Abstract
Climate change might alter annual snowfall patterns and modify the duration and magnitude of snow cover in temperate regions with resultant impacts on soil microclimate and soil CO2 efflux (Fsoil). We used a 5-year time series of Fsoil measurements from a mid-elevation forest to assess the effects of naturally changing snow cover. Snow cover varied considerably in duration (105–154 days) and depth (mean snow depth 19–59 cm). Periodically shallow snow cover (<10 cm) caused soil freezing or increased variation in soil temperature. This was mostly not reflected in Fsoil which tended to decrease gradually throughout winter. Progressively decreasing C substrate availability (identified by substrate induced respiration) likely over-rid the effects of slowly changing soil temperatures and determined the overall course of Fsoil. Cumulative CO2 efflux from beneath snow cover varied between 0.46 and 0.95 t C ha−1 yr−1 and amounted to between 6 and 12% of the annual efflux. When compared over a fixed interval (the longest period of snow cover during the 5 years), the cumulative CO2 efflux ranged between 0.77 and 1.18 t C ha−1 or between 11 and 15% of the annual soil CO2 efflux. The relative contribution (15%) was highest during the year with the shortest winter. Variations in snow cover were not reflected in the annual CO2 efflux (7.44–8.41 t C ha−1) which did not differ significantly between years and did not correlate with any snow parameter. Regional climate at our site was characterized by relatively high amounts of precipitation. Therefore, snow did not play a role in terms of water supply during the warm season and primarily affected cold season processes. The role of changing snow cover therefore seems rather marginal when compared to potential climate change effects on Fsoil during the warm season.
Keywords: soil CO2 efflux, snow, winter, substrate availability, temperature sensitivity, C cycling
Introduction
Climate change may alter annual snowfall patterns and modify the duration and magnitude of snow cover in temperate regions of the Northern Hemisphere (Laternser & Schneebeli, 2003; IPCC, 2007; Liu et al., 2012). Changing snow cover can affect ecosystem processes, such as carbon (C) cycling (Sommerfeld et al., 1993). The insulating snow cover decouples soil from air temperatures and generates a specific microclimate in the top-soil (Sommerfeld et al., 1993; Winston et al., 1995). The beneath-snow microclimate determines the activity of decomposing soil organisms and thereby largely controls the rate of the CO2 efflux from soil (Fsoil) (Monson et al., 2006b). Wintertime Fsoil is an important component of the annual C cycle in cold ecosystems as a substantial part of the C assimilated during the growing season can be lost during the following winter (Monson et al., 2002; Suni et al., 2003). Earlier research focused on high-latitude and high-altitude sites, whereas information on wintertime Fsoil from low to mid-elevation temperate forests remained relatively scarce (e.g., Hirano, 2005; Mo et al., 2005; Groffman et al., 2006; Schindlbacher et al., 2007; Muhr et al., 2009; Aanderud et al., 2013). Mid-elevation temperate sites experience less harsh winters and wintertime air temperatures more often fluctuate around freezing. Climate warming could therefore reduce the amount of precipitation fallen as snow, increase the frequency of mild (>0 °C) periods and/or prepone snowmelt during spring (Laternser & Schneebeli, 2003). In reverse, harsher winters could produce deeper snow cover and delay snow melt during spring (Liu et al., 2012). These factors could directly affect wintertime Fsoil, but the duration and magnitude of snow cover may as well have implications on spring and summer processes such as net ecosystem exchange (NEE) (Monson et al., 2002; Hu et al., 2009), water table depth (Dunn et al., 2007), or soil CO2 efflux (Muhr et al., 2009). Therefore, effects of changing snow cover could be diverse and the assessment of their influence on Fsoil is challenging. Aside from artificial manipulation (removal or addition of snow), time series of annual and wintertime Fsoil measurements under naturally changing snow cover can provide information about the potential effects of future changing climate. However, with the exception of the high elevation, subalpine Niwot Ridge Ameri-Flux site, where eddy flux measurements were accompanied by extensive wintertime Fsoil measurements and microbial studies (e.g., Monson et al., 2002, 2006a,b; Schmidt et al., 2009), most studies focusing on CO2 efflux from beneath snow cover were confined to short periods (one or two winters). Here, we present Fsoil data from a mid-elevation (ca. 900 m a.s.l.) temperate mountain forest which were gathered from November 2007 until December 2012. The five consecutive winters varied in their duration as well as in snow properties. As snow cover insulates soil from low wintertime air temperatures, we hypothesized that (I) duration, thickness, and persistency of snow cover determined soil temperatures and thereby controlled Fsoil rates which were considered to be over-proportionally temperature sensitive during the cold season. Regarding the annual variations in the durations of winter (= cold season) and snow cover we hypothesized that (II) the annual soil CO2 efflux was lower during years with longer winters and longer lasting snow cover than during years with shorter winters and shorter snow cover. To assess other potential drivers of wintertime Fsoil than soil temperature, the role of substrate availability was investigated. Aside from our periodical Fsoil survey, the actual temperature sensitivity of Fsoil around freezing was assessed by a high-frequency measurement campaign while soil temperatures fluctuated around 0 °C.
Materials and methods
Site description
The study site was located at 910 m a.s.l. on a north-north-east slope of a mountain in the Northern Limestone Alps, Achenkirch, Austria (47° 34′ 50′’ N; 11° 38′ 21′’ E). The 125-year-old forest was dominated by Norway spruce (Picea abies), with interspersed European beech (Fagus sylvatica) and silver fir (Abies alba). The understory mainly consisted of naturally regenerating beech. The bedrock was formed of dolomite. The soils were a mosaic of shallow Chromic Cambisols and Rendzic Leptosols. Soils showed high small scale variability in soil types as well as horizon depths. Mull was the dominant humus form and the depths of the litter and O-layer reached from 0 to 5 cm. A-horizons thickness varied from 10 up to 40 cm. Organic C stocks were estimated to be ca. 10 t ha−1 in the organic layer and ca. 120 t ha−1 in the mineral soil (Schindlbacher et al., 2010).
The site was characterized by a cool humid climate. Local mean annual air temperature and precipitation were 6.9 °C and 1506 mm (1992–2012), respectively (Achenkirch village; ca. 7 km away at similar altitude; ZAMG data). Precipitation was evenly distributed throughout the seasons with 20–40% falling as snow. The duration of snow-cover recorded at Achenkirch village was on average 122 days yr−1 (1992–2012), ranging from a minimum of 73 days in 1994 to a maximum of 163 days in 1999. Permanent snow cover built up during November/December and lasted until March/April.
Measurement of climate parameters and soil CO2 efflux
The field site hosted a long-term climate change manipulation experiment (Schindlbacher et al., 2009, 2012). For this study, the untreated control plot data from November 2007 until December 2012 were used. The untreated control plots had a size of 2 × 2 m and were replicated three times. At each of the three plots, soil temperature and soil moisture were measured at 5 cm and 15 cm soil depth. Soil temperature (PT100 temperature sensors; EMS, Brno) and soil moisture (ECH2O-10 soil moisture probes; Decagon, Washington) records were stored on different data loggers (Campbell CR 10×, Campbell Scientific, Inc., North Logan; Delta-T DL2, Delta-T Devices Ltd, Cambridge; MiniCube, EMS, Brno) as half hourly averages. Air temperature (ST1 sensor, Delta-T Devices Ltd, Cambridge) and relative air humidity (OTM-592 C sensor, Sommer, Koblach) were measured in close vicinity to the plots at 2 m height and stored as half hourly averages. Precipitation was measured with an ombrometer (NIWA/MED-K505, Sommer, Koblach) at 1-min resolution at a meteorological station ca. 100 m away from the site in an open area. Snow depth was measured manually during each wintertime CO2 measurement campaign. The measured snow depth onsite showed good fit with the snow depth measured at Achenkirch village (Fig S1). We therefore used the daily snow depth record from Achenkirch village to fill the gaps in the on-site record.
