Abstract
Background
Pediatric rhabdomyosarcoma (RMS) is highly curable, however, cure may come with significant radiation related toxicity in developing tissues. Proton therapy (PT) can spare excess dose to normal structures, potentially reducing the incidence of adverse effects.
Methods
Between 2005 and 2012, 54 patients were enrolled on a prospective multi-institutional phase II trial using PT in pediatric RMS. As part of the protocol, intensity modulated radiation therapy (IMRT) plans were generated for comparison with clinical PT plans.
Results
Target coverage was comparable between PT and IMRT plans with a mean CTV V95 of 100% for both modalities (p=0.82). However, mean integral dose was 1.8 times higher for IMRT (range 1.0-4.9). By site, mean integral dose for IMRT was 1.8 times higher for H&N (p<0.01) and GU (p=0.02), 2.0 times higher for trunk/extremity (p<0.01), and 3.5 times higher for orbit (p<0.01) compared to PT. Significant sparing was seen with PT in 26 of 30 critical structures assessed for orbital, head and neck, pelvic, and trunk/extremity patients.
Conclusions
Proton radiation lowers integral dose and improves normal tissue sparing when compared to IMRT for pediatric RMS. Correlation with clinical outcomes is necessary once mature long-term toxicity data are available.
Keywords: Pediatrics, Rhabdomyosarcoma, Protons
INTRODUCTION
Pediatric RMS accounts for 3.8% of solid malignancies in children under the age of 19 years and is the most common soft tissue sarcoma in childhood1,2. Advances in systemic and local therapy have led to increased survival rates, with more than 70% of children becoming long term survivors3,4. Radiation therapy (RT) is an integral component of treatment in many of these patients but can be associated with both short and long-term morbidity, depending upon the volume treated and the dose delivered5-10. RMS may occur at almost any site in the body, and acute toxicity and late complications from radiation therapy depend on the location being treated.
Proton radiotherapy can decrease normal tissue doses by a factor of 2-3 and therefore holds promise in reducing the toxicity of treatment11,12. Previous dosimetric studies comparing proton therapy and IMRT in RMS and other cancers have demonstrated greater sparing of the ipsilateral and contralateral critical structures in both head and neck and genitourinary sites13-18. This sparing occurs through the specific physical properties of protons that both eliminate exit dose to normal tissues and reduce entrance dose at depth.
Since 2005, Massachusetts General Hospital (MGH) and MD Anderson Cancer Center (MDACC) have enrolled pediatric patients on a joint phase II trial, incorporating proton RT into standard RMS treatment regimens. As part of the trial, each child receives both a proton RT plan used for treatment as well as an IMRT plan for dosimetric comparison. In this study, we report the dosimetric results for those pediatric RMS patients treated on study.
MATERIALS AND METHODS
From February 2005 to October 2012, 54 pediatric RMS patients were treated with passively scattered proton RT on study. Patient characteristics are presented in the supplemental section (Table 1, supplement).
For radiation planning, patients were placed in a customized site-specific immobilization device in the treatment position and computed tomographic simulation provided images at 1.25-2.5mm for head and neck and orbital patients and at 2.5-mm for tumors below the neck. The gross tumor volume (GTV) included the primary tumor and any pathologically involved or enlarged regional lymph nodes, and was contoured by a pediatric radiation oncologist. The clinical treatment volume (CTV) was generated manually to cover areas of suspected microscopic involvement. For protons, the planning target volume (PTV) was achieved by using a 3mm “smear” for compensator calculations and an additional margin to the aperture edge, range, and modulation (2-5mm depending on anatomic site) to account for uncertainty in the path length and patient set up. A uniform 3mm PTV was added to IMRT plans. When possible, an MRI scan was anatomically registered to the planning treatment CT scan to facilitate target delineation. Normal tissue structures were contoured and/or checked by the treating pediatric radiation oncologist and centrally reviewed for consistency. All patient plans were reviewed and approved by the treating physician prior to treatment. Target and normal tissue volumes were held constant for both proton and IMRT planning. The dose delivered by protons is expressed as GyRBE which uses a relative biologic effectiveness (RBE) of 1.1 for protons to convert physical to biologic dose, based upon estimates of relative biologic effectiveness of protons relative to Cobalt-6019. For ease of presentation, proton doses in this paper are expressed as Gy.
At MGH, the XiO planning system™ (CMS, Inc., St. Louis, MO) was used for both proton and IMRT planning. At MDACC, an Eclipse treatment planning system™ (Varian Medical Systems, Palo Alto, CA) was utilized for proton therapy planning and Pinnacle treatment planning system™ (Philips Medical Systems, Fitchburg, WI) for IMRT comparison plans. Target volume and normal tissue constraints were derived from the Children's Oncology Group protocols for RMS (www.childrensoncologygroup.org). Dose–volume histograms were generated and compared for organs at risk (OAR). The percent of normal tissue spared by using protons was calculated using the following equation:
The integral dose (Dint), defined as the total energy deposited in patients, was calculated by summing the energy deposited in each individual voxel (Edepi) of the patient CT image. Edepi was computed using the voxel volume (Vi), CT Hounsfield unit data (HU) to calculate the voxel density (ρi), and the voxel dose (Di) using the following equation:
Event free survival (EFS), overall survival (OS), and local control (LC) rates were estimated by the Kaplan-Meier method. Continuous dosimetry values were compared using paired t-tests, while Fisher's exact test was used for categorical comparisons. Two-sided tests were employed and p<0.05 was used to determine statistical significance. Data analysis was performed using SAS version 9.2.
