Abstract
Adrenocortical carcinoma (ACC) is an aggressive malignancy, which lacks an effective systemic treatment. Abnormal activation of insulin-like growth factor receptor 1 (IGF1R) has been frequently observed. Preclinical studies demonstrated that pharmacological inhibition of IGF1R signaling in ACC has antiproliferative effects. A previous phase I trial with an IGF1R inhibitor has demonstrated biological activity against ACC. The objective of this study is to assess the efficacy of the combination of the IGF1R inhibitor cixutumumab (IMC-A12) in association with mitotane as a first-line treatment for advanced/metastatic ACC. We conducted a multicenter, randomized double-arm phase II trial in patients with irresectable recurrent/metastatic ACC. The original protocol included two treatment groups: IMC-A12 + mitotane and mitotane as a single agent, after an initial single-arm phase for safety evaluation with IMC-A12 + mitotane. IMC-A12 was dosed at 10 mg/kg intravenously every 2 weeks. The starting dose for mitotane was 2 g daily, subsequently adjusted according to serum levels/symptoms. The primary endpoint was progression-free survival (PFS) according to RECIST (Response Evaluation Criteria in Solid Tumors). This study was terminated before the randomization phase due to slow accrual and limited efficacy. Twenty patients (13 males, 7 females) with a median age of 50.2 years (range 21.9–79.6) were enrolled for the single-arm phase. Therapeutic effects were observed in 8/20 patients, including one partial response and seven stable diseases. The median PFS was 6 weeks (range 2.66–48). Toxic events included two grade 4 (hyperglycemia and hyponatremia) and one grade 5 (multiorgan failure). Although the regimen demonstrated activity in some patients, the relatively low therapeutic efficacy precluded further studies with this combination of drugs.
Keywords: Temsirolimus, Adrenocortical Carcinoma, Mitotane, IGF1R Signaling, IGF1R Inhibitor
Introduction
Adrenocortical carcinoma (ACC) is a rare tumor, with an estimated worldwide annual prevalence of 0.5 to 2 cases per million [14]. About half of newly diagnosed ACC patients present with advanced/metastatic disease [8]. In this scenario, the 5-year survival rates are dismal, usually less than 15 % [8]. High recurrence rates are observed even in early-stage patients in whom a complete resection could be accomplished [17]. Therapeutic options for advanced disease are associated with adverse effects and do not clearly improve survival [9]. Mitotane remains the only FDA-approved drug for metastatic ACC. The reported response rates for mitotane as a single agent are based on uncontrolled trials and small case series averaging 32 % [23]. The progression-free survival (PFS) of patients treated with mitotane only remains unknown. Recently, a phase III prospective trial compared the efficacy of two multidrug regimens: streptozotocin plus mitotane (Sz + M) and cisplatin, etoposide, doxorubicin plus mitotane (EDP + M), favoring EDP + M as the first-line option [10]. However, the majority of the patients experienced a rapid and inexorable progression. Therefore, new therapies for advanced ACC are urgently needed.
In recent years, molecular-targeted therapies have been proposed as therapeutic options for different types of cancer. In ACC, several studies have demonstrated a significant role for insulin-like growth factor system activation in tumorigenesis. High expression levels of insulin-like growth factor 2 (IGF2) have been demonstrated in 80–90 % of ACCs [2, 11, 13, 16]. The mitogenic effects of IGF2 are mediated by the insulin-like growth factor receptor 1 (IGF1R), which is also highly expressed in ACC [4, 11]. IGF1R is a membrane tyrosine kinase-associated receptor (RTK) that upon ligand binding forms a dimer with other IGF1R, leading to transphosphorylation and recruitment of insulin receptor substrates (IRS) and Src homology adaptor proteins. Signaling transduction occurs by activation of the phosphoinositide-3-kinase/v-akt murine thymoma viral oncogene homolog (PI3K/AKT) and RAS/RAF/mitogen-activated protein kinase (MAPK) pathways [12]. In addition to IGF1R, IGF2 has also high affinity for the short isoform of the insulin receptor (IR-A) [5]. Unlike the long isoform (IR-B), which is preferentially expressed in adult tissues and mediates metabolic effects, IR-A is more prevalent in fetal tissues and its activation promotes cell proliferation. High IR-A expression levels have also been documented in some cancer types and may induce resistance to IGF1R inhibitors since these drugs do not target IR-A [5]. Preclinical studies have demonstrated that inhibition of IGF1R signaling significantly reduces cell proliferation and enhances apoptosis [2, 4]. Moreover, preclinical data have shown that inhibition of IGF1R potentiates mitotane cytotoxic activity [2, 4]. A phase I trial of a monoclonal antibody targeting IGF1R for advanced ACC has demonstrated good tolerability and activity against the disease [15].