Soil CO2 efflux was measured fortnightly during the snow free seasons and every third week during snow cover. During the snowfree season Fsoil was measured from permanently installed chambers (20 cm diameter, 10 cm height). Three chambers were randomly distributed at each plot and inserted 1 cm into the mineral soil to establish an airtight seal. Chambers were closed with a stainless steel lid for 300 sec. CO2 concentrations were measured every 30 sec using an EGM4 infrared gas analyzer (PP-Systems, Amesbury). The CO2 concentration increase in the chamber headspace during the last 120 sec was used to calculate the flux (linear fit). A detailed description of the system and measurement procedure can be found in (Schindlbacher et al., 2009). During snow cover Fsoil was estimated according to Schindlbacher et al. (2007). On each plot, three CO2 concentration profiles were determined. CO2 concentration profiles in the snow cover were measured with a 2 m long aluminum probe (outer diameter 6 mm, inner diameter 3 mm) which was directly connected to the CO2 analyzer (EGM 4; PP-Systems, Amesbury) by a flexible 1.5 m long tube. Prior to inserting the probe into the snow, CO2 was measured ca. 1 cm above the snow surface to assess the atmospheric CO2 concentration. Then, the CO2 concentration in the snow profile was measured every 20 cm until the soil surface was reached. During periods with <20 cm snow depth, CO2 concentration was measured at ca. 1 cm above the snow surface and at the soil surface. Soil surface CO2 efflux through the snow was estimated using Fick’s law of diffusion, following Musselman et al. (2005) and Hubbard et al. (2005):
| 1 |
where Fsoil is the gas flux (μmol m−2 s−1), D is the diffusion coefficient for CO2 in air (0.1381(10−4) m2 s−1) (Massman, 1998), P0/RT0 is the molecular density of CO2 at STP (44.613), f is snow pack porosity (unitless), τ is tortuosity (unitless), T is the average snow pack temperature (K). The gradient function dg/dz was derived from fitting a linear regression through the measured CO2 concentration profile. Probe measurements were only performed at windless conditions to avoid disturbance by pressure pumping effects. Snow pack parameters were estimated from three snow pits, which were dug at each sampling date. The snow pits were dug at a distance of about 3 m to the plots to avoid any interference with the measured snow profile. At each pit, we measured snow density and temperature in 10 cm increments for the entire depth of the snow profile. The mean density of the snow pack (kg m−3) was estimated from a weighted average of the 10 cm layers. Porosity was calculated from the mean density (ρ) as f = 1 – ρ/973 where 973 is the density of ice (kg m−3). Tortuosity was estimated as a function of porosity (Millington, 1959; Millington & Shearer, 1971) as τ = f 1/3. The gradient method did not yield reliable CO2 fluxes at snow depth <7 cm. Therefore, chamber measurements were performed during periods of <7 cm snow depth. Snow was removed from and around the chambers 30 min prior to CO2 flux measurements which were performed as described above. After measurements, snow was redistributed into and around the chambers. A comparison of CO2 flux estimates by snow probe and chamber measurements showed that the two methods were comparable when snow depths were shallow (Fig S2). In general, the gradient method gives rather conservative estimates of Fsoil. It was recently shown that the gradient method produced lower soil respiration estimates as eddy covariance measurements under certain winter conditions (Sullivan et al., 2012; Merbold et al., 2013).
A high-frequency measurement campaign was conducted from February 13 to February 15 2008 to assess the temperature sensitivity of soil CO2 efflux at soil temperatures around freezing. On February 12, snow (ca. 15 cm) was removed from three adjacent plots, releasing six chambers for CO2 flux measurements. Flux measurements started the next day 10:30 and were repeated every fourth hour until February 15 07:00 (from 22:00 until 06:30, no measurements were undertaken). The timing of the campaign was chosen because air and top-soil temperature showed strong diurnal fluctuation during these days. During each CO2 flux measurement, soil temperature was measured with a handheld probe at 1 cm, 5 cm, and 10 cm soil depth. CO2 efflux was measured as described above for the snow free season.
Substrate induced respiration (SIR)
To assess the effect of potential substrate limitation on wintertime soil CO2 efflux, a modified SIR experiment was carried out in the field (Göttlicher et al., 2006). The aim of the method was to test how decomposers responded to an artificial increase in labile substrate at the beginning and toward the end of winter. A C4 sucrose solution (100 g m−2) was added to three adjacent plots in November 2007 and February 2008. Before adding the solution, snow was removed from the plots. All 2 × 2 m plots were used in a preliminary experiment and were already equipped with plastic chambers (20 cm diameter, 10 cm height) for CO2 measurements, as described above for the snow free season. Each plot was equipped with three chambers. One chamber was used for sucrose amendment in November and another in February. The distance between two chambers was always larger than 1 m to avoid a contamination from the previous sucrose amendment. On squares of 0.5 × 0.5 m around the chambers, sucrose solution was added. One hundred and fifty grams of cane sugar (VERIVAL, EP Naturprodukte AG, Austria) was dissolved in 3-L snow water. With a syringe, 0.5 L of sucrose solution was added per chamber (63 ml directly into the camber). On three adjacent control plots, the same amount of untreated melted snow was suspended in the same way as the sugar treatment to quantify potential side-effects of the procedure. CO2 efflux was measured prior to sucrose amendment (1 h after snow removal). After the initial CO2 efflux measurements and the sucrose amendment, the snow pit was refilled and opened again after 24 h, allowing for measurements of the substrate induced soil respiration. After the SIR measurements, CO2 efflux from the plots was measured periodically until the end of winter (ca. every 20 days).
To assess the source of SIR (indigenous C3-C or C4-C from the added sugar), the isotopic signature of the soil CO2 efflux was determined. After SIR-efflux measurements, all chambers were closed with lids and air samples (12 ml) for mass spectrometry were taken at 0, 15, 30, 45, and 60 min from the chambers. Samples were taken with a glass syringe and injected into previously evacuated 12-ml glass vials (Exetainer, Labco Ltd, High Wycombe, UK). The stable carbon isotopic ratio of soil-respired CO2 was then analyzed by continuous-flow isotope-ratio mass spectrometry on a Thermo Finnigan Delta V Advantage Mass Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) coupled to a Finnigan GasBench. Sample CO2 concentrations were measured with the same system after calibration with reference gases with 400 and 1000 ppm CO2 (Air Liquide, Vienna, Austria). To assess the isotopic composition of soil respired CO2, the Keeling plot approach was used (Keeling, 1958). The intercept of a linear regression of δ13C of sampled CO2 vs. 1/[CO2] provided an estimate of δ13C of soil-respired CO2 (where [CO2] was the CO2 concentration in%). Although the CO2 efflux during the two winter sampling dates was low, the fit of the linear regression was overall sufficient for keeling plot analysis (November 2007 r2 from 0.97 to 0.99; February 2008 treated plots r2 from 0.92 to 0.99; control plots r2 from 0.90 to 0.99).
The fraction (f) of C4-C-derived CO2 in Fsoil was calculated as follows:
| 2 |
where fC4 is the proportion that can be attributed to the respiration of C4-substrate, δ13CSIR is the C isotope composition (‰) of soil respired CO2 in sucrose-amended plots, δ13Ccontrol is the C isotope composition in CO2 from control plots and, δ13C4suc is the C isotope composition (−10.97 ± 0.06‰) of the C4 sucrose solution applied.
Data analysis
The duration of snow cover was defined as the period during which a permanent, closed, snow-layer covered the soil surface. In addition, the duration of the most persistent snow cover throughout the study (7th November to 8th April) was defined as ‘maximal snow cover’. This fixed time frame allowed for a direct quantitative comparison of Fsoil during the period. Winter (= freezing period) was defined as the period during which the smoothed 5-day mean air temperature remained below 0 °C for at least five consecutive days. The year, as the basis for annual values, was defined from 7th of November (the earliest beginning of snow cover during the study) to 6th of November the following year. Using the calendar year (January 01 to December 31) would have split winters in two parts and precluded a reasonable analysis. The warm season was defined as the period of the year during which the smoothed 5-day mean air temperature remained above 0 °C for at least five consecutive days.