RESULTS
Median follow up for all 54 patients was 3.9 years. The 3/5 year event free survival and overall survival was 69%/65% and 80%/77% respectively. Local control at 3 and 5 years was 78%/78%. Toxicity was favorable with only 3 patients developing late grade 3 toxicity. These consisted of a unilateral cataract (orbital primary), chronic otitis (PM mastoid primary), and retinopathy with decreased visual acuity (orbital primary). No toxicities higher than grade 3 were observed. To date there have been no reported secondary malignancies. A complete description of toxicity for this trial is given in a separate publication discussing clinical outcomes20.
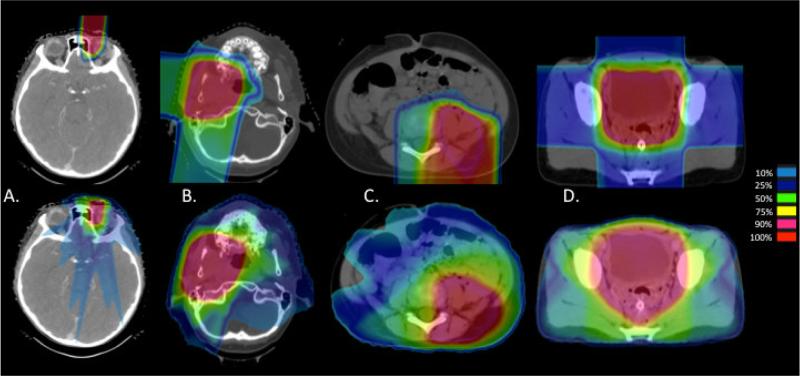
A median of 7 beams were used in IMRT plans (range 4-9), and for proton plans the median was 3 beams (range 1-7) (Table 1, supplement). Coverage of target volumes was equivalent between PT and IMRT plans. Due to the difference in PTV generation with PT, CTV was used to compare coverage. The mean CTV V95 (percent volume receiving at least 95% of the prescription dose) was 100% for both modalities (range 97-100% for PT and 98-100% for IMRT) (p=0.82). The mean CTV V100 was 98% for PT (range 95-100%) and 99% for IMRT (range 97-100%) (p=0.64). The mean maximum dose (DMax) was 107% (range 101-112%) for PT and 106% (range 103-110%) for IMRT (p = 0.17). Comparative dosimetry for PT and IMRT plans are shown in Figure 1.
Figure 1.
Comparative proton (above) and IMRT (below) dosimetry for primaries of the (A) orbit, (B) parameninges, (C) trunk, and (D) pelvis
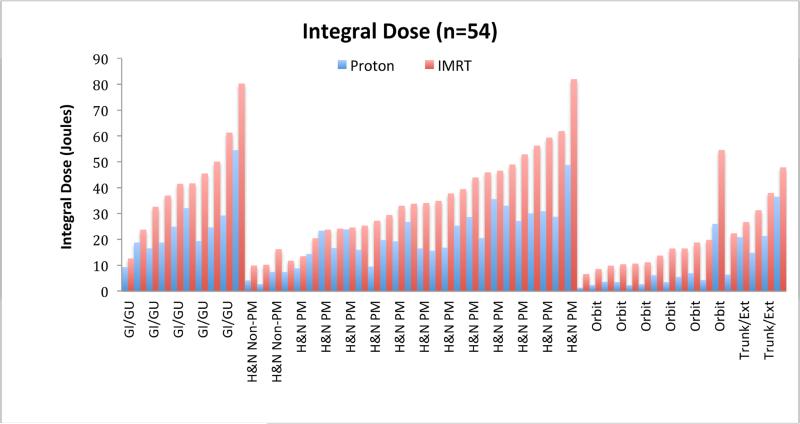
In all 54 cases, the integral dose was calculated for IMRT and PT plans. The integral dose represents the total energy deposited in a patient and is given in joules rather than gray because of the latter's dependence on patient weight (Gy = J/Kg). Integral dose was 18 J for PT and 32 J for IMRT (p=<0.01) with a mean integral dose 1.8 times higher for IMRT (range 1.0-4.9). By site, mean integral dose for IMRT was 1.8 times higher for genitourinary (p=0.02) and head and neck sites (p<0.01), 2.0 times higher for trunk and extremity sites (p<0.01), and 3.5 times higher for orbital sites (p=<0.01) when compared to PT. Individual results are shown in Figure 2.
Figure 2.
Integral dose values in joules for proton and IMRT plans for each of the 54 patients on study
Statistically significant sparing was seen with PT in all disease sites with 3 or more patients and in 26 of 30 OARs assessed. Results are presented as mean dose and in volume percent at clinically significant intervals.
There were 27 patients with non-orbital head and neck (H&N) tumors and of these 24 were parameningeal sites. Tumors were classified as “central” in 9 cases and “lateral” in 18 cases. For central sites, dose to paired organs such as the parotid glands was recorded individually for each gland and then pooled for analysis without assigning laterality. In the lateral cases, paired organs were designated as “ipsilateral” or “contralateral” and results are reported as such. Median RT dose was 50.4 Gy (range 36-52.2 Gy). Complete dosimetric results for head and neck patients are presented in table 1.
Table 1.
