Abstract
Mitochondrial protein Nfu1 plays an important role in the assembly of mitochondrial Fe-S clusters and intracellular iron homeostasis in the model yeast Saccharomyces cerevisiae. In this study, we identified the Nfu1 ortholog in the human fungal pathogen Cryptococcus neoformans. Our data showed that C. neoformans Nfu1 localized in the mitochondria and influenced homeostasis of essential metals such as iron, copper and manganese. Marked growth defects were observed in the mutant lacking NFU1, which suggests a critical role of Nfu1 in Fe-S cluster biosynthesis and intracellular metal homeostasis in C. neoformans.
Keywords: Cryptococcus neoformans, Fe-S cluster, Metals, Mitochondria, Nfu1
Fe-S clusters are ubiquitous cofactors present in most organisms. Proteins containing the Fe-S cluster play critical roles in various cellular processes, including respiration and regulation of gene expression. Fe-S cluster biosynthesis is highly conserved in prokaryotes and eukaryotes. Three systems are involved in the assembly of Fe-S clusters in prokaryotes. Nitrogen fixation is responsible for the assembly of the Fe-S cluster for nitrogenase in nitrogen-fixing bacteria, and the Fe-S clusters and sulfur-mobilization assembly machineries synthesize Fe-S clusters required for proteins with house-keeping functions and stress response [1, 2, 3]. In eukaryotes, mitochondrial Fe-S cluster assembly, Fe-S cluster export and cytosolic Fe-S cluster assembly systems are three key machineries for Fe-S cluster biosynthesis. Mitochondrial functions of the Fe-S cluster assembly are particularly important for cellular Fe-S cluster biosynthesis, whereas Fe-S cluster export and cytosolic Fe-S cluster assembly systems are only involved in cytosolic Fe-S cluster biosynthesis. In the model yeast Saccharomyces cerevisiae, 14 proteins have been identified to be required for the mitochondrial Fe-S cluster assembly system [4].
Fe-S cluster biosynthesis is not only critical for enzymes required for house-keeping functions but is also involved in virulence in a number of bacterial pathogens, including Shigella flexneri and Erwinia chrysanthemi [5, 6]. Any direct contribution of Fe-S cluster biosynthesis in the virulence of fungal pathogens has not been demonstrated. However, recent studies have shown that Fe-S cluster biosynthesis is mainly regulated by the conserved Hap protein complex, and that deletion of the Hap protein homolog influences the virulence of fungal pathogens Candida albicans, Aspergillus fumigatus, and Cryptococcus neoformans [7, 8, 9].
The basidiomycete fungus C. neoformans is the causative agent of life-threatening cryptococcosis and mostly infects patients who are immunocompromised owing to human immunodeficiency virus infection, cancers or organ transplantation [10, 11]. Like most other microbial pathogens, iron acquisition and regulation play essential roles in survival and the expression of virulence factors of C. neoformans within the host [12, 13]. Transcription factors that are responsible for the regulation of iron acquisition have been identified, and their involvement in controlling the expression of genes required for Fe-S cluster biosynthesis have been suggested [9, 14]. The protein encoded by the gene CNAG_03395 in the genome of C. neoformans var. grubii H99 strain (serotype A) is an example. This protein has a putative function in Fe-S cluster biosynthesis, and its transcript levels were found to be significantly down-regulated in a mutant lacking the gene encoding the iron regulatory protein HapX [9]. The protein encoded by CNAG_03395 is highly homologous to NifU of nitrogen-fixing bacteria. Its C-terminal domain (amino acids 189-249) shows 38% identity to Azotobacter vinelandii NifU, which functions in Fe-S cluster assembly for the maturation of nitrogenase components [15, 16, 17]. S. cerevisiae also possesses the NifU homolog Nfu1, which also shows 38% identity to A. vinelandii NifU. Nfu1 displays synthetic lethality with Ssq1, which is a mitochondrial heat shock protein 70 and is involved in Fe-S cluster assembly [18]. In addition to Nfu1, two other bacterial NifU-like proteins, Isu1 and Isu2, have been found in S. cerevisiae. Furthermore, it has been suggested that Nfu1 localizes in mitochondria, and together with Isu1, plays roles in mitochondrial Fe-S cluster assembly and iron homeostasis in S. cerevisiae [16].