Cixutumumab (IMC-A12) is a recombinant human IgG1 monoclonal antibody directed at the IGF1R. IMC-A12 binds IGF1R with high affinity, blocking the interaction with its ligands. IMC-A12 has demonstrated tumor growth inhibition in experimental models and clinical trials in a wide variety of human cancers [6, 1, 3]. In addition, the combination of IMC-A12 with other agents and radiation therapy has demonstrated synergistic effects [19, 24, 7, 18]. The aim of the present study was to assess the therapeutic efficacy of the combination of IMC-A12 with mitotane as a first-line treatment in patients with recurrent/metastatic ACC.
Materials and Methods
Patients
Adults with progressive, unresectable, or metastatic histologically proven ACC were eligible for this study. Main inclusion criteria were at least one measurable tumor lesion; no previous treatments with cytotoxic or molecular-targeted drugs; ECOG performance status ≤2; adequate hematopoietic, renal, and hepatic functions; and previous mitotane treatment for no more than 8 weeks with good tolerance. This trial was performed according to the principles embodied in the Declaration of Helsinki and was approved by the IRB of each participating institution; all enrolled patients signed an informed consent. This trial was registered on ClinicalTrials.gov (NCT00778817).
Study Design
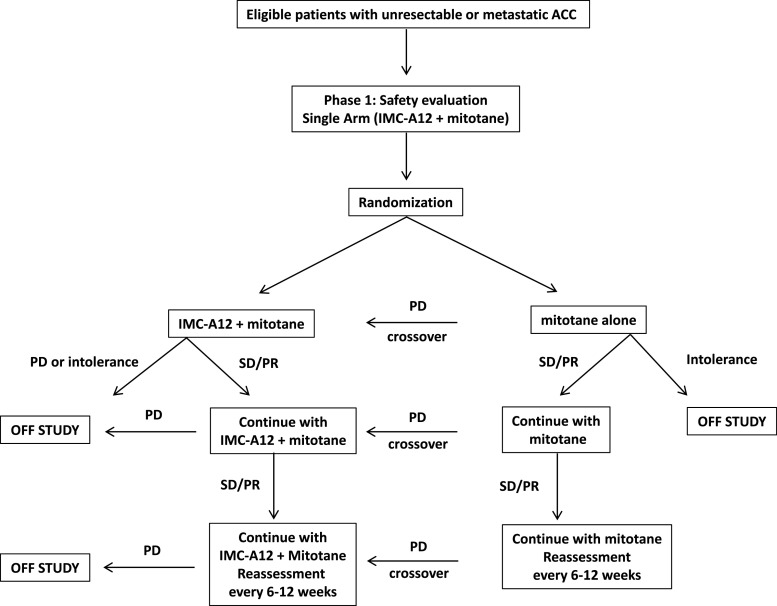
A flowchart with the study protocol is shown in Fig. 1. The original design included a phase I run-in single arm with the combined treatment (mitotane + IMC-A12), followed by a randomized, open-label, double-arm phase II trial in which patients would be randomized to receive mitotane as a single agent or the combined regimen (mitotane + IMC-A12). The main goal of phase II was to assess whether the combination regimen (mitotane + IMC-A12) could provide additional benefits over monotherapy with mitotane, mirroring the synergistic effects observed in vitro [4]. Since the combination of mitotane and IMC-A12 had not been previously tested in patients and it was being proposed as a first-line treatment, it was important to provide preliminary evidence of activity and to assure no unexpected toxicities. For this reason, the original design of the study included a phase I single arm in 20 patients with the combined treatment (mitotane + IMC-A12). A review of the data following the run-in phase was planned to assess the efficacy and tolerability of the regimen although no formal stopping rules were defined. The primary endpoint was PFS according to RECIST (Response Evaluation Criteria in Solid Tumors) criteria. Patients were allowed to have received mitotane for no longer than 8 weeks before enrollment. Due to slow accrual and limited antitumor activity, the study was terminated before the randomization phase commenced.