OAR doses for all head and neck patients including parameningeal primaries
| Head and Neck | Central H&N Primary (n=9) | Lateral H&N Primary (n=18) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Structure | Dose | Proton | IMRT | % Spared | P Value | Proton | IMRT | % Spared | P Value |
| Chiasm | Mean (Gy) | 26 | 28 | 7 | 0.35 | 15 | 24 | 38 | < 0.01 |
| V45 (%) | 35 | 37 | 5 | 0.11 | 5 | 14 | 64 | 0.34 | |
| Pituitary | Mean (Gy) | 32 | 35 | 9 | 0.02 | 25 | 33 | 24 | < 0.01 |
| V30 (%) | 64 | 67 | 4 | 0.17 | 49 | 63 | 22 | 0.03 | |
| Hypothalamus | Mean (Gy) | 9 | 15 | 40 | < 0.01 | 7 | 15 | 53 | < 0.01 |
| V16 (%) | 27 | 46 | 41 | 0.02 | 19 | 35 | 46 | 0.03 | |
| Brainstem | Mean (Gy) | 9 | 18 | 50 | < 0.01 | 8 | 18 | 56 | < 0.01 |
| V30 (%) | 8 | 21 | 62 | 0.07 | 9 | 22 | 59 | 0.02 | |
| Cerebellum | Mean (Gy) | 3 | 12 | 75 | < 0.01 | 5 | 15 | 67 | < 0.01 |
| V20 (%) | 1 | 18 | 94 | 0.05 | 7 | 26 | 73 | < 0.01 | |
| Maxilla | Mean (Gy) | 22 | 30 | 27 | < 0.01 | 15 | 30 | 50 | < 0.01 |
| V20 (%) | 48 | 71 | 32 | < 0.01 | 32 | 70 | 54 | < 0.01 | |
| V30 (%) | 38 | 55 | 31 | < 0.01 | 26 | 44 | 41 | < 0.01 | |
| Mandible | Mean (Gy) | 11 | 19 | 42 | < 0.01 | 12 | 24 | 50 | < 0.01 |
| V20 (%) | 25 | 47 | 47 | < 0.01 | 24 | 50 | 52 | < 0.01 | |
| V30 (%) | 17 | 28 | 39 | 0.08 | 20 | 33 | 39 | < 0.01 | |
| Thyroid | Mean (Gy) | 2 | 4 | 50 | 0.12 | 2 | 3 | 33 | 0.09 |
| V10 (%) | 6 | 11 | 45 | 0.22 | 5 | 8 | 38 | 0.17 | |
| Optic Nerves | Mean (Gy) | 30 | 30 | 0 | 0.60 | -- | -- | -- | -- |
| V50 (%) | 27 | 34 | 21 | 0.36 | -- | -- | -- | -- | |
| Optic NerveIpsi | Mean (Gy) | -- | -- | -- | -- | 25 | 31 | 19 | 0.01 |
| V50 (%) | -- | -- | -- | -- | 11 | 6 | −45 | 0.45 | |
| Optic NerveContra | Mean (Gy) | -- | -- | -- | -- | 9 | 21 | 57 | < 0.01 |
| Temporal Lobes | Mean (Gy) | 6 | 10 | 40 | < 0.01 | -- | -- | -- | -- |
| V20 (%) | 10 | 18 | 44 | 0.01 | -- | -- | -- | -- | |
| V30 (%) | 8 | 9 | 11 | 0.01 | -- | -- | -- | -- | |
| Temp LobeIpsi | Mean (Gy) | -- | -- | -- | -- | 12 | 21 | 43 | < 0.01 |
| V20 (%) | -- | -- | -- | -- | 23 | 44 | 48 | < 0.01 | |
| V30 (%) | -- | -- | -- | -- | 18 | 31 | 42 | < 0.01 | |
| Temp LobeContra | Mean (Gy) | -- | -- | -- | -- | 2 | 9 | 78 | < 0.01 |
| V20 (%) | -- | -- | -- | -- | 4 | 10 | 60 | 0.05 | |
| V30 (%) | -- | -- | -- | -- | 1 | 3 | 67 | 0.04 | |
| Lens | Mean (Gy) | 3 | 5 | 40 | 0.02 | -- | -- | -- | -- |
| V5 (%) | 20 | 48 | 58 | 0.01 | -- | -- | -- | -- | |
| LensIpsi | Mean (Gy) | -- | -- | -- | -- | 2 | 9 | 78 | 0.01 |
| V5 (%) | -- | -- | -- | -- | 16 | 63 | 75 | < 0.01 | |
| LensContra | Mean (Gy) | -- | -- | -- | -- | 0.2 | 6 | 97 | < 0.01 |
| V5 (%) | -- | -- | -- | -- | 0 | 46 | 100 | < 0.01 | |
| Retina | Mean (Gy) | 16 | 18 | 11 | 0.47 | -- | -- | -- | -- |
| V45 (%) | 8 | 6 | −25 | 0.88 | -- | -- | -- | -- | |
| RetinaIpsi | Mean (Gy) | -- | -- | -- | -- | 13 | 19 | 32 | 0.01 |
| V45 (%) | -- | -- | -- | -- | 8 | 5 | −38 | 0.12 | |
| RetinaContra | Mean (Gy) | -- | -- | -- | -- | 3 | 11 | 73 | < 0.01 |
| Cochlea | Mean (Gy) | 19 | 19 | 0 | 0.57 | -- | -- | -- | -- |
| V36 (%) | 17 | 4 | −76 | 0.18 | -- | -- | -- | -- | |
| CochleaIpsi | Mean (Gy) | -- | -- | -- | -- | 36 | 39 | 8 | 0.24 |
| V36 (%) | -- | -- | -- | -- | 62 | 63 | 2 | 0.83 | |
| CochleaContra | Mean (Gy) | -- | -- | -- | -- | 5 | 17 | 71 | < 0.01 |
| V20 (%) | -- | -- | -- | -- | 12 | 32 | 63 | 0.06 | |
| Lacrimal Gland | Mean (Gy) | 6 | 11 | 45 | < 0.01 | -- | -- | -- | -- |
| V20 (%) | 9 | 25 | 64 | 0.02 | -- | -- | -- | -- | |
| LacrimalIpsi | V30 (%) | 4 | 4 | 0 | 0.25 | -- | -- | -- | -- |