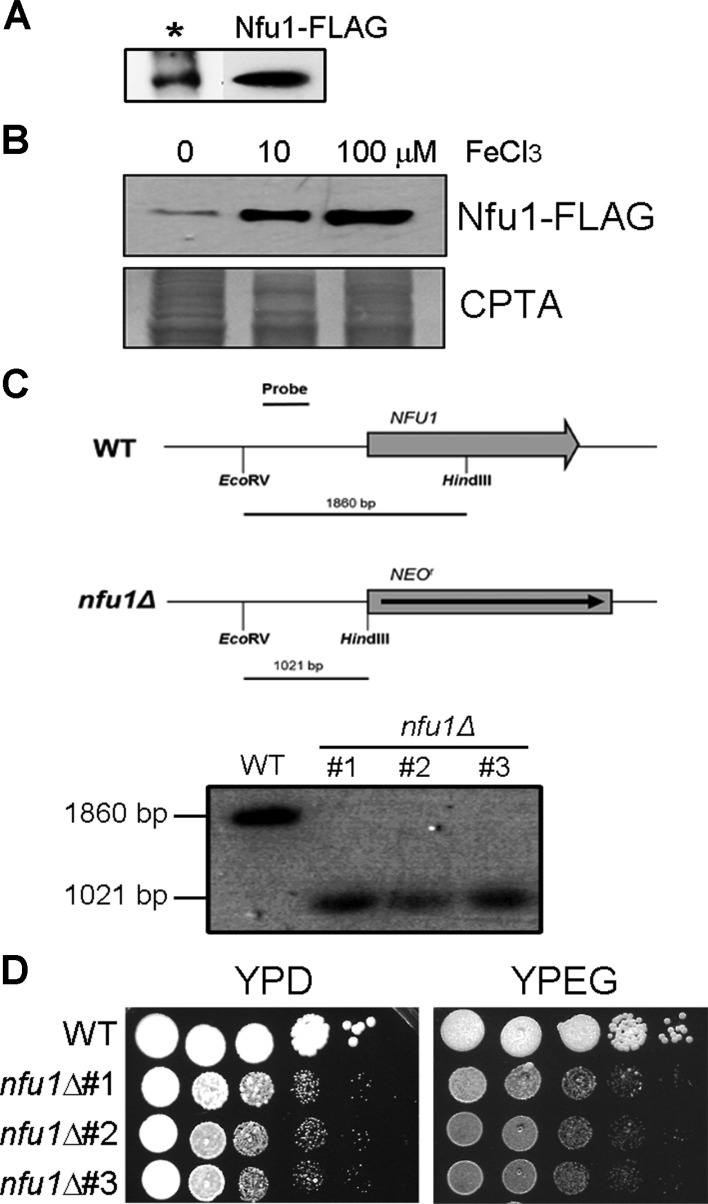
Our previous findings and the importance of Nfu1 in S. cerevisiae led us to characterize a function of the Nfu1 ortholog in C. neoformans. Because S. cerevisiae Nfu1 plays a role in mitochondria, we first investigated the localization of the protein. The C-terminal of Nfu1 was fused with the 3×FLAG epitope tag (DYKDDDDK) and introduced into the C. neoformans var. grubii H99 strain by homologous recombination. Cryptococcal cells expressing the Nfu1-FLAG fusion protein were grown in yeast extract peptone destrose medium, followed by isolation of the mitochondrial fraction as described elsewhere, and western blot analysis was performed using the anti-FLAG antibody [19]. As shown in Fig. 1A, the western blot data using the isolated mitochondrial fraction suggested that Nfu1 localizes in mitochondria in C. neoformans. Moreover, our data revealed that Nfu1-FLAG protein levels are also influenced by iron levels in the medium (Fig. 1B).
Fig. 1.
