Abstract
During 2011~2013, a total of 729 samples for 19 types of medicinal plant were collected from Seoulyekryungsi in Seoul, Korea, and investigated for the presence of aflatoxins. The samples were analyzed using immunoaffinity column cleanup and high-performance liquid chromatography coupled to a fluorescence detector after post-column derivatization. Aflatoxins were found in 124 out of the 729 analyzed samples: 65 containing aflatoxin B1 (AFB1), 24 with aflatoxin B2 (AFB2), 15 with aflatoxin G1 (AFG1), and 20 samples with aflatoxin G2 (AFG2). The ranges for positive samples were 0.1~404.7 µg/kg for AFB1, 0.1~10.0 µg/kg for AFB2, 0.1~635.3 µg/kg for AFG1, 0.1~182.5 µg/kg for AFG2, and 0.1~1,043.9 µg/kg for total aflatoxins. Most of the medicinal plant samples (721, 98.9%) were below legal limits, but 8 samples exceeded the legal limits of 10 and 15 µg/kg established by the Korean standard for AFB1 and total aflatoxins (the sum of AFB1, AFB2, AFG1 and AFG2), respectively.
Keywords: Aflatoxins, High-performance liquid chromatography, Total aflatoxins
In general, while the plants are harvested, processed, stored, and transported, they could become contaminated with a variety of molds spoilage, resulting in the production of mycotoxins. Because herbal medicine is based on the basic properties of the plant, they could easily become contaminated by fungi.
With respect to aflatoxins standards in medicinal herbs in Korea, the standards for 9 items were set in 2008, 19 in 2009, and 20 in 2012 of the 546 herbal medicines generally consumed. Korea strengthened the standard limits of aflatoxin B1 (AFB1; below 10 µg/kg) and total aflatoxins (TAFs; the sum of AFB1, aflatoxin B2 [AFB2], aflatoxin G1 [AFG1], and aflatoxin G2 [AFG2], 15 µg/kg or less) [1, 2, 3].
Mycotoxins are difurano-coumarins [4], toxic secondary metabolites produced by species of filamentous fungi which grow on food before harvest and during storage in hot climate with high humidity. Although only AFB1, AFB2, AFG1, and AFG2 are normally found in the natural environment. AFB1 and AFB2 are produced by Aspergillus flavus, while all four isoforms (B1, B2, G1, G2) are produced by Aspergillus parasiticus, and ranking in order of AFB1 > AFG1 > AFB2 > AFG2 for toxicity [5].
AFB is commonly found in contact with air, and often occurs in the leaves and flower parts due to A. flavus, while AFB and AFG are found in soil to produce better adaptations to the environment, for which production is limited to A. parasiticus [6]. Previous works showed that the levels of aflatoxins are not reduced by domestic cooking by either a microwave or conventional gas oven heating, and that aflatoxins do not decompose at the temperature of boiling water during preparation of beverages [7], allowing them to persist to cause chronic disease [8]. The international agency for research on cancer [9] has acknowledged that there is sufficient evidence in humans of the carcinogenicity of naturally occurring AFB1 and mixtures of the aflatoxins.
The International Food and Agricultural Organization [10] announced that about 25% of global food is contaminated with fungus, making aflatoxins contamination a global problem.
Regulations on the aflatoxins allowed in medicinal plants have been made in some countries, such as Argentina, China, and a few countries who manage it with a couple of rules [11]. Korea [1, 2, 3] set the maximum permissible limits 10 µg/kg for AFB1 and 15 µg/kg for TAFs, while Germany and Italy [12] set the maximum permissible limits to 2 and 5 µg/kg for AFB1, and 4 and 10 µg/kg for the sum of AFB1, AFB2, AFG1, and AFG2 in medicinal plants, respectively. In addition, the European Pharmacopeia set limits for AFB1 and TAFs at 2 and 4 µg/kg, respectively, for some medicinal herbs [13]. The World Health Organization announced that at least 50% of Europe's population has used herbal medicines more than once [14]. Recently, the use of medicinal herbs for prophylactic and therapeutic purposes has gained interest in Western countries, subsequently causing increased consumption of aflatoxins through contamination, giving rise to a number of studies [15].
