Abstract
Makgeolli, also known as Takju, is a non-filtered traditional Korean alcoholic beverage that contains various floating matter, including yeast cells, which contributes to its high physiological functionality. In the present study, we assessed the levels of β-glucan and glutathione in various yeast strains isolated from traditional Korean Nuruk and selected a β-glucan- and glutathione-rich yeast strain to add value to Makgeolli by enhancing its physiological functionality through increased levels of these compounds. Yeast β-glucan levels ranged from 6.26% to 32.69% (dry basis) and were strongly species-dependent. Dried Saccharomyces cerevisiae isolated from Nuruk contained 25.53 µg/mg glutathione, 0.70 µg/mg oxidized glutathione, and 11.69 µg/g and 47.85 µg/g spermidine and L-ornithine monohydrochloride, respectively. To produce functional Makgeolli, a β-glucan- and glutathione-rich yeast strain was selected in a screening analysis. Makgeolli fermented with the selected yeast strain contained higher β-glucan and glutathione levels than commercial Makgeolli. Using the selected yeast strain to produce Makgeolli with high β-glucan and glutathione content may enable the production of functional Makgeolli.
Keywords: β-Glucan, Glutathione, Makgeolli, Yeast
Glucan is a component of polysaccharides that consists of glucose molecules chemically bound by glycosidic bonds. They are classified as α-glucans and β-glucans according to the bonds connecting the monosaccharides (α- and β-glycosidic, respectively). β-Glucan is known to exert excellent immune-modulatory effects by activating the non-specific immune response of macrophages and promoting cell proliferation. β-Glucan also has various other functional effects, such as a cholesterol lowering effect [1], postprandial hypoglycemic effect [2], and anti-cancer and antioxidant effects [3, 4, 5]. Therefore, it has been used in cosmetics and as an anti-cancer drug and food additive. Recently it has gained focused as five functional polysaccharides with hyaluronic acid, chitosan and so on [6, 7]. β-Glucans are divided into β-1,3; β-1,4; and β-1,6 glucans based on the glycosidic bonds, and their functions have been reported to vary based on these structural differences. The most important are the β-1,3 glucans, which have immune regulation functions and anti-cancer effects. β-Glucans are synthesized by both prokaryotic and eukaryotic organisms. Some are secreted from cells, but most exist as cell wall components in microorganisms [8]. In the yeast Saccharomyces cerevisiae, the cell wall contains β-(1,3)-D-glucan, β-(1,6)-D-glucan, chitin, and mannoproteins. Yeast β-glucans have the basic structure of (1→3),(1→6)-β-D-glucan. Due to the structural and molecular weight differences between yeast β-glucan and the β-glucans from plants, yeast β-glucan is a well-known immunomodulator that has a strong positive effect on human and animal immune systems [8, 9].
Glutathione is a tripeptide of three amino acids: glutamic acid, cysteine, and glycine, and it is the most abundant non-protein thiol compound in almost all eukaryotic cells. It has important biological roles, including protection against oxidative stress, control of redox potential, detoxification of toxins, protein folding, and organic sulfur storage and transport [10], as well as immune reinforcement effects [11, 12]. Because of these biological effects, glutathione is used in the food industry as an additive to improve the nutritional value of products. Although glutathione is widely distributed in nature, many researchers have attempted to mass-produce it using yeast [13, 14]. Thus, glutathione from yeast and β-glucan are regarded as good functional foods for the prevention of diseases, although they are still relatively expensive compared to other functional materials.
