Abstract
We report the case of a 52-year-old female with spinocerebellar ataxia (SCA) 13. She presented with complaints of insomnia and had a history of restless legs syndrome. Her polysomnogram revealed that she had a significantly elevated periodic limb movement index, mild obstructive sleep apnea, and the absence of REM sleep. Sleep disorders have previously been described in patients with SCA 1, SCA 2, SCA 3, and SCA 6. To our knowledge, this is the first description of sleep disorders in a patient with SCA 13.
Citation:
Kapoor M, Greenough G. Spectrum of sleep disorders in a patient with spinocerebellar ataxia 13. J Clin Sleep Med 2015;11(2):177–179.
Keywords: spinocerebellar ataxia, sleep, insomnia, restless legs syndrome, periodic limb movements, obstructive sleep apnea
Spinocerebellar ataxia (SCA) consists of a group of autosomal dominant disorders in which there is degeneration of the cerebellum and its connections. There are several different genetically heterogeneous subtypes, but all are characterized by ataxia and may have other overlapping clinical features such as dysarthria, incoordination, and nystagmus.1,2 No clinical sign is specific for any one subtype.1 Patients with SCA 13 can have a variable presentation ranging from slowly progressive childhood-onset ataxia along with mild intellectual disability to adult-onset progressive ataxia.3 The underlying defect is a mutation in the KCNC3 gene4 as opposed to CAG trinucleotide repeats, as is the case in patients with SCA 1, SCA 2, SCA 3, and SCA 6.5 The clinical features of SCA 13 overlap with those of the other SCAs and the correct diagnosis can only be established by genetic testing for mutations in the KCNC3 gene.3
Several authors have previously described sleep changes and disorders in patients with SCAs (Table 1). There is a significantly increased prevalence of insomnia and restless legs syndrome (RLS) in patients with SCA 2 and SCA 3.6,7 REM sleep behavior disorder (RBD) has been associated with SCA 3.8–10 Polysomnographic studies have shown a significant decrease in sleep efficiency and REM sleep as well as a significant increase in the periodic limb movement index and REM without atonia in patients with SCA 2 and SCA 3.6,9 A significant decrease in sleep efficiency and REM sleep has also been reported in asymptomatic SCA 2 gene carriers.11 Sleep disorders have also been reported in patients with SCA 112,13 and SCA 6.14 Patients with SCA 6 have been reported to score higher on the Epworth Sleepiness Scale and the Pittsburgh Sleep Quality Index.15
Table 1.
Sleep related findings in the SCAs.
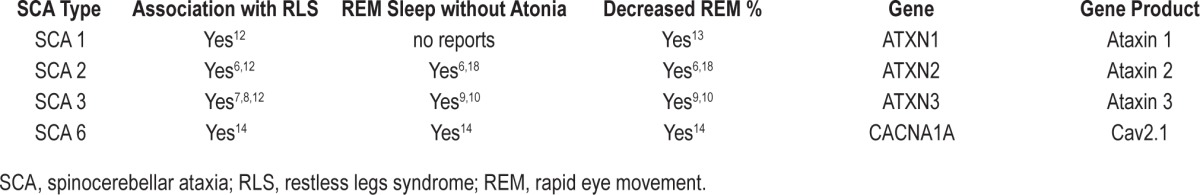
To our knowledge, there are no previous descriptions of sleep disorders in patients with SCA 13. We present the case of a patient with this condition and describe her associated comorbid sleep disorders.
REPORT OF CASE
A 52-year-old female was referred to the Dartmouth Hitchcock Sleep Disorders Center for the evaluation of her sleep onset and maintenance insomnia. She reported typically getting in to bed at 10 pm and then having 45- to 60-minute sleep latency. She attributed this prolonged latency to having difficulty shutting her mind off. She had symptoms suggestive of RLS and felt that at times this discomfort would interfere with her ability to fall asleep. She had between none to three night time awakenings during the night. She was not sure what would awaken her in the night. Once awake, she would again have difficulty shutting her mind off and it would take her approximately one hour to fall back asleep. She typically would wake up for the day at around 5 am. In regard to daytime symptoms, she scored 8 on the Epworth Sleepiness Scale. She reported having some daytime fatigue and sleepiness. She denied taking any planned naps during the day or feeling sleepy while driving. She admitted to having around two episodes of involuntary dozing per week in the evening and each episode would last for around 30 minutes. Review of systems was negative for snoring, witnessed apneas, nocturnal gasping, dream enactment, sleep walking, sleep related hallucinations, sleep paralysis, and cataplexy.
