Abstract
BACKGROUND
The cause of death in burn patients after 48 hours of hospitalization has been reported to be bacterial infections. Recently, due to the compounds accelerating the healing process and the intense reduction of treatment side effects, medicinal plants are used to cure burn wound infections. This study aims to investigate the medicinal effect of the ethanolic extract of Scrophularia striata on burn wound infection in in-vivo and in-vitro in comparison with silver sulfadiazine (SSD).
METHODS
One hundred and fifty male Sprague Dawley rats were divided into 3 equal groups. A hot plate of 1×1cm was used to create second degree burn wounds. The ethanolic extract of S. striata was provided through percolation method. Group 1 was treated with SSD, group 2 with S. striata, and group 3 was considered as control group. All animals were infected to Pseudomonas aeruginosa. On days 3, 7, 10, 14, and 21 after burn wound injury, the animals were euthanized and were evaluated histologically. The MIC and MBC were determined using the micro dilution method.
RESULTS
The rate of wound healing was significantly greater in S. striata group in comparison to SSD and control groups.
CONCLUSION
S. striata contains was shown to have anti-bacterial and wound healing effects while this effect was significantly more than SSD denoting to its use when needed for burn wounds infected to P. aeruginosa.
Key Words: Scrophularia striata, Wound, Healing, Silver sulfadiazine, Pseudomonas aeruginosa
INTRODUCTION
Burn wound infection is the major cause of disability and mortality affecting all ages groups in both developed and developing countries.1,2 Death in the first 48 hours of hospitalization is caused by organ failures and burn shocks which are dependent upon the type of burn and total body surface area (TBSA). However, the causes of death after 48 hours are nosocomial infections, wound infections, and septicemia.3 Disappearance of the primary defenses provides favorable conditions for implantation and invasion of opportunistic pathogens that can cause infection in a short time.4 The most common infectious bacterium that causes septicemia is Pseudomonas aeruginosa.5
Wound healing is widely discussed in the medical literature. To treat burn wound infections, a variety of common ointments, vaccines and antibiotics are used. Despite high side effects, topical medications like silver sulfadiazine (SSD) are most frequently used in healthcare centers.6,7 In topical burn therapy, SSD was introduced as the gold standard having antibacterial properties too.8 Using conventional antibiotics has led to an increase in multi-drug resistant strains of Pseudomonas aeruginosa, which is one of the most worrying opportunistic factors in nosocomial infections.9
Numerous studies were carried out to develop more sophisticated dressings to expedite healing process and diminish bacterial burden in wounds. Even medicinal plants were introduced in wound healing of burned injuries, traditional forms of medicine, especially herbal products, which have been employed for centuries in Africa and Asia are under scientific investigation for their roles in wound treatment.10-14
In western states of Iran, a plant of Snapdragon with the scientific name of Scrophularia striata Boiss has traditional medical usage. Different extracts of this plant are traditionally used in treating infectious diseases. Pharmaceutical activities ranging from reduced edema, reduced infiltration, and proliferation in connection with T-cells have been studied. The ingredients of this medicinal plant prevent the release of inflammatory factors such as PGE-2, IL-4 and IL-1B. These ingredients are used in treating inflammatory diseases.15,16 Probably, the anti-bacterial effects of this plant are related to its phenolic, flavonoid, and flavonol compounds, the highest amount of which having been isolated in the ethanolic extract. These components are attached to the bacterial outer membrane proteinsand deactivate the matrix metalloproteinase. The research conducted on this subject indicate that the anti-bacterial effect of this plant is considerably higher than that of conventional antibiotics.17-19
Regarding the deadly risks of Pseudomonas infections, long-lasting therapy period, and side effects of treatment with SSD, a therapy based on medicinal plants with multiple anti-bacterial and healing effects can be a suitable alternative.11,14 Regarding to curative properties that have been suggested, we decided to investigate the anti-bacterial and healing effect of the ethanolic extract of the plant S. striata on P. aeruginosa burn wound infection in comparison to SSD in experimental rats.