Fig. 1.
Flowchart representing the original study protocol
Treatment
IMC-A12 was dosed at 10 mg/kg intravenously as a single 1-h infusion every 2 weeks. The starting dose for mitotane was 2 g orally on a daily basis. Dose adjustments were based on symptoms and serum levels. Glucocorticoid replacement therapy (oral hydrocortisone 10–30 mg every morning and 5–15 mg every evening) was initiated concomitantly with mitotane. Fludrocortisone replacement was initiated if a patient presented signs of mineralocorticoid deficiency. Treatment was withheld for hematological toxicity grade ≥3 or nonhematological toxicity grade ≥2 and resumed after recovery for grade ≤2 for hematological and ≤1 for nonhematological. IMC-A12 dose reductions to 8 and 6 mg/kg were allowed.
Efficacy and Safety Evaluation
Clinical evaluation and toxicity assessment were performed every 2 weeks for the first 12 weeks and every 6 weeks thereafter. Biochemical exams included a complete blood count, serum electrolytes, blood urea nitrogen, serum creatinine, aspartate-aminotransferase (AST), alanine-aminotransferase (ALT), alkaline phosphatase, random blood sugar, and HbA1c.
Response Assessment
Tumor response assessment was performed with appropriate imaging methods (CT scans or MRI) every 6 weeks according to RECIST criteria.
Results
Patient Characteristics
From December 2008 to January 2012, a total of 20 chemotherapy-naïve patients (13 males, 7 females) with recurrent or unresectable/metastatic ACC were enrolled on the study. The median of the age of the participants was 50.2 years (range 21.9–79.6). Patient characteristics are summarized in Table 1.
Table 1.
Demographic characteristics at baseline and best response to treatment of individual patients
| Patient | Age (years) | Sex | ECOG status | Affected sites | Primary tumor Tx | Sum of LD (target lesions) | Best response | Best response | Number of weeks | Reason for termination |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48.8 | M | 0 | Peritoneum, retroperitoneum | Surgery + adj. RxTx | 10.3 | 5 | SD | 12 | PD |