Her past medical and surgical history was significant for anemia, dyslipidemia, depression, tonsillectomy, esophageal reflux, and SCA 13, the diagnosis of which had been confirmed by genetic testing. The latter had revealed a DNA sequence variant in her KCNC3 gene of an adenosine to guanine transition at nucleotide position 1771. She reported having mild problems with her balance and hand dexterity attributed to her SCA. With regard to her anemia, lab work revealed that her iron saturation was 31% and serum ferritin was 33 mcg/L. In regard to medication, she was taking gabapentin 300 mg twice a day and this had been started around 6 months prior for her RLS. She was also on duloxetine 30 mg once a day for her depression. This medication had been started one month prior to evaluation. Prior to this, she had been on sertraline. Her mood had improved with the duloxetine, but she did not find any improvement in her sleep with this medication or previously with the sertraline. Other pertinent medications included lorazepam 1 mg at bedtime, which helped somewhat with her insomnia and ferrous gluconate 324 mg twice daily for anemia. Two of her sisters had spinocerebellar ataxia, and one of these sisters had also been diagnosed with obstructive sleep apnea. On examination, our patient weighed 130 lb and had a BMI of 21.63 kg/m2. She had a Mallampati grade III oropharynx, a deviated nasal septum, and a neck circumference of 11.5 inches. She also had minimal dysarthria and mild dysmetria.
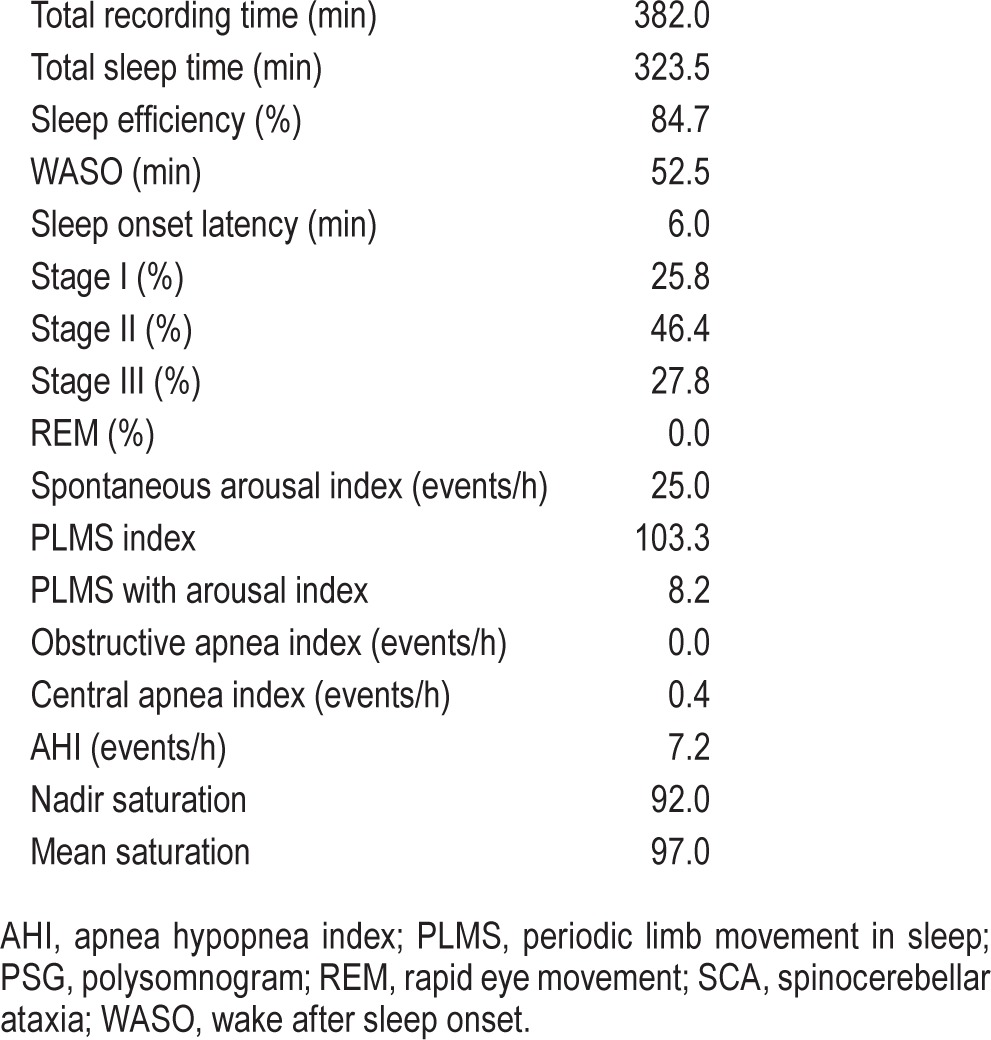
She was diagnosed with psychophysiologic insomnia. Formal cognitive behavioral therapy for insomnia (CBTI) was offered to her, but she declined. An overnight polysomnogram was performed (Table 2). The most notable findings were the absence of REM sleep, an elevated periodic limb movement index, and a modest elevation in the apnea hypopnea index (AHI). Options for further management were discussed with her. She deferred CPAP but was interested in optimizing the treatment of her RLS. She was started on pramipexole 0.125 mg with dinner, and this was gradually increased to 0.375 mg once a day. During a follow-up visit, she reported a significant improvement in her RLS symptoms and her sleep onset and maintenance insomnia.