In this study, we investigated a total of 729 samples for 19 types of medicinal plant for aflatoxins contamination.
Materials and Methods
Regents and material
A mixture of aflatoxins (B1, B2, G1, and G2; Supelco, Bellefonte, PA, USA) with acetonitrile (Merck, Darmstadt, Germany) was prepared as a standard solution. An immunoaffinity column (Aflaochra; Vicam, Watertown, MA, USA) was employed for purification. Ultrapure water used for the high-performance liquid chromatography (HPLC) mobile phase and all analytical steps was produced in an 18.2 MQ Gradient water purification system (Millipore, Temecula, CA, USA).
Samples
The samples contained the following: Glycyrrhizae Radix et Rhizoma (119), Cassiae Semen (41), Trichosanthis Radix (37), Testudinis plastrum (5), Persicae Semen (58), Chaenomelis fructus (73), Pinelliae Tuber (17), Thujae Semen (13), Batryticatus Bombyx (3), Dolichoris Semen (29), Areca Semen (60), Zizyphi Semen (29), Nelumbinis Semen (66), Curcumae Radix (16), Polygalae Radix (62), Myristicae Semen (8), Armeniacae Semen (32), Carthami flos (59), and Hovenia dulis Thumb (2).
Standard solution
The authentic standard solution Mix kit-M was purchased (B1, 1 µg/mL; B2, 0.3 µg/mL; G1, 1 µg/mL; and G2, 0.3 µg/mL) and diluted into 5, 10, and 20 ng/mL solutions of AFB1 and AFG1, and into 1.5, 3, and 6 ng/mL solutions of AFB2 and AFG2.
Calibration curve
The linearity of the calibration curve (R2 > 0.99) was confirmed for each of the four aflatoxins. Three replications were carried out for each concentration. Each aflatoxin peak in the chromatogram was identified by comparing the retention times with those of corresponding reference standards. The quantity of aflatoxins in injected eluate was determined by comparison to the respective standard curves of aflotixin B1, B2, G1, and G2.
Extraction and clean-up procedure
To quantify the aflatoxins test solutions for testing the process of residual contamination such as the corporate herbal test method [2], about 500 g of sample was homogenized, and the crushed sample was weighed accurately to about 5.0 g. It was then extracted for 1 hr in a water·methanol mixture (3 : 7) of 100mL. Forty milliliters of the extracted solution was passed through an immunoaffinity column with 10mL of water twice at a flow rate of 3mL/min. The column was then washed for 10 sec in a syringe, passed through, and air-dried. The column was eluted with 1.0 mL dry methanol. If the eluent was not clear, it was passed through a 0.45 µm filter.
HPLC-FLD analysis
HPLC analysis was performed with a Water e2695 system consisting of a 2475 multi fluorescence detector (Waters 2475 Multi Fluorescence Detector; Waters Corp., Millford, MA, USA) fitted with a liquid chromatograph (Water e2695 Seperation Module; Waters Corp.). A Symmetry (R) C18 column was employed (5 µm, 4.6 × 250 mm; Waters Corp.). The wavelengths for excitation and emission were 365 nm and 435 nm, respectively. Post-column derivatization was carried out with a photochemical reactor for enhanced detection (photochemical reaction apparatus, PHRED) (Photochemical Reactor; Aura Industries, New York, NY, USA) and the column temperature was maintained at 40℃ with a flow rate of 1.2mL/min. The mobile phase was acetonitrile : methanol : water (15 : 25 : 60).