| Mean (Gy) | -- | -- | -- | -- | 9 | 15 | 40 | < 0.01 | |
| V20 (%) | -- | -- | -- | -- | 18 | 29 | 38 | 0.02 | |
| V30 (%) | -- | -- | -- | -- | 11 | 19 | 42 | 0.08 | |
| LacrimalContra | Mean (Gy) | -- | -- | -- | -- | 1 | 8 | 88 | < 0.01 |
| Parotid Gland | Mean (Gy) | 18 | 26 | 31 | 0.08 | -- | -- | -- | -- |
| V36 (%) | 22 | 21 | −5 | 0.33 | -- | -- | -- | -- | |
| ParotidIpsi | Mean (Gy) | -- | -- | -- | -- | 37 | 39 | 5 | 0.06 |
| V36 (%) | -- | -- | -- | -- | 66 | 64 | −3 | 0.66 | |
| ParotidContra | Mean (Gy) | -- | -- | -- | -- | 2 | 11 | 82 | < 0.01 |
| Skin | DMax (Gy) | 32 | 32 | 0 | 0.75 | 44 | 44 | 0 | 0.63 |
Abbreviations: ipsi, ipsilateral; contra, contralateral, temp; temporal.
For CNS structures, significant sparing for PT was seen in all OARs examined with the greatest sparing in the hypothalamus, temporal lobes, brainstem, and cerebellum. Moderate dose reductions were also noted in the optic nerves, optic chiasm, and pituitary. In non-CNS structures, the most significant sparing occurred in the lens, maxilla, mandible, and lacrimal gland. Doses to the retina, skin, parotid gland, and thyroid showed no or minimal differences.
There were 12 patients with orbital rhabdomyosarcoma, 9 with a left-sided primary and 3 with a right-sided primary. Median RT dose was 45 Gy (range 45-50.4 Gy). Both ipsilateral and contralateral temporal lobes and lacrimal glands showed significant sparing with PT plans, as did the hypothalamus, pituitary, and maxilla. Contralateral lens dose and retina was also spared with PT. Minimal differences were seen in the ipsilateral optic nerve dose and skin doses were similar. Complete dosimetric results are shown in table 2.
Table 2.
OAR doses for all orbital primary patients
| Orbit | Orbital Primary (n=12) | ||||
|---|---|---|---|---|---|
| Structure | Dose | Proton | IMRT | % Spared | P Value |
| LensIpsi | Mean (Gy) | 26 | 32 | 19 | 0.19 |
| V5 (%) | 89 | 99 | 10 | 0.10 | |
| LensContra | Mean (Gy) | 0 | 3 | 100 | < 0.01 |
| V5 (%) | 0 | 23 | 100 | 0.05 | |
| RetinaIpsi | Mean (Gy) | 33 | 40 | 18 | < 0.01 |
| V45 (%) | 34 | 48 | 0.09 | ||
| RetinaContra | Mean (Gy) | 0.1 | 8 | 99 | < 0.01 |
| V20 (%) | 0 | 11 | 100 | 0.04 | |
| Optic NerveIpsi | Mean (Gy) | 31 | 37 | 16 | 0.03 |
| V45 (%) | 33 | 40 | 18 | 0.27 | |
| Lacrimal GlandIpsi | Mean (Gy) | 19 | 35 | 46 | < 0.01 |
| V30 (%) | 31 | 65 | 52 | < 0.01 | |
| Lacrimal GlandContra | Mean (Gy) | 0 | 5 | 100 | < 0.01 |
| Hypothalamus | Mean (Gy) | 1 | 8 | 88 | < 0.01 |
| V16 (%) | 3 | 13 | 77 | 0.02 | |
| Pituitary | Mean (Gy) | 4 | 15 | 73 | < 0.01 |
| V20 (%) | 5 | 19 | 74 | 0.10 | |
| Temp LobeIpsi | Mean (Gy) | 1 | 9 | 89 | < 0.01 |
| V20 (%) | 2 | 10 | 80 | < 0.01 | |
| Temp LobeContra | Mean (Gy) | 0 | 4 | 100 | < 0.01 |
| V10 (%) | 0 | 7 | 100 | 0.03 | |
| Maxilla | Mean (Gy) | 7 | 12 | 42 | 0.02 |
| V20 (%) | 15 | 25 | 40 | 0.05 | |
| Skin | DMax (Gy) | 43 | 42 | −2 | 0.68 |
Abbreviations: ipsi, ipsilateral; contra, contralateral, temp; temporal.
Only one patient on study had a primary in the chest region, a left shoulder primary that received 50.4 Gy. All OARs showed improvement with PT, though statistical significance could not be calculated for a single case. (Table 2, supplement).
Two patients presented with abdominal tumors, one biliary primary and one left paraspinal primary, both treated to 50.4 Gy. Due to small numbers (n=2), statistical significance between the two groups could not be shown. Despite the limited numbers, notable reductions in ipsilateral and contralateral kidney dose were seen in both cases (Table 3, supplement).