Table 2.
PSG findings in a patient with SCA 13.
DISCUSSION
Our case adds to the existing body of knowledge on sleep disorders in patients with spinocerebellar ataxia. This patient with SCA 13 had a history suggestive of a psychophysiologic insomnia and RLS. Her polysomnogram was most notable for frequent PLMS, the absence of REM sleep and mild sleep apnea. Clinically, she responded well to a dopamine agonist with improvement in her RLS symptoms and insomnia.
This patient with SCA 13 had similar sleep complaints (RLS and insomnia) to patients with some of the other spinocerebellar ataxias. She did have a ferritin level less than 50 mcg/L and was on duloxetine, both of which could have contributed to her RLS. She could also have had a neuropathy, though we did not perform an EMG/NCS on her. That being said, neuropathy is not considered a typical feature of SCA 13. It has also been reported that the prevalence of EMG/NCS abnormalities is the same in SCA 1, SCA 2, and SCA 3 patients with and without RLS.7,12 Studies have shown a reduction in striatal dopamine D2 receptor binding potential in the later stages of SCA 1, SCA 2, and SCA 3, and dopamine transporter (DAT) density in patients with SCA 3 but have failed to show a correlation between these findings and the presence of RLS in these patients.16,17
Of note was the absence of REM sleep on the polysomnogram of this patient with SCA 13. The significance of this is unclear. The absence of REM sleep in this patient could be secondary to her medications (duloxetine and lorazepam), a first night effect, or possibly because of her underlying neurologic condition. REM sleep has been reported as decreased in SCA 2, 3, and 6.6,9,10,14,18 The absence of REM sleep in this patient is consistent with the aforementioned findings in the other SCAs.
Obstructive sleep apnea, albeit of a mild degree was found in our subject. Her sister, who also has SCA 13, has obstructive sleep apnea as well. It is possible the occurrence of obstructive sleep apnea in our patient and her sister was coincidental. Snoring and witnessed apneas have been reported to occur together more frequently in SCA 3 patients.7 Along these same lines, patients with SCA 1, SCA 2, SCA 3, and SCA 6 have been reported to have sleep disordered breathing confirmed by polysomnography.10,13,14,18,19 It must be noted, though, that studies using polysomnography in patients with SCA 3 have failed to show a significantly higher incidence of sleep disordered breathing and a significantly different average respiratory disturbance index (RDI) or average apnea hypopnea index (AHI) in these patients as compared to healthy controls.9,10 The cerebellum plays an underappreciated role in the sleep wake cycle20 and breathing,21–23 and its involvement in patients with SCA might lead to some of their sleep and respiratory problems.