MATERIALS AND METHODS
One hundred and fifty male Sprague Dawley rats (250±30 grams) from the Laboratory Animal Center of Shiraz University of Medical Sciences, Shiraz, Iran were enrolled. The whole stages of the investigation were completed based on the rules posed by the Animal Care Committee of Veterinary Organization of Iran and confirmed by this committee too. All the rats were kept in separate racks at 22-25˚C, 50% humidity and 12-hour cycle of light–darkness and these were fed ad libitum.
S. striata was collected from the natural habitat of the plant in west of Iran (Ilam Provine) with voucher number of 24998 from the Herbarium Department of the Medicinal Plants Research Center of Shiraz University. Plants were dried out and the ethanolic extract was provided through percolation method, condensed in vacuum by distillation.
The animals were divided into two treatment groups of SSD (Razi Pharmaceutical Company, Iran) and S. striata and one control group. First, the animal was anesthetized using ketamine (100 mg/kg) and xylazine (10 mg/kg). After removing the hair at the animal’s back with a shaver, the skin was washed with cotton and savlon for disinfection. In order to create second-degree burn wounds, a hot plate of 1×1cm was used. The created burn was confirmed by a pathologist based on TBSA.20 All the rats were kept in separate cages. The rats were all divided into 6 equal subgroups. In SSD group, half a gram of the 1% ointment; in S. striata group, 1 ml of the 10% S. striata dilution; and in the control groups, only topical base gel were administered on the wound surfaces.
In this study, a standard strain (ATCC27853) and also a clinical strain of P. aeruginosa isolated from a patient with the infection caused by second-degree burn wounds (from Ghotb-Al-Din Burn Hospital, Shiraz, Iran) were used. From both standard and clinical strains, the fresh standard McFarland dilution 1 [108 colony forming units (CFU)] was provided to be injected to the burn area. To infect the animals, each rat was injected with 0.1 ml of bacterial dilution in the wound area topically. After 24 hours, the wound area was sampled using a sterile swab to confirm P. aeruginosa infection. The bacterial strains and the infection caused by wound were confirmed by a bacteriologist throughout the study.20
To determine the wound healing, two methods of dimension determination and histological evaluation were used. In histological study, the animals were euthanized in time intervals of 14 and 21 days after therapy and tissue samples were taken from the edges of the infected wounds. The sample was then transferred to the laboratory in a 10% neutral-buffered formalin dilution for histological evaluation and investigated in terms of scoring criteria such as epithelialization, collagenization, granulation, inflammation, and neovascularization illustrated by Abramov et al.21
To determine the dimensions of the wound from the day of burn (day 0) to the 21st day, each treatment-infection group in the interval subgroup of 21 day was measured with ruler based on mm in the whole time intervals of the wound (Figure 1). The data entered the EXCEL program and each wound dimension was determined using the horizontal diameter×vertical diameter×Л formula. Using the following formula, the wound healing percentage in each rat was determined:
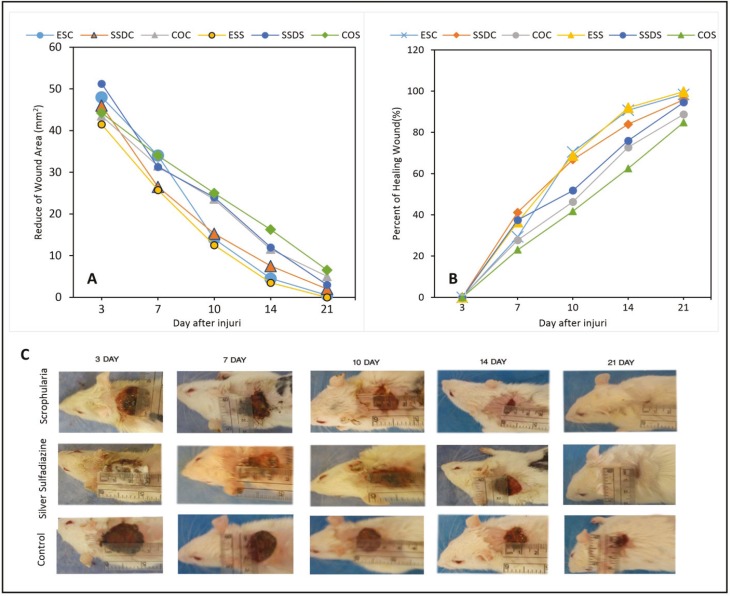
Fig. 1.