There were 12 patients with primaries in the pelvic region, 7 prostate or bladder primaries, 3 extremity tumors in the groin or thigh, and 2 perianal primaries. Median dose was 50.4 Gy (range 36-50.4 Gy). Doses to the testes were reported together due to minimal dose variation between each testicle, while the doses to the left and right ovaries and growth plates are presented individually. Important reductions in gonadal doses (ovaries and testes) were seen with the use of PT. The growth plates and pelvic bones were also spared significant dose with PT plans. Femoral head dose was improved with PT, though doses with both modalities were generally low. Dose reductions with PT were also seen in the vagina, uterus, and penile bulb with variable in significance (Table 4, supplement).
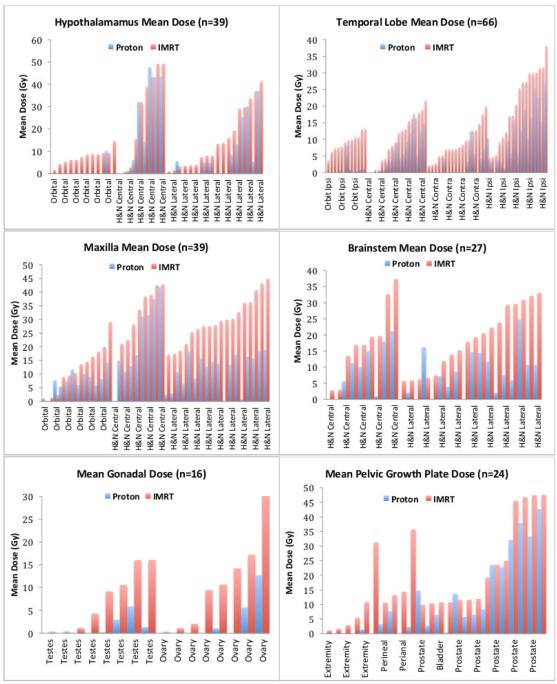
Mean dose and mean percent volumes for OARs are useful for describing general trends in a large data set such as ours, but tend to wash out significant individual case differences. Figure 3 presents the individual dosimetric results for select critical structures. To demonstrate comparative risks for late effects in tissues with well-established dose tolerances, the number of patients with OARs exceeding clinically significant levels for PT vs IMRT are provided in Table 3.
Figure 3.
Individual patient dose for select OARs (organs at risk). (A) Hypothalamic mean dose for all orbital and head and neck (H&N) patients. (B) Temporal lobe mean doses for orbital (ipsilateral lobe only) and H&N patients (ipsilateral and contralateral lobes). (C) Maxillary mean dose for all orbital and H&N patients. (D) Brainstem mean dose for H&N patients only. (E) Mean gonad doses for the 12 pelvic patients (paired testicles displayed as a single OAR, paired ovaries as separate OARs). (D) Growth plate mean doses for the 12 pelvic patients
Table 3.
OAR dose levels for critical structures
| Organ At Risk | Patients Above Dose Level | ||
|---|---|---|---|
| Mean Dose | Protons (%) | IMRT (%) | |
| Lens (n=78) | > 2 Gy | 25 (32) | 64 (81) |
| > 6 Gy | 16 (21) | 36 (46) | |
| > 12 Gy | 10 (13) | 13 (17) | |
| Hypothalamus (n=39) | > 5 Gy | 13 (33) | 27 (69) |
| > 16 Gy | 6 (15) | 12 (30) | |
| > 35 Gy | 1 (3) | 5 (15) | |
| Pituitary (n=39) | > 20 Gy | 16 (41) | 21 (54) |
| > 30 Gy | 15 (38) | 18 (46) | |
| > 40 Gy | 10 (26) | 15 (38) | |
| Testes (n=16) | Any Dose | 12 (75) | 16 (100) |
| > 2 Gy | 4 (25) | 10 (63) | |
| > 12 Gy | 0 (0) | 6 (38) | |
| Ovaries (n=8) | Any Dose | 3 (38) | 8 (100) |
| > 6 Gy | 2 (25) | 5 (63) | |
| > 12 Gy | 1 (13) | 3 (38) | |
| Lacrimal Gland (n=78) | > 20 Gy | 11 (14) | 21 (27) |
| > 35 Gy | 3 (4) | 9 (12) | |
| > 45 Gy | 3 (4) | 5 (6) | |
| Growth Plates (n=24) | > 10 Gy | 8 (33) | 19 (79) |
| > 20 Gy | 6 (25) | 8 (33) | |
| > 30 Gy | 4 (17) | 6 (25) | |
DISCUSSION
This study represents the first comparison of proton vs photon dosimetry for patients enrolled on a prospective clinical trial, and with 54 patients it also stands as the largest published dosimetric series for RMS. Rather than selecting patients for comparison based on tumor location, as has been done in prior studies, we present the results for every RMS patient on study over the course of 7 years. In doing so, our data more closely resembles the demographics for pediatric RMS patients treated at a high volume center. Although IMRT plans were not used for treatment, multiple iterations were generated in the majority of cases to achieve optimal coverage while respecting the tissue tolerance of critical structures. In some cases, target volume coverage was altered to improve sparing of these critical structures, as would be done if the plans were used for actual treatment. Prior dosimetric studies for select patients with parameningeal, orbital, and genitourinary RMS showed similar benefits with proton RT compared to IMRT, and this study adds confirmation to these results on a larger scale15,16,18.