Preceding studies indicated a strong positive relationship between soil temperature and Fsoil at the study site. Accordingly, a temperature function was fitted to the Fsoil data of the five seasons to obtain seasonal and annual flux estimates. A Gaussian function was fitted to the Fsoil data of each plot (Fig.1) by means of a least square fitter (SigmaPlot 10.0) and the daily CO2 efflux from each plot was modeled according to the function parameters. Compared to simple exponential functions, the Gaussian function has the opportunity of varying temperature sensitivity (Q10) over the temperature range. The modeled daily average CO2 efflux of the three individual plots was summed for and a corresponding mean cumulative CO2 efflux was calculated by averaging the periodical plot estimates. Alternatively to the modeled efflux, the cumulative CO2 efflux during seasons and years was estimated by linear interpolation between the measurement dates (below the CO2 data points in Fig.2). Although this method misses temporal variations in Fsoil between measurements, it efficiently reflects the efflux during periods when soil temperature does not primarily drive Fsoil. Due to the low number of replicates (n = 3 plots) the spatial variability in Fsoil at the site was not fully covered. For a temporal analysis such as the comparison of seasons and years, the replication was sufficient because Fsoil was always measured at the same locations. Potential variations of cumulative seasonal CO2 efflux, as well as the relative contribution of wintertime efflux during the five periods (2007/08 until 2011/12) were statistically tested by means of one-way anova. anova was only applied for the linear interpolation estimates. Testing the modeled estimates would actually have meant testing for differences in soil temperature. Correlation analysis was used to test for potential correlation of cumulative soil CO2 efflux with duration, amount, and average depth of snow cover as well as with the duration of winter and mean air and soil temperature during snow cover.
Figure 1.
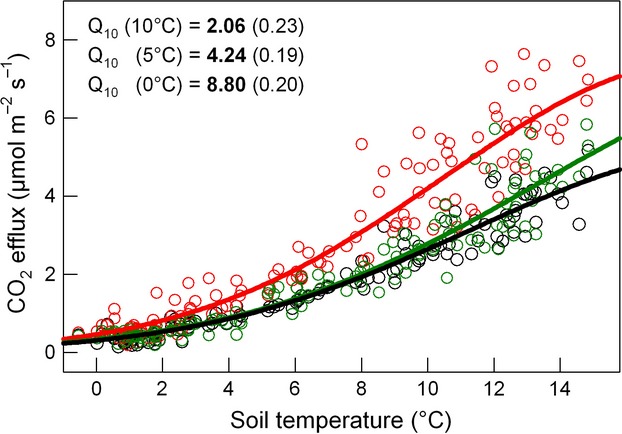
Relationship between soil temperature at 5 cm soil depth and the manually measured soil CO2 efflux during the 5 years observation. The three plots are shown in different colors. Curves show the fit of a Gaussian function (Eqn 3). Temperature sensitivities of soil CO2 efflux are shown as Q10 values. According the Gaussian function, the temperature sensitivity varies with soil temperature. Mean Q10 values (± SE, n = 3) are shown for 10, 5, and 0 °C soil temperature.
Figure 2.
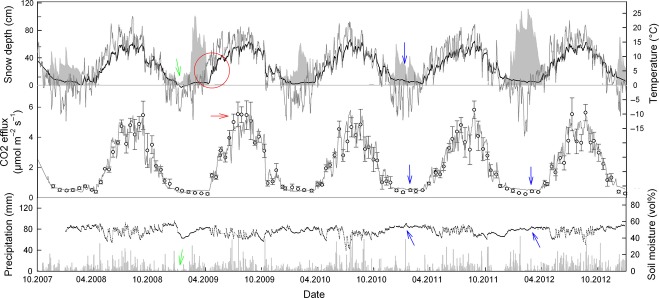
Course of daily air temperature (gray line, upper panel), soil temperature (black line, upper panel), and soil moisture (black line, lower panel). Gray areas in the upper panel show the depth of the snow cover. Manually measured soil CO2 efflux (open circles; means ± SE, n = 3) and the mean daily CO2 efflux modeled with a simple temperature function (gray line) are shown in the medium panel. Gray bars in the lower panels are precipitation in mm. The red circle indicates a period during which snow cover delayed the increase of soil temperature during spring; the red arrow indicates underestimated CO2 efflux by the temperature model; blue arrows indicate rising soil CO2 efflux after periods of snow-melt; green arrows indicate periods with less precipitation. The exact meaning of arrows and cycles is explained in the results and discussion.
Temperature sensitivity of the overall soil CO2 efflux was calculated for each plot from the 5 years data record by deriving Q10 from the Gaussian function (Tuomi et al., 2008)
| 3 |
describing the soil CO2 efflux rates R(T) as function of soil temperature T. R, a, and b are constants (R > 0, a > 0, b < 0). The function was found to best describe the temperature dependence of heterotrophic soil respiration during the annual course of soil temperature (Tuomi et al., 2008). Q10, the relative growth of soil CO2 efflux as the temperature increases by 10 °C from the initial temperature T, was derived as
| 4 |
For wintertime, during which soil temperature varied in a narrow range, a simple exponential function
| 5 |
was fitted. Q10 represents the relative growth of soil CO2 efflux as the temperature increases by 10 °C while R10 is the soil CO2 efflux at 10 °C soil temperature. Eq. 5 was applied for all CO2 efflux measurements beneath snow (Fig.3) and for the higher frequency measurement campaign (Fig.4).
Figure 3.
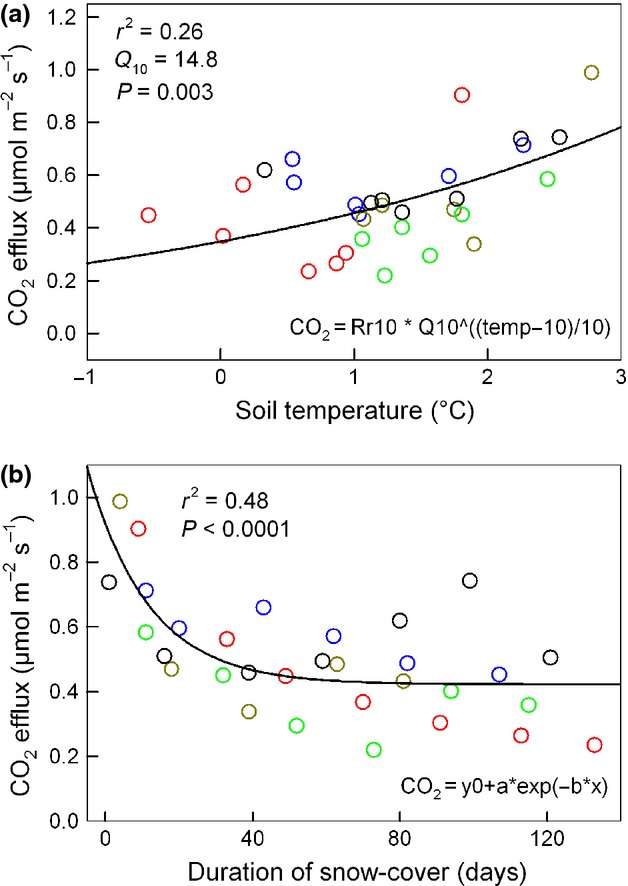
(a) Relationship between soil temperature at 5 cm soil depth and mean soil CO2 efflux during snow cover (efflux measurements with snow probe). The different colors represent different seasons (2007/2008 black, 2008/2009 red, 2009/2010 blue, 2010/2011 yellow, 2011/2012 green). (b) Relationship between soil CO2 efflux and duration of snow cover (same colors as for a). The functions of the fitted curves are shown in the right lower corners.