Nfu1 localized in mitochondria, and the mutant lacking NFU1 showed reduced growth. A, Western blot analysis was performed with isolated mitochondrial fractions using the anti-FLAG antibody. The asterisk indicates the mitochondrial Lys4-FLAG fusion protein, which was used as a reference; B, The abundance of the Nfu1-FLAG protein in the cells grown in medium containing different concentrations of FeCl3 (0, 10, and 100 mM) was evaluated by western blot analysis using the anti-FLAG antibody. The same protein samples were stained with copper phthalocyanine-3,4',4'',4'''- tetrasulfonic acid tetrasodium (CPTA) to show equal loading of each sample. The results of Southern blot analysis; C, Genomic DNA of the wild type and nfu1 mutants were digested with EcoRV and HindIII, and were hybridized with the probe indicated; D, The growth of three independent nfu1 mutants in media containing different carbon sources is shown. Ten-fold serial dilutions of cells (starting at 105 cells) were spotted onto the plates and incubated at 30℃ for 2 days. YPD, yeast extract peptone destrose; YPEG, yeast extract peptone ethanol glycerol.
For functional characterization of Nfu1 in C. neoformans var. grubii H99, a gene-specific deletion cassette was prepared by overlap PCR using primers Nfu1-KO1 (CTCCAGCACAATATATGCCCTGGTTAC), Nfu1-KO2 (AATTCTGCAGATATCCATCACACTGGCGGCCGAAATGGGCGGACATATGGCAATATC), Nfu1-KO3 (AATTCCAGCACACTGGCGGCCGTTACTAGTGGGTTCGCTGGTTTTGCGACCTAATAC), Nfu1-KO4 (GCGACGCCAAACCCATCTTTCACAATATG), Nfu1-KO5 (TCAAGATGTGACCAGCACCGATTGAC) and Nfu1-KO6 (GGAGCCCCAATATCTCCCTTTTTGAC) with genomic DNA and the plasmid pJAF1 containing the neomycin resistance marker as templates [20, 21]. The wild-type strain was biolistically transformed with the amplified gene deletion cassette as described previously [22]. Three independent positive transformants were selected, confirmed by Southern blot analysis and used throughout this study (Fig. 1C). For construction of the strain with reintroduced NFU1, the wild-type NFU1 gene was amplified by PCR using primers Nfu1-Re1 (GGCGACCTCCGAACATTGTAATTGTCATC) and Nfu1-Re2 (GAACAGGAACTCTCGAAGATGGATCAG), cloned into the plasmid pCH233 containing the nourseothricin resistance marker, and transformed into two independent nfu1 mutants. Positive transformants containing wild-type NFU1 at its original locus were identified by PCR and included in this study.
Growth of the nfu1 mutants was first compared with that of the wild-type parental strain. Three independent mutants were viable but showed marked growth defects in the medium containing glucose as a carbon source, suggesting that Nfu1 plays an essential role in the physiology of C. neoformans. Similar growth defects were observed when the mutants grew in medium containing non-fermentable carbon sources glycerol and ethanol (Fig. 1D). These reduced growth phenotypes of the C. neoformans nfu1 mutants differed from the phenotype of the S. cerevisiae nfu1 mutant, which showed wild-type level growth in medium containing either a fermentable or non-fermentable carbon source. In S. cerevisiae, only double deletion of NFU1 and ISU1 caused considerable growth retardation when the cells were grown in medium containing a non-fermentable carbon source [16]. These previous observations and our current data imply that the role of Nfu1 in C. neoformans differs from that of Nfu1 in S. cerevisiae.
Mitochondrial Fe-S cluster synthesis is known to contribute to iron homeostasis. Therefore, we determined iron levels in the nfu1 mutants and compared them with that of the wild type to find if Nfu1 influences iron homeostasis in C. neoformans. Intracellular concentrations of other essential metals such as zinc, manganese and copper were also measured because substantial evidence has suggested that iron uptake and metabolism are linked with other essential metals in fungi [23, 24, 25]. The results from the analysis using inductively coupled plasma-atomic emission spectroscopy showed that the intracellular concentration of iron, manganese and copper were reduced in the nfu1 mutant. Interestingly, however, zinc concentration in the nfu1 mutant was unchanged compared to the wild-type cells (Table 1). These data suggested that while Nfu1 influences not only homeostasis of iron, manganese and copper, zinc homeostasis is independent of Nfu1 function.