Arguments against the widespread adoption of proton therapy, as highlighted by De Ruysscher et al. and others, have stemmed from the contention that the main benefit of protons, the reduction in the medium and low dose regions, is of little clinical significance to patients21,22. Our data, summarized in table 4, show that these reductions lead to important sparing by PT in multiple structures with well-defined tolerances. Growth hormone deficiency from RT to the hypothalamus has been shown at an incidence of 50% at 16 Gy and 99% at 35 Gy23. In our study hypothalamic dose with PT was lower for 90% of all orbital and H&N patients and doses above 16 Gy were seen in 6 PT patients (15%) and 12 IMRT patients (30%) and above 35 Gy in 1 PT patient (3%) and 5 (15%) IMRT patients. Growth hormone deficiency in children has been linked to multiple co-morbidities including poor growth, altered energy metabolism and body composition, cognitive impairment, cardiovascular disease, and diminished quality of life 23-27. Furthermore, growth hormone monitoring and replacement for pediatric patients comes at an annual cost of over $13,00028.
Memory functions are largely localized to the temporal regions of the brain and Armstrong et al. found an increased risk for memory difficulties and task efficiency with increasing dose above ≥30 Gy to the temporal lobes29,30. Survivors who received temporal region irradiation also experienced significantly more difficulty in social functioning, including lower overall wage earning and marriage rates in these studies. In our cohort, PT spared significant temporal lobe dose for H&N and orbital patients with a mean temporal lobe V20 2.0 times higher and V30 1.7 higher for IMRT plans. The most significant sparing was seen in lateralized H&N tumors where the ipsilateral V20 and V30 were reduced by 44% and 31% respectively.
Dry eye syndrome (DES) following RT has been linked to doses delivered to lacrimal glands. Tolerance doses for the entire gland with conventional fractionation are estimated to be in the range of 30 to 40 Gy and Mendenhall et al. found that DES occurred at a rate of 6% with 35–40 Gy to the lacrimal gland and a rate of 50% at 45 Gy or higher31-33. In our study, lacrimal doses above 35 Gy occurred in 3 PT patients (4%) and 9 IMRT patients (12%) and above 45 Gy in 3 PT patients (4%) and 5 IMRT patients (6%).
Orbital and H&N patients were spared significant lens dose with PT as well. Lens dose of >6 Gy was seen in 16 (21%) of PT patients and 35 (45%) of IMRT patients and a lens dose of >12 Gy was seen in 10 (13%) of PT patients and 13 (17%) of IMRT patients. The majority of those > 12 Gy had orbital tumors. Data in adult patients shows a 33% risk of progressive cataracts after 2.5 to 6.5 Gy, and 66% after 6.5 to 11.5 Gy32. Increased sensitivity in younger patients is suspected and Hall et al. found a risk of opacities at lens doses of <0.5 Gy and calculated a 35–50% increase in the risk of opacity development per unit of Gray during childhood34. Cataracts in younger children can lead to astigmatism and visual complications if left untreated, but the decision to undertake surgical repair is not trivial. Pediatric patients are subject to a higher rate of complications than their adult counterparts and a risk of blindness and enucleation exists due to infection, hemorrhage, and retinal detachment following repair35,36.
Fertility preservation is an area of great interest in pediatric RMS and recent COG trials have attempted to decrease cyclophosphamide doses to this end. Radiation also plays a role and fractionated RT doses of 2 Gy to the testes and 6 Gy to the ovaries are thought to represent a 50% risk of sterility, while doses above 12 Gy the testes and 8 Gy to the ovaries likely represents a 100% risk37-39. In our pelvic cohort, testicular doses above 2 Gy were seen in 4 cases with PT (25%) and 10 IMRT cases (63%). No patients had a testicular dose over 12 Gy with PT while 6 patients (38%) exceeded this dose with IMRT. Ovarian doses above 6 Gy/12 Gy were seen in 25%/13% of PT patients and 63%/38% of IMRT patients.
Finally, recent clinical data from a mixed pediatric and adult population of over 1000 patients has suggested a reduction in second tumor rates in a proton treated population40. No difference was noted in “in field malignancies” and therefore it is likely the reduction of integral dose to normal tissues outside the target volume that leads to this improvement. In our cohort, integral dose was reduced by 1.8 times for all sites using PT and one could expect a similar reduction in second cancers for pediatric RMS.
Significant OAR sparing by PT was seen in all tumor sites and in 26 of 30 structures examined (excluding the sites with <3 patients each). While significant sparing was observed for both central and lateral primaries, the lateral tumors provided for the greatest dose savings to structures compared with more central tumors. Similarly, serial organs (chiasm, brainstem, spinal cord) showed less benefit with PT and the maximum dose was often lower with IMRT, highlighting IMRT's ability for conformality in the high dose regions. In contrast, parallel organs (temporal lobes, mandible/maxilla, pelvic bones) as well as organs sensitive to low doses of RT (hypothalamus, gonads) consistently showed benefit. Few patients had abdominal and chest tumors but kidney, lung, and heart doses were lower with PT. Proton studies in patients with Hodgkins disease and soft tissue sarcoma have shown similar benefit in these regions41-43. It should be noted that all patients in this study were treated with passively scattered proton RT. As scanned beam capabilities improve and become more widely available, the use of IMPT should augment these observed benefits to a greater extent.