| 2 | 48.9 | F | 1 | Liver, lungs | Surgery | 22.1 | −5.8 | SD | 10 | Withdrew |
| 3 | 48.6 | M | 0 | Liver, lungs | Surgery | 3.5 | – | PD (interval progression documented by PET-CT) | 6 | PD |
| 4 | 21.9 | M | 0 | Liver | Surgery | 1.1 | 64 | PD | 6 | PD |
| 5 | 38 | F | 0 | Lungs | Surgery | 4.4 | 23 | PD | 6 | PD |
| 6 | 23.1 | M | 0 | Liver, lungs | – | 20.2 | 7 | PD | 4 | Clinical progression |
| 7 | 35.9 | M | 0 | Liver, lungs, lymph nodes | Surgery | 5.9 | – | PD (new lesion) | 6 | PD |
| 8 | 55.1 | M | 0 | Liver | – | 21 | – | PD (clinical progression) | 2.6 | PD |
| 9 | 55.5 | F | 1 | Liver | Surgery | 25.5 | 10.6 | SD | 12 | PD |
| 10 | 79.6 | M | 0 | Liver, peritoneum, lymph nodes | Surgery + adj. RxTx | 11.8 | 18.9 | SD | 12 | PD |
| 11 | 29.6 | M | 0 | Liver, peritoneum | Surgery | 5.9 | 49 | PD | 6 | PD |
| 12 | 52.7 | F | 0 | Lungs | Surgery | 2.2 | −18 | SD | 18 | PD |
| 13 | 68 | M | 1 | Liver, lymph nodes | – | 22.2 | 0 | SD | 6.2 | Adverse events |
| 14 | 23.2 | F | 0 | Kidney | Surgery | 1.9 | 20 | PD | 6 | PD |
| 15 | 51.9 | M | 1 | Liver, lungs | Surgery | 2.8 | 71 | PD | 6 | PD |
| 16 | 74.5 | M | 0 | Liver, lungs, peritoneum | Surgery | 16.8 | 25 | PD | 6 | PD |
| 17 | 51.3 | M | 1 | Liver, lungs, peritoneum | Surgery | 14 | −49 | PR | 18.4 | Adverse events |
| 18 | 53.9 | F | 1 | Lungs, peritoneum, spleen | Surgery | 26.1 | −18 | SD | 48 | Withdrew |
| 19 | 74 | F | 1 | Liver | Surgery | 11.8 | – | PD (new lesion) | 6 | PD |
| 20 | 49.2 | M | 1 | Liver, lymph nodes, pleura | Surgery | 6.7 | – | PD (clinical progression) | 3 | Clinical progression |
PD progressive disease, PR partial response, SD stable disease, LD largest diameters
Antitumor Activity
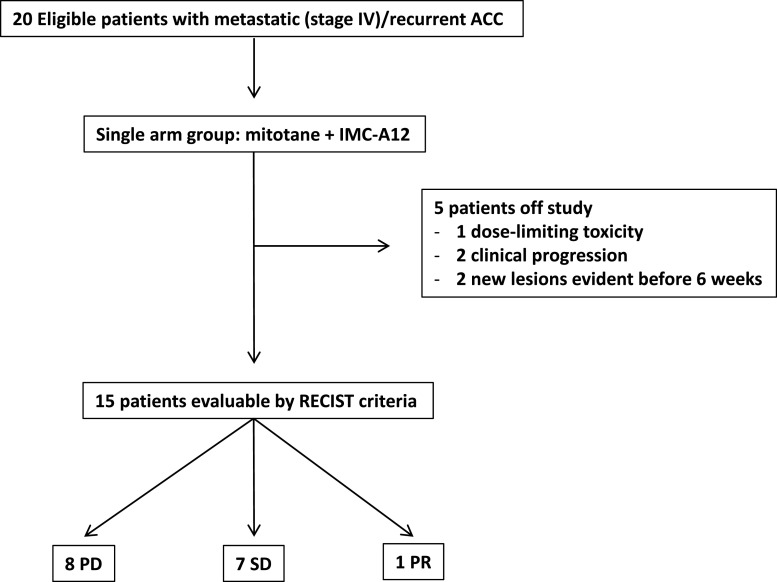
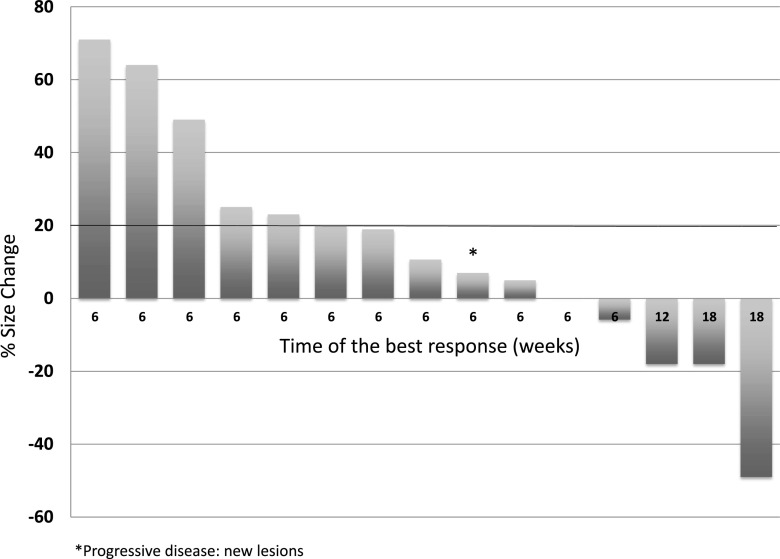
A partial response was observed in one patient (objective response rate 5 %; [95 % CI 0.89–23.6]). In addition, SD was observed in seven patients. The median PFS was 6 weeks (range 2.66–48). A consort diagram including patient outcomes is shown in Fig. 2. The best responses for the 15 study patients who were evaluable by RECIST are shown in Fig. 3.