Makgeolli is a traditional Korean alcoholic beverage made by simultaneous two-step fermentation using grains and yeast. After fermentation, it becomes white and cloudy in appearance due to the presence of unfiltered rice material, and it has evenly blended sweet, acidic, and bitter flavors. Makgeolli has higher health functionality than Yakju, a Korean traditional alcoholic beverage similar to Japanese Sake that is filtered after fermentation [15, 16, 17]. These suspended solids in Makgeolli include microorganisms, such as yeast and lactic acid bacteria, as well as starches, oligosaccharides, organic acids, peptides, and various other biologically useful substances [15]. In a study aimed at increasing Makgeolli functionality, Kang et al. reported that Makgeolli peptides showed angiotensin I-converting enzyme (ACE) inhibitory activity [18]. In the present study, we investigated the levels of β-glucan and glutathione in yeast strains isolated from Nuruk, a Korean traditional fermentation starter that is produced by the natural proliferation of fungi on crushed grain and is used in the fermentation of Makgeolli. We selected a β-glucan- and glutathione-rich yeast to add value to Makgeolli by increasing the levels of these physiologically functional compounds. The results of this research may be used to increase the functionality of yeast-containing products, such as bakery products or other foods containing whole yeast cells.
MATERIALS AND METHODS
Isolation and identification of wild yeast strains
Wild yeast strains were isolated from Korean traditional Nuruk collected from several provinces in South Korea. The microorganisms were sent to Macrogen, Inc. (Seoul, Korea) for identification via PCR using a PTC-225 Peltier Thermal Cycler (MJ Research, Reno, NV, USA) and primers ITS1 (5'-TCCGTAGGTGAACCTGCGG-3') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3'). The 18S rRNA genes were sequenced using these same primers and the ABI PRISM Big Dye Terminator Cycle Sequencing Kit in an ABI PRISM 3730XL DNA Analyzer (Applied Biosystems, Foster City, CA, USA). The sequences obtained were used in a BLAST search of GenBank (www.ncbi.mlm.nih.gov/BLAST), and then edited using BioEdit software [19].
Quantitative analysis of the β-glucan content in yeast cells
Yeast strains were cultured in potato dextrose agar (PDA) broth medium at 25℃ for 48 hr. Cultured cells were harvested by centrifugation and lyophilized. β-Glucan content in dried yeast cells was quantified using a Megazyme kit (Megazyme Inc., Wicklow, Ireland).
Intracellular glutathione content analysis of yeast cells
Yeast strains were cultured in PDA medium at 25℃ for 72 hr, and then freeze-dried. The dried yeast (20 mg) was transferred to a bottle containing beads, and then 1 mL of 50% methanol was added. The yeast cells were homogenized using an automated homogenizer (Precellys 24 lysis/homogenizer; Bertin Technologies, Montigny-le-Bretonneux, France) three times at 6,500 rpm for 10 sec each. After the cell pellet was extracted for 2 hr at room temperature, the supernatant of the final sample was obtained by centrifugation (10,000 ×g, 5 min, 4℃) and was analyzed by ultra-high performance liquid chromatograph (UHPLC).
Glutathione and its metabolites were analyzed with a UPLC system (Agilent 1290 Infinity; Agilent, Santa Clara, CA, USA) interfaced with an accurate-mass quadrupole time-of-flight instrument (Agilent 6520 with Jet Stream Technology). The QTOF-MS instrument was operated with an electrospray ion source (Agilent Jet Stream Technology) in positive mode. It was set at 350℃ with drying gas at a flow rate of 12 L/mL, a nebulizer pressure of 45 psi, Vcap of 2,000 V, skimmer voltage of 65 V, and fragmentor voltage of 170 V. The mobile phase was in gradient mode with 0.1% formic acid in distilled water (A) and 0.1% formic acid in acetonitrile (B), with a flow rate of 0.9mL/min. The analysis was performed with an ACE Excel C18 column (3 µm, 4.6 × 150 mm) at a column temperature of 30℃ and an injection volume of 0.3 µL.
Makgeolli production using selected yeast
The white rice (Dongsongnonghyup; Cheorwon O-Dae Rice, Cheorwon, Korea), Cozy (saccharification: 60 sp.; Joeungoksik, Gyeonggi-do, Korea), and purified enzyme (saccharification: 300 sp.; Hangukhyoso, Hwaseong, Korea) used for Makgeolli production were purchased and used as is. The yeast strains (S. cerevisiae) used in this study were isolated from preserved Nuruk collected from various regions of Korea [19]. Lactobacillus (Lactobacillus plantarum JS1) strains were isolated and purified from commercial Makgeolli. The first soak was fermented for 2 days at 25℃ at a water ratio of 200% with Cozy, lactic acid bacteria, and yeast in a bottle. In the second soak, the same water ratio was used, and the Cozy and ground rice steeped in water were added and then incubated for 5 days at 25℃. Cozy and purified yeast were added at 35 sp./g of rice. Yeast and lactic acid bacteria were added at 0.06% and 0.012% of the Makgeolli mash, respectively.
