Abstract
Purpose
The aim of the present study aimed to evaluate the effect of testosterone on cardiovascular disease by using the Framingham Risk Score (FRS) in patients with sexual dysfunction.
Materials and Methods
A total of 308 men with sexual dysfunction were enrolled in this study. Clinical assessments included the 15-item International Index of Erectile Function (IIEF), blood pressure measurement, and clinical laboratory indexes. The FRS, which predicts the incidence rate of cardiovascular diseases in the next 10 years, was calculated on the basis of age, gender, total cholesterol, smoking status, high density lipoprotein cholesterol, and systolic blood pressure.
Results
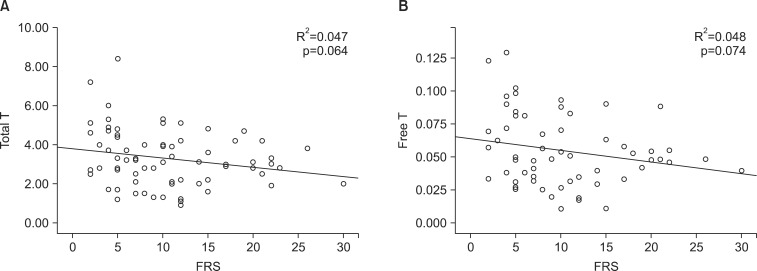
The mean age of the 308 enrolled patients was 49.42±10.73 years, and the patients' mean body mass index (kg/m2) was 25.07±3.14. The mean total IIEF score was 28.44±18.06. The median total testosterone concentration was 3.2 ng/mL (interquartile range [IQR]: 2.3~3.2 ng/mL). The median calculated free and bioavailable testosterone concentrations were 0.052 ng/mL (IQR 0.039~0.070 ng/mL) and 1.30 ng/mL (IQR: 1.00~1.76 ng/mL), respectively. The mean FRS was 10.47±6.45. The FRS tended to show a negative correlation with the total and calculated free testosterone levels, but this was not significant (p=0.064 and p=0.074, respectively). In the multiple linear regression analysis, a significant negative correlation was observed between the total testosterone level and the FRS (p=0.048).
Conclusions
The results suggest that the testosterone level is related to the FRS and that a high testosterone level may decrease the risk of cardiovascular disease.
Keywords: Cardiovascular diseases, Sexual dysfunction, Testosterone
INTRODUCTION
With the increase in the average human life span, the morbidity of chronic diseases such as hypertension and diabetes has also increased [1]. In addition, testosterone levels in men decrease with age as a result of declining adrenal and testicular function [2]. Consequently, studying the impact of this decrease in testosterone on chronic diseases has become important. Testosterone decreases in men induce decreases in muscle mass and increases in the amount of fat, particularly visceral fat [3]. As a result, abnormalities in glucose and lipid metabolism develop along with increased insulin resistance [4,5]. This series of events is generally referred to as the metabolic syndrome and is known to increase the incidence rate of cardiovascular diseases.
Cardiovascular diseases are among the most common causes of death in older adults [6,7]. Past studies comparing males and females in similar age ranges have shown that high testosterone levels are related to the occurrence of cardiovascular diseases [8,9,10]. Despite a few negative results, recent large-scale cohort analyses suggest that low testosterone levels are associated with coronary artery disease [11,12]. Type-2 diabetes and obesity are also associated with low testosterone levels and contribute to increased cardiovascular disease risk. Lifestyle habits such as drinking and smoking, additional factors that increase incidence rates of cardiovascular disease, also induce low testosterone levels in men [13,14,15].
The Framingham risk score (FRS), a scoring system derived using data from the Framingham heart study conducted in the United States (US), is an instrument that uses simple past medical history and gender-specific cholesterol levels in asymptomatic patients to predict the incidence rate of cardiovascular diseases in the next 10 years [16,17]. In the present study, we used the FRS to study the effect of testosterone levels on cardiovascular disease risk in patients with sexual dysfunction.
MATERIALS AND METHODS
This retrospective study included patients who visited the urology clinic between 2003 and 2013. A total of 308 patients with completed surveys, high fidelity medical records, and planned laboratory tests were selected and analyzed. This study was approved by the Institutional Review Board of Hallym University Kangdong Sacred Heart Hospital.
History taking and a physical examination including the 15-item International Index of Erectile Function (IIEF) were performed on all patients. Mean blood pressure was recorded after three measurements with the patient in a stabilized state. Laboratory tests included complete blood cell count, liver function tests, hemoglobin A1c, total testosterone, sexual hormone binding globulin (SHBG), and serum prolactin. Because testosterone fluctuates throughout the day, samples were collected before 10:00 a.m. irrespective of the season. Vermeulen's formula (available at http://www.issam.ch/freetesto.htm) was used to calculate free testosterone and bioavailable testosterone levels.