Figure 4.
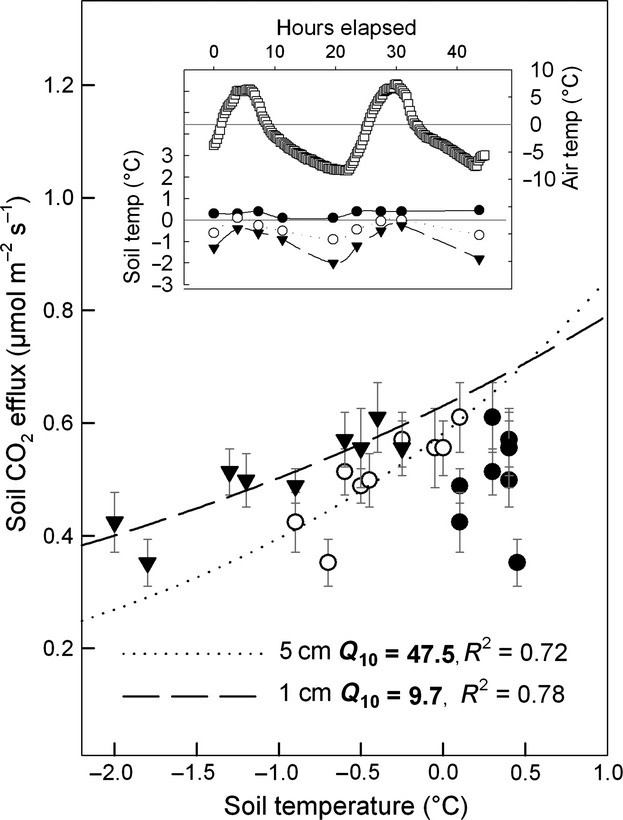
Temperature sensitivity of soil CO2 efflux during a three days measurements campaign in February 2008. Black triangles show CO2 efflux vs. soil temperature measured at 1 cm soil depth. Open and full circles show CO2 efflux vs. soil temperatures measured at 5 cm and 10cm soil depth, respectively. An exponential function (Eqn. 5) was fitted. Q10 values as a measure of temperature sensitivity are shown for 1 cm and 5 cm depth. At 10 cm soil depth, the relationship was not significant. The inner panel shows the course of air and soil temperature during CO2 efflux measurements (same symbols plus open squares for air temperature).
The effect of sucrose addition on the soil CO2 efflux (SIR) was tested by repeated measures anova. Early winter treatment and late winter treatment were tested interpedently because measurements were made at different chambers. All CO2 efflux rates were temperature corrected prior to statistical analysis because soil temperature varied (2.6–0.5 °C) between measurement dates. All flux values were adjusted to a soil temperature of 2.6 °C using the parameters of the temperature function of Plot B in Fig.1. Differences in δ13C values were tested using a t-test. All statistics were carried out with sas 9.2 (SAS Institute, Cary, NC, USA, www.sas.com) at a significance level of 95%.
Results
Annual snow cover showed considerable variations in duration and thickness (Table1, Fig.2). The average depth of the snow cover ranged from 19 cm in the first, to 59 cm in the last year. Snow cover lasted longest in the first year (154 days), whereas the shortest snow cover was 105 days in 2010/11. The build-up of snow cover fell well within the beginning of winter during most years, except for the last year 2011/12 where build-up of snow cover was delayed due to lack in precipitation during early winter. The duration of winter and the duration of snow cover were strongly correlated (r = 0.926, P = 0.02) but snow cover always lasted into the following spring; particularly when late winter snow packs were thick (Table1, Fig.2). Mean air temperature during snow cover varied between −0.3 °C and −2.7 °C and was positively correlated with the duration of snow cover (Table2). Mean soil temperatures beneath snow cover ranged from 0.8 °C to 1.6 °C, but did not correlate with any snow parameter (duration, mean depth, and amount). Variations in annual air and soil temperatures were comparatively small (Table1).
Table 1.
Snow and climate parameters
| Period | 2007–2008 | 2008–2009 | 2009–2010 | 2010–2011 | 2011–2012 |
|---|---|---|---|---|---|
| Snow cover | Nov 07–Apr 08 | Nov 22–Apr 08 | Dec 04–Mar 23 | Nov 23–Mar 07 | Dec 06–Apr 01 |
| Duration (days) | 154 | 138 | 110 | 105 | 118 |
| Average snow depth (cm) | 19 | 30 | 23 | 22 | 59 |
| Amount (average depth × days) | 2967 | 4117 | 2516 | 2322 | 6568 |
| Mean air temp (°C) | −0.3 | −1.1 | −2.7 | −1.8 | −1.6 |
| Mean soil temp (°C) | 1.6 | 0.8 | 1.0 | 1.6 | 1.6 |
| Precipitation (mm) | 529 | 362 | 345 | 386 | 548 |
| Max. snow cover (Nov 07–Apr 08) | |||||
| Mean air temp (°C) | −0.3 | −0.6 | −0.7 | −0.3 | −0.8 |
| Mean soil temp (°C) | 1.6 | 1.2 | 1.9 | 2.2 | 2.0 |
| Winter (5 days mean air temp <0 °C) | Nov 12–Mar 25 | Nov 21–Mar 23 | Dec 12–Mar 15 | Nov 23–Mar 3 | Nov 15–Mar 11 |
| Duration (days) | 135 | 124 | 95 | 102 | 118 |
| Mean air temp (°C) | −1.0 | −1.8 | −3.5 | −2.7 | −2.4 |
| Mean soil temp (°C) | 1.5 | 0.8 | 0.9 | 1.5 | 1.8 |
| Annual (Nov 08–Nov 07) | |||||
| Mean air temp (°C) | 6.9 | 6.8 | 6.2 | 6.6 | 6.5 |
| Mean soil temp (°C) | 6.3 | 6.2 | 6.4 | 6.6 | 6.5 |
| Precipitation (mm) | 1602 | 1665 | 1636 | 1439 | 2001 |
| Annual (Jan 01–Dec 31) | 2008 | 2009 | 2010 | 2011 | 2012 |
| Mean air temp (°C) | 7.1 | 6.9 | 5.8 | 6.9 | 6.5 |
| Mean soil temp (°C) | 6.3 | 6.3 | 6.3 | 6.6 | 6.6 |
| Precipitation (mm) | 1554 | 1763 | 1661 | 1478 | 2037 |
The duration of snow cover was defined as the period during which a permanent, closed, snow-layer covered the soil surface. Winter was defined as the period during which the smoothed 5-days mean air temperature was below 0 °C for at least 5 consecutive days. The year was defined from November 07 to November 06 of the next calendar year to avoid splitting apart the winter seasons. For periodical comparison, climate parameters during the period of most persistent snow cover (November 07–April 08) during the 5 years are shown (= max. snow cover). The lower panel shows annual values based on calendar years 2008–2012 for comparison.
Table 2.
Correlation coefficients between different snow and climate parameters and the soil CO2 efflux (Fsoil) during snow cover (Fsoil snow), without snow cover (Fsoil no snow) and annually (Fsoil annual) from 2007/08 until 2011/12
| Snow duration | Snow depth | Snow amount | Air temp. (snow) | Soil temp. (snow) | Fsoil snow | Fsoil no snow | Fsoil annual | |
|---|---|---|---|---|---|---|---|---|
| Snow duration | 1.000 | |||||||
| Snow depth | −0.191 | 1.000 | ||||||
| Snow amount | 0.068 | 0.964 | 1.000 | |||||
| Air temp. (snow) | 0.899 | −0.082 | 0.150 | 1.000 | ||||
| Soil temp. (snow) | −0.071 | 0.177 | 0.103 | 0.239 | 1.000 | |||
| Fsoil snow | 0.628 | −0.459 | −0.313 | 0.503 | −0.026 | 1.000 | ||
| Fsoil no snow | 0.023 | −0.051 | −0.033 | 0.024 | −0.115 | 0.586 | 1.000 | |
| Fsoil annual | 0.104 | −0.107 | −0.072 | 0.089 | −0.109 | – | – | 1.000 |
Correlation is significant. Pearson (n = 9 chambers).