In a large scale, multi-institutional prospective phase II study, proton radiotherapy for pediatric RMS demonstrates improved normal tissue sparing compared to IMRT. Further correlation with clinical outcomes is needed once our data matures to determine whether the dosimetric benefits observed translate into reduced rates of late toxicity.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict Of Interest Statement: Dr. Tarbell reports having stock options (of zero value) in the ProCure corporation as well as an immediate family member on the advisory board of ProCure. The authors report no other conflicts of interest.
References
- 1.Ries LAG, SEER Program (National Cancer Institute (U.S.)) In: Cancer incidence and survival among children and adolescents : United States SEER program 1975-1995/ Gloecker Ries Lynn A., editor. National Cancer Institute, SEER Program; Bethesda, MD: 1999. [Google Scholar]
- 2.Miller RW, Young JL, Jr., Novakovic B. Childhood cancer. Cancer. 1995;75:395–405. doi: 10.1002/1097-0142(19950101)75:1+<395::aid-cncr2820751321>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: children's oncology group study D9803. J Clin Oncol. 2009;27:5182–8. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raney RB, Walterhouse DO, Meza JL, et al. Results of the Intergroup Rhabdomyosarcoma Study Group D9602 protocol, using vincristine and dactinomycin with or without cyclophosphamide and radiation therapy, for newly diagnosed patients with low-risk embryonal rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J Clin Oncol. 2011;29:1312–8. doi: 10.1200/JCO.2010.30.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merks JH, De Salvo GL, Bergeron C, et al. Parameningeal rhabdomyosarcoma in pediatric age: results of a pooled analysis from North American and European cooperative groups. Ann Oncol. 2014;25:231–6. doi: 10.1093/annonc/mdt426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breneman J, Meza J, Donaldson SS, et al. Local control with reduced-dose radiotherapy for low-risk rhabdomyosarcoma: a report from the Children's Oncology Group D9602 study. Int J Radiat Oncol Biol Phys. 2012;83:720–6. doi: 10.1016/j.ijrobp.2011.06.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minn AY, Lyden ER, Anderson JR, et al. Early treatment failure in intermediate-risk rhabdomyosarcoma: results from IRS-IV and D9803--a report from the Children's Oncology Group. J Clin Oncol. 2010;28:4228–32. doi: 10.1200/JCO.2010.29.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolden SL, Anderson JR, Crist WM, et al. Indications for radiotherapy and chemotherapy after complete resection in rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Studies I to III. J Clin Oncol. 1999;17:3468–75. doi: 10.1200/JCO.1999.17.11.3468. [DOI] [PubMed] [Google Scholar]
- 9.Rubin P, Constine L, Williams J. Late effects of cancer treatment: radiation and drug toxicity., in Principles and practice of radiation oncology. In: Perez CBL, editor. Principles and practice of radiation oncology. Lippincott-Raven Publishers; Philadelphia: 1998. pp. 155–211. [Google Scholar]
- 10.Mulhern RK, Merchant TE, Gajjar A, et al. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 11.Miralbell R, Lomax A, Cella L, et al. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys. 2002;54:824–9. doi: 10.1016/s0360-3016(02)02982-6. [DOI] [PubMed] [Google Scholar]
- 12.Cotter SE, McBride SM, Yock TI. Proton radiotherapy for solid tumors of childhood. Technol Cancer Res Treat. 2012;11:267–78. doi: 10.7785/tcrt.2012.500295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langendijk JA, Lambin P, De Ruysscher D, et al. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother Oncol. 2013;107:267–73. doi: 10.1016/j.radonc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Simone CB, 2nd, Ly D, Dan TD, et al. Comparison of intensity-modulated radiotherapy, adaptive radiotherapy, proton radiotherapy, and adaptive proton radiotherapy for treatment of locally advanced head and neck cancer. Radiother Oncol. 2011;101:376–82. doi: 10.1016/j.radonc.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozak KR, Adams J, Krejcarek SJ, et al. A dosimetric comparison of proton and intensity-modulated photon radiotherapy for pediatric parameningeal rhabdomyosarcomas. Int J Radiat Oncol Biol Phys. 2009;74:179–86. doi: 10.1016/j.ijrobp.2008.06.1942. [DOI] [PubMed] [Google Scholar]
- 16.Yock T, Schneider R, Friedmann A, et al. Proton radiotherapy for orbital rhabdomyosarcoma: clinical outcome and a dosimetric comparison with photons. Int J Radiat Oncol Biol Phys. 2005;63:1161–8. doi: 10.1016/j.ijrobp.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 17.Childs SK, Kozak KR, Friedmann AM, et al. Proton radiotherapy for parameningeal rhabdomyosarcoma: clinical outcomes and late effects. Int J Radiat Oncol Biol Phys. 2012;82:635–42. doi: 10.1016/j.ijrobp.2010.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotter SE, Herrup DA, Friedmann A, et al. Proton radiotherapy for pediatric bladder/prostate rhabdomyosarcoma: clinical outcomes and dosimetry compared to intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2011;81:1367–73. doi: 10.1016/j.ijrobp.2010.07.1989. [DOI] [PubMed] [Google Scholar]