The FRS is calculated on the basis of age, gender, total cholesterol, smoking status, high density lipoprotein (HDL) cholesterol, and systolic blood pressure (SBP). The FRS was calculated using an online calculator provided by the National Heart, Lung, and Blood Institute (available at http://cvdrisk.nhlbi.nih.gov/calculator.asp). An individual with an FRS of 12 is considered to have a 10% risk of coronary heart disease in the next 10 years. An FRS of 15 indicates a 20% rate, and an FRS of 17 indicates an incidence rate of greater than 30%.
Statistical analysis was performed using IBM SPSS Statistics version 19 (IBM Co., Armonk, NY, USA). Spearman's correlation analysis was carried out for clinicopathological data relevant to testosterone. A multiple linear regression analysis was used to identify hormones and clinicopathological parameters that affected the FRS. All statistical results showed a 95% confidence interval and were considered significant when the p value was below 0.05.
RESULTS
The mean age of the 308 enrolled patients was 49.42±10.73 years, and the mean body mass index (kg/m2) was 25.07±3.14. The patients' mean total IIEF score was 28.44±18.06; the mean scores on the individual domains of the IIEF are presented in Table 1. The median total testosterone level was 3.2 ng/mL (interquartile range [IQR]: 2.3~3.2 ng/mL), and the median SHBG level was 39.06 nmol/L (IQR: 26.93~56.00 nmol/L). The median calculated free testosterone level was 0.052 ng/mL (IQR: 0.039~0.070 ng/mL), and the median calculated bioavailable testosterone level was 1.30 ng/mL (IQR: 1.00~1.76 ng/mL).
Table 1.
Demographics and other baseline characteristics of male patients with sexual dysfunction
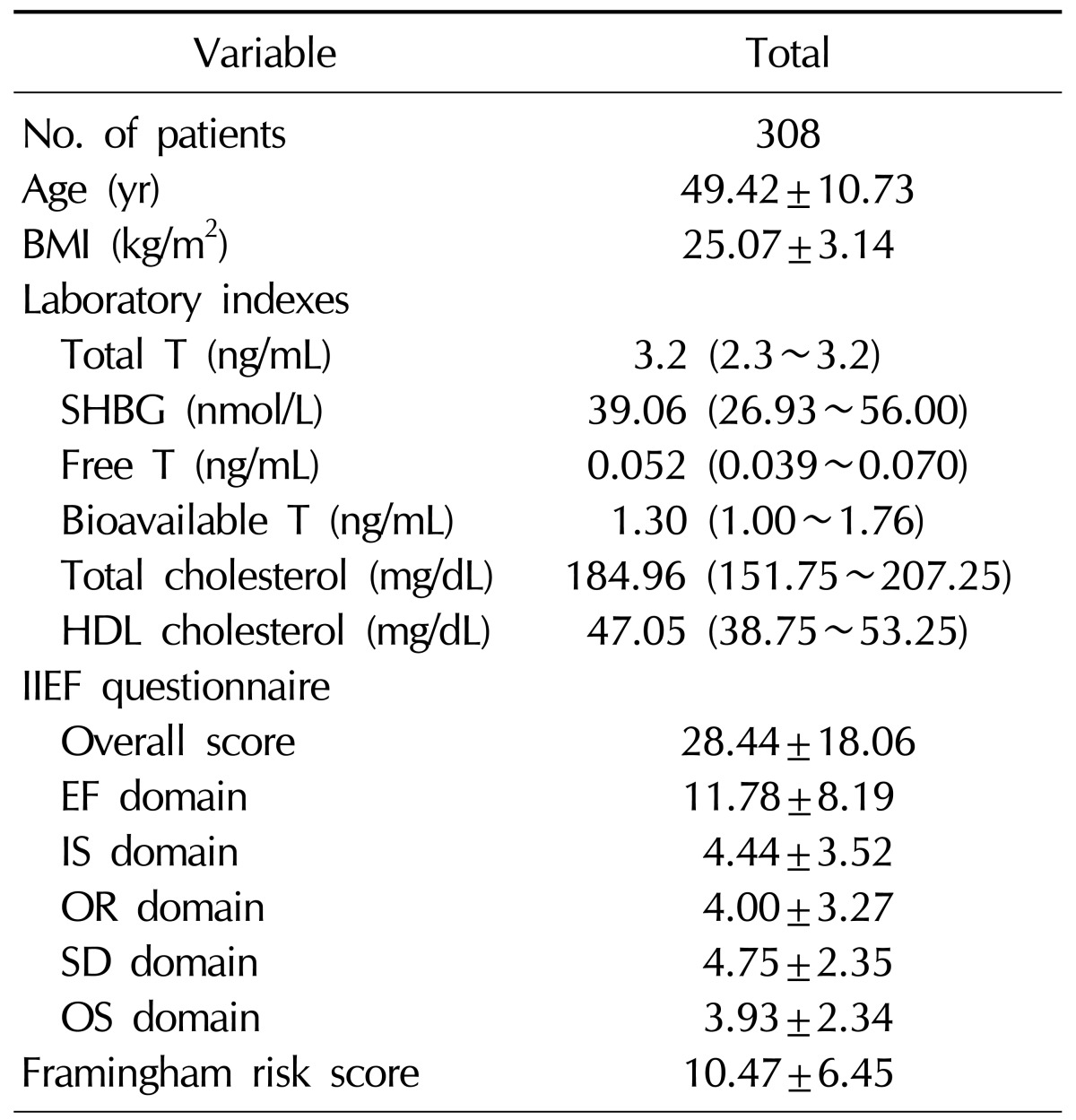
Values are presented as number only, mean±standard deviation, or median (interquartile range).
