Abstract
The beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) is difficult to control using chemical insecticides because of the development of insecticide resistance. Several pest control agents are used to control the beet armyworm. Entomopathogenic fungi are one of the candidates for eco-friendly pest control instead of chemical control agents. In this study, among various entomopathogenic fungal strains isolated from soil two isolates were selected as high virulence pathogens against larva of beet armyworm. Control efficacy of fungal conidia was influenced by conidia concentration, temperature, and relative humidity (RH). The isolates Metarhizium anisopliae FT83 showed 100% cumulative mortality against second instar larvae of S. exigua 3 days after treatment at 1 × 107 conidia/mL and Paecilomyces fumosoroseus FG340 caused 100% mortality 6 days after treatment at 1 × 104 conidia/mL. Both M. anisopliae FT83 and P. fumosoroseus FG340 effectively controlled the moth at 20~30℃. M. anisopliae FT83 was significantly affected mortality by RH: mortality was 86.7% at 85% RH and 13.4% at 45% RH. P. fumosoroseus FG340 showed high mortality as 90% at 45% RH and 100% at 75% RH 6 days after conidia treatments. These results suggest that P. fumosoroseus FG340 and M. anisopliae FT83 have high potential to develop as a biocontrol agent against the beet armyworm.
Keywords: Beet armyworm, Entomopathogenic fungi, Metarhizium anisopliae, Paecilomyces fumosoroseus, Spodoptera exigua
The beet armyworm (Spodoptera exigua Hüber) is a widely distributed polyphagous pest for many economically important crops, such as cotton, tomato, celery, lettuce, cabbage, alfalfa and so on [1, 2]. In Korea, beet armyworm occurs 4~5 generations annually in open fields. In greenhouses, this pest can survive throughout the year and causes year-round damage to crops across the country [3].
The first and second instar larvae of beet armyworm are gregarious and devour plant leaves. Early stage of S. exigua is difficult to control because it feeds in hidden parts of plants, for example, inside of welsh onion and the heart of Chinese cabbage which may be less exposed to insecticides [4]. The late larvae of beet armyworm could not be controlled with chemical insecticides because it develops resistance towards insecticides such as spinosad, chlorinated hydrocarbon, organophosphates, carbamates, pyrethroids, and benzoylphenylureas, etc. [5, 6, 7, 8, 9, 10, 11].
Studies on the biocontrol of S. exigua have mainly focused on nuclear polyhedrosis viruses (NPV) [12, 13] and Bacillus thuringiensis which are now commercially available many countries. However, S. exigua have developed B. thuringiensis resistance [14]. Mass production of NPV required much time and cost and productivity is so low. Therefore, it is important to develop alternative biocontrol agents to control S. exigua.
There are more than 700 species of fungi belonging to 90 genera which isolated from various insect species [15, 16]. At least 12 species or subspecies of fungi have been used as active ingredients for mycoinsecticides. One hundred seventy-six mycopesticides were developed in several countries using Beauveria bassiana, Metarhizium anisopliae, Isaria fumosorosea, and B. brongniartii to control several agricultural pests. Among these only ten mycopesticides (seven B. bassiana, two M. anisopliae and one mixture of two or more species) have been used to control Noctuidae [17].
Several fungal isolates including B. brongniartii, Nomuraea rileyi, I. fumosorosea, and B. bassiana were reported the pathogenicity to S. litura in China [18] and M. anisopliae and I. fumosorosea in Pakistan [19]. But there are few studies against S. exigua [20].
Therefore we studied and selected two fungal isolates having high virulence to control beet armyworm [21]. In this study, we conducted several tests at various conidial concentrations, temperatures and relative humidities (RHs) to find effective control condition of the two selected isolates for commercialization.
MATERIALS AND METHODS
Beet armyworm rearing.
Beet armyworms were obtained from the Crop Protection Division, National Academy of Agricultural Science, Rural Development Administration in South Korea. Larvae were reared on artificial diet (#F9219B, mixing direction; Bio-Serv, San Diego, CA, USA) and maintained at 25 ± 1℃ with a 14 : 10 h (L : D) photoperiod. Newly molted second instar larva (7 days after hatching) were used for bioassays.