Table 1.
Metal content in the nfu1 mutant
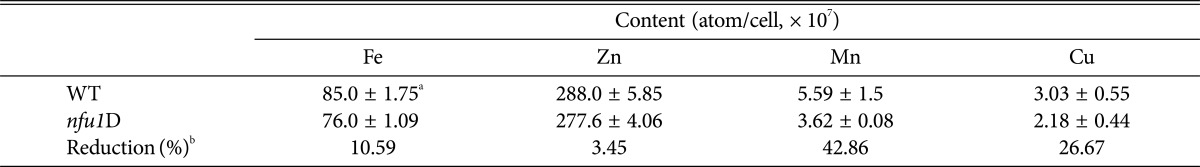
aAll values (atoms per cell) were obtained from three biological replicates and are expressed with standard deviations.
bPercentage reduction from the wild type.
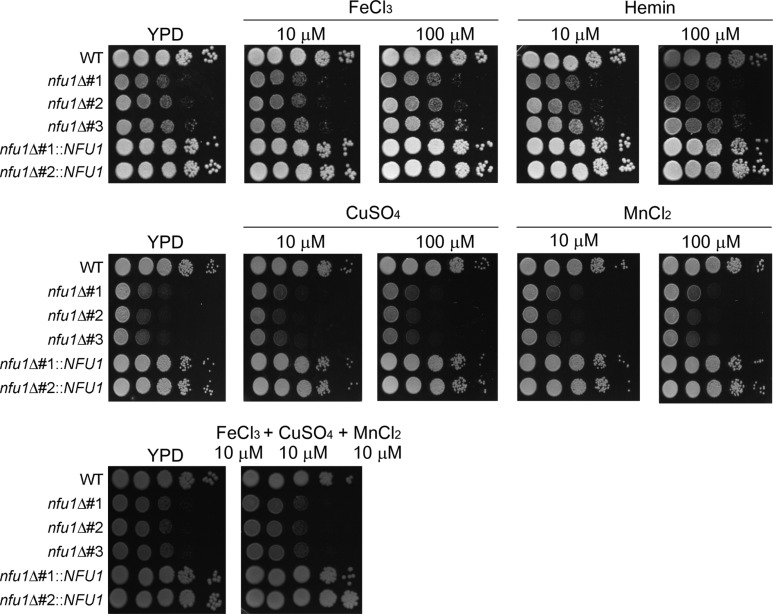
Failure in intracellular homeostasis of iron, copper and manganese causes a detrimental effect in the physiology of fungal cells. Therefore, we attributed the growth defects of the nfu1 mutant to the reduced cellular levels of essential metals and investigated if supplementation of each metal restored the growth of the nfu1 mutant. As shown in Fig. 2, supplementation with individual metal did not restore the growth of the nfu1 mutant. Moreover, addition of iron together with copper and manganese also did not support growth of the nfu1 mutant. These results suggested that growth deficiency of the mutants was caused not only by reduced levels of essential metals but also by other defects such as dysfunctional mitochondrial Fe-S assembly in the cell caused by NFU1 deletion. Mitochondrial functions are of particular interest in fungal pathogens, including C. neoformans. Considerable evidence implies mitochondrial functions in essential metabolic functions such as respiration and lipid biosynthesis, as well as in susceptibility to antifungal drugs [26]. Moreover, the contribution of mitochondrial functions to virulence in the closely related species Cryptococcus gattii has been described [26, 27, 28].
Fig. 2.
Reduced growth of the nfu1 mutants was not restored by metal supplementation. The growth of three independent nfu1 mutants in media containing different essential metals is shown. Hemin was also added as an alternative iron source. Ten-fold serial dilutions of cells (starting at 105 cells) were spotted onto the plates and incubated at 30℃ for 2 days. YPD, yeast extract peptone destrose.