Soil CO2 efflux was closely related to soil temperature at 5 cm soil depth (Fig.1). The Gaussian function (Eqn. 3) showed a good fit (Fig.1 and 2) and the modeled data explained 95% of the variance in Fsoil throughout the 5 years. The temperature sensitivity (Q10) of Fsoil obtained from all Fsoil measurements ranged from 2.06 (± 0.23) at 10 °C soil temperature to 8.80 (± 0.20) at 0 °C soil temperature (Fig.1). The Q10 of Fsoil during the high-frequency measurement campaign in winter (February) 2008 ranged from 9.70 to 47.50 and strongly depended on soil temperature measurement depth (Fig.4). The Q10 of 47.50 is unrealistically high for a physiological process and indicates that most of the measured variation in Fsoil occurred from above the corresponding temperature sensor depth (5 cm). Overall, the low Fsoil rates during winter were well reproduced by the Gaussian model. However, the temporal course of Fsoil beneath snow did not always match with the model output (Fig.2). Fig.3a shows that the relationship between Fsoil and soil temperature beneath the snow cover was rather weak. During three out of 5 winters, there even was no relationship between soil temperature and Fsoil at all. During winter 2008/2009, a period of minor precipitation led to shallow (2–5 cm) snow cover, causing a mild freezing down to 5 cm soil depth (Fig.2, green arrows). The freezing did however not affect Fsoil rates. During all other winters, soil temperature at 5 cm depth remained above freezing. During late winter 2007/08 a warm period caused a short gap in snow cover and increased soil temperatures as well as Fsoil rates (Fig.2). Deep snow cover during spring 2008/09 delayed the increase in soil temperature, compared to that of air temperature (Fig.2, red circle). No such effect was observed in 2011/12 where spring air temperatures were lower. Generally, beneath snow Fsoil gradually declined throughout winters (Fig.3b). The decline was periodically interrupted by short-term increases in Fsoil; mostly after mild periods and snow-melt triggered increases in soil moisture (Fig.2, blue arrows). The addition of sucrose significantly affected the CO2 efflux during early and late winter (repeated measures anova, P < 0.05). Fsoil rates increased significantly the day after sucrose was added (Fig.5). The increase was more pronounced in late winter (absolute increase in Fsoil early winter = 80%, late winter = 135%) but absolute flux rates after sucrose addition were nearly equal during early and late winter (Fig.5). After sucrose addition, the δ13C value was slightly (not significantly) less negative in early winter (Fig.5a). The difference in δ13C corresponded to a roughly 25% contribution of CO2 from sucrose to the observed increase in Fsoil (SIR) in early winter. The δ13C value significantly changed from −22.24 ‰ to −18.70 ‰ in late winter (Fig.5b) corresponding to a roughly 50% contribution of the added source to the SIR in late winter. Both, the more pronounced response of Fsoil as well as the higher contribution of the added C source to Fsoil during late winter indicate a temporal decrease in substrate availability throughout snow cover.
Figure 5.
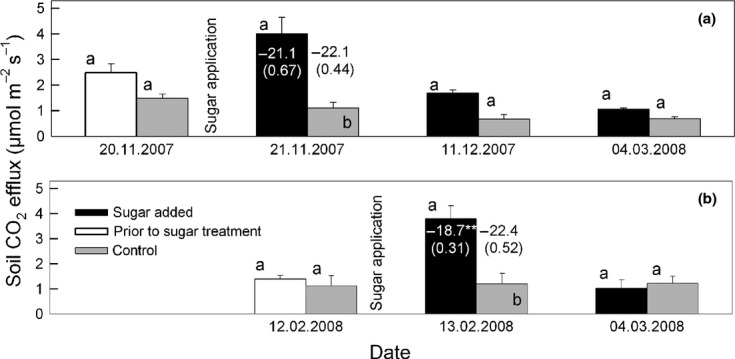
Substrate induced respiration (SIR). Bars show the mean CO2 efflux ± SE (n = 3) from chambers prior to sugar amendment (white bars), after sugar amendment (black bars), and from corresponding control plots (gray bars). Sugar was added to different chambers in early winter (a) and late winter (b). Different letters indicate statistically significant differences between control and sugar treatment (Student-Newman-Keuls’ Post hoc test, repeated measures anova) for each measurement date. Values in and above bars show the δ13C of soil CO2 efflux in ‰ ± SE (n = 3; significantly difference between treatment and control is indicated by **). All flux values were temperature corrected.
Cumulative C efflux during snow cover was between 0.46 and 0.95 t ha−1, but did not differ significantly between years (Table3). Cumulative C efflux during snow cover was positively correlated with the duration of snow cover, but did not correlate with average snow depth, average winter air temperature, or average beneath snow soil temperature (Table2). The C efflux during snow cover amounted between 6.4% and 11.9% of the annual C efflux. The relative contribution during the year with the longest lasting winter and snow cover (11.9%) was statistically significantly higher than all others (Table3). During the period of maximal snow cover (November 07–April 08) cumulative C efflux was between 0.77 t ha−1 and 1.18 t ha−1 but differences between years were not statistically significant either (Table3). When set into relation to the corresponding annual soil C efflux, C efflux during maximal snow cover ranged from 10.5% to 15.1%. The contribution during the year with the shortest winter (15.1%) was statistically significantly higher than in years with longer snow cover (Table3). Annual C efflux ranged between 8.02 t ha−1 and 7.44 t ha−1 when estimated by linear interpolation and between 8.14 t ha−1 and 7.91 t ha−1 when estimated by the model output (Table3). Annual soil C efflux did not differ significantly between years and was not correlated with the duration of snow cover, average snow depth, or air and soil temperature during snow cover (Table2).
Table 3.