Quantitative analysis of β-glucan content in Makgeolli
To obtain a pellet of the Makgeolli, after shaking to mix, a 150-mL aliquot was obtained and centrifuged at 3,000 rpm for 10 min. The β-glucan content of the collected pellets was analyzed by using the Megazyme β-glucan assay kit. The water content of the pellets was measured with an infrared moisture analyzer (MAC 50/NH; RADWAG, Radom, Poland). The final β-glucan content in Makgeolli is presented as a dry basis.
Statistical analysis
The raw data were analyzed to assess the significance of differences between samples using analysis of variance (ANOVA), and means were compared using Duncan's multiple range test (honestly significant differences). All statistical analyses were conducted with SAS 9.2 statistical software (SAS Institute Inc., Cary, NC, USA). p-values less than 0.05 were considered significant. Glutathione data are presented as box-and-whisker plots.
RESULTS AND DISCUSSION
Identification of wild yeast strains isolated from Nuruk and β-glucan content analysis
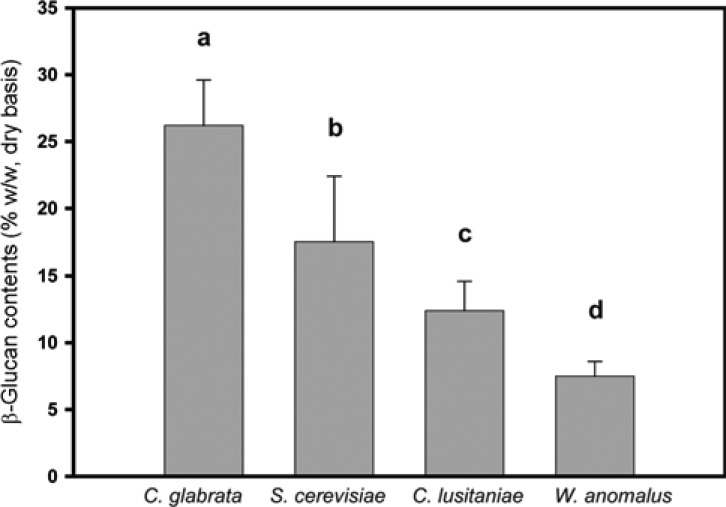
A list of the wild yeast strains isolated form Korean traditional Nuruk and their identification information are presented in Table 1. The isolated yeast strains identified by 18S rRNA sequencing included 30 strains of Saccharomyces sp., 11 strains of Candida sp., 21 strains of Wickerhamomyces sp., 1 strain of Pichia sp., and 1 strain of Torulaspora sp. Yeast β-glucans containing (1,3)-(1,6)-β bonds are typically insoluble, and they have been shown to possess a variety of pharmacological activities, including anti-cholesterolemic activity, hypoglycemic activity, acceleration of heavy metal excretion, and immune system stimulation [20, 21, 22, 23]. In an attempt to identify strains with high glucan content, we assessed the β-glucan levels in the isolated wild yeast strains, and the results are shown in Table 1. The levels ranged from 6.26% to 32.68%. Among the 64 yeast strains tested, strain 230-9, identified as Candida glabrata, showed the highest β-glucan content (32.68%). The strain with the lowest β-glucan content (6.41%) was 197-12, which was identified as Wickerhamomyces anomalus. Among the identified S. cerevisiae strains, the highest β-glucan content was observed in strain 89-3-1 (25.95%), whereas the lowest content was found in strain 115-1 (7.68%). S. cerevisiae is the most common yeast, and it is widely used as a food and beverage starter. In addition, it is also sold as a source of β-glucan due to its nutraceutical and biological effects [20, 24, 25]. It is interesting to note that the yeast β-glucan levels were strongly dependent on the species. In the present study, the β-glucan content of the different yeast species varied, and C. glabrata > S. cerevisiae > Candida lusitaniae > W. anomalus (Fig. 1). This suggested that β-glucan content is influenced by the yeast species.