- 19.Paganetti H, Niemierko A, Ancukiewicz M, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53:407–21. doi: 10.1016/s0360-3016(02)02754-2. [DOI] [PubMed] [Google Scholar]
- 20.Ladra MS, Szymonifka J, Yock TI, et al. Preliminary Results of a Phase II Trial of Proton Radiotherapy for Pediatric Rhabdomyosarcoma. Journal of Clincal Oncology. 2014 doi: 10.1200/JCO.2014.56.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Ruysscher D, Mark Lodge M, Jones B, et al. Charged particles in radiotherapy: a 5-year update of a systematic review. Radiother Oncol. 2012;103:5–7. doi: 10.1016/j.radonc.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Goitein M. Trials and tribulations in charged particle radiotherapy. Radiother Oncol. 2010;95:23–31. doi: 10.1016/j.radonc.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Merchant TE, Rose SR, Bosley C, et al. Growth hormone secretion after conformal radiation therapy in pediatric patients with localized brain tumors. J Clin Oncol. 2011;29:4776–80. doi: 10.1200/JCO.2011.37.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vance ML, Mauras N. Growth hormone therapy in adults and children. N Engl J Med. 1999;341:1206–16. doi: 10.1056/NEJM199910143411607. [DOI] [PubMed] [Google Scholar]
- 25.Gilchrist FJ, Murray RD, Shalet SM. The effect of long-term untreated growth hormone deficiency (GHD) and 9 years of GH replacement on the quality of life (QoL) of GH-deficient adults. Clin Endocrinol (Oxf) 2002;57:363–70. doi: 10.1046/j.1365-2265.2002.01608.x. [DOI] [PubMed] [Google Scholar]
- 26.Wass JA, Reddy R. Growth hormone and memory. J Endocrinol. 2010;207:125–6. doi: 10.1677/JOE-10-0126. [DOI] [PubMed] [Google Scholar]
- 27.Nieves-Martinez E, Sonntag WE, Wilson A, et al. Early-onset GH deficiency results in spatial memory impairment in mid-life and is prevented by GH supplementation. J Endocrinol. 2010;204:31–6. doi: 10.1677/JOE-09-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mailhot Vega RB, Kim J, Bussiere M, et al. Cost effectiveness of proton therapy compared with photon therapy in the management of pediatric medulloblastoma. Cancer. 2013;119:4299–307. doi: 10.1002/cncr.28322. [DOI] [PubMed] [Google Scholar]
- 29.Budson AE. Understanding memory dysfunction. Neurologist. 2009;15:71–9. doi: 10.1097/NRL.0b013e318188040d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101:946–58. doi: 10.1093/jnci/djp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bessell EM, Henk JM, Whitelocke RA, et al. Ocular morbidity after radiotherapy of orbital and conjunctival lymphoma. Eye (Lond) 1987;1(Pt 1):90–6. doi: 10.1038/eye.1987.14. [DOI] [PubMed] [Google Scholar]
- 32.Jeganathan VS, Wirth A, MacManus MP. Ocular risks from orbital and periorbital radiation therapy: a critical review. Int J Radiat Oncol Biol Phys. 2011;79:650–9. doi: 10.1016/j.ijrobp.2010.09.056. [DOI] [PubMed] [Google Scholar]
- 33.Bhandare N, Moiseenko V, Song WY, et al. Severe dry eye syndrome after radiotherapy for head-and-neck tumors. Int J Radiat Oncol Biol Phys. 2012;82:1501–8. doi: 10.1016/j.ijrobp.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Hall P, Granath F, Lundell M, et al. Lenticular opacities in individuals exposed to ionizing radiation in infancy. Radiat Res. 1999;152:190–5. [PubMed] [Google Scholar]
- 35.Brooks HL, Jr., Meyer D, Shields JA, et al. Removal of radiation-induced cataracts in patients treated for retinoblastoma. Arch Ophthalmol. 1990;108:1701–8. doi: 10.1001/archopht.1990.01070140055028. [DOI] [PubMed] [Google Scholar]
- 36.Hoehn ME, Irshad F, Kerr NC, et al. Outcomes after cataract extraction in young children with radiation-induced cataracts and retinoblastoma. J AAPOS. 2010;14:232–4. doi: 10.1016/j.jaapos.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Green DM, Sklar CA, Boice JD, Jr., et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2374–81. doi: 10.1200/JCO.2008.21.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedman DC,L. Late Effects of Cancer Treatment. In: Halperin E, editor. Pediatric Radiation Oncology. Lippincott Williams and Wilkins; Philadelphia, PA: 2011. pp. 353–395. [Google Scholar]
- 39.Ash P. The influence of radiation on fertility in man. Br J Radiol. 1980;53:271–8. doi: 10.1259/0007-1285-53-628-271. [DOI] [PubMed] [Google Scholar]
- 40.Chung CS, Yock TI, Nelson K, et al. Incidence of second malignancies among patients treated with proton versus photon radiation. Int J Radiat Oncol Biol Phys. 2013;87:46–52. doi: 10.1016/j.ijrobp.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 41.Hoppe BS, Flampouri S, Su Z, et al. Effective dose reduction to cardiac structures using protons compared with 3DCRT and IMRT in mediastinal Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2012;84:449–55. doi: 10.1016/j.ijrobp.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 42.Hoppe BS, Flampouri S, Su Z, et al. Consolidative involved-node proton therapy for Stage IA-IIIB mediastinal Hodgkin lymphoma: preliminary dosimetric outcomes from a Phase II study. Int J Radiat Oncol Biol Phys. 2012;83:260–7. doi: 10.1016/j.ijrobp.2011.06.1959. [DOI] [PubMed] [Google Scholar]
- 43.Weber DC, Trofimov AV, Delaney TF, et al. A treatment planning comparison of intensity modulated photon and proton therapy for paraspinal sarcomas. Int J Radiat Oncol Biol Phys. 2004;58:1596–606. doi: 10.1016/j.ijrobp.2003.11.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.