Analytical quality parameter
The limit of detection (LOD) and limit of quantification (LOQ) were determined using spiked samples based on a signal-to-noise ratio of 3 : 1 for LOD and 10 : 1 for LOQ. Aflatoxin-free samples that were previously examined by a laboratory certified for the aflatoxins analysis of herbal medicines were used in the recovery tests. The herbal medicines employed in the recovery test were Zizyphi Semen, Persicae Semen, Pinelliae Tuber, Glycyrrhizae Radix et Rhizoma, Trichosanthis Semen, Chaenomelis Fructus, Arecae Semen, and Nelumbinis Semen. AFB1 and AFG1 to a final concentration of 10 µg/kg, along with AFB2 and AFG2 to a final concentration of 3 µg/kg were added to the samples for analysis, which was carried out in triplicate. In addition, four calibration curves for each aflatoxin were prepared, testing the following aflatoxins concentrations (µg/kg): AFB1, 2.66, 5.32, 10.6, 21.36, and 106.30; AFB2, 0.76, 1.52, 3.03, 6.06, and 30.30; AFG1, 2.71, 5.43, 10.85, 21.70, and 108.50; and AFG2, 0.69, 1.38, 2.75, 5.50, and 27.50.
Results
Analytical method performance
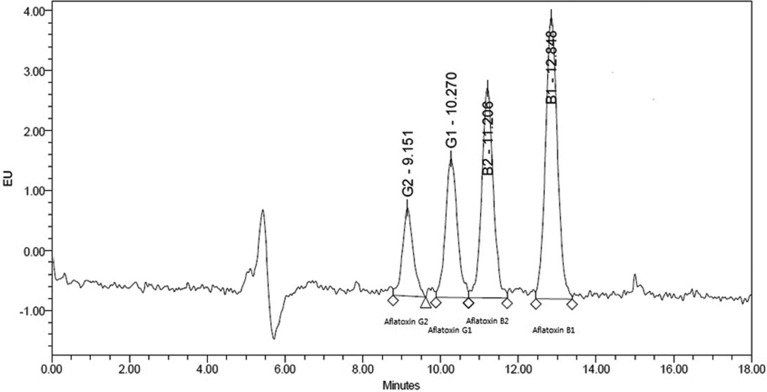
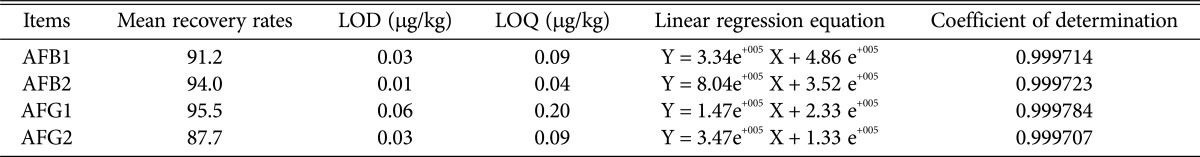
Aflatoxins detection limits, quantification limits, and recovery rates are shown in Table 1. Liquid chromatography spectra are shown in Fig. 1. The peaks for AFB1, AFB2, AFG1, and AFG2 were obtained clearly, resulting in a linear calibration curve for each standard solution, as shown in Fig. 2.
Table 1.
Recovery rates, LOD and LOQ of aflatoxins in herbal medicines by HPLC
LOD, limit of detection; LOQ, limit of quantification; HPLC, high-performance liquid chromatography; AFB1, aflatoxin B1; AFB2, aflatoxin B2; AFG1, aflatoxin G1; AFG2, aflatoxin G2.
Fig. 1.
Chromatogram of aflatoxin standard solution B1, B2, G1, and G2.
Fig. 2.
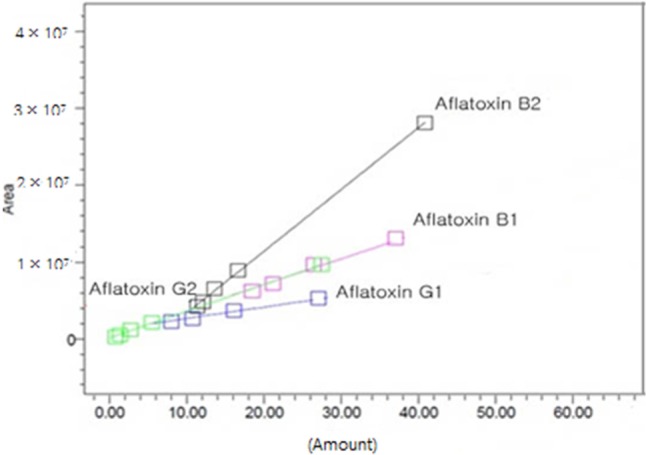
Calibration curve of aflatoxin standard solution B1, B2, G1, and G2.