Fungal strains and preparation of conidial suspensions.
Two fungal isolates, M. anisopliae FT83 [21] and P. fumosoroseus FG340 isolated from soil of agricultural fields using Tenebrio molitor and Galleria mellonella in Korea, were selected as high pathogenicity isolates to control beet armyworm. These fungi were cultivated at 25 ± 1℃ on potato dextrose agar (PDA) medium for 14 days. Conidia were harvested by adding 5 mL of sterilized 0.05% Tween 80 and scraping with spreader. The suspensions were vortexed for 3 min and then filtered through four layers of sterilized cheese cloth. Conidial concentrations were measured using a hemacytometer. The suspensions were diluted to a range of concentrations for each bioassay.
Bioassay at various conidial concentrations, temperatures and RHs.
Chinese cabbages for the bioassays were grown in a greenhouse for approximately 30 days. Leaf discs (9 cm diameter) were placed in a 90-mm-diameter insect-breeding dish. Six hundred microliters of conidial suspension at 6 different concentrations (1 × 104, 1 × 105, 1 × 106, 1 × 107, 1 × 108, and 1 × 109 conidia/mL) of M. anisopliae FT83 and P. fumosoroseus FG340 was sprayed onto each sides of the leaf disc infested 10 second instar larvae of beet armyworm inside the breeding dishes using a Plexiglass spray box (90 × 90 × 90 cm). The spray box was installed a polyvinyl acetal cone nozzle (1.5 mm diameter) on the top layer and the nozzle was connected to a vacuum pump which was fixed at 100 kPA. To avoid cross contamination among the sprayed isolates, the sprayer head and line were rinsed with 1 mL of 70% ethyl alcohol and 0.05% Tween 80. After air drying, dishes containing the leaf discs + larva were incubated in a Plexiglass cage at 25 ± 1℃ with > 90% RH and a 16L : 8D photoperiod. Mortality was recorded daily for six days, and dead insects were transferred to Petri dishes with dampened filter paper. Mycosis cadavers were counted daily for 1 wk. Spore viability was measured by inoculating 100 µL drops of spore suspension (106 conidia/mL) onto 1.5% water agar in 35-mm-diameter Petri dish, and incubating for 24-hr incubation at 25℃, as described by Goettel and Inglis [22]. The spore viabilities were 93.4% and 53.6% for P. fumosoroseus FG340 and M. anisopliae FT83, respectively. Each bioassay was conducted three different times. There are 3 replicate dishes with 30 larvae per treatment in each trial.
To study the effect of temperature and RH on insect mortality, dishes treated with M. anisopliae FT83 and P. fumosoroseus FG340 (1 × 108 conidia/mL) were incubated at different temperatures (15℃, 20℃, 25℃, 30℃, and 35℃) and different RH (45%, 75%, 85%, and 95%) at 25℃ using the bioassay conditions described above. Constant humidities of 45%, 75%, 85%, and 95% were achieved with saturated solutions of potassium carbonate, sodium chloride, potassium chloride and potassium sulphate, respectively [22]. When the leaf disc of Chinese cabbage sprayed with the conidia suspension dried or were entirely consumed, the larvae were provided artificial diet.
Statistical analysis.
Normally distributed data were compared using one-way ANOVA (Proc GLM; SAS ver. 9.2, 2010, SAS Institute Inc., Cary, NC, USA). Median lethal times (LT50) were estimated using the LIFEREG procedure, and the data were fitted to a Weibull distribution (SAS ver. 9.2). Goodness of fit was estimated using the Pearson chi-squared test.
RESULTS
Infection symptoms. Cardaver infected with the isolate M. anisopliae FT83 was covered with white mycelia 3 days after treatment and changed to dark green color by conidia from creamy white mycelia 5 days after treatment. Infection with P. fumosoroseus FG340 caused noticeable hyphal growth on the surface of cuticle 3 days after treatment, and the cadavers covered by white to brown colored conidia 5 days after treatment (Fig. 1).
Fig. 1.