A: Comparison of wound healing according to mm2 in different time intervals and various treatment groups of Scrophularia striata, silver sulfadiazine and the control; B: Comparison of the percentage of burn wound healing in different time intervals and various groups; C: The macroscopic wound healing process in treatment groups with respect to the time interval
Treatment percentage=100–wound percentage
To determine the amount of Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC), the modified micro dilution method suggested by NCCLS (National Committee for Clinical Laboratory Standards) was used.22 First, a suspension of both bacterial strains with the density equal to 0.5 McFarland was provided in Trypticase Soy Broth medium (Merck, Homburg, Germany). Then, as a result of continuous dilutions by S. striata and SSD in a density range of 20–200 mg/ml (20, 40, 60, 80, 100, 120, 140, 160, 180, 200), the two strains were heated at a temperature of 37˚C. After overnight incubation, the amount of MIC was determined due to the lowest concentration of anti-microbial agent that entirely inhibited the growth of the bacterium. The amount of MBC was determined by subculturing of the last clear MIC tube on to MacConkey agar and evaluation for bacterial growth. Finally, the results obtained were compared descriptively.23
Statistical analysis was done by SPSS siftware (Version 21, Chicago, IL, USA). Logistic-Regression, Chi-Square, Fisher’s exact and Kruskal-Wallis tests were used to analyze the data. While using the χ2 test was not possible, the Monte Carlo method was used to compare the p values. To compare neovascularization based on time and group, two-way ANOVA was used. Statistical values p<0.05 were considered significant.
RESULTS
The highest increment of regular and complete reepithelialization, particularly in the time periods of 14 and 21 days, was created in S. striata subgroups. This rate was 17% and 24%, respectively, that was higher than that of SSD subgroups (12% and 12.5%, respectively) (Figure 2). The formation of epithelium in the control on the 14th and 21st days was either poor or partial (Figure 2). We found a significant association between the time periods and epithelialization (p<0.001).
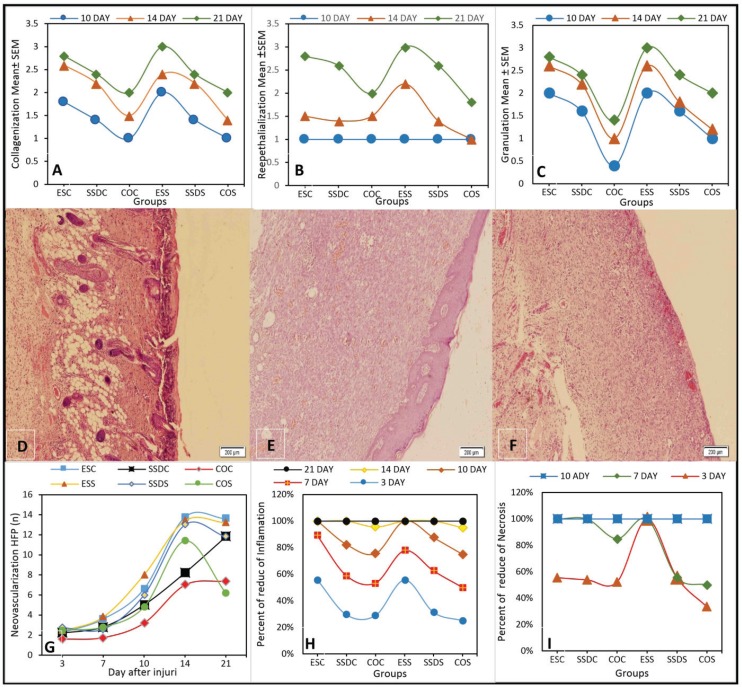
Fig. 2.
A-C: The values of remodeling criteria including collagenization, reepethelialization and granulation in different time intervals of 14, and 21 days and various treatment groups of Scrophularia striata, silver sulfadiazine and the control. D-F: Fourteen days after burn wound infection in various treatment groups of Scrophularia striata, silver sulfadiazine and the control, respectively (H&E×100); G: The increase of HPF (High Power Field) formation with respect to different time periods in various treatment groups of Scrophularia striata, silver sulfadiazine and the control; H and I: Comparison of inflammation and necrosis in various treatment groups of Scrophularia striata, silver sulfadiazine and the control at different time intervals.