As shown in Table 1, the limits of detection of AFB1, AFB2, AFG1, and AFG2 were 0.03, 0.01, 0.06, and 0.03 µg/kg, respectively, and the quantitative limits were 0.09, 0.04, 0.20, and 0.09 µg/kg, respectively. The average recoveries of AFB1, AFB2, AFG1, and AFG2 were 91.2%, 94.0%, 95.5%, and 87.7%, respectively.
Aflatoxin analysis
The amount and incidence rate of aflatoxins in herbal medicines were examined, the results of which are shown in Table 2. The amounts of AFB1, AFB2, AFG1, AFG2 and TAFs in all the samples were in the ranges of 0.1~404.7, 0.1~10.0, 0.1~635.3, 0.1~182.5, and 0.1~1,043.9 µg/kg, respectively. The average amount of each aflatoxin detected was 1.7 µg/kg for AFB1, 0.1 µg/kg for AFB2, 1.1 µg/kg for AFG1, 0.3 µg/kg for AFG2, and 3.2 µg/kg for TAFs occurring in the order AFB1 > AFG1 > AFG2 > AFB1. As shown in Table 2, the incidence rate of the aflatoxins was 65 samples (8.9%) with AFB1, 24 (3.3%) with AFB2, 15 (2.1%) with AFG1, and 20 (2.7%) with AFG2, in order of which the rate of occurrence was AFB1 > AFB2 > AFG2 > AFG1.
Table 2.
The level of aflatoxins (µg/kg) detected in herbal medicines in Korea
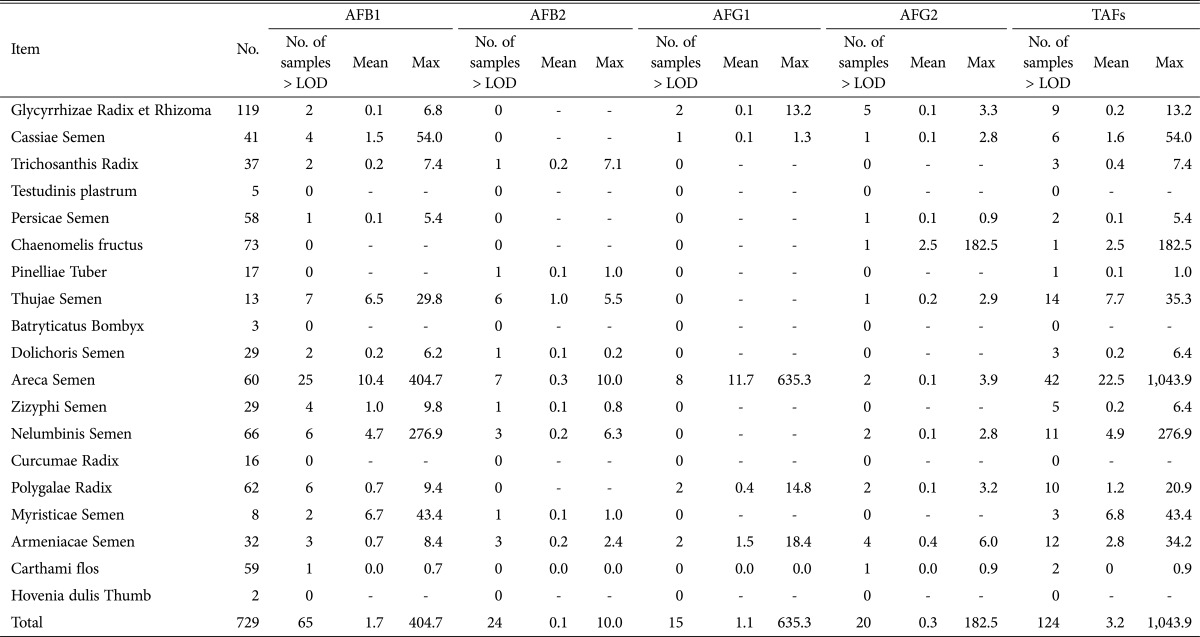
AFB1, aflatoxin B1; AFB2, aflatoxin B2; AFG1, aflatoxin G1; AFG2, aflatoxin G2; TAFs, total aflatoxins; LOD, limit of detection.