Fig. 2.
Consort diagram representing patient enrollment and the outcomes observed during the trial
Fig. 3.
Waterfall plot showing best response by RECIST in 15 patients
Toxicity
Table 2 summarizes frequently observed toxic events. The most common events were neurological/psychiatric and gastrointestinal, mirroring the toxicity profile of mitotane. Grade 4 events included hyperglycemia (one event) and hyponatremia (one event). One patient was excluded from the study at 12 weeks due to persistent grade 3 ALT elevation. One patient died from multiorgan failure in the course of the treatment. This patient had extensive metastatic disease and was exhibiting significant tumor shrinkage at the time of death. The underlying conditions that caused multiorgan failure could not be established.
Table 2.
Common treatment-related toxic effects
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Gastrointestinal | |||||
| Diarrhea | 13 | 1 | |||
| Nausea | 8 | 3 | |||
| Vomiting | 2 | 1 | |||
| Abdominal pain | 2 | ||||
| Abdominal distension | 1 | ||||
| Flatulence | 2 | 1 | |||
| Neuro/psychiatric | |||||
| Confusion | 2 | 1 | |||
| Dizziness | 5 | ||||
| Cognitive disturbance | 1 | ||||
| Depressed level of consciousness | 1 | ||||
| Dysphasia | 1 | ||||
| Headache | 1 | 4 | |||
| Memory impairment | 3 | ||||
| Peripheral sensory neuropathy | 2 | ||||
| Tinnitus | 8 | ||||
| Tremor | 4 | ||||
| General | |||||
| Anorexia | 2 | ||||
| Chills | 1 | ||||
| Fatigue | 7 | 3 | |||
| Fever | 2 | 1 | |||
| Flu-like symptoms | 1 | ||||
| Multiorgan failure | 1 | ||||
| Weight loss | 2 | 1 | |||
| Investigational | |||||
| Increased ALT | 4 | 5 | 1 | ||
| Increased AST | 8 | 3 | |||
| Increased alkaline phosphatase | 2 | 1 | |||
| Increased bilirubin | 1 | ||||
| Anemia | 2 | 1 | |||
| Hypoglycemia | 1 | ||||
| Hyperglycemia | 5 | 2 | 4 | 1 | |
| High cholesterol | 6 | 1 | 1 | ||
| High creatinine | 1 | ||||
| Hypertriglyceridemia | 6 | 2 | |||
| Hypoalbuminemia | 1 | ||||
| Hyponatremia | 1 | ||||
| Hypophosphatemia | 1 | ||||
| Lymphopenia | 1 | ||||
| Platelet count decreased | 2 | ||||
| Other | |||||
| Allergic reaction | 1 | ||||
| Dehydration | 1 | ||||
| Generalized muscle weakness | 2 | ||||
| Hematuria | 2 | ||||
| Hypotension | 1 | ||||
| Injection site reaction | 1 | ||||
| Nail loss | 1 | ||||
| Pruritus | 2 | ||||
| Maculopapular rash | 3 | 1 | |||
Because of the limited efficacy, slow accrual, and observed toxicities, the study was terminated after completion of the single-arm run-in.