In this study, we identified the ortholog of S. cerevisiae Nfu1, which is involved in the assembly of mitochondrial Fe-S clusters and intracellular iron homeostasis in C. neoformans. Nfu1 localized in mitochondria and influenced the homeostasis of essential metals such as iron, copper and manganese. Unlike S. cerevisiae, significant growth defects were observed in the mutant lacking NFU1. Overall, our results suggest that Nfu1 plays essential roles in Fe-S cluster biosynthesis and intracellular metal homeostasis in C. neoformans.
ACKNOWLEDGEMENTS
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning NRF-2013R1A1A1A05007037.
References
- 1.Zheng L, Cash VL, Flint DH, Dean DR. Assembly of ironsulfur clusters: identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J Biol Chem. 1998;273:13264–13272. doi: 10.1074/jbc.273.21.13264. [DOI] [PubMed] [Google Scholar]
- 2.Frazzon J, Dean DR. Biosynthesis of the nitrogenase ironmolybdenum-cofactor from Azotobacter vinelandii. Met Ions Biol Syst. 2002;39:163–186. [PubMed] [Google Scholar]
- 3.Rees DC, Howard JB. Nitrogenase: standing at the crossroads. Curr Opin Chem Biol. 2000;4:559–566. doi: 10.1016/s1367-5931(00)00132-0. [DOI] [PubMed] [Google Scholar]
- 4.Lill R, Dutkiewicz R, Elsässer HP, Hausmann A, Netz DJ, Pierik AJ, Stehling O, Urzica E, Mühlenhoff U. Mechanisms of iron-sulfur protein maturation in mitochondria, cytosol and nucleus of eukaryotes. Biochim Biophys Acta. 2006;1763:652–667. doi: 10.1016/j.bbamcr.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Runyen-Janecky L, Daugherty A, Lloyd B, Wellington C, Eskandarian H, Sagransky M. Role and regulation of ironsulfur cluster biosynthesis genes in Shigella flexneri virulence. Infect Immun. 2008;76:1083–1092. doi: 10.1128/IAI.01211-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rincon-Enriquez G, Crété P, Barras F, Py B. Biogenesis of Fe/S proteins and pathogenicity: IscR plays a key role in allowing Erwinia chrysanthemi to adapt to hostile conditions. Mol Microbiol. 2008;67:1257–1273. doi: 10.1111/j.1365-2958.2008.06118.x. [DOI] [PubMed] [Google Scholar]
- 7.Schrettl M, Beckmann N, Varga J, Heinekamp T, Jacobsen ID, Jöchl C, Moussa TA, Wang S, Gsaller F, Blatzer M, et al. HapX-mediated adaption to iron starvation is crucial for virulence of Aspergillus fumigatus. PLoS Pathog. 2010;6:e1001124. doi: 10.1371/journal.ppat.1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu PC, Yang CY, Lan CY. Candida albicans Hap43 is a repressor induced under low-iron conditions and is essential for iron-responsive transcriptional regulation and virulence. Eukaryot Cell. 2011;10:207–225. doi: 10.1128/EC.00158-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung WH, Saikia S, Hu G, Wang J, Fung CK, D'Souza C, White R, Kronstad JW. HapX positively and negatively regulates the transcriptional response to iron deprivation in Cryptococcus neoformans. PLoS Pathog. 2010;6:e1001209. doi: 10.1371/journal.ppat.1001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett KH, Kidd SE, Kronstad JW. The emergence of Cryptococcus gattii in British Columbia and the Pacific Northwest. Curr Infect Dis Rep. 2008;10:58–65. doi: 10.1007/s11908-008-0011-1. [DOI] [PubMed] [Google Scholar]