The samples above the standard limits are shown in Table 3. When the legal limits of aflatoxins were analyzed, all samples were about 98.9% lower than the acceptable valves. The remaining 1.1% of samples exceeded the limits with 8 samples of five different herbs: Cassiae Semen, Thujae Semen, Dollichoris Semen, Dollichoris Semen, and Myristicae Semen. The incidence rate the AFB1, AFB2, and AFG1 were highest in Dollichoris Semen, while the incidence rate of AFG2 was highest in Glycyrrhizae Radix et Rhizoma by item. In addition, one or more of the four aflatoxins were detected in 15 of the 19 plants tested, but AFB1, AFB2, AFG1, and AFG2 were not detected at all in Testudinis plastrum, Bombysis Corpus, Curcumae Radix and Hovenia dulis Thumb.
Table 3.
The level of aflatoxins above the Korea safety guidelines in herbal medicines in Korea
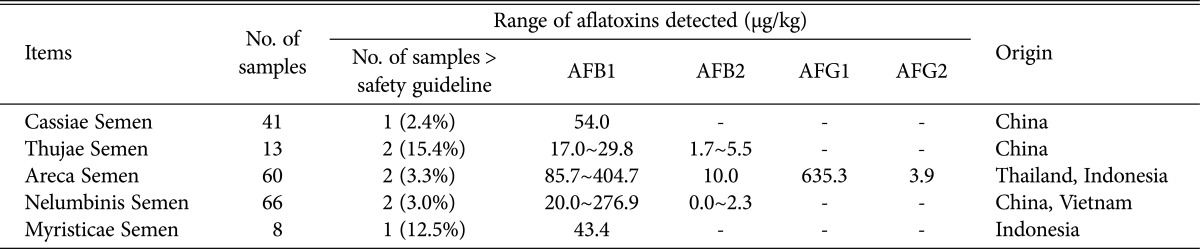
Values in parentheses were detected rates over than Korea safety guideline in herbal medicines.
AFB1, aflatoxin B1; AFB2, aflatoxin B2; AFG1, aflatoxin G1; AFG2, aflatoxin G2.
As shown in Table 4, according to the parts of sampled, the samples were classified into flowers, animals, roots, seeds, fruit and trunk. AFB1, AFB2, AFG1, and AFG2 were detected in the seeds of 57 samples (15.3%), 24 samples (6.4%), 12 samples (3.2%), and 12 samples (3.2%), respectively. In the roots, AFB1, AFG1, and AFG2 were detected in 8 samples (4.1%), 4 samples (2.0%), and 8 samples (4.1%), respectively. In the flowers, AFB1 and AFG1 were detected in only 1 sample (1.7%) each. Finally, AFG2 was detected in 1.3% of the fruit samples, while AFB2 was detected in 5.9% of the stem parts.
Table 4.
The incidence and amounts of aflatoxins in herbal medicines according to used parts (µg/kg)
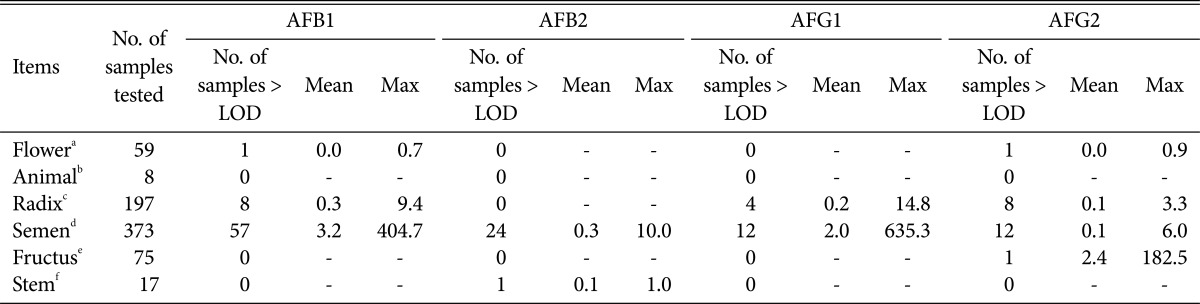
AFB1, aflatoxin B1; AFB2, aflatoxin B2; AFG1, aflatoxin G1; AFG2, aflatoxin G2; LOD, limit of detection.