BMI: body mass index, T: testosterone, SHBG: sex hormone binding globulin, HDL: high- density lipoprotein, IIEF: International Index of Erectile Function, EF: erectile function, IS: intercourse satisfaction, OR: orgasmic function, SD: sexual desire, OS: overall satisfaction.
The study included 88 current smokers (38.6%) and 52 (22.8%) ex-smokers. The patients' mean SBP based on three measurements at a stable state was 126.00±16.76 mmHg. The median total cholesterol level was 184.96 mg/dL (IQR: 151.75~207.25 mg/dL), and the median HDL cholesterol level was 47.05 mg/dL (IQR: 38.75~53.25 mg/dL). The mean FRS for the study population on the basis of these values was 10.47±6.45 (Table 1).
The FRS showed a tendency toward a negative correlation with total testosterone and calculated free testosterone. However, these negative correlations were not statistically significant in Spearman's correlation analysis (p=0.064 for total testosterone, Fig. 1A; p=0.074 for free testosterone, Fig. 1B). A correlation between the total testosterone level and the total IIEF score was also not statistically significant (p=0.311), nor was the correlation between the FRS and the IIEF (p=0.143).
Fig. 1.
Representation of the correlation analysis. (A) There was a trend toward a negative correlation between the FRS and the total T level. (B) There was a trend toward a negative correlation between the FRS and the calculated free T level. T: testosterone, FRS: Framingham risk score.
In the multiple linear regression analysis that included total testosterone and free testosterone, a significant negative correlation was observed between the total testosterone level and the FRS (p=0.017, Table 2). However, the free testosterone level was not significantly correlated with the FRS in the multiple linear regression analysis.
Table 2.
Results of multiple linear regression analysis of the Framingham risk score
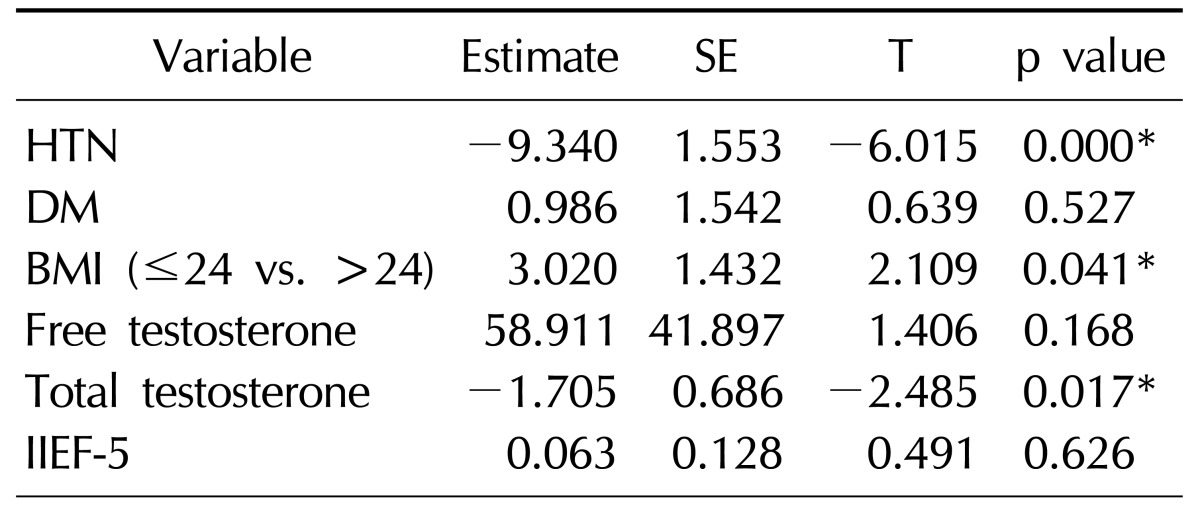
HTN: hypertension, DM: diabetes mellitus, BMI: body mass index, IIEF-5: five-item International Index of Erectile Function, SE: standard error, T: Test statistic.