The treatment groups and time periods under investigation had a significant relationship with collagenization (p<0.001). The highest collagenization was seen in S. striata subgroupsafter 14 and 21 days (28% and 30%, respectively). This rate was much higher than that of SSD subgroups after 14 and 21 days (12% and 13%, respectively) (Figure 2).
Immature granolation was seen in the control group on the 14th day. The highest amount of granolation in different time periods was seen in the S. striata subgroups. It was only the S. striata group in which moderate mature granolation was seen on the 14th day and full mature on the 21st day (Figure 2). We found a significant relationship for granulation among different groups (p<0.001).
Vessel formation in the wound area in the S. striata subgroups was more than others. This rate was completed in the two S. striata subgroups in a time period of 14 days. A significant relationship was seen between neovascularization and different time intervals and the subgroups (p=0.03). We found a significant association between the neovascularization changes and different time intervals and subgroups (p<0.001) (Figure 2).
In this study, the rate of necrosis and inflammation in the S. striata subgroups was very low, while it was seen in the SSD and control groups until the 14th day. In the S. striata subgroups, no inflammation was seen after 14 days, while it was seen in the control group in the 21st day. Considering the statistical results, there was a significant relationship between inflammation and the groups in the investigation times (p<0.001) (Figure 2). The necrotic changes were significant in different times in various subgroups (p=0.02) (Figure 2).
We found a significant relationship between the healing percentage and time interval and treatment subgroups. Also, the healing rates in different groups were significant in different time intervals (p<0.001). According to the results, wound dimension mean in the treatment subgroups at different time intervals efficiently showed that the decrease rate of wound dimension in the S. striata subgroups was much more than that of the control and SSD subgroups (Figure 1).
Our results indicated that the MIC for S. striata and SSD groups were respectively 45 mg/ml and 90 mg/ml, respectively while the MBC for these two were respectively 55 mg/ml and 100 mg/ml.
DISCUSSION
Severe tissue damage caused by burns results in damage or loss of the tissue involved in some cases. Different organs have different healing powers.24As the skin is exposed to high temperature, the tissue collagen protein loses its spiral mode and turns into gelatin.25The SSD with anti-bacterial effects is widely used in burn centers. In treatment with SSD, the duration of therapy is long and undergoes tissue damages due to the prevention of keratinocyte and fibroblast proliferation and toxicity of these substances to cells.6
Use of conventional antibiotics has resulted into an increase in multi-drug resistant strains of P. aeruginosa, which is one of the most worrying opportunistic factors in nosocomial infections.9
Various studies were done to develop more effective dressings to improve the healing process and decrease the bacterial burden in burn wounds. Even medicinal plants were introduced in wound healing of burned injuries, traditional forms of medicine, especially herbal products, which were employed for centuries are under scientific investigation for their roles in wound healing.10-14
S. striata extract was shown to have antiseptic effects in treating infections caused by gram positive and negative bacteria and virus.15,1719 Our results indicate that unlike SSD, the effect of S. striata extract on P. aeruginosa infection in in-vivo conditions is much more than in-vitro conditions. We found a decrease in infection due to the effects of flavonol and flavonoid compounds of the S. striata extract in combination with cell wall structures or extracellular proteins.19 Our data indicate that epithelial formation was completed in the S. striata group before the 21st day, while irregular epithelialization was seen in the control group in the last time interval.
These results can be due to the presence of iridoid glycoside compounds of S. striata that are capable of fibroblast growth and causing more collagen release and faster healing which is quite in contrast to the cytotoxic effects of SSD.16 In the current study, the degree of necrosis and inflammation in the S. striata group was very low. The anti-inflammatory effect can be due to the presence of phenyl propanoid glycosides seen in S. striata species which inhibited the activities of macrophages and production of chemical mediators and consequently reduced inflammation and subsequently promotes organization.16,26
The value of tissue repair in wounds treated with S. striata is much faster than other groups. In the S. striata group, the epidermis, collagen, hair follicles, and sebaceous gland were organized completely and systematically on the 14th day. Full recovery resulting from healing and anti-microbial properties of this extract led to a complete remission on the 14th day in a way that the decrease in infection reduced the inflammation in the wound area. However, the presence of bacterial infection was a cause of inflammation increase, which resulted into tissue destruction and more inflammation in the wound area.19
Considering our findings, we can suggest that the anti-bacterial effects in synergy with the activating compounds of fibroblasts and keratinocytes were the most important factors in wound healing. The difference between anti-bacterial effects of S. striata and SSD in in-vivo and in-vitro showed the synergistic anti-bacterial and constructive effect of S. striata with respect to SSD. However, SSD had cytotoxic effects along with anti-microbial effects and its use led to an increased length of treatment.24 This study indicated that the ethanolic extract of S. striata with an anti-bacterial effect decreased the inflammation and necrosis in the burn wound areas and lead to collagen release and complete epithelialization in wound healing.