aCarthami flos.
bTestudinis plastrum. Batryticatus Bombyx.
cGlycyrrhizae Radix et Rhizoma, Curcumae Radix, Polygalae Radix.
dCassiae Semen, Trichosanthis Radix, Persicae Semen, Thujae Semen, Dolichoris Semen, Areca Semen, Zizyphi Semen, Nelumbinis Semen, Myristicae Semen, Armeniacae Semen.
eChaenomelis fructus, Hovenia dulis Thumb.
fPinelliae Tuber.
Discussion
The use of herbs contaminated with aflatoxins is contrary to their purpose, and can cause serious illness. It is important to use herbs that are not contaminated with aflatoxins.
Analytical method performance
In this study, the average recoveries of AFB1, AFB2, AFG1, and AFG2 were 91.2%, 94.0%, 95.5%, and 87.7%, respectively. Romagnoli et al. [16], Arranz et al. [17] and Zhang et al. [18] previously obtained the ranges of 76.1~78.1%, 98~103%, and 93~97%, respectively, which were different from the results obtained in the present study. Reiter et al. [6] reported that the recovery of aflatoxins depends on the type of substrate, purification method, and type of device used for derivatization. Ip and Che [19] suggested that the recovery of aflatoxins depends on the sample substrate and the method of analysis, according to the temperament, and that the estimated difference, particularly when using an immunoaffinity column for purification of aflatoxins, depends on the binding affinity determined by the pH and salt concentration of the buffer used.
Aflatoxin analysis
Many differences were observed in the amounts of AFB1, AFB2, AFG1, and AFG2 between the samples, but with the exception of some, they all occurred at low levels. The mean incidence rates of the four kinds of aflatoxins were less than 10%. Recent research on medicinal plants has shown aflatoxins contamination, such as the report by Rizzo et al. [20] on 152 samples showing AFB2 and AFB1 ranging from 10~2,000 µg/kg. Roy and Chourasia [21] also reported AFB1 from 170~670 µg/kg, while Chourasia et al. [22] reported AFB1 of 910.0 µg/kg, and Tassaneeyakul et al. [23] reported aflatoxins of 1.7~14.3 µg/kg. Romagnoli et al. [16] reported that no aflatoxins were detected. In addition, the frequency of contamination was reported by Santos et al. [24] to occur in 53 of 84 samples (63.1%), and by Rizzo et al. [20] to occur in 41 of 152 samples (27.0%). Romagnoli et al. [16] detected no aflatoxins in a set of 27 samples. The previous research showed many differences in the amount and incidence rate of aflatoxins production. In this case, Cotty and Jaime-Garcia [25] suggested the samples to be contaminated more often by A. flavus than A. parasiticusis, or by both strains at the same time. With respect to the frequency of occurrence, aflatoxins was reported by the Food and Agriculture Organization of the United Nations (FAO) [26] to be present in food and feed at ratio of 1 : 0.8 for AFB1 compared to AFB2 + AFG1 + AFG2, and a ratio of 4 : 1 for AFB1 versus AFB2. Reddy et al. [27] reported the occurrence rates of AFB1, AFB2, AFG1 and AFG2 to be different depending on the substrate, but generally AFB1 and AFB2 occurred at a ratio of 1.0 : 0.1, while the 4 kinds of toxins occurred at ratios of 1.0 : 0.1 : 0.3 : 0.03 while the production of AFB1 was reported to be the highest. Some differences in the incidence rate compared to FAO [26] and Reddy et al. [27] were observed for Armeniacae Semen for the ratios of the four aflatoxins. The occurrence of AFB1 and AFB2 at a ratio of 3.5 : 1.0 was similar to the findings of the FAO, but the incidence rate of AFB1 : AFB2 : AFG1 : AFG2 was 1.0 : 0.3 : 2.1 : 0.5, while AFB1 to AFB2 + AFG1 + AFG2 was 1.0 : 2.8. These differences