Table 1.
Identification and β-glucan contents analysis of wild yeast strains
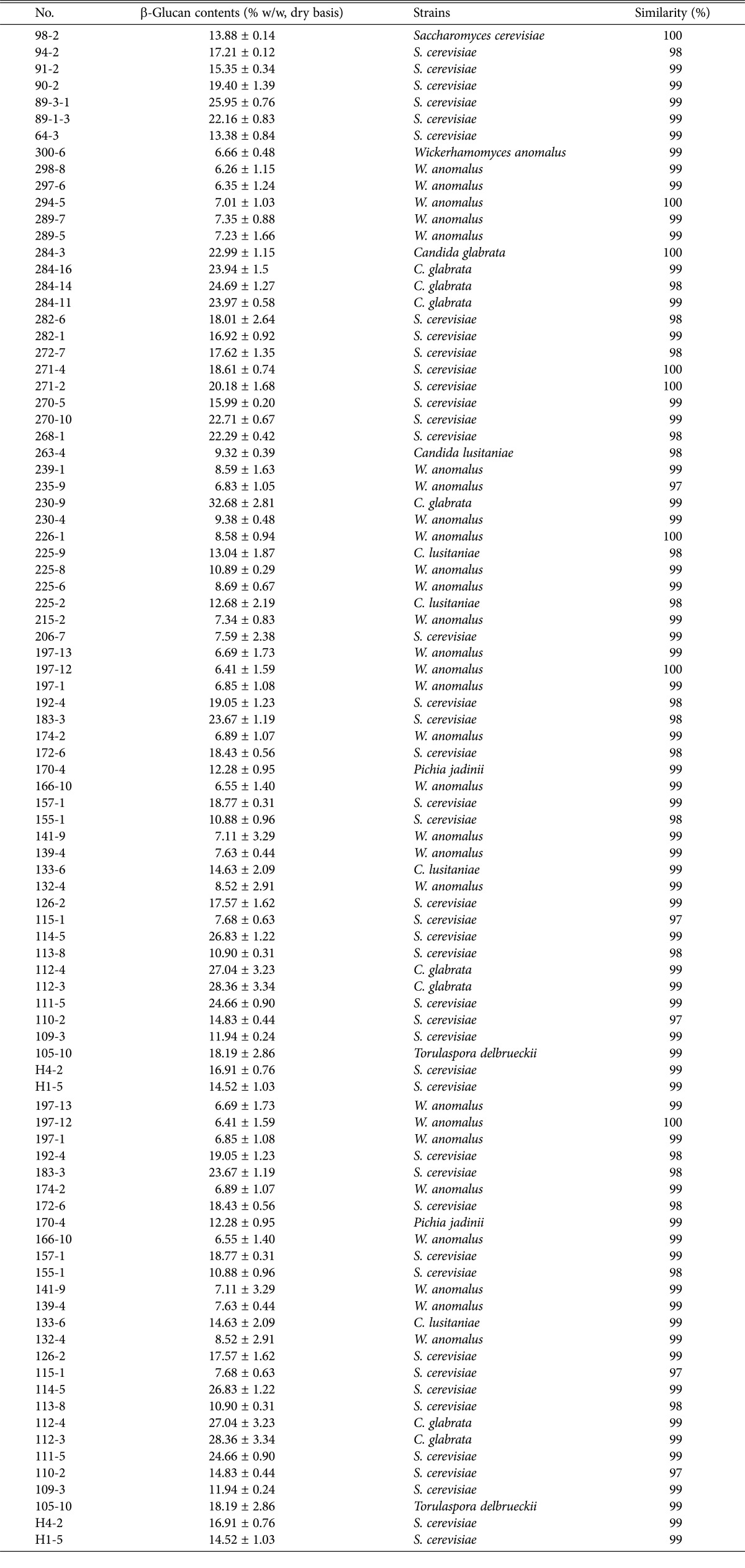
Fig. 1.