*p<0.05.
DISCUSSION
We found a negative correlation between the total testosterone levels in men and the risk of cardiovascular disease. Testosterone is a steroid hormone from the androgen group [18]. Although a small amount is produced in tissues other than the testicles, most testosterone is produced in the male testicles [19]. Many studies to date have reported a negative correlation between testosterone levels and the metabolic syndrome, whereby patients with low testosterone levels show elevated levels of serum triglycerides, total cholesterol, lower density lipoprotein cholesterol, apolipoprotein B, and fasting and 2-hour plasma insulin concentrations, along with decreased levels of HDL cholesterol [20]. Such changes in the lipid profile have been known to increase the risk of cardiovascular disease [21].
The IIEF is a globally used questionnaire for determining both the diagnosis and the treatment effect of sexual dysfunction. However, because the IIEF was developed to assess the impact of phosphodiesterase-5 inhibitors on erectile dysfunction, its results may not be relevant to the actual measurement of serum testosterone levels [22,23]. No significant relationship between the serum total testosterone level and the IIEF was observed in this study. This result contradicts the results of a recently published study showing a weak association between the IIEF and the total testosterone level [24]. This discrepancy may be the result of the differences in inclusion criteria. The above study targeted patients with a measured total testosterone concentration of less than 3.5 ng/mL, whereas we enrolled patients irrespective of their testosterone concentration.
The FRS is a gender-specific scoring system used to predict an individual's cardiovascular disease risk in the next 10 years [16,17]. The FRS was revised in 2008 to include cerebrovascular events, peripheral artery disease, and heart failure. The benefit of the FRS is the ability to calculate the 10-year cardiovascular disease risk by using simple medical history and cholesterol levels and to be able to advise patients to stop smoking and change their diets in order to lower their cardiovascular risk by reducing their cholesterol levels. However, because the FRS targeted the upper middle-class population of Massachusetts in the US, there is a tendency for risk overestimation in Asian (including Korean) and European contemporary populations [25]. The recent recalibration of the FRS as a coronary heart disease prediction model in Korea could be helpful for future studies of the relationship between testosterone and cardiovascular diseases [26].
Although a negative correlation between testosterone levels and the coronary artery score has been reported [21], few studies have evaluated decreases in cardiovascular disease risk following testosterone replacement therapy. In a recent study, no definite proof of reduced cardiovascular disease risk was shown in patients who received testosterone replacement therapies when compared with a placebo group [27]. Thus, a prospective study of the effect of testosterone replacement therapy is necessary. The thesis of a lowered cardiovascular disease risk through a changed metabolic profile (via changes in central obesity, insulin resistance, and lipid profile) and through changed testosterone concentrations suggests that scoring systems such as the FRS may be very helpful for future studies.
This study had the limitations of being a single-institute, retrospective study. Second, the study only included patients who visited the urology department for erectile dysfunction between 2003 and 2013. Because this may induce a selection bias, an additional broader study is thought necessary. However, patients with relevant medical records were selected for participation, and the amount of data lost was controlled as much as possible. Third, the FRS used in this study may overestimate the cardiovascular risk in Koreans because it was developed for a US population. Finally, we lacked longitudinal data on changes in the FRS or lipid profile after testosterone replacement therapy.
CONCLUSIONS
The present study found a negative correlation between the total testosterone level and the FRS. This result suggests that high testosterone levels are associated with a low 10-year risk of cardiovascular disease. However, these findings are based on data from 308 patients with erectile dysfunction and thus, require further exploration in prospective larger-scale studies.