observed for the medicinal plants likely depends on their origin [28], the type and number of fungi which produce toxins, and the ability to create a mold of the environmental and geographical conditions. Sweeney and Dobson [29] reported that mold generating mycotoxins usually produce more than one kind of toxin, and Santos et al. [24] reported that most medicinal plants were contaminated with at least two types of mycotoxins, while 87% of plants were contaminated with 3~4 kinds of mycotoxins at the same time. Cross contamination of fungal toxins may lead to a synergistic effect, increasing the health risk due to the ingestion of mycotoxins, thus requiring a great deal of attention [8]. One or more toxins were detected in 124 of the samples (17.0%). The toxicity of aflatoxins varies depending on age, gender, ethnicity, nutritional status, and exposure time. As herbal medicine is mainly used in the elderly with reduced immune function, more concern should be given to the risks.
It is necessary to take steps during the production, manufacture and distribution of the samples which were above the limit by implementing thorough safety management. In particular, because most of the medicinal plants consumed in the country were imported in the form of raw materials, medicinal plants contaminated with aflatoxins should be banned. Second, as packaging and distribution can also contribute to contamination with aflatoxins, strategic measures need to be established to provide stricter guidelines regarding medicinal plants.
References
- 1.Korea Food and Drug Administration. Food code, KFDA notification No 2008-4. Seoul: Korea Food and Drug Administration; 2008. [Google Scholar]
- 2.Korea Food and Drug Administration. Food code, KFDA notification No 2009-104. Seoul: Korea Food and Drug Administration; 2009. [Google Scholar]
- 3.Korea Food and Drug Administration. Food code, KFDA notification No 2011-42. Seoul: Korea Food and Drug Administration; 2011. [Google Scholar]
- 4.Saleemullah, Iqbal A, Khalil IA, Shah H. Aflatoxin contents of stored and artificially inoculated cereals and nuts. Food Chem. 2006;98:699–703. [Google Scholar]
- 5.European Food Safety Authority. Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to the potential increase of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds, hazelnuts and pistachios and derived products. No. ESFA-Q-2006-174. EFSA J. 2007;446:1–127. [Google Scholar]
- 6.Reiter E, Zentek J, Razzazi E. Review on sample preparation strategies and methods used for the analysis of aflatoxins in food and feed. Mol Nutr Food Res. 2009;53:508–524. doi: 10.1002/mnfr.200800145. [DOI] [PubMed] [Google Scholar]
- 7.Midio AF, Campos RR, Sabino M. Occurrence of aflatoxins B1, B2, G1 and G2 in cooked food components of whole meals marketed in fast food outlets of the city of Sao Paulo, SP, Brazil. Food Addit Contam. 2001;18:445–448. doi: 10.1080/02652030120070. [DOI] [PubMed] [Google Scholar]
- 8.Speijers GJ, Speijers MH. Combined toxic effects of mycotoxins. Toxicol Lett. 2004;153:91–98. doi: 10.1016/j.toxlet.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer (IARC) Monographs on the evaluation of the carcinogenic risks of chemicals to humans. Lyon: IARC; 1993. Some naturally occurring substances: food items and constituents: heterocyclic aromatic amines and mycotoxin. Vol. 56; pp. 245–521. [Google Scholar]
- 10.Food and Agriculture Organization of United Nations (FAO) Manual on the application of the HACCP system in mycotoxin prevention and control, No. 73. FAO Food and Nutrition Paper. Rome: FAO; 2001. [Google Scholar]