Soil CO2 efflux (cumulative; tons C ha−1 ± SE (n = 3) in parenthesis)
| Period | 2007–2008 | 2008–2009 | 2009–2010 | 2010–2011 | 2011–2012 |
|---|---|---|---|---|---|
| Snow cover (model) | 0.91 (0.13) | 0.67 (0.09) | 0.56 (0.08) | 0.71 (0.13) | 0.69 (0.17) |
| Snow cover (linear) | 0.95 (0.11) ns | 0.68 (0.11) ns | 0.63 (0.15) ns | 0.53 (0.10) ns | 0.46 (0.04) ns |
| Max. snow cover (model) | 0.91 (0.13) | 0.87 (0.12) | 1.04 (0.15) | 1.13 (0.18) | 1.05 (0.15) |
| Max. snow cover (linear) | 0.95 (0.11) ns | 0.90 (0.14) ns | 1.18 (0.25) ns | 0.97 (0.18) ns | 0.77 (0.06) ns |
| Winter (model) | 0.79 (0.11) | 0.59 (0.08) | 0.52 (0.07) | 0.61 (0.09) | 0.74 (0.11) |
| Winter (linear) | 0.83 (0.10) ns | 0.65 (0.10) ns | 0.54 (0.12) ns | 0.51 (0.10) ns | 0.52 (0.03) ns |
| Annual (model) | 7.97 (1.17) | 8.04 (1.18) | 7.91 (1.17) | 8.06 (1.22) | 8.14 (1.20) |
| Annual (linear) | 8.02 (1.06) ns | 8.41 (0.98) ns | 7.74 (1.38) ns | 7.87 (1.36) ns | 7.44 (1.16) ns |
| % snow cover (model) | 11.4 (0.1) | 8.4 (0.1) | 7.0 (0.7) | 8.9 (0.1) | 8.6 (0.1) |
| % snow cover (linear) | 11.9 (0.5) a | 8.0 (0.3) b | 8.0 (1.0) b | 6.7 (0.1) b | 6.4 (0.6) b |
| % max snow cover (model) | 11.4 (0.1) | 10.8 (0.1) | 13.0 (0.1) | 14.1 (0.5) | 13.1 (0.1) |
| % max snow cover (linear) | 11.9 (0.5) a | 10.6 (0.5) a | 15.1 (0.7) b | 12.3 (0.3) a | 10.5 (0.7) a |
| % winter (model) | 9.8 (0.1) | 7.4 (0.1) | 5.8 (0.1) | 7.6 (0.6) | 9.3 (0.1) |
| % winter (linear) | 10.4 (0.5) a | 7.6 (0.3) b | 6.9 (0.7) b | 6.5 (0.1) b | 7.2 (0.7) b |
| Annual (Jan 01 – Dec 31) | 2008 | 2009 | 2010 | 2011 | 2012 |
| Annual (model) | 7.97 (1.17) | 8.11 (1.19) | 7.91 (1.17) | 8.06 (1.20) | 8.14 (1.20) |
| Annual (linear) | 8.20 (1.13) ns | 8.43 (0.99) ns | 7.58 (1.36) ns | 7.84 (1.27) ns | 7.42 (1.22) ns |
Cumulative values were calculated from daily model output (model) and by linear interpolation between the single CO2 efflux data points in Fig.2 (linear). Percent values show the contribution to the annual CO2 efflux (November 7 to November 6; = 100%). The lower panel shows annual values based on the calendar years 2008–2012 for comparison. Statistically significant differences between linear interpolation estimates during the periods the 2007/08 until 2011/12 are indicated by different letters (ns, not significant).
Discussion
Serving as a temporary insulation layer, snow cover largely regulated wintertime soil temperatures. According to our hypothesis (I), snowpack parameters affected wintertime soil temperatures. It turned out that the onset of snow cover was a decisive factor because it determined the starting point of soil insulation. If the first freezing coincided with snowfall, snow cover built up early and the beneath snow soil temperatures remained relatively high when compared to years with delayed snowfall. Snow depth did not affect soil temperatures as long as a minimal insulation layer was present. A shallow (∼ 10 cm) snow layer was enough to keep wintertime soil temperatures above freezing at our site. At high-elevation sites, below zero soil temperatures were measured even beneath deep snowpacks (Mast et al., 1998; Monson et al., 2006a,b). Snow depth therefore might have considerable more impact on wintertime Fsoil at high-elevation or at other colder sites than at comparatively warmer mid-elevation sites. This may also explain why Monson et al. (2006b) found a clear relationship between annual amount of snow and Fsoil in a subalpine forest at Niwot Ridge while we did not find such a relationship at our mid-elevation site. Inconsistencies in snow cover (periods of very shallow snow or temporary gaps) strongly affected soil temperature. Lack of sufficient snow cover during freezing periods caused periodic soil frost or low soil temperatures while gaps in snow cover during warmer periods increased soil temperatures. Accordingly, the temporal variability of soil temperatures was higher during winters with inconstant snow conditions. During winters with permanent snow cover soil temperatures gradually decreased throughout snow cover. Surprisingly, the temporal trend of soil temperature was often not reflected in the course of Fsoil which tended to steadily decrease throughout snow cover (Fig.3). As Fsoil is a temperature dependent process (Lloyd & Taylor, 1994) with even higher temperature sensitivity at low temperatures [Fig.1, Fig.4; (Mikan et al., 2002)], other processes must have over-ridden the effects of slowly changing soil temperature. Changes in microbial biomass or community structure can affect Fsoil (Schmidt et al., 2009) but such changes are rather unlikely according to previous studies conducted at the same plots. Microbial biomass was at least as high during winter as during the warm season (Schindlbacher et al., 2011). In contrary to high latitude and altitude sites where substantial shifts in the microbial community structure occur between winter and summer (Lipson et al., 2002; Schadt et al., 2003), we did not observe such a pronounced shift in microbial community structure at our site (Schindlbacher et al., 2011; Kuffner et al., 2012). Another reason for the steady decrease of Fsoil throughout snow cover could have been shortage of available substrate for microbial decomposition. A gradual decrease in substrate availability during winter has been reported before (Zimov et al., 1996; Brooks et al., 2004) and was supported by our SIR data indicating a substantial decrease in substrate availability from early to late winter (Fig.5). Such a change in substrate availability could have easily over-ridden the effects of soil temperature considering that the absolute change in soil temperature beneath snow was small (<3 °C) and slow. We found evidence that the decrease in substrate availability might be related to the rather static soil conditions beneath snow. Active lateral transport of substrate should have been largely hindered as long as no fresh water entered the soil. Immobile decomposers might have suffered from increasing substrate shortage in their microsites. The little peaks in Fsoil after intermediate snow melt (Fig.2) support this explanation. Incoming meltwater could have mobilized labile substrate, thereby causing a temporary increase in Fsoil. Similar observations after snowmelt events were made by Hirano (2005). Another cause of substrate shortage could have been decreasing root and mycorrhiza activity throughout winter (Muhr et al., 2009; Subke et al., 2011). Temporarily increased Fsoil after warm melting periods may as well be explained by temporarily increased C supply from roots or mycorrhiza. Taken together, our hypothesis (I) that snow cover determined wintertime soil temperatures and thereby drove Fsoil was only partly confirmed. Snow cover determined soil temperatures but Fsoil was also controlled by substrate availability (overall low Fsoil during winter was for sure determined by overall low soil temperature).
During the 5 years, cumulative gaseous C efflux from beneath snow cover varied between 0.46 and 0.95 t ha−1 yr−1 and amounted to between 6% and 12% of the annual efflux. These values were well within the range of observations from other studies at mid-elevation forest sites (Mariko et al., 2000; Hirano, 2005; Mo et al., 2005; Groffman et al., 2006) and confirm a first assessment of wintertime Fsoil (ca. 12% of annual soil CO2 efflux) at the same site during the snow-rich winter 2005/06 (Schindlbacher et al., 2007). The cumulative C efflux from beneath the snow cover was largely determined by the duration of snow cover and was highest during the year with longest snow cover. However, for a quantitative assessment of the effects of changing snow cover, it was necessary to compare not only the absolute C loss during snow cover (which varied in duration) but also the soil CO2 efflux during a fixed period of time. We chose the longest duration of snow cover during the 5 years; the ‘maximum snow cover’ (154 days, 7th November–8th April) as a timeframe for quantitative comparison. It allowed us to assess how shorter and longer lasting snow cover affected the soil CO2 efflux during this fixed period. The cumulative CO2 efflux during the period of “maximal snow cover” ranged between 0.77 and 1.18 t C ha−1 yr−1 or between 11 and 15% of the annual CO2 efflux. In absolute terms, the cumulative soil CO2 efflux during ‘maximal snow cover’ did not differ significantly between years, but the relative contribution (15%) to the annual soil CO2 efflux was largest during the year with the shortest winter. This is as an indication that shorter winters and shorter snow cover will increase the soil CO2 efflux during the currently cold season. Especially as climate change may, on the long run, impose much milder winters as observed in this study. The impact of variations in current wintertime Fsoil seems however marginal when related to the annual soil CO2 efflux. For instance, the C efflux during the longest period of snow cover (2007/08) was only 0.21 t ha−1 lower than the C efflux during the same period in the year with shortest snow cover (2010/11). Considering an average annual C efflux of ca. 8 t ha−1 this was less than 3%. Accordingly, we did not detect any relation between snow cover duration and annual soil CO2 efflux. Our hypothesis (II) that the annual soil CO2 efflux was lower during years with long winters and long lasting snow cover than during years with short winters and shorter snow cover was not verified. Like the duration of snow cover, there were no effects of snow amount or snow depth on the annual CO2 efflux either. We had expected that springtime Fsoil would be depressed by thawing of deep snowpacks and associated delayed increase in soil temperature. In spring 2009 thawing of a deep snowpack indeed kept soil temperatures low and thereby lowered the springtime CO2 efflux. However, in the following summer, the measured Fsoil rates temporarily exceeded the modeled rates (Fig. 2, red arrow). This came a bit surprising as the model otherwise fitted very well. The reason for the discrepancy in measured and modeled Fsoil could have been that the easily decomposable substrate which was left over from winter/spring was then decomposed during the following summer. However, this potentially close link between winter and warm season processes remains speculative but may deserve further investigation. In general, variations in snow cover had comparatively low impact on Fsoil from our mid-elevation forest when compared to high-elevation (Monson et al., 2006a,b; Hu et al., 2009) or boreal forest sites (Dunn et al., 2007). Beside the less harsh winter-climate at the lower elevation, our site in the northern Alps was also characterized by frequent precipitation. Hence, at our site snow did not play a dominant role for hydrology throughout the year. In the drier Inner Alps, snow water could already be more critical in determining annual Fsoil and NEE. Our observations also deviate from the usually more drastically effects observed during snow-manipulation experiments; for example, the strong decrease in winter and warm season Fsoil after snow-removal observed by Muhr et al. (2009) in a similar forest. However, in most manipulation experiments snow was removed during very cold conditions. Such a combination might at least at our site naturally not be the case in future as cold conditions and precipitation always produce snow cover.