Discussion and Conclusion
Therapeutic options for metastatic ACC remain very limited. Recently, EDP + M was recommended as a first-line treatment based on the results of a phase III trial [10]. However, EDP + M is highly toxic, and majority of the patients will experience disease progression. Clearly, better systemic treatments are urgently needed. Recently, molecular-targeted drugs have emerged as promising therapeutic options for advanced cancer. For ACC, different studies have demonstrated that activation of the IGF system is the most frequent molecular abnormality. Thus, targeting the IGF1R would be a logical approach to treat advanced ACC. In fact, data from preclinical studies have shown promising results. In addition, a synergistic effect with mitotane was observed [2, 4]. A phase I trial with figitumumab, a monoclonal antibody that inhibits the IGF1R, has demonstrated activity in some patients with advanced ACC, with few toxic effects [15]. However, the patients enrolled in this trial had been heavily pretreated with multiple lines of systemic agents, including mitotane. Thus, the potential synergistic effects specific to mitotane and IGF1R inhibition could not be evaluated. In the current study, we evaluated the efficacy of the combination of IMC-A12 and mitotane as a first-line therapy for advanced ACC in mitotane-naïve patients. This trial was specifically designed to assess whether the synergistic effects between mitotane and IGF1R inhibitors, as demonstrated by preclinical studies, also occur in the clinical setting. Despite being initially designed as a phase II trial aiming to compare the efficacy of the combination regimen versus mitotane alone, this study was terminated in the safety evaluation phase because of slow patient accrual and relatively poor response rates. The reasons for the low accrual rates observed in this trial were probably related to the rarity of patients that met inclusion criteria (chemotherapy-naïve). Other reasons may include competition with other concurrent trials/oncology centers. In addition, PD was documented in the majority of patients at 6 weeks and median PFS in patients treated with mitotane + IMC-A12 was lower than that reported in patients treated with EDP + M (6 versus 20 weeks) [10]. Compared to historical data involving the use of mitotane as a single agent in which tumor shrinkage is observed in 32 % of patients [23], the addition of IMC-A12 did not seem to be of clinical benefit. One of the reasons that may explain the low efficacy of the regimen is related to the pharmacodynamics of mitotane. Since the therapeutic response of mitotane therapy is strictly dependent on its serum level and in most cases it takes several weeks to achieve therapeutic concentrations [9], it might be possible that the majority of the patients in this trial had subtherapeutical levels when the progression was documented. Unfortunately, mitotane serum levels were not available for the majority of the patients since most of them presented disease progression before the time point that mitotane serum levels are usually measured. In addition, we observed significant toxic effects, including two grade 4 and one grade 5. Except for hyperglycemia (a common toxic effect of IMC-A12), most of the commonly observed adverse reactions are known to be mitotane-related. Whether IMC-A12 could have potentiated these adverse effects could not be assessed since the study was terminated before the randomization phase.
A definitive conclusion regarding the benefits of the combined regimen over mitotane alone could not be drawn. However, some observations can be made. First, the regimen seems to be active in a small subset of patients. This result reproduces the findings of a previous trial involving an IGF1R inhibitor in ACC [15]. Similarly to our results, trials involving IGF1R inhibition in other tumors, such as NSCLC and Ewing sarcoma, were also disappointing, in spite of preclinical studies that provided encouraging results [21, 22, 26]. The challenge for future trials is to correctly identify subgroups of patients that may benefit from IGF1R inhibitors. In our study, due to the limited number of patients, no conclusion can be drawn regarding specific clinical characteristics that might predict response. Abnormalities in IGF signaling pathway have been shown to mediate resistance to IGF1R inhibitors. Particularly, abnormally high expression of IR-A has been observed in different cancer types and is associated to resistance to IGF1R inhibitors [5]. Whether this phenomenon was responsible for therapeutical failure in ACC is currently unknown. Unfortunately, in this trial, biosamples were not available to test this hypothesis.