- 11.Cho SY, Kang SJ, Jung J, Jeong BO, Jeong CS. Co-contamination of aflatoxins with ochratoxin A and zearalenone in Thuja orientalis Semen. Toxicol Res. 2009;25:125–131. doi: 10.5487/TR.2009.25.3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Pharmacopoeia. European Directorate for the Quality of Medicines and Health Care (EDQM) 7th ed. Strasbourg: Council of Europe; 2011. [Google Scholar]
- 14.World Health Organization. WHO guidelines for assessing quality of herbal medicines with reference to contaminants and residues. Geneva: WHO; 2007. [Google Scholar]
- 15.Ashiq S, Hussain M, Ahmad B. Natural occurrence of mycotoxins in medicinal plants: a review. Fungal Genet Biol. 2014;66:1–10. doi: 10.1016/j.fgb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Romagnoli B, Menna V, Gruppioni N, Bergamini C. Aflatoxins in spices, aromatic herbs, herb-teas and medicinal plants marketed in Italy. Food Control. 2007;18:697–701. [Google Scholar]
- 17.Arranz I, Sizoo E, Van Egmond H, Kroeger K, Legarda TM, Burdaspal P, Reif K, Stroka J. Determination of aflatoxin B1 in medical herbs: interlaboratory study. J AOAC Int. 2006;89:595–605. [PubMed] [Google Scholar]
- 18.Zhang X, Liu H, Chen J. Immunoaffinity column cleanup with liquid chromatography using post-column bromination for aflatoxins in medicinal herbs and plant extracts. J Chromatogr Sci. 2005;43:47–51. doi: 10.1093/chromsci/43.1.47. [DOI] [PubMed] [Google Scholar]
- 19.Ip SP, Che CT. Determination of aflatoxins in Chinese medicinal herbs by high-performance liquid chromatography using immunoaffinity column cleanup improvement of recovery. J Chromatogr A. 2006;1135:241–244. doi: 10.1016/j.chroma.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Rizzo I, Vedoya G, Maurutto S, Haidukowski M, Varsavsky E. Assessment of toxigenic fungi on Argentinean medicinal herbs. Microbiol Res. 2004;159:113–120. doi: 10.1016/j.micres.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Roy AK, Chourasia HK. Mycoflora, mycotoxin producibility and mycotoxins in traditional herbal drugs from India. J Gen Appl Microbiol. 1990;36:295–302. [Google Scholar]
- 22.Chourasia HK. Mycobiota and mycotoxins in herbal drugs of Indian pharmaceutical industries. Microbiol Res. 1995;99:697–703. [Google Scholar]
- 23.Tassaneeyakul W, Razzazi-Fazeli E, Porasuphatana S, Bohm J. Contamination of aflatoxins in herbal medicinal products in Thailand. Mycopathologia. 2004;158:239–244. doi: 10.1023/b:myco.0000041892.26907.b4. [DOI] [PubMed] [Google Scholar]
- 24.Santos L, Marín S, Sanchis V, Ramos AJ. Screening of mycotoxin multicontamination in medicinal and aromatic herbs sampled in Spain. J Sci Food Agric. 2009;89:1802–1807. [Google Scholar]
- 25.Cotty PJ, Jaime-Garcia R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int J Food Microbiol. 2007;119:109–115. doi: 10.1016/j.ijfoodmicro.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 26.Food and Agriculture Organization of United Nations (FAO) Worldwide regulations for mycotoxins in food and feed in 2003. Rome: FAO; 2004. [Google Scholar]
- 27.Reddy KR, Salleh B, Saad B, Abbas HK, Abel CA, Shier WT. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010;29:3–26. [Google Scholar]
- 28.Liu L, Jin H, Sun L, Ma S, Lin R. Determination of aflatoxins in medicinal herbs by high-performance liquid chromatographytandem mass spectrometry. Phytochem Anal. 2012;23:469–476. doi: 10.1002/pca.2343. [DOI] [PubMed] [Google Scholar]
- 29.Sweeney MJ, Dobson AD. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int J Food Microbiol. 1998;43:141–158. doi: 10.1016/s0168-1605(98)00112-3. [DOI] [PubMed] [Google Scholar]