Regarding the potential effects of climate change on snow cover at our mid-elevation temperate site, we conclude that warmer climate likely reduces the duration of winter and snow cover and thereby increases the soil CO2 efflux during the present cold season. Since the soil CO2 efflux during the cold season is almost an order lower than during the warm season, the quantitative effects on the annual soil CO2 efflux are expected to be rather marginal when they are compared to potential climate change effects during the growing season (Schindlbacher et al., 2012). Effects of changing snow cover at our mid-elevation temperate forest seem of minor relevance when compared to the potential effects of changing snow cover on soil C at high altitude and high latitude biomes which are characterized by a much longer cold season, lower winter temperatures and the occurrence of permafrost (Welker et al., 2000; Zhang et al., 2001; Schimel et al., 2004; Liptzin et al., 2009; Schuur et al., 2009; Natali et al., 2011).
Acknowledgments
The study was financed by the Austrian Science Fund – FWF (project P 23222). We thank Hans Pausch and Thomas Gigele for periodical CO2 measurements during summers and Wernfried Zainer, Barbara Kitzler, Peter Winkelbauer, and Roland Mahr for occasional help during winters. The database was handled by Hans Hauer. Technical problems at site were eliminated by Christian Holtermann. We acknowledge the support of climate data by Hydrographischer Dienst Tirol.
Supporting Information
Figure S1. Relation between snow depth measurements at Achenkirch village and at the field site. Linear regression (black line) and 1 : 1 line (red).
Figure S2. Comparison of snow probe and chamber technique at low snow depth. Measurements were performed on a single day at a snow depth of 9–11 cm. Snow probe measurements were performed directly in the permanent chambers. Subsequently, snow was removed from inside and from around the chambers and the CO2 efflux was estimated by the closed dynamic chamber method. Linear regression (black line), 1 : 1 line (red), and 95% confidence bands (blue).
References
- Aanderud ZT, Jones SE, Schoolmaster DR, Jr, Fierer N, Lennon JT. Sensitivity of soil respiration and microbial communities to altered snowfall. Soil Biology and Biochemistry. 2013;57:217–227. [Google Scholar]
- Brooks PD, Mcknight D, Elder K. Carbon limitation of soil respiration under winter snowpacks: potential feedbacks between growing season and winter carbon fluxes. Global Change Biology. 2004;11:231–238. [Google Scholar]
- Dunn AL, Barford CC, Wofsy SC, Goulden ML, Daube BC. A long-term record of carbon exchange in a boreal black spruce forest: means, responses to interannual variability, and decadal trends. Global Change Biology. 2007;13:577–590. [Google Scholar]
- Göttlicher S, Steinmann K, Betson NR, Högberg P. The dependence of soil microbial activity on recent photosynthate from trees. Plant and Soil. 2006;287:85–94. [Google Scholar]
- Groffman PM, Hardy JP, Driscoll CT, Fahey TJ. Snow depth, soil freezing, and fluxes of carbon dioxide, nitrous oxide and methane in a northern hardwood forest. Global Change Biology. 2006;12:1748–1760. [Google Scholar]
- Hirano T. Seasonal and diurnal variations in topsoil and subsoil respiration under snowpack in a temperate deciduous forest. Global Biogeochemical Cycles. 2005;19:GB2011. [Google Scholar]
- Hu JIA, Moore DJP, Burns SP, Monson RK. Longer growing seasons lead to less carbon sequestration by a subalpine forest. Global Change Biology. 2009;16:771–783. [Google Scholar]
- Hubbard RM, Ryan MG, Kelly E, Rhoades CC. Seasonal patterns in soil surface CO2 flux under snow cover in 50 and 300 year old subalpine forests. Biogeochemistry. 2005;73:93–107. [Google Scholar]
- Ipcc. Climate Change 2007: The Physical Science Basis. Contribution of Working group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press; 2007. [Google Scholar]
- Keeling CD. The concentration and isotopic abundances of atmospheric carbon dioxide in rural areas. Geochimica et Cosmochimica Acta. 1958;13:322–334. [Google Scholar]
- Kuffner M, Hai B, Rattei T, et al. Effects of season and experimental warming on the bacterial community in a temperate mountain forest soil assessed by 16S rRNA gene pyrosequencing. FEMS Microbiology Ecology. 2012;82:551–562. doi: 10.1111/j.1574-6941.2012.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laternser M, Schneebeli M. Long-term snow climate trends of the Swiss Alps (1931–99) International Journal of Climatology. 2003;23:733–750. [Google Scholar]
- Lipson DA, Schadt CW, Schmidt SK. Changes in soil microbial community structure and function in an alpine dry meadow following spring snow melt. Microbial Ecology. 2002;43:307–314. doi: 10.1007/s00248-001-1057-x. [DOI] [PubMed] [Google Scholar]
- Liptzin D, Williams MW, Helmig D, Seok B, Filippa G, Chowansky K, Hueber J. Process-level controls on CO2 fluxes from a seasonally snow-covered subalpine meadow soil, Niwot Ridge, Colorado. Biogeochemistry. 2009;95:151–166. [Google Scholar]
- Liu J, Curry JA, Wang H, Song M, Horton RM. Impact of declining Arctic sea ice on winter snowfall. Proceedings of the National Academy of Sciences. 2012;109:4074–4079. doi: 10.1073/pnas.1114910109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd J, Taylor JA. On the temperature dependence of soil respiration. Functional Ecology. 1994;8:315–323. [Google Scholar]
- Mariko S, Nishimura N, Mo W, Matsui Y, Kibe T, Koizumi H. Winter CO2 flux from soil and snow surfaces in a cool-temperate deciduous forest, Japan. Ecological Research. 2000;15:363–372. [Google Scholar]
- Massman WJ. A review of the molecular diffusivities of H2O, CO2, CH4, CO, O3, SO2, NH3, N2O, NO, and NO2 in air, O2 and N2 near STP. Atmospheric Environment. 1998;32:1111–1127. [Google Scholar]
- Mast MA, Wickland KP, Striegl RT, Clow DW. Winter fluxes of CO2 and CH4 from subalpine soils in Rocky Mountain National Park, Colorado. Global Biogeochemical Cycles. 1998;12:607–620. [Google Scholar]
- Merbold L, Steinlin C, Hagedorn F. Winter greenhouse gas emissions (CO2, CH4 and N2O) from a sub-alpine grassland. Biogeosciences Discuss. 2013;10:401–455. [Google Scholar]
- Mikan CJ, Schimel JP, Doyle AP. Temperature controls of microbial respiration in arctic tundra soils above and below freezing. Soil Biology & Biochemistry. 2002;34:1785–1795. [Google Scholar]
- Millington RJ. Gas diffusion in porous media. Science. 1959;130:100–102. doi: 10.1126/science.130.3367.100-a. [DOI] [PubMed] [Google Scholar]