This patient had a mutation in her KCNC3 gene which encodes a type of voltage gated potassium channel called Kv3.3 and is expressed throughout the brain with marked expression in the Purkinje cells of the cerebellum.24 It has been suggested that mutations in this gene can lead to altered calcium homeostasis and cell death.25 This gene is also expressed in areas of the brain involved in the sleep wake cycle like the reticular nucleus of the thalamus and the reticular formation.24,26 One would thus expect that mutations in this gene would lead to alterations in sleep. To test this hypothesis, Espinosa and colleagues conducted a study on mice in which this gene and another closely related gene called the KCNC1 gene had been knocked out.27 The KCNC1 gene encodes a type of voltage gated potassium channel called Kv3.1 and is co-expressed with the KCNC3 gene in several areas of the brain.28 The authors found that Kv3.1−/− Kv3.3−/− (or double knock out) mice and Kv3.1−/− Kv3.3+/+ mice slept significantly less compared to Kv3.1+/+ Kv3.3+/+ (or wild-type [WT]) mice. This same study also showed that Kv3.1−/− Kv3.3−/− and Kv3.1−/− Kv3.3+/+ mice are more hyperactive than WT mice. The authors suggested that the KCNC3 gene may have a minor effect on sleep as compared to the KCNC1 gene which appeared to have a major effect. There have been subsequent studies in mice which have looked at the effects of the KCNC1 and KCNC3 gene on sleep, but it has been difficult to discern from these studies what role defects in the KCNC3 gene have on sleep.26,28
Comorbid sleep disorders in the SCA population can further reduce the functional level and quality of life of these patients. Providers should be aware of the potentially increased incidence of sleep disorders in SCA patients. This patient was on multiple medications including duloxetine, gabapentin, and lorazepam, which could have contributed to or altered some of her sleep related symptoms and PSG findings. These abnormalities, however, could also have been secondary to her underling SCA 13. The presence of similar abnormalities in other SCA types would suggest a relationship. Further study of larger populations of patients with SCA 13 would be required to see what associations could be definitively made between SCA 13 and these sleep disorders. Identifying and managing these early on can help these patients have an improved quality of life and improved functional status as well as prevent the development of further complications.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Schols L, Bauer P, Schmidt T, Schulte T, Riess O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3:291–304. doi: 10.1016/S1474-4422(04)00737-9. [DOI] [PubMed] [Google Scholar]
- 2.Subramony SH. Overview of autosomal dominant ataxias. Handb Clin Neurol. 2012;103:389–98. doi: 10.1016/B978-0-444-51892-7.00024-3. [DOI] [PubMed] [Google Scholar]
- 3.Pulst SM. Spinocerebellar Ataxia Type 13. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews. Seattle, WA: University of Washington; 1993-2014. Available from http://www.ncbi.nlm.nih.gov/books/NBK1116/ [PubMed] [Google Scholar]
- 4.Figueroa KP, Minassian NA, Stevanin G, et al. KCNC3: phenotype, mutations, channel biophysics-a study of 260 familial ataxia patients. Hum Mutat. 2010;31:191–6. doi: 10.1002/humu.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gatchel JR, Zoghbi HY. Diseases of unstable repeat expansion: mechanisms and common principles. Nat Rev Genet. 2005;6:743–55. doi: 10.1038/nrg1691. [DOI] [PubMed] [Google Scholar]
- 6.Velazquez-Perez L, Voss U, Rodriguez-Labrada R, et al. Sleep disorders in spinocerebellar ataxia type 2 patients. Neurodegener Dis. 2011;8:447–54. doi: 10.1159/000324374. [DOI] [PubMed] [Google Scholar]
- 7.D'Abreu A, Franca M, Jr., Conz L, et al. Sleep symptoms and their clinical correlates in Machado-Joseph disease. Acta Neurol Scand. 2009;119:277–80. doi: 10.1111/j.1600-0404.2008.01092.x. [DOI] [PubMed] [Google Scholar]
- 8.Pedroso JL, Braga-Neto P, Felicio AC, et al. Sleep disorders in machado-joseph disease: frequency, discriminative thresholds, predictive values, and correlation with ataxia-related motor and non-motor features. Cerebellum. 2011;10:291–5. doi: 10.1007/s12311-011-0252-7. [DOI] [PubMed] [Google Scholar]
- 9.Iranzo A, Munoz E, Santamaria J, Vilaseca I, Mila M, Tolosa E. REM sleep behavior disorder and vocal cord paralysis in Machado-Joseph disease. Mov Disord. 2003;18:1179–83. doi: 10.1002/mds.10509. [DOI] [PubMed] [Google Scholar]