Other possible molecular mechanism that may contribute for resistance to IGF1R inhibitors is the crosstalk and functional redundancy between different oncogenic pathways [25]. Preclinical studies and clinical trials have suggested that the combination of different targeted agents could be an interesting approach to overcome drug resistance [25]. An interesting drug regimen that has emerged is the combination of the mammalian target of rapamycin (mTOR) and IGF1R inhibitors. mTOR is a downstream target of the PI3K–AKT pathway, and therefore, is also activated by IGF1R signaling. Interestingly, mTOR inhibitors are known to cause feedback increase in AKT signaling by an IGF1R-independent mechanism, which overcomes its antiproliferative effects. More recently, it has been demonstrated that treatment with IGF1R inhibitors may paradoxically increase mTOR signaling [27]. Thus, pharmacological inhibition of both IGF1R and mTOR may have complementary effects on inhibiting proliferative cell signaling. The efficacy of this approach has been demonstrated in clinical trials. In ACC, a recent extension study of phase 1 trial assessed the combination of IMC-A12 and temsirolimus. Stable disease lasting more than 6 months was observed in 42 % of the participants, suggesting that the dual-blockage strategy is an attractive and viable option [20].
In conclusion, we tested for the first time a combination regimen consisting of mitotane and cixutumumab as a first-line treatment for inoperable/metastatic ACC. Although biological activity was demonstrated in some patients, the relatively low therapeutic efficacy along with potentially fatal toxic effects precluded further studies with this combination of drugs. Although the results of IGF1R inhibitors as single agents in ACC have been disappointing, these drugs may still have a role in selected subgroups of patients who are sensitive and in combination regimens with either cytotoxic chemotherapy or other targeted agents. However, biomarkers of therapeutic response to IGF1R in ACC remain unknown, as does the optimal combination of drugs. Future trials should be designed to specifically address these questions. Unfortunately, efficacious treatment protocols for advanced ACC remain elusive.
Acknowledgments
This study was supported by the following grants provided by NCI: HHSN261201100070C and HHSN261201100071C.
Conflict of Interest
AML, FPW, CAR, EAH, WMS, MHS, EA, and KR have nothing to disclose. GH is a shareholder of Atterocor, Orphagen, and Embara. He is a consultant for ISIS, Orphagen, Embara, and Atterocor.
References
- 1.Abou-Alfa GK, Capanu M, O’Reilly EM, Ma J, Chou JF, Gansukh B, Shia J, et al. A phase II study of cixutumumab (IMC-A12, NSC742460) in advanced hepatocellular carcinoma. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida MQ, Fragoso MC, Lotfi CF, Santos MG, Nishi MY, Costa MH, Lerario AM, et al. Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumors. J Clin Endocrinol Metab. 2008;93(9):3524–3531. doi: 10.1210/jc.2008-0065. [DOI] [PubMed] [Google Scholar]
- 3.Attias-Geva Z, Bentov I, Ludwig DL, Fishman A, Bruchim I, Werner H. Insulin-like growth factor-I receptor (IGF-IR) targeting with monoclonal antibody cixutumumab (IMC-A12) inhibits IGF-I action in endometrial cancer cells. Eur J Cancer. 2011;47(11):1717–1726. doi: 10.1016/j.ejca.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Barlaskar FM, Spalding AC, Heaton JH, Kuick R, Kim AC, Thomas DG, Giordano TJ, Ben-Josef E, Hammer GD. Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab. 2009;94(1):204–212. doi: 10.1210/jc.2008-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30(6):586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 6.Burtrum D, Zhu Z, Lu D, Anderson DM, Prewett M, Pereira DS, Bassi R, et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63(24):8912–8921. [PubMed] [Google Scholar]
- 7.Cohen BD, Baker DA, Soderstrom C, Tkalcevic G, Rossi AM, Miller PE, Tengowski MW, et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res. 2005;11(5):2063–2073. doi: 10.1158/1078-0432.CCR-04-1070. [DOI] [PubMed] [Google Scholar]
- 8.Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, Beuschlein F, Terzolo M, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer. 2009;115(2):243–250. doi: 10.1002/cncr.24030. [DOI] [PubMed] [Google Scholar]
- 9.Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98(12):4551–4564. doi: 10.1210/jc.2013-3020. [DOI] [PubMed] [Google Scholar]