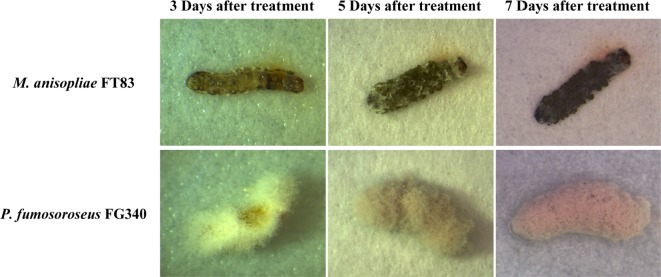
Symptoms of cadavers of Spodoptera exigua infected by Metarhizium anisopliae FT83 and Paecilomyces fumosoroseus FG340. Cadaver infected by M. anisopliae FT83 were covered with mycelia 3 days after treatment and changed to dark green color by conidia from creamy white mycelia 5 days after treatment. Infection with P. fumosoroseus FG340 caused noticeable hyphal growth on the surface of cuticle 3 days after treatment, and the cadaver covered with conidia from white to brown color 5 days after treatment.
Virulence of entomopathogenic fungi.
Larval mortality with M. anisopliae FT83 and P. fumosoroseus FG340 differed significantly at different conidial concentrations: the mortality caused by each fungus increased with conidial concentration. Mortality by M. anisopliae FT83 was 49.8%, 65.3%, 85.8%, 100%, 100%, and 100% at 1 × 104, 1 × 105, 1 × 106, 1 × 107, 1 × 108, and 1 × 109 conidia/mL, respectively (F = 40.93, df = 6, 76, p < 0.0001). The mortality caused by P. fumosoroseus FG340 was 100% all concentrations from 1 × 104 conidia/mL to 1 × 109 conidia/mL (F = Infty, df = 6, 35, p < 0.0001) (Table 1). P. fumosoroseus FG340 showed higher mortality (100% 6 days after treatment) than about 50% of M. anisopliae FT83 at 1 × 104 conidia/mL. The median lethal time (LT50) of S. exigua larvae at low concentration from 104 to 106 was shorter by P. fumosoroseus FG340 as 4.2, 3.8, and 3.2 days compared to 5.7, 4.7, and 3.6 days by M. anisopliae FT83, but above 107 LT50 value was reverse. The median lethal times decreased with increase in conidial concentration.
Table 1.
Mortality of second instar larvae of Spodoptera exigua 6 days after treatments of different concentrations of Metarhizium anisopliae FT83 and Paecilomyces fumosoroseus and their median lethal time (LT50)
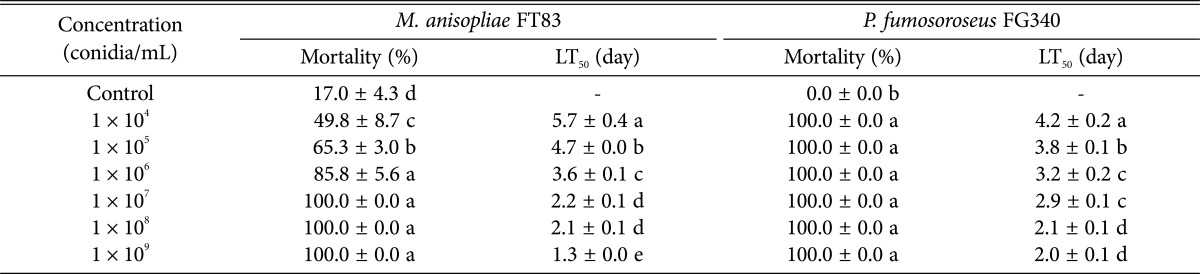
Values are prersented as mean ± SE. Means within the same column followed by the same letter are not significantly different using Duncan's multiple range test within the same column.
Efficacy of entomopathogenic fungi at different temperatures and humidities.
Mycelial growth of M. anisopliae FT83 was fastest at 25℃ (32.4 ± 2.0 mm) (F = 282.92, df = 4, 8, p < 0.0001) and P. fumosoroseus FG340 was 30℃ (29.04 ± 0.3 mm) (F = 60.53, df = 3, 32, p < 0.0001) among the tested five different temperatures (Table 2). Mortality caused by M. anisopliae FT83 and P. fumosoroseus FG340 was temperature-dependent and increased from 20 to 30℃ but decreased at 35℃ (Fig. 2). The median lethal time (LT50) of M. anisopliae FT83 was 2.6, 2.2, 2.0, and 27.7 days at 20℃, 25℃, 30℃, and 35℃, respectively, and the LT50 of P. fumosoroseus FG340 was 3.0, 2.5, 2.0, and 79.7 days at 20℃, 25℃, 30℃, and 35℃, respectively. Both fungi showed the highest mortality at 30℃.