Our results indicated that ethanolic extract of Scrophularia striata could accelerate the wound healing and may be a good candidate for treating infected burn wounds because of strong anti-bacterial and healing effects.
ACKNOWLEDGMENT
This work was supported financially by Shiraz Burn Research Center and the authors wish to thank Dr. Nasrin Shokrpour at Center for Development of Clinical Research of Nemazee Hospital for editorial assistance.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Note
Please cite this paper as:
Tanideh N, Haddadi MH, Rokni-Hosseini MH, Hossienzadeh M, Mehrabani D, Sayehmiri K, Koohi-Hossienabadi O. The Healing Effect of Scrophularia Striata on Experimental Burn Wounds Infected to Pseudomonas Aeruginosa in Rat. World J Plast Surg 2015;4(1):16-22.
References
- 1.Mohammadi AA, Amini M, Mehrabani D, Kiani Z, Seddigh A. A survey on 30 months electrical burns in Shiraz University of Medical Sciences Burn Hospital. Burns. 2008;34:111–3. doi: 10.1016/j.burns.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Pasalar M, Mohammadi AA, Rajaeefard AR, Neghab M, Tolidie HR, Mehrabani D. Epidemiology of burns during pregnancy in southern Iran: Effect on maternal and fetal outcomes. World Appl Sci J. 2013;28:153–8. [Google Scholar]
- 3.Rezaei E, Safari H, Motamedolshariati SM, Afzal Aghaei M. Analysis of mortality in a burn center. J Mashad Univ Med Sci. 2010;52:239–43. [Google Scholar]
- 4.Cakir B, Yegen BC. Systemic responses to burn injury. Turkish J Med Sci. 2004;34:215–26. [Google Scholar]
- 5.Karimi Estahbanati H, Pourkashani P, Ghanaatpisheh F. Frequency of Pseudomonas aeruginosa serotypes in burn wound infections and their resistance to antibiotics. Burns. 2002;28:340–8. doi: 10.1016/s0305-4179(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 6.Cho Lee AR, Leem H, Lee J, Chan Park K. Reversal of silver sulfadiazine-impaired wound healing by epidermal growth factor. Biomaterials. 2005;26:4670–6. doi: 10.1016/j.biomaterials.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 7.Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infectioncontrol and healing: review of the literature. Burns. 2007;33:139–48. doi: 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Hosseini SV, Tanideh N, Kohanteb J, Ghodrati Z, Mehrabani D, Yarmohammadi H. Comparison between Alpha and silver sulfadiazine ointments in treatment of Pseudomonas infections in 3rd degree burns. Int J Surg. 2007;5:23–6. doi: 10.1016/j.ijsu.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Dai T, Huang YY, Sharma SK, Hashmi JT, Kurup DB, Hamblin MR. Topical antimicrobials for burn wound infections. Recent Pat Antiinfect Drug Discov. 2010;5:124–51. doi: 10.2174/157489110791233522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amini M, Kherad M, Mehrabani D, Azarpira N, Panjehshahin MR, Tanideh N. Effect of Plantago major on burn wound healing in rat. J Appl Anim Res. 2010;37:53–6. [Google Scholar]
- 11.Hazrati M, Mehrabani D, Japoni A, Montasery H, Azarpira N, Hamidian-Shirazi AR, Tanideh N. Effect of honey on healing of Pseudomonas aeruginosa infected burn wounds in rat. J Appl Anim Res. 2010;37:106–10. [Google Scholar]
- 12.Hosseini SV, Niknahad H, Fakhar N, Rezaianzadeh A, Mehrabani D. The healing effect of honey, putty, vitriol and olive oil in Psudomonas areoginosa infected burns in experiental rat model. Asian J Anim Vet Adv. 2011;6:572–9. [Google Scholar]