- 10.Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366(23):2189–2197. doi: 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 11.Fottner C, Hoeflich A, Wolf E, Weber MM. Role of the insulin-like growth factor system in adrenocortical growth control and carcinogenesis. Horm Metab Res. 2004;36(6):397–405. doi: 10.1055/s-2004-814563. [DOI] [PubMed] [Google Scholar]
- 12.Furstenberger G, Senn HJ. Insulin-like growth factors and cancer. Lancet Oncol. 2002;3(5):298–302. doi: 10.1016/S1470-2045(02)00731-3. [DOI] [PubMed] [Google Scholar]
- 13.Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, Bauersfeld J, Sanders D, Thomas DG, Doherty G, Hammer G. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res. 2009;15(2):668–676. doi: 10.1158/1078-0432.CCR-08-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golden SH, Robinson KA, Saldanha I, Anton B, Ladenson PW. Clinical review: prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94(6):1853–1878. doi: 10.1210/jc.2008-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haluska P, Worden F, Olmos D, Yin D, Schteingart D, Batzel GN, Paccagnella ML, de Bono JS, Gualberto A, Hammer GD. Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemother Pharmacol. 2010;65(4):765–773. doi: 10.1007/s00280-009-1083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaton JH, Wood MA, Kim AC, Lima LO, Barlaskar FM, Almeida MQ, Fragoso MC, et al. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and beta-catenin. Am J Pathol. 2012;181(3):1017–1033. doi: 10.1016/j.ajpath.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Icard P, Goudet P, Charpenay C, Andreassian B, Carnaille B, Chapuis Y, Cougard P, Henry JF, Proye C. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg. 2001;25(7):891–897. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 18.Kurmasheva RT, Dudkin L, Billups C, Debelenko LV, Morton CL, Houghton PJ. The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res. 2009;69(19):7662–7671. doi: 10.1158/0008-5472.CAN-09-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma CX, Suman VJ, Goetz M, Haluska P, Moynihan T, Nanda R, Olopade O, et al. A phase I trial of the IGF-1R antibody cixutumumab in combination with temsirolimus in patients with metastatic breast cancer. Breast Cancer Res Treat. 2013;139(1):145–153. doi: 10.1007/s10549-013-2528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naing A, Lorusso P, Fu S, Hong D, Chen HX, Doyle LA, Phan AT, Habra MA, Kurzrock R. Insulin growth factor receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with metastatic adrenocortical carcinoma. Br J Cancer. 2013;108(4):826–830. doi: 10.1038/bjc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olmos D, Postel-Vinay S, Molife LR, Okuno SH, Schuetze SM, Paccagnella ML, Batzel GN, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing’s sarcoma: a phase 1 expansion cohort study. Lancet Oncol. 2010;11(2):129–135. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pappo AS, Patel SR, Crowley J, Reinke DK, Kuenkele KP, Chawla SP, Toner GC, et al. R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research Through Collaboration study. J Clin Oncol. 2011;29(34):4541–4547. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phan AT. Adrenal cortical carcinoma—review of current knowledge and treatment practices. Hematol Oncol Clin North Am. 2007;21(3):489–507. doi: 10.1016/j.hoc.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Riesterer O, Yang Q, Raju U, Torres M, Molkentine D, Patel N, Valdecanas D, Milas L, Ang KK. Combination of anti-IGF-1R antibody A12 and ionizing radiation in upper respiratory tract cancers. Int J Radiat Oncol Biol Phys. 2011;79(4):1179–1187. doi: 10.1016/j.ijrobp.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodon J, DeSantos V, Ferry RJ, Jr, Kurzrock R. Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: lessons from the first clinical trials. Mol Cancer Ther. 2008;7(9):2575–2588. doi: 10.1158/1535-7163.MCT-08-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scagliotti GV, Novello S. The role of the insulin-like growth factor signaling pathway in non-small cell lung cancer and other solid tumors. Cancer Treat Rev. 2012;38(4):292–302. doi: 10.1016/j.ctrv.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Shin DH, Min HY, El-Naggar AK, Lippman SM, Glisson B, Lee HY. Akt/mTOR counteract the antitumor activities of cixutumumab, an anti-insulin-like growth factor I receptor monoclonal antibody. Mol Cancer Ther. 2011;10(12):2437–2448. doi: 10.1158/1535-7163.MCT-11-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]