References
- 1.Lim S, Park KS, Lee HK, Cho SI Korean National Health and Nutrition Examination Surveys. Changes in the characteristics of metabolic syndrome in Korea over the period 1998-2001 as determined by Korean National Health and Nutrition Examination Surveys. Diabetes Care. 2005;28:1810–1812. doi: 10.2337/diacare.28.7.1810. [DOI] [PubMed] [Google Scholar]
- 2.Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 3.Vermeulen A. Ageing, hormones, body composition, metabolic effects. World J Urol. 2002;20:23–27. doi: 10.1007/s00345-002-0257-4. [DOI] [PubMed] [Google Scholar]
- 4.Björntorp P. Portal adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10:493–496. [PubMed] [Google Scholar]
- 5.Després JP, Lemieux S, Lamarche B, Prudhomme D, Moorjani S, Brun LD, et al. The insulin resistance-dyslipidemic syndrome: contribution of visceral obesity and therapeutic implications. Int J Obes Relat Metab Disord. 1995;19(Suppl 1):S76–S86. [PubMed] [Google Scholar]
- 6.Srinivas-Shankar U, Roberts SA, Connolly MJ, O'Connell MD, Adams JE, Oldham JA, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–650. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 7.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsson B, Bengtsson C, Björntorp P, Lapidus L, Sjöström L, Svärdsudd K, et al. Is abdominal body fat distribution a major explanation for the sex difference in the incidence of myocardial infarction? The study of men born in 1913 and the study of women, Göteborg, Sweden. Am J Epidemiol. 1992;135:266–273. doi: 10.1093/oxfordjournals.aje.a116280. [DOI] [PubMed] [Google Scholar]
- 9.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111:383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 10.Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116:2694–2701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 11.Tsujimura A. The relationship between testosterone deficiency and men's health. World J Mens Health. 2013;31:126–135. doi: 10.5534/wjmh.2013.31.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oskui PM, French WJ, Herring MJ, Mayeda GS, Burstein S, Kloner RA. Testosterone and the cardiovascular system: a comprehensive review of the clinical literature. J Am Heart Assoc. 2013;2:e000272. doi: 10.1161/JAHA.113.000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, Pols HA. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002;87:3632–3639. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- 14.English KM, Mandour O, Steeds RP, Diver MJ, Jones TH, Channer KS. Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur Heart J. 2000;21:890–894. doi: 10.1053/euhj.1999.1873. [DOI] [PubMed] [Google Scholar]
- 15.Alexandersen P, Haarbo J, Christiansen C. The relationship of natural androgens to coronary heart disease in males: a review. Atherosclerosis. 1996;125:1–13. doi: 10.1016/0021-9150(96)05864-9. [DOI] [PubMed] [Google Scholar]
- 16.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 17.DAgostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 18.Habert R, Lejeune H, Saez JM. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol Cell Endocrinol. 2001;179:47–74. doi: 10.1016/s0303-7207(01)00461-0. [DOI] [PubMed] [Google Scholar]
- 19.King SR, Manna PR, Ishii T, Syapin PJ, Ginsberg SD, Wilson K, et al. An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. J Neurosci. 2002;22:10613–10620. doi: 10.1523/JNEUROSCI.22-24-10613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saad F. The role of testosterone in type 2 diabetes and metabolic syndrome in men. Arq Bras Endocrinol Metabol. 2009;53:901–907. doi: 10.1590/s0004-27302009000800002. [DOI] [PubMed] [Google Scholar]
- 21.Rosano GM, Sheiban I, Massaro R, Pagnotta P, Marazzi G, Vitale C, et al. Low testosterone levels are associated with coronary artery disease in male patients with angina. Int J Impot Res. 2007;19:176–182. doi: 10.1038/sj.ijir.3901504. [DOI] [PubMed] [Google Scholar]
- 22.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 23.Kang S, Park HJ, Park NC. Serum total testosterone level and identification of late-onset hypogonadism: a community-based study. Korean J Urol. 2013;54:619–623. doi: 10.4111/kju.2013.54.9.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang JI, 2nd, Ham BK, Oh MM, Kim JJ, Moon du G. Correlation between serum total testosterone and the AMS and IIEF questionnaires in patients with erectile dysfunction with testosterone deficiency syndrome. Korean J Urol. 2011;52:416–420. doi: 10.4111/kju.2011.52.6.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Hong Y, D'Agostino RB, Sr, Wu Z, Wang W, Sun J, et al. Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese Multi-Provincial Cohort Study. JAMA. 2004;291:2591–2599. doi: 10.1001/jama.291.21.2591. [DOI] [PubMed] [Google Scholar]
- 26.Jee SH, Jang Y, Oh DJ, Oh BH, Lee SH, Park SW, et al. A coronary heart disease prediction model: the Korean Heart Study. BMJ Open. 2014;4:e005025. doi: 10.1136/bmjopen-2014-005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corona G, Rastrelli G, Morelli A, Vignozzi L, Mannucci E, Maggi M. Hypogonadism and metabolic syndrome. J Endocrinol Invest. 2011;34:557–567. doi: 10.3275/7806. [DOI] [PubMed] [Google Scholar]