- 10.Chi NF, Shiao GM, Ku HL, Soong BW. Sleep disruption in spinocerebellar ataxia type 3: a genetic and polysomnographic study. J Chin Med Assoc. 2013;76:25–30. doi: 10.1016/j.jcma.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Labrada R, Velazquez-Perez L, Ochoa NC, et al. Subtle rapid eye movement sleep abnormalities in presymptomatic spinocerebellar ataxia type 2 gene carriers. Mov Disord. 2011;26:347–50. doi: 10.1002/mds.23409. [DOI] [PubMed] [Google Scholar]
- 12.Abele M, Burk K, Laccone F, Dichgans J, Klockgether T. Restless legs syndrome in spinocerebellar ataxia types 1, 2, and 3. J Neurol. 2001;248:311–4. doi: 10.1007/s004150170206. [DOI] [PubMed] [Google Scholar]
- 13.Dang D, Cunnington D. Excessive daytime somnolence in spinocerebellar ataxia type 1. J Neurol Sci. 2010;290:146–7. doi: 10.1016/j.jns.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Boesch SM, Frauscher B, Brandauer E, Wenning GK, Poewe W, Hogl B. Restless legs syndrome and motor activity during sleep in spinocerebellar ataxia type 6. Sleep Med. 2006;7:529–32. doi: 10.1016/j.sleep.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Howell MJ, Mahowald MW, Gomez CM. Evaluation of sleep and daytime somnolence in spinocerebellar ataxia type 6 (SCA6) Neurology. 2006;66:1430–1. doi: 10.1212/01.wnl.0000210485.37521.0b. [DOI] [PubMed] [Google Scholar]
- 16.Reimold M, Globas C, Gleichmann M, et al. Spinocerebellar ataxia type 1, 2, and 3 and restless legs syndrome: striatal dopamine D2 receptor status investigated by [11C]raclopride positron emission tomography. Mov Disord. 2006;21:1667–73. doi: 10.1002/mds.20978. [DOI] [PubMed] [Google Scholar]
- 17.Pedroso JL, Braga-Neto P, Felicio AC, et al. Sleep disorders in Machado-Joseph disease: a dopamine transporter imaging study. J Neurol Sci. 2013;324:90–3. doi: 10.1016/j.jns.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Tuin I, Voss U, Kang JS, et al. Stages of sleep pathology in spinocerebellar ataxia type 2 (SCA2) Neurology. 2006;67:1966–72. doi: 10.1212/01.wnl.0000247054.90322.14. [DOI] [PubMed] [Google Scholar]
- 19.Boesch SM, Frauscher B, Brandauer E, Wenning GK, Hogl B, Poewe W. Disturbance of rapid eye movement sleep in spinocerebellar ataxia type 2. Mov Disord. 2006;21:1751–4. doi: 10.1002/mds.21036. [DOI] [PubMed] [Google Scholar]
- 20.de Andres I, Garzon M, Reinoso-Suarez F. Functional anatomy of non-REM sleep. Front Neurol. 2011;2:70. doi: 10.3389/fneur.2011.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu F, Frazier DT. Role of the cerebellar deep nuclei in respiratory modulation. Cerebellum. 2002;1:35–40. doi: 10.1080/147342202753203078. [DOI] [PubMed] [Google Scholar]
- 22.Chen ML, Witmans MB, Tablizo MA, et al. Disordered respiratory control in children with partial cerebellar resections. Pediatr Pulmonol. 2005;40:88–91. doi: 10.1002/ppul.20225. [DOI] [PubMed] [Google Scholar]
- 23.Lee A, Chen ML, Abeshaus S, Poliakov A, Ojemann JG. Posterior fossa tumors and their impact on sleep and ventilatory control: a clinical perspective. Respir Physiol Neurobiol. 2013;189:261–71. doi: 10.1016/j.resp.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Chang SY, Zagha E, Kwon ES, et al. Distribution of Kv3.3 potassium channel subunits in distinct neuronal populations of mouse brain. J Comp Neurol. 2007;502:953–72. doi: 10.1002/cne.21353. [DOI] [PubMed] [Google Scholar]
- 25.Stevanin G, Durr A. Spinocerebellar ataxia 13 and 25. Handb Clin Neurol. 2012;103:549–53. doi: 10.1016/B978-0-444-51892-7.00035-8. [DOI] [PubMed] [Google Scholar]
- 26.Joho RH, Marks GA, Espinosa F. Kv3 potassium channels control the duration of different arousal states by distinct stochastic and clock-like mechanisms. Eur J Neurosci. 2006;23:1567–74. doi: 10.1111/j.1460-9568.2006.04672.x. [DOI] [PubMed] [Google Scholar]
- 27.Espinosa F, Marks G, Heintz N, Joho RH. Increased motor drive and sleep loss in mice lacking Kv3-type potassium channels. Genes Brain Behav. 2004;3:90–100. doi: 10.1046/j.1601-183x.2003.00054.x. [DOI] [PubMed] [Google Scholar]
- 28.Espinosa F, Torres-Vega MA, Marks GA, Joho RH. Ablation of Kv3.1 and Kv3.3 potassium channels disrupts thalamocortical oscillations in vitro and in vivo. J Neurosci. 2008;28:5570–81. doi: 10.1523/JNEUROSCI.0747-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