- 12.Kronstad J, Saikia S, Nielson ED, Kretschmer M, Jung W, Hu G, Geddes JM, Griffiths EJ, Choi J, Cadieux B, Caza M, Attarian R. Adaptation of Cryptococcus neoformans to mammalian hosts: integrated regulation of metabolism and virulence. Eukaryot Cell. 2012;11:109–118. doi: 10.1128/EC.05273-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung WH, Do E. Iron acquisition in the human fungal pathogen Cryptococcus neoformans. Curr Opin Microbiol. 2013;16:686–691. doi: 10.1016/j.mib.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Jung WH, Sham A, White R, Kronstad JW. Iron regulation of the major virulence factors in the AIDS-associated pathogen Cryptococcus neoformans. PLoS Biol. 2006;4:e410. doi: 10.1371/journal.pbio.0040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dos Santos PC, Johnson DC, Ragle BE, Unciuleac MC, Dean DR. Controlled expression of nif and isc iron-sulfur protein maturation components reveals target specificity and limited functional replacement between the two systems. J Bacteriol. 2007;189:2854–2862. doi: 10.1128/JB.01734-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schilke B, Voisine C, Beinert H, Craig E. Evidence for a conserved system for iron metabolism in the mitochondria of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1999;96:10206–10211. doi: 10.1073/pnas.96.18.10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson MR, Cash VL, Weiss MC, Laird NF, Newton WE, Dean DR. Biochemical and genetic analysis of the nifUSVWZM cluster from Azotobacter vinelandii. Mol Gen Genet. 1989;219:49–57. doi: 10.1007/BF00261156. [DOI] [PubMed] [Google Scholar]
- 18.Strain J, Lorenz CR, Bode J, Garland S, Smolen GA, Ta DT, Vickery LE, Culotta VC. Suppressors of superoxide dismutase (SOD1) deficiency in Saccharomyces cerevisiae: identification of proteins predicted to mediate iron-sulfur cluster assembly. J Biol Chem. 1998;273:31138–31144. doi: 10.1074/jbc.273.47.31138. [DOI] [PubMed] [Google Scholar]
- 19.Gregg C, Kyryakov P, Titorenko VI. Purification of mitochondria from yeast cells. J Vis Exp. 2009;24:1417. doi: 10.3791/1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson RC, Blankenship JR, Kraus PR, de Jesus Berrios M, Hull CM, D'Souza C, Wang P, Heitman J. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology. 2002;148(Pt 8):2607–2615. doi: 10.1099/00221287-148-8-2607. [DOI] [PubMed] [Google Scholar]
- 21.Yu JH, Hamari Z, Han KH, Seo JA, Reyes-Domínguez Y, Scazzocchio C. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet Biol. 2004;41:973–981. doi: 10.1016/j.fgb.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Toffaletti DL, Rude TH, Johnston SA, Durack DT, Perfect JR. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J Bacteriol. 1993;175:1405–1411. doi: 10.1128/jb.175.5.1405-1411.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasmin S, Abt B, Schrettl M, Moussa TA, Werner ER, Haas H. The interplay between iron and zinc metabolism in Aspergillus fumigatus. Fungal Genet Biol. 2009;46:707–713. doi: 10.1016/j.fgb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Harris ED. Iron-copper interactions: some new revelations. Nutr Rev. 1994;52:311–315. doi: 10.1111/j.1753-4887.1994.tb01462.x. [DOI] [PubMed] [Google Scholar]
- 25.Chang A, Fink GR. Metal ion metabolism: the copper-iron connection. Curr Biol. 1994;4:532–533. doi: 10.1016/s0960-9822(00)00116-0. [DOI] [PubMed] [Google Scholar]
- 26.Shingu-Vazquez M, Traven A. Mitochondria and fungal pathogenesis: drug tolerance, virulence, and potential for antifungal therapy. Eukaryot Cell. 2011;10:1376–1383. doi: 10.1128/EC.05184-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narasipura SD, Chaturvedi V, Chaturvedi S. Characterization of Cryptococcus neoformans variety gattii SOD2 reveals distinct roles of the two superoxide dismutases in fungal biology and virulence. Mol Microbiol. 2005;55:1782–1800. doi: 10.1111/j.1365-2958.2005.04503.x. [DOI] [PubMed] [Google Scholar]
- 28.Ma H, Hagen F, Stekel DJ, Johnston SA, Sionov E, Falk R, Polacheck I, Boekhout T, May RC. The fatal fungal outbreak on Vancouver Island is characterized by enhanced intracellular parasitism driven by mitochondrial regulation. Proc Natl Acad Sci U S A. 2009;106:12980–12985. doi: 10.1073/pnas.0902963106. [DOI] [PMC free article] [PubMed] [Google Scholar]