- Millington RJ, Shearer RC. Diffusion in aggregated porous media. Soil Science. 1971;111:372. [Google Scholar]
- Mo W, Lee M, Uchida M, Inatomi M, Saigusa N, Mariko S, Koizumi H. Seasonal and annual variations in soil respiration in a cool-temperate deciduous broad-leaved forest in Japan. Agricultural and Forest Meteorology. 2005;134:81–95. [Google Scholar]
- Monson RK, Turnipseed AA, Sparks JP, Harley PC, Scott-Denton LE, Sparks K, Huxman TE. Carbon sequestration in a high-elevation, subalpine forest. Global Change Biology. 2002;8:459–478. [Google Scholar]
- Monson RK, Burns SP, Williams MW, Delany AC, Weintraub M, Lipson DA. The contribution of beneath-snow soil respiration to total ecosystem respiration in a high-elevation, subalpine forest. Global Biogeochemical Cycles. 2006a;20:GB3030. [Google Scholar]
- Monson RK, Lipson DL, Burns SP, Turnipseed AA, Delany AC, Williams MW, Schmidt SK. Winter forest soil respiration controlled by climate and microbial community composition. Nature. 2006b;439:711–714. doi: 10.1038/nature04555. [DOI] [PubMed] [Google Scholar]
- Muhr JAN, Borken W, Matzner E. Effects of soil frost on soil respiration and its radiocarbon signature in a Norway spruce forest soil. Global Change Biology. 2009;15:782–793. [Google Scholar]
- Musselman RC, Massman WJ, Frank JM, Korfmacher JL. The temporal dynamics of carbon dioxide under snow in a high elevation Rocky Mountain subalpine forest and meadow. Arctic, Antarctic, and Alpine Research. 2005;37:527–538. [Google Scholar]
- Natali SM, Schuur EAG, Trucco C, Hicks Pries CE, Crummer KG, Baron Lopez AF. Effects of experimental warming of air, soil and permafrost on carbon balance in Alaskan tundra. Global Change Biology. 2011;17:1394–1407. [Google Scholar]
- Schadt CW, Martin AP, Lipson DA, Schmidt SK. Seasonal dynamics of previously unknown fungal lineages in tundra soils. Science. 2003;301:1359–1361. doi: 10.1126/science.1086940. [DOI] [PubMed] [Google Scholar]
- Schimel JP, Bilbrough C, Welker JM. Increased snow depth affects microbial activity and nitrogen mineralization in two Arctic tundra communities. Soil Biology and Biochemistry. 2004;36:217–227. [Google Scholar]
- Schindlbacher A, Zechmeister-Boltenstern S, Glatzel G, Jandl R. Winter soil respiration from an Austrian mountain forest. Agricultural and Forest Meteorology. 2007;146:205–215. [Google Scholar]
- Schindlbacher A, Zechmeister-Boltenstern S, Jandl R. Carbon losses due to soil warming: do autotrophic and heterotrophic soil respiration respond equally? Global Change Biology. 2009;15:901–913. [Google Scholar]
- Schindlbacher A, De Gonzalo C, Díaz-Pinés E, et al. Temperature sensitivity of forest soil organic matter decomposition along two elevation gradients. Journal of Geophysical Research. 2010;115:G03018. [Google Scholar]
- Schindlbacher A, Rodler A, Kuffner M, Kitzler B, Sessitsch A, Zechmeister-Boltenstern S. Experimental warming effects on the microbial community of a temperate mountain forest soil. Soil Biology & Biochemistry. 2011;43:1417–1425. doi: 10.1016/j.soilbio.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindlbacher A, Wunderlich S, Borken W, Kitzler B, Zechmeister-Boltenstern S, Jandl R. Soil respiration under climate change: prolonged summer drought offsets soil warming effects. Global Change Biology. 2012;18:2270–2279. [Google Scholar]
- Schmidt SK, Wilson KL, Monson RK, Lipson DA. Exponential growth of ‘snow molds’ at sub-zero temperatures: an explanation for high beneath-snow respiration rates and Q10 values. Biogeochemistry. 2009;95:13–21. [Google Scholar]
- Schuur EAG, Vogel JG, Crummer KG, Lee H, Sickman JO, Osterkamp TE. The effect of permafrost thaw on old carbon release and net carbon exchange from tundra. Nature. 2009;459:556–559. doi: 10.1038/nature08031. [DOI] [PubMed] [Google Scholar]
- Sommerfeld RA, Moiser AR, Musselman RC. CO2, CH4, and N2O flux through a Wyoming snowpack and implications for global budgets. Nature. 1993;361:140–142. [Google Scholar]
- Subke J-A, Voke NR, Leronni V, Garnett MH, Ineson P. Dynamics and pathways of autotrophic and heterotrophic soil CO2 efflux revealed by forest girdling. Journal of Ecology. 2011;99:186–193. [Google Scholar]
- Sullivan B, Dore S, Montes-Helu M, Kolb T, Hart S. Pulse emissions of carbon dioxide during snowmelt at a high-elevation site in Northern Arizona, U.S.A. Arctic, Antarctic, and Alpine Research. 2012;44:247–254. [Google Scholar]
- Suni T, Berninger F, Markkanen T, Keronen P, Rannik Ü, Vesala T. Interannual variability and timing of growing-season CO2 exchange in a boreal forest. Journal of Geophysical Research. 2003;108:4265. [Google Scholar]
- Tuomi M, Vanhala P, Karhu K, Fritze H, Liski J. Heterotrophic soil respiration - Comparison of different models describing its temperature dependence. Ecological Modelling. 2008;211:182–190. [Google Scholar]
- Welker JM, Fahnestock JT, Jones MH. Annual CO2 in try and moist arctic tundra: field responses to increases in summer temperatures and winter snow depth. Climatic Change. 2000;44:139–150. [Google Scholar]
- Winston GC, Stephens BB, Sundquist ET, Hardy JP, Davis RE. Seasonal variability in CO2 transport through snow in a boreal forest. In: Tonnessen Ka, Williams Mw, Tranter M., editors. Biogeochemistry of Seasonally Snow-Covered Catchments. Vol. 228. IAHS Publication: 1995. pp. 61–70. [Google Scholar]
- Zhang T, Barry RG, Haeberli W. Numerical simulations of the influence of the seasonal snow cover on the occurrence of permafrost at high latitudes. Norsk Geografisk Tidsskrift - Norwegian Journal of Geography. 2001;55:261–266. [Google Scholar]
- Zimov SA, Davidov SP, Voropaev YV, Prosiannikov SF, Semiletov IP, Chapin MC, Chapin FS. Siberian CO2 efflux in winter as CO2 source and cause of seasonality in atmospheric CO2. Climatic Change. 1996;33:111–120. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Relation between snow depth measurements at Achenkirch village and at the field site. Linear regression (black line) and 1 : 1 line (red).
Figure S2. Comparison of snow probe and chamber technique at low snow depth. Measurements were performed on a single day at a snow depth of 9–11 cm. Snow probe measurements were performed directly in the permanent chambers. Subsequently, snow was removed from inside and from around the chambers and the CO2 efflux was estimated by the closed dynamic chamber method. Linear regression (black line), 1 : 1 line (red), and 95% confidence bands (blue).