Table 2.
Mycelial growth of Metarhizium anisopliae FT83 and Paecilomyces fumosoroseus FG340 at different temperatures after 7-day cultivation on potato dextrose agar media
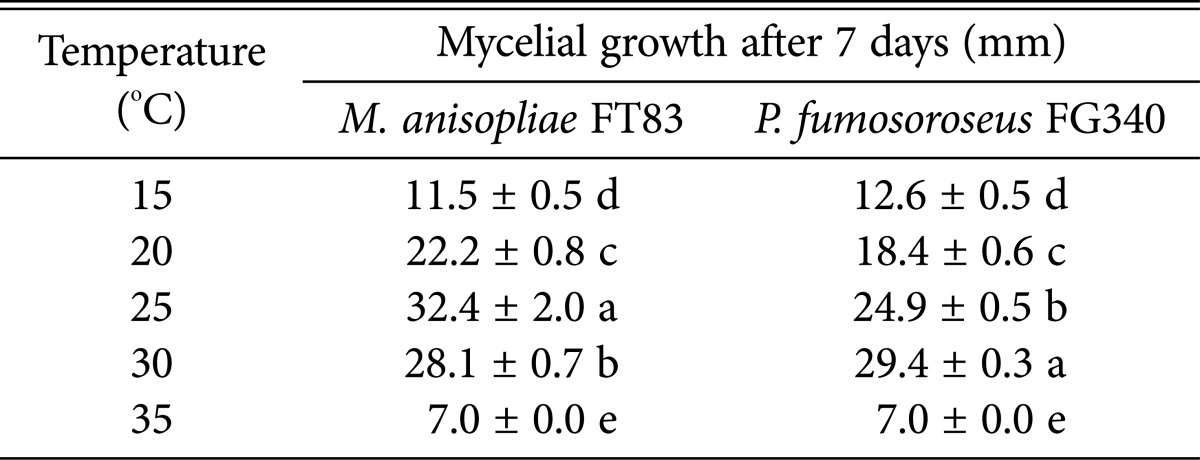
Values are prersented as mean ± SE. Means within the same column followed by the same letter are not significantly different using Duncan's multiple range test.
Fig. 2.
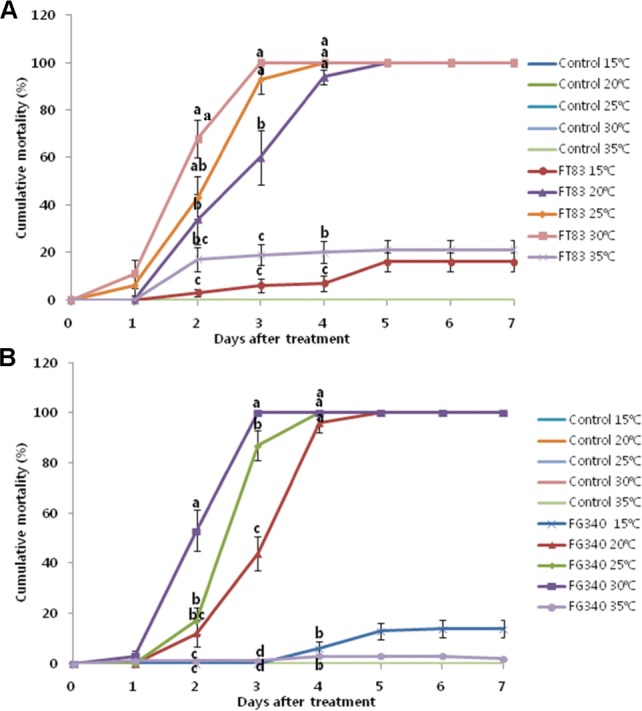
Cumulative mortality of Spodoptera exigua larvae treated with Metarhizium anisopliae FT83 (A) and Paecilomyces fumosoroseus FG340 (B) at different temperatures: 15℃, 20℃, 25℃, 30℃, and 35℃. The conidial concentration used for each treatment was 1 × 108 conidia/mL. Control was treated with 0.01% Tween 80. Means above the line followed by the same letter are not significantly different using Duncan's multiple range test (p > 0.05).