β-Glucan contents of yeast strains isolated from Nuruk. C. glabrata, Candida glabrata; S. cerevisiae, Saccharomyces cerevisiae; C. lusitaniae, Candida lusitaniae; W. anomalus, Wickerhamomyces anomalus.
Glutathione contents of wild yeast strains
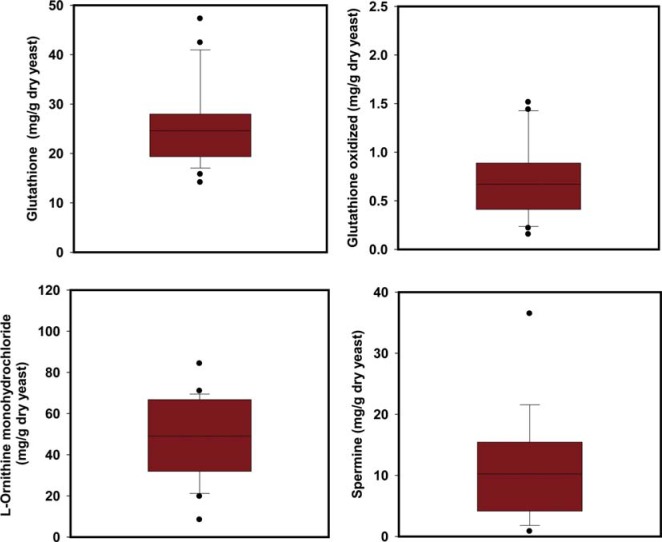
Glutathione (GSH) has various derivatives. GSH is oxidized by exposure to hydroperoxides, and redox cycling of GSH is central to the cellular response to oxidative stress. Polyamines (putrescine, spermidine, and spermine), which were analyzed in this study, are a family of molecules that are derived from ornithine through a decarboxylation process [26, 27, 28, 29, 30, 31, 32]. The amount of GSH and GSH derivatives in wild yeast cells has not yet been reported. In the present study, the glutathione and GSH derivative contents in yeast isolated from Nuruk were quantitatively analyzed with a UHPLC system, and their distribution was confirmed (Table 2, Fig. 2). The S. cerevisiae isolated from Nuruk contained 25.53 µg/mg GSH, 0.70 µg/mg oxidized glutathione, and 11.69 µg/g and 47.85 µg/g spermidine and L-ornithine monohydrochloride, respectively. In the present study, the glutathione content of S. cerevisiae ranged from 14.14 mg/g to 47.25 mg/g. For comparison, Zechner-Krpan et al. [23] reported GSH levels of 0.1~1% dry cell weight, Whistler et al. [28] reported 80~4,320 mg/L GSH, and Penninckx [30] reported high GSH concentrations of up to 10mM in most living cells (from prokaryotes to eukaryotes). The levels of GSH and GSH derivatives in S. cerevisiae strains isolated from Nuruk are presented as box-and-whisker plots (Fig. 2). The range of GSH and GSH oxidized contents was 30 and 1, respectively, and the interquartile ranges (IQR) were 9 and 0.4, respectively. However, the ranges for L-ornithine monohydrochloride and spermidine were approximately 70 and 35, respectively, and the IQR were 35 and 10, respectively. The distributions of GSH, oxidized GSH, and spermidine contents skewed to the right, whereas L-ornithine monohydrochloride content was symmetric.
Table 2.
Glutathione and its derivatives contents in Saccharomyces cerevisiae isolated from Nuruk
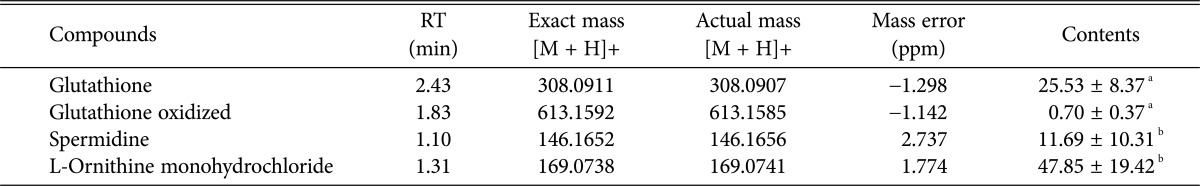
Values are presented as number or mean ± SD.
aµg/mg, bµg/g in dried yeast.
Fig. 2.
Box-and-whisker plots of the levels of glutathione derivatives in Saccharomyces cerevisiae strains isolated from Nuruk.
β-Glucan- and glutathione-rich Makgeolli production using a selected wild yeast strain
In order to develop an improved functional Makgeolli, we produced Makgeolli (SY-1 Makgeolli) using the yeast strain 89-3-1, which contained high β-glucan and glutathione contents. Then, the β-glucan and glutathione contents of the SY-1 Makgeolli were compared to those of commercial Makgeolli, and the results are presented in Fig. 3. The levels of β-glucan and glutathione in the SY-1 Makgeolli were significantly higher than those of commercial sterilized and non-sterilized Makgeolli. In fact, the β-glucan level in SY-1 Makgeolli was six times higher than that in commercial Makgeolli, and the glutathione level was two times higher. It has been reported that commercial Makgeolli containing high levels of β-glucan showed high ACE inhibitory activity [1]. Therefore, SY-1 Makgeolli fermented with 89-3-1 yeast would be expected to have superior physiological functionality.
Fig. 3.
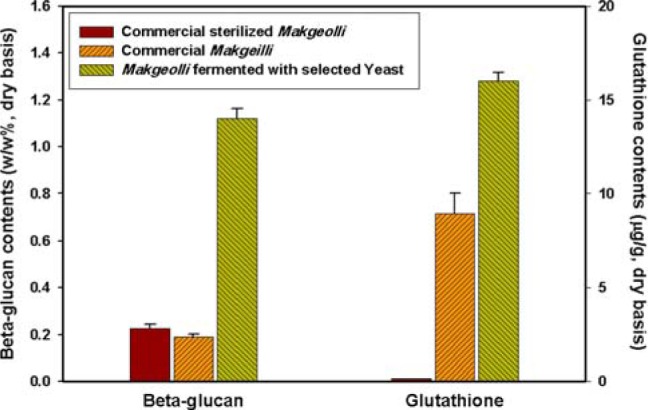
β-Glucan and glutathione contents of commercial Makgeolli and Makgeolli produced with the selected high β-glucan and glutathione yeast strain.
In sterilized Makgeolli, only β-glucan was detected, and glutathione was not. It was thought that the β-glucan was not completely destroyed in the sterilization process due to swelling and agglomeration of the glucan particles, whereas glutathione, a tripeptide, was modified or destroyed by sterilization [27]. Thus, from a functional standpoint, non-sterile Makgeolli, which contains higher amounts of functional substances, has higher functionality than sterilized Makgeolli. Based on the results presented here, production of Makgeolli with the selected yeast, which has high β-glucan and glutathione contents, can enhance its functionality; therefore, it may be possible to produce functional Makgeolli. Further studies are needed to evaluate the physiological effects of Makgeolli with high β-glucan and glutathione contents and to identify the underlying mechanisms of its health functions.
ACKNOWLEDGEMENT
This study was carried out with the support of "Cooperative Research Program for Agricultural Science & Technology Development (Project No. PH009070)".
References
- 1.Min JH, Kim YH, Kim JH, Choi SY, Lee JS, Kim HK. Comparison of microbial diversity of Korean commercial Makgeolli showing high β-glucan content and high antihypertensive activity, respectively. Mycobiology. 2012;40:138–141. doi: 10.5941/MYCO.2012.40.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae IY, Kim SM, Lee S, Lee HG. Effect of enzymatic hydrolysis on cholesterol-lowering activity of oat β-glucan. N Biotechnol. 2010;27:85–88. doi: 10.1016/j.nbt.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Gunn L, Hansen R, Yan J. Combined yeast-derived β-glucan with anti-tumor monoclonal antibody for cancer immunotherapy. Exp Mol Pathol. 2009;86:208–214. doi: 10.1016/j.yexmp.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Gunn L, Hansen R, Yan J. Yeast-derived β-glucan in combination with anti-tumor monoclonal antibody therapy in cancer. Recent Pat Anticancer Drug Discov. 2009;4:101–109. doi: 10.2174/157489209788452858. [DOI] [PubMed] [Google Scholar]
- 5.Driscoll M, Hansen R, Ding C, Cramer DE, Yan J. Therapeutic potential of various β-glucan sources in conjunction with anti-tumor monoclonal antibody in cancer therapy. Cancer Biol Ther. 2009;8:218–225. doi: 10.4161/cbt.8.3.7337. [DOI] [PubMed] [Google Scholar]
- 6.Dziezak JD. Yeasts and yeast derivatives: definitions, characteristics, and processing. Food Technol. 1987;41:104–121. [Google Scholar]
- 7.Donzis BA. Substantially purified beta (1,3) finely ground yeast cell wall glucan composition with dermatological and nutritional uses [Internet] United States Patent; 1996. [cited 2014 Sep 24]. Available from: http://www.google.com/patents/US5576015. [Google Scholar]
- 8.Ha CH. Preparation and analysis of yeast cell wall components, mannoproteins and β-glucan, from wild type and cell wall mutants, Saccharomyces cerevisiae [dissertation] Seoul: Korea University; 2005. [Google Scholar]
- 9.Jaehrig SC, Rohn S, Kroh LW, Wildenauer FX, Lisdat F, Fleischer LG, Kurz T. Antioxidative activity of (1 -> 3), (1 -> 6)-β-D-glucan from Saccharomyces cerevisiae grown on different media. LWT Food Sci Technol. 2008;41:868–877. [Google Scholar]
- 10.Chen HH, Kuo MT. Role of glutathione in the regulation of cisplatin resistance in cancer chemotherapy. Met Based Drugs. 2010;2010:430939. doi: 10.1155/2010/430939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdalla MY, Hassan IM, Mustafa NH, Tahtamouni LH, Ahmad IM. Total body glutathione depletion induces oxidative stress and disrupts the immune function in mice. Toxicol Environ Chem. 2011;93:157–170. [Google Scholar]
- 12.Rakhshandeh A, Holliss A, Karrow NA, de Lange CF. Immune system stimulation and sulfur amino acid intake alter the pathways of glutathione metabolism at transcriptional level in pigs. J Anim Sci. 2010;88(Suppl 2):256. [Google Scholar]
- 13.Jang HY, Oh CH, Oh NS. Production of glutathione by the yeast mutant Saccharomyces cerevisiae Sa59. Korean J Food Sci Technol. 2013;45:801–804. [Google Scholar]
- 14.Musatti A, Devesa V, Calatayud M, Vélez D, Manzoni M, Rollini M. Glutathione-enriched baker's yeast: production, bioaccessibility and intestinal transport assays. J Appl Microbiol. 2014;116:304–313. doi: 10.1111/jam.12363. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Kim JH, Jung YW, Park S, Shin WC, Park CS, Hong S, Kim GW. Composition of organic acids and physiological functionality of commercial Makgeolli. Korean J Food Sci Technol. 2011;43:206–212. [Google Scholar]
- 16.Kim YH, Min JH, Kang MG, Kim JH, Ahn BH, Kim HK, Lee JS. Physicochemical properties, lactic acid bacteria content and physiological funtionalities of Korean commercial Makgeolli. Korean J Microbiol Biotechnol. 2012;40:325–332. [Google Scholar]
- 17.Kim BK, Kang MS, Jeon MJ, Lee SH, Kim M. Effects of Makgeolli and Makgeolli precipitate on hepatotoxicity and serum lipid content in rats. J Life Sci. 2013;23:282–289. [Google Scholar]
- 18.Kang MG, Kim JH, Ahn BH, Lee JS. Characterization of new antihypertensive angiotensin I-converting enzyme inhibitory peptides form Korean traditional rice wine. J Microbiol Biotechnol. 2012;22:339–342. doi: 10.4014/jmb.1109.09015. [DOI] [PubMed] [Google Scholar]
- 19.Kim HR, Kim JH, Bae DH, Ahn BH. Characterization of Yakju brewed from glutinous rice and wild-type yeast strains isolated from Nuruks. J Microbiol Biotechnol. 2010;20:1702–1710. [PubMed] [Google Scholar]
- 20.Chan GC, Chan WK, Sze DM. The effects of β-glucan on human immune and cancer cells. J Hematol Oncol. 2009;2:25. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klis FM, Boorsma A, De Groot PW. Cell wall construction in Saccharomyces cerevisiae. Yeast. 2006;23:185–202. doi: 10.1002/yea.1349. [DOI] [PubMed] [Google Scholar]
- 22.Williams DL. Overview of (1→3)-β-D-glucan immunobiology. Mediators Inflamm. 1997;6:247–250. doi: 10.1080/09629359791550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zechner-Krpan V, Petravić-Tominac V, Gospodarić I, Sajli L, Đaković S, Filipović-Grčić J. Characterization of β-glucans isolated from brewer's yeast and dried by different methods. Food Technol Biotechnol. 2010;48:189–197. [Google Scholar]
- 24.Vetvicka V, Vetvickova J. Physiological effects of different types of β-glucan. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151:225–231. doi: 10.5507/bp.2007.038. [DOI] [PubMed] [Google Scholar]
- 25.Hwang MS. Physical properties of the enzymatic hydrolysates of yeast β-glucan [dissertation] Seoul: Korea University; 2005. [Google Scholar]
- 26.Rodríguez-Bencomo JJ, Andújar-Ortiz I, Moreno-Arribas MV, Simó C, González J, Chana A, Dávalos J, Pozo-Bayón MÁ. Impact of glutathione-enriched inactive dry yeast preparations on the stability of terpenes during model wine aging. J Agric Food Chem. 2014;62:1373–1383. doi: 10.1021/jf402866q. [DOI] [PubMed] [Google Scholar]
- 27.Deacon-Shaw MG, Koenig R. Methods for sterilizing glucans [Internet] United States Patent; 2008. [cited 2014 Sep 24]. Available from: http://www.google.com/patents/US20110028709. [Google Scholar]
- 28.Whistler RL, Bushway AA, Singh PP. Noncytotoxic, antitumor polysaccharides. Adv Carbohydr Chem Biochem. 1976;32:235–275. doi: 10.1016/s0065-2318(08)60338-8. [DOI] [PubMed] [Google Scholar]
- 29.Pucciarelli S, Moreschini B, Micozzi D, De Fronzo GS, Carpi FM, Polzonetti V, Vincenzetti S, Mignini F, Napolioni V. Spermidine and spermine are enriched in whole blood of nona/centenarians. Rejuvenation Res. 2012;15:590–595. doi: 10.1089/rej.2012.1349. [DOI] [PubMed] [Google Scholar]
- 30.Penninckx MJ. An overview on glutathione in Saccharomyces versus nonXMLLink_XYZconventional yeasts. FEMS Yeast Res. 2002;2:295–305. doi: 10.1016/S1567-1356(02)00081-8. [DOI] [PubMed] [Google Scholar]
- 31.Lee JC, Straffon MJ, Jang TY, Higgins VJ, Grant CM, Dawes IW. The essential and ancillary role of glutathione in Saccharomyces cerevisiae analysed using a grande gsh1 disruptant strain. FEMS Yeast Res. 2001;1:57–65. doi: 10.1111/j.1567-1364.2001.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 32.Styblo M, Serves SV, Cullen WR, Thomas DJ. Comparative inhibition of yeast glutathione reductase by arsenicals and arsenothiols. Chem Res Toxicol. 1997;10:27–33. doi: 10.1021/tx960139g. [DOI] [PubMed] [Google Scholar]