- 13.Tanideh N, Rokhsari P, Mehrabani D, Mohammadi Samani S, Sabet Sarvestani F, Ashraf MJ, Koohi Hosseinabadi O, Shamsian S, Ahmadi N. The healing effect of licorice on Pseudomonas aeruginosa infected burn wounds in experimental rat model. World J Plast Surg. 2014;3:1–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Edraki M, Akbarzadeh A, Hosseinzadeh M, Tanideh N, Salehi A, Koohi-Hosseinabadi O. Healing effect of sea buckthorn, olive oil, and their mixture on full-thickness burn wounds. Adv Skin Wound Care. 2014;27:317–23. doi: 10.1097/01.ASW.0000451061.85540.f9. [DOI] [PubMed] [Google Scholar]
- 15.Bahrami A. The effectiveness of Scrophularia striata on Newcastle disease. Australian J Basic App Sci. 2011;5:2883–8. [Google Scholar]
- 16.Azadmehr A, Afshari A, Baradaran B, Hajiaghaee R, RezazadehS , Monsef-Esfahani H. Suppression of nitric oxide productioin activated murine peritoneal macrophages in vitro and exvivo by Scrophularia striata ethanolic extract. J Ethnopharmacol. 2009;124:166–9. doi: 10.1016/j.jep.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 17.Bahrami A, Valadi A. Effect of Scrophularia ethanolic leaves extracts on Staphylococcus aureus. Int J Pharm. 2010;6:393–6. [Google Scholar]
- 18.Hajiaghaee R, Monsef-Esfahani HR, Khorramizadeh MR, Saadat F, Shahverdi AR, Attar F. Inhibitory effect of aerial parts of Scrophularia striata on matrix metalloproteinases expression. Phytother Res. 2007;21 doi: 10.1002/ptr.2221. [DOI] [PubMed] [Google Scholar]
- 19.Mahboubi M, Kazempour N, Nazar ARB. Total phenolic, total flavonoids, antioxidant and antimicrobial activities of Scrophularia striata Boiss extracts. Jundishapur J Natural Pharmaceut Products. 2013;8:15. [PMC free article] [PubMed] [Google Scholar]
- 20.Manafi A, Kohanteb J, Mehrabani D, Japoni A, Amini M, Naghmachi M, Zaghi AH, KhaliliN Active immunization using exotoxin aconfers protection against pseudomonas aeruginosa infection in a mouse burn model. BMC J Microbiol. 2009;9:23–7. doi: 10.1186/1471-2180-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abramov Y, Golden B, Sullivan M, Botros SM, Miller JJ, Alshahrour A, Goldberg RP, Sand PK. Histologic characterization of vaginal vs. abdominal surgical wound healing in a rabbit model. Wound Repair Regen. 2007;15:80–6. doi: 10.1111/j.1524-475X.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standard, authors. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard Order No M7-A1990; pp. 1-31.
- 23.Sharafati-Chaleshtori R, Rafieian-Kopaei M. Screening of antibacterial effect of the Scrophularia striata against E. coli in vitro. J HerbMed Pharmacol. 2014;3:31–4. [Google Scholar]
- 24.Trudel G, Doherty GP, Koike Y, Ramachandran N, Lecompte M, Dinh L, Uhthoff HK. Restoration of strength despite low stress and abnormal imaging after achilles injury. Med Sci Sports Exerc . 2009;41:2009–16. doi: 10.1249/MSS.0b013e3181a706f0. [DOI] [PubMed] [Google Scholar]
- 25.Anuar NS, Zahari SS, Taib IA, Rahman MT. Effect of green and ripe Carica papaya epicarp extracts on wound healing and during pregnancy. Food Chemical Toxicol. 2008;46:2384–9. doi: 10.1016/j.fct.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 26.Diaz AM, Abadb MJ. Phenylpropanoidglycosides from Scrophularia scorodonia:in vitro anti-inflammatory activity. Life Sci. 2004;74:2515–26. doi: 10.1016/j.lfs.2003.10.008. [DOI] [PubMed] [Google Scholar]