. doi: 10.1186/1741-7015-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gupta MA. Review of somatic symptoms in post-traumatic stress disorder. Int Rev Psychiatry. 2013;25:86–99. doi: 10.3109/09540261.2012.736367. [DOI] [PubMed] [Google Scholar]
- 95.Karaca Z, Ismailogullari S, Korkmaz S, et al. Obstructive sleep apnoea syndrome is associated with relative hypocortisolemia and decreased hypothalamo-pituitary-adrenal axis response to 1 and 250mug ACTH and glucagon stimulation tests. Sleep Med. 2013;14:160–4. doi: 10.1016/j.sleep.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 96.Ferini-Strambi L, Marelli S, Galbiati A, Castronovo C. Effects of continuous positive airway pressure on cognitition and neuroimaging data in sleep apnea. Int J Psychophysiol. 2013;89:203–12. doi: 10.1016/j.ijpsycho.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 97.Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology. 2013;18:61–70. doi: 10.1111/j.1440-1843.2012.02255.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PubMed search strategy.
Included studies.
Excluded studies.
Reasons for exclusion.
Studies of the prevalence of OSA in individuals with schizophrenia and psychotic disorders.24,25,30,31
Studies of the prevalence of OSA in individuals with depressive disorders (MDD).24–26,30,32,35–39
Case studies of interventions for individuals with schizophrenia and psychotic disorders and OSA.45–54
ANCOLI-ISRAEL 1999
CARNEY 2006
DELDIN 2006
HATTORI 2009
HRUBOS-STROM 2012
KELLY 2013
KINOSHITA 2012
KRAKOW 2006
LEVINE 2001
MYSLIWIEC 2013
MYSLIWIEC 2013B
ONG 2009
SHARAFKHANEH 2005
SUMMERS 2010
VAN LIEMPT 2013
WINKELMAN 2001
YESAVAGE 2012
COLLEN 2012
DAHLOF 2000
EL-SHERBINI 2011
EL-SOHL 2010
GHARAIBEH 2013
HABUKAWA 2010
KRYSTAL 2011
MACKINGER 2004
TAKAESU 2012