RH significantly affected infection by M. anisopliae FT83. The mortality of larvae treated with M. anisopliae FT83 (1 × 108 conidia/mL) at various RH was higher as RH increases: the mortality was 13.4%, 55.6%, 86.7%, and 97.2% at 45%, 75%, 85%, and 95% RH, respectively. P. fumosoroseus FG340 showed high control effects in whole ranges of RHs we conducted tests from 45% to 95% RHs; mortality of second instar larva of S. exigua treated with P. fumosoroseus FG340 (1 × 108 conidia/mL) at 45%, 75%, 85%, and 95% RH was 90.0%, 100%, 100%, and 100%, respectively (F = 137.7, df = 11, 275, p < 0.0001) (Table 3).
Table 3.
Mortality and LT50 of Spodoptera exigua second instar larvae treated with 1 × 108 conidia/mL of Metarhizium anisopliae FT83 and Paecilomyces fumosoroseus FG340 at different relative humidities
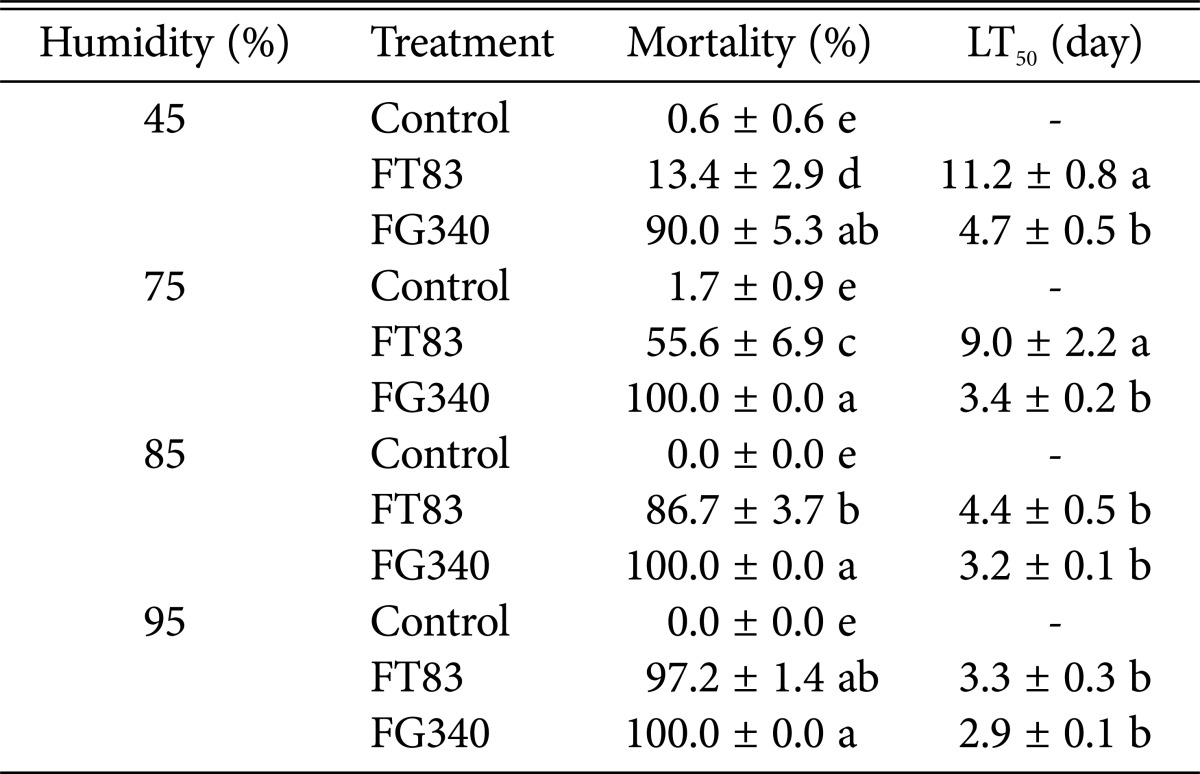
Values are prersented as mean ± SE. Means within the same column followed by the same letter are not significantly different using Duncan's multiple range test.
DISCUSSION
Entomopathogenic fungi are important factors regulating insect populations. M. anisopliae, I. fumosorosea, B. bassiana and Lecanicillium sp. are important natural control agents and sources of mycopecticides for many Noctuidae pests management worldwide [19]. Several studies have examined their potential use as biological control agents. Lin et al. [18] compared the pathogenicity of several fungal species against S. litura. B. brongniartii and N. rileyi showed 100% and 95.2% mortality after treatment with 8 × 107 conidia/mL and LT50 was 3.0 and 4.1 days against larvae of S. litura, respectively. The cumulative mortality of S. litura exposed to I. fumosorosea and B. bassiana was 85.7% and 71.4%, respectively, and the LT50 values were 4.9 and 6.3 days, respectively. Asi et al. [19] also examined the susceptibility of S. litura to M. anisopliae and I. fumosorosea. These two fungi caused mortalities of 53.5% and 41.2%, respectively after 10 days of treatment at a concentration 107 conidia/mL. Dose-mortality assays using these two isolates revealed that the mortality of third instar larvae was 15%, 21%, 52%, and 58% when applied with I. fumosorosea at 105, 106, 107, and 108 conidia/mL, respectively, and 9.3%, 12.0%, 36.2%, and 43.0% with M. anisopliae 10 days after treatment. We observed that M. anisopliae FT83 and P. fumosoroseus FG340 which are used in this study, showed higher mortality than other results. All second instar larvae of S. exigua died after exposure to 107 conidia/mL of M. anisopliae FT83 and 104 conidia/mL of P. fumosoroseus FG340 within 6 days after application.
The pathogenicity of the fungi is primarily mediated by entry through the external larval integument [23]. Conidia attachand germinate on cuticle and penetrate into insect body. Upon entry into the hemocoel, the mycelia grow and spread throughout the whole body and then form hyphae and produce blastospores. Host death often occurs due to a combination of fungal toxins, physical obstruction of blood circulation, nutrient depletion and organ invasion. Efficacy of control agents against insect pests is influenced by abiotic environmental factors such as temperature, RH, water, and solar radiation which have effect on germination, vegetative growth and viability of entomopathogenic fungi. In this study, mycelial growth of the M. anisopliae FT83 and P. fumosoroseus FG340 on PDA media and control efficacy to beet armyworm were affected by temperature but influence of RHs on control efficacy was differed from two isolates.
Several studies have examined the insecticidal activity of metabolites produced by entomopathogenic fungi including M. anisopliae and P. fumosoroseus. Beauvericin, beauverolides, 2,6-pyridinecarboxylic acid and dipicolinic acid are metabolites isolated from P. fumosoroseus [24, 25, 26]. Beauvericin is a cyclic hexadepsipeptide that has insecticidal activity against Aedes aegypti mosquito larvae [27]. Dipicolinic acid is toxic to the blowfly Calliphora erythrocephala [28] and third instar nymphs of Bemisia tabaci type B [29]. The toxic crude proteins produced by I. fumosorosea have insecticidal and antifeedant activity against Plutella xylostella [30]. The toxic crude proteins showed 83.3% mortality of third instar larvae 6 days after treatment. In general, entomopathogenic fungi have low control efficacy in low RH or dry condition such as 45% RH or under 75% RH. For example, L. attenuatum having high pathogenicity against cotton aphid showed 49% mortality at 85% RH but 97% mortality at 97% RH [31]. M. anisopliae FT83 also showed low mortality (13.4%) at 45% RH and high mortality (97.2%) at 95% RH. However P. fumosoroseus FG340 showed high mortality (90%) at 45% RH compared with other fungal isolates. We suppose P. fumosoroseus FG340 at low RH was influenced by direct fungal infection as well as the secondary metabolites for high mortality.
Based on our results, we suggest that M. anisopliae FT83 and P. fumosoroseus FG340 have good potential to develop mycopesticides to control beet armyworm. Furthermore, we will conduct further study to test the control efficacy of these fungal isolates in greenhouses to control S. exigua larva.
ACKNOWLEDGEMENTS
This study was supported by the research grant (PJ00865202) of Rural Development Administration (RDA) of Korea.
References
- 1.Metcalf CL, Flint WP. Destructive and useful insects: their habits and control. 4th ed. San Francisco: McGrawhill; 1962. [Google Scholar]
- 2.Capinera J. Beet armyworm, Spodoptera exigua (Hubner) (Insecta: Lepidoptera: Noctuidae). Publication No. EENY-105 [Internet] Gainesville: IFAS Extension, University of Florida; 2006. [cited 2014 Nov 1]. Available from: http://edis.ifas.ufl.edu/in262. [Google Scholar]
- 3.Kang EJ, Kang MG, Seo MJ, Park SN, Kim CU, Yu YM, Youn YN. Toxicological effects of some insecticides against Welsh onion beet armyworm (Spodoptera exigua) Korean J Appl Entomol. 2008;47:155–162. [Google Scholar]
- 4.Yoshida HA, Parrella MP. Chrysanthemum cultivar preferences exhibited by Spodoptera exigua (Lepidoptera : Noctuidae) Environ Entomol. 1991;20:160–165. [Google Scholar]
- 5.Moulton JK, Pepper DA, Dennehy TJ. Studies of resistance of beet armyworm (Spodoptera exigua) to spinosad in field populations from the southern USA and southeast Asia [Internet] Arizona: University of Arizona College of Agriculture 1999 Vegetable Report; 1999. [cited 2014 Nov 1]. Available from: http://extension.arizona.edu/sites/extension.arizona.edu/files/pubs/az1143_21.pdf. [Google Scholar]
- 6.Meinke LJ, Ware GW. Tolerance of three beet armyworm strains in Arizona to methomyl. J Econ Entomol. 1978;71:645–646. [Google Scholar]
- 7.Chaufaux J, Ferron P. Different susceptibility of two populations of Spodoptera exigua Hub. (Lepid., Noctuidae) to baculoviruses and pyrethroid insecticides. Agronomie. 1986;6:99–104. [Google Scholar]
- 8.Delorme R, Fournier D, Chaufaux J, Cuany A, Bride JM, Auge D, Berge JB. Esterase metabolism and reduced penetration are causes of resistance to deltamethrin in Spodoptera exigua HUB (Noctuidea: Lepidoptera) Pestic Biochem Physiol. 1988;32:240–246. [Google Scholar]
- 9.Brewer MJ, Trumble JT. Beet armyworm resistance to fenvalerate and methomyl: resistance variation and insecticide synergism. J Agric Entomol. 1994;11:291–300. [Google Scholar]
- 10.Laecke VK, Degheele D. Synergism of diflubenzuron and teflubenzuron in larvae of beet armyworm (Lepidoptera: Noctuidae) J Econ Entomol. 1991;84:785–789. [Google Scholar]
- 11.Layton MB. The 1993 beet armyworm outbreak in Mississippi a future management guidelines; Proceedings of Beltwide Cotton Conference; 1993 Jan 13-14; New Orleans, LA, USA. Memphis: National Cotton Council; 1994. pp. 854–856. [Google Scholar]
- 12.Kondo A, Yamanoto M, Takashi S, Maeda S. Isolation and characterization of nuclear polyhedrosis viruses from the beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) found in Shiga, Japan. Appl Entomol Zool. 1994;29:105–111. [Google Scholar]
- 13.Caballero P, Zuidema D, Santiago-Alvarez C, Vlak JM. Biochemical and biological characterization of four isolates of Spodoptera exigua nuclear polyhedrosis virus. Biocontrol Sci Technol. 1992;2:145–157. [Google Scholar]
- 14.Moar WJ, Pusztai-Carey M, Faassen HV, Bosch D, Frutos R, Rang C, Luo K, Adang MJ. Development of Bacillus thuringiensis CryIC resistance by Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae) Appl Environ Microbiol. 1995;61:2086–2092. doi: 10.1128/aem.61.6.2086-2092.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inglis GD, Goettel MS, Butt TM, Strasser H. Use of hyphomycetous fungi for managing insect pests. In: Butt TM, Jackson C, Magan N, editors. Fungi as biocontrol agents: progress, problems and potential. Wallingford: CABI International/AAFC; 2001. pp. 23–69. [Google Scholar]
- 16.Roberts DW, Humber RA. Entomogenous fungi. In: Cole GT, Kendrick B, editors. Biology of conidial fungi. New York: Academic Press; 1981. pp. 201–236. [Google Scholar]
- 17.de Faria MR, Wraight SP. Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control. 2007;43:237–256. [Google Scholar]
- 18.Lin HF, Yang XJ, Gao YB, Li SG. Pathogenicity of several fungal species on Spodoptera litura. J Appl Ecol. 2007;18:937–940. [PubMed] [Google Scholar]
- 19.Asi MR, Bashir MH, Afzal M, Zia K, Akram M. Potential of entomopathogenic fungi for biocontrol of Spodoptera litura Fabricius (Lepidoptera: Noctuidae) J Anim Plant Sci. 2013;23:913–918. [Google Scholar]
- 20.Freed S, Saleem MA, Khan MB, Naeem M. Prevalence and effectiveness of Metarhizium anisopliae against Spodoptera exigua (Lepidoptera: Noctuidae) in southern Punjab, Pakistan. Pak J Zool. 2012;44:753–758. [Google Scholar]
- 21.Han JH, Kim H, Leem HT, Kim JJ, Lee SY. Characteristics and virulence assay of entomopathogenic fungus Metarhizium anisopliae for the microbial control of Spodoptera exigua. Korean J Pestic Sci. 2013;17:454–459. [Google Scholar]
- 22.Goettel MS, Inglis GD. Laboratoy techniques used for entomopathogenic fungi: Hypocreales. In: Lacey LA, editor. Manual of techniques in invertebrate pathology. 2nd ed. Oxford: Academic Publisher; 2012. pp. 189–253. [Google Scholar]
- 23.Zimmermann G. The entomopathogenic fungi Isaria farinosa (formerly Paecilomyces farinosus) and the Isaria fumosorosea species complex (formerly Paecilomyces fumosoroseus): biology, ecology and use in biological control. Biocontrol Sci Technol. 2008;18:865–901. [Google Scholar]
- 24.Bernardini M, Carilli A, Pacioni G, Santurbano B. Isolation of beauvericin from Paecilomyces fumoso-roseus. Phytochemistry. 1975;14:1865. [Google Scholar]
- 25.Jegorov A, Sedmera P, Matha V, Simek P, Zahradnicková H, Landa Z, Eyal J. Beauverolides L and La from Beauveria tenella and Paecilomyces fumosoroseus. Phytochemistry. 1994;37:1301–1303. doi: 10.1016/s0031-9422(00)90402-3. [DOI] [PubMed] [Google Scholar]
- 26.Shima M. On the metabolic products of the silkworm muscardines. Bull Sericult Exp Stn. 1955;14:427–449. [Google Scholar]
- 27.Grove JF, Pople M. The insecticidal activity of beauvericin and the enniatin complex. Mycopathologia. 1980;70:103–105. [Google Scholar]
- 28.Claydon N, Grove JF. Insecticidal secondary metabolic products from the entomogenous fungus Verticillium lecanii. J Invertebr Pathol. 1982;40:413–418. [Google Scholar]
- 29.Asaff A, Cerda-García-Rojas C, de la Torre M. Isolation of dipicolinic acid as an insecticidal toxin from Paecilomyces fumosoroseus. Appl Microbiol Biotechnol. 2005;68:542–547. doi: 10.1007/s00253-005-1909-2. [DOI] [PubMed] [Google Scholar]
- 30.Freed S, Feng-Liang J, Naeem M, Shun-Xiang R, Hussian M. Toxicity of proteins secreted by entomopathogenic fungi against Plutella xylostella (Lpidoptera: Plutellidae) Int J Agric Biol. 2012;14:291–295. [Google Scholar]
- 31.Kim JJ, Kim KC. Selection of a highly virulent isolate of Lecanicillium attenuatum against cotton aphid. J Asia Pac Entomol. 2008;11:1–4. [